Abstract
Objective
COVID-19 in people with diabetes is associated with a disproportionately worse prognosis. DKA is an acute complication of diabetes with a mortality rate of approximately 0.67%. Little is known about the natural history of DKA in the presence of COVID-19. This study aimed to explore the effects of COVID-19 on presentation, clinical course and outcome in patients presenting with DKA.
Design
Retrospective cohort study.
Methods
All patients treated for DKA between 1 March 2020 and 30 May 2020 were included. Patients were categorised as COVID-positive or COVID-negative based on the swab test. A pre-COVID group was established using data from 01 March 2019 to 30 May 2019 as external control. Data regarding demographics, diabetes type, pH, bicarbonate, lactate, glucose, DKA duration, complications and outcome were collected.
Results
A total of 88 DKA episodes were included in this study. There was no significant difference in the severity or duration of DKA between the three groups. COVID-positive T1DM were more hyperglycaemic on admission compared to COVID-negative and pre-COVID patients. There was an over representation of T2DM in COVID-positive patients with DKA than in pre-COVID or COVID-negative groups.
Conclusion
COVID-19 appears to influence the natural history of DKA differently in T1DM and T2DM. Patients with T1DM and COVID-19 presented with more hyperglycaemia (60 mmol/L (35.9–60.0) vs 31.4 mmol/L (28.0–39.1) vs 24 mmol/L (20.2–33.75), respectively). Patients with T2DM were unusually presenting in DKA when infected with COVID-19 with greater ICU need and higher mortality rates. A collaborative, multi-centre study is needed to provide more definitive results.
Keywords: diabetes, DKA, COVID-19
Introduction
People with diabetes are disproportionately affected by COVID-19. They are more likely to be admitted to the intensive care unit (ICU) compared to those without diabetes (1). A recent UK cohort study has also demonstrated two times greater mortality from COVID-19 for those with type 2 diabetes (T2DM) and 3.5 times in those with type 1 diabetes mellitus (T1DM) compared to those without diabetes (2). Importantly, the majority of these deaths occurred in the elderly, and the absolute risk of death in the lower age groups was small (2).
Diabetic ketoacidosis (DKA) is an acute complication of diabetes characterised by hyperglycaemia, metabolic acidosis and ketosis (3, 4). The management of DKA includes aggressive re-hydration, insulin, and potassium monitoring (3, 5). The mortality of DKA is 0.67% outside the context of COVID-19 (6). Mortality rates associated with DKA in the presence of COVID-19 are unknown. Chamorro–Pareja et al. report a mortality rate of 50% in patients with diabetes and COVID-19, while Alkundi et al. suggest that the development of DKA in patients with diabetes and COVID-19 is associated with a greater survival compared to those who do not develop DKA (7, 8).
Preliminary literature has suggested that an overall higher proportion of those with newly diagnosed T1DM is presented in DKA, with or without COVID-19 (9, 10). This perhaps represents delayed access of healthcare services. However, Beliard et al. found no difference in the proportion of those with newly diagnosed T1DM presenting with DKA between COVID-19 and non-COVID-19 groups (P = 0.81) (9). There remains uncertainty surrounding the clinical presentation and management of DKA in those with COVID-19. Case series have demonstrated that COVID-19 can precipitate DKA in those with pre-existing and undiagnosed diabetes (11, 12, 13). It is clear larger-scale studies are needed to improve insight into the clinical characteristics and relationship between COVID-19 and DKA.
The aim of this study was to explore whether the presentation, clinical course, and outcome of DKA are altered in the presence of COVID-19 and whether there are any differences between patients with T1DM and T2DM.
Methods
We undertook a retrospective cohort study of all patients with DKA in our institution from 01 March 2020 to 30 May 2020. Based on the SARS-coronavirus-PCR test results, patients were categorised into COVID-positive and COVID-negative groups. Further, to enable comparison with non-COVID associated DKA, we collected data on patients admitted with DKA from the corresponding period last year, that is, 01 March 2019 to 30 May 2019. People with other causes of acidosis (i.e. respiratory acidosis associated with hyperglycaemia) and mixed pictures of HHS and DKA were excluded from the study.
For all patients, we recorded demographic data, diabetes type, admission pH, bicarbonate, lactate, and glucose. Additionally, we also collected data on serum electrolytes, urea and creatinine at the time of admission, time to resolution of acidosis, time to resolution of ketosis, need for admission to ICU length of stay and final outcome.
DKA was diagnosed as per Joint British Diabetes Society guidelines in the UK (3). Rates of hypoglycaemia (glucose < 4 mmol/L), hypokalaemia (K+ < 3.5 mmol/L) and hyperkalaemia (K+ > 5.5 mmol/L) during DKA episodes were also recorded. In cases where the same patient was included twice in the data set for separate DKA episodes, the episodes were considered discrete if they were spaced 12 or more hours following biochemical resolution of the original DKA.
The data were analysed using GraphPad Prism Version 6.07. To determine the distribution of baseline characteristics, the Shapiro–Wilk test was used to test for normality. One-way ANOVA was used to compare the differences between groups. P-values provided are two-tailed, and a value of <0.05 was considered as statistically significant. This report is part of an ongoing service improvement programme registered with the department of clinical governance in University Hospitals Birmingham NHS Foundation Trust to improve the care of people with DKA (Clinical Audit Registration and management system number: 12074).
Results
A total of 103 episodes were identified for the study. Nine cases were excluded as they had acidosis from respiratory failure associated with hyperglycaemia. A further six cases were excluded because the outcome for the COVID test was not available. Eighty-eight cases were included in the final analysis. These were further sub-classified into T1DM and T2DM. We are unsure of the type of diabetes in one patient who is currently undergoing further investigation (Table 1). Six COVID positive (all T2DM), eleven COVID negative (eight T1DM, three T2DM), and one pre-COVID (T2DM) had hypertension as a comorbidity. Six patients in COVID-positive and one person in COVID-negative group were on SGLT2 inhibitors; none of the people in the pre-COVID period were on this class of drug.
Table 1.
Characteristics of all patients included in the study analysis.
| COVID positive | COVID negative | Pre-COVID | |
|---|---|---|---|
| All patients | |||
| n | 20 | 31 | 37 |
| T2DM, n | 15/20 | 2/31 | 8/37 |
| Median length of hospitalisation, days (IQR) | 7.0 (5.2–19.5) | 5 (1.9–8.2) | 4.9 (2.3–11.4) |
| Median age, years (IQR) | 59.8 (43.0–69.0) | 50.9 (33.3–60.9) | 31 (24.4–54.2) |
| Male gender, n | 14/20 | 19/31 | 22/37 |
| Median weight, kg (IQR) | 76.2 (51.7–86.1) | 65.4 (58.9–70.7) | 63 (55.1–75.0) |
| Median height, m (IQR) | 1.73 (1.7–1.8) | 1.6 (1.6–1.7) | 1.7 (1.6–1.8) |
| SGLT2 inhibitors, n | 6/20 | 1/31 | 0/37 |
| ACEi/ARB, n | 6/20 | 1/31 | 1/37 |
| ITU admission, n | 6/20 | 4/31 | 0/37 |
| Deaths, n | 4/20 | 1/31 | 0/37 |
| Episodes of hypokalaemia, n (IQR) | 0 (0–0) | 1 (0–4) | 0 (0–0) |
| Episodes of hyperkalaemia, n (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–0) |
| T1DM | |||
| n | 5 | 29 | 29 |
| Median length of hospitalisation, days (IQR) | 4.5 (4.3–4.8) | 5 (1.9–8.0) | 3.6 (2.1–7.8) |
| Median age, years (IQR) | 30.9 (27.0–45.0) | 47.4 (33.2–58.9) | 26.5 (23.9–49.8) |
| Male gender, n | 2/5 | 17/29 | 16/29 |
| Median weight, kg (IQR) | 67.9 (49.4–86.7) | 65.7 (58.5–71.1) | 60.7 (55.1–71.1) |
| Median height, m (IQR) | 1.7 (1.7–1.8) | 1.6 (1.6–1.7) | 1.7 (1.6–1.7) |
| SGLT2 inhibitors, n | 0/5 | 0/29 | 0/29 |
| ACEi/ARB, n | 0/5 | 1/29 | 0/29 |
| ITU admission, n | 2/5 | 4/29 | 0/29 |
| Deaths, n | 0/5 | 1/29 | 0/29 |
| Episodes of hypokalaemia, n (IQR) | 0 (0–2) | 0.5 (0–3) | 0 (0–0) |
| Episodes of hyperkalaemia, n (IQR) | 1 (0–1) | 0 (0–0.75) | 0 (0–0) |
| T2DM | |||
| n | 15 | 2 | 8 |
| Median length of hospitalisation, days (IQR) | 10.0 (6.0–20.0) | 10.8 (7.4–14.2) | 11.2 (8.1–18.7) |
| Median age, years (IQR) | 63.0 (58.8–75.0) | 72.7 (70.9–74.6) | 58.0 (50.5–70.4) |
| Male gender, n | 12/15 | 2/2 | 6/8 |
| Median weight, kg (IQR) | 76.2 (64.8–83.4) | 60.0 (60.0–60.0) | 98.0 (59.4–114.0) |
| Median height, m (IQR) | 1.7 (1.7–1.7) | 1.7 (1.7–1.7) | 1.8 (1.7–1.8) |
| SGLT2 inhibitors, n | 6/15 | 1/2 | 0/8 |
| ACEi/ARB, n | 6/15 | 0/2 | 1/8 |
| ITU admission, n | 4/15 | 0/2 | 1/8 |
| Deaths, n | 4/15 | 0/2 | 0/8 |
| Episodes of hypokalaemia, n (IQR) | 0 (0–0) | 2.5 (1–4) | 0 (0–0.75) |
| Episodes of hyperkalaemia, n (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–0) |
Compared to the other two groups, COVID-positive patients were older, mainly driven by the number of T2DM patients in this group. Across all groups, there was a slight male preponderance. There was also a higher proportion of COVID-positive T2DM on treatment with sodium glucose transporter 2 inhibitors (SGLT2-i) (Table 1).
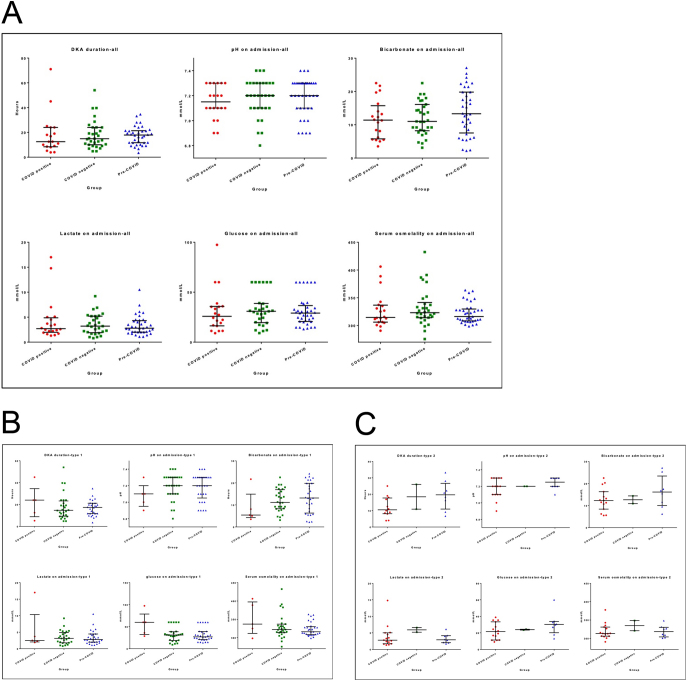
There was no significant difference in the severity of DKA at presentation in terms of pH, bicarbonate, glucose, lactate and serum osmolality. There was also no significant difference between the groups for the DKA duration (Fig. 1A).
Figure 1.
Differences in the duration and severity of DKA (pH, bicarbonate, glucose, lactate and serum osmolality) between three groups in all patients (A), T1DM (B) and T2DM (C).
COVID-positive patients with T1DM were more hyperglycaemic compared to COVID-negative and pre-COVID patients at admission (60 mmol/L (35.9–60.0) vs 31.4 mmol/L (28.0–39.1) vs 24 mmol/L (20.2–33.75), respectively). There was, however, no difference in pH, bicarbonate, lactate, and serum osmolality on admission between the three groups (Fig. 1B). Also, there was no significant difference between the three groups for the fixed rate i.v. Also, there was no significant difference in the fixed rate of i.v. insulin infusion during DKA (COVID positive (n = 6/20): 7.5 units/h (5.6–9.1) vs COVID negative (n = 26/31): 7 units/h (5.6–7.1) vs pre-COVID (n = 37/37): 6 units/h (5.2–7.6)).
In patients with T2DM, no major differences were seen between groups in pH, bicarbonate, glucose, lactate and serum osmolality on admission or duration of DKA (Fig. 1C).
COVID-negative patients had more episodes of hypokalaemia and hyperkalaemia during DKA compared to other two groups (Hypokalaemia: COVID-negative vs COVID-positive group: P => 0.0037; COVID-negative vs pre-COVID group: P = 0.0005; Hyperkalaemia: COVID-negative vs COVID-positive group: P => 0.1026; COVID-negative vs pre-COVID group: P = 0.0043). There was no significant difference between the number of episodes of hypokalaemia in the COVID-positive compared to the pre-COVID group (P > 0.9999). In patients with T2DM, hypokalaemia was more common in COVID-negative patients (COVID-negative vs COVID-positive group: P => 0.0016; COVID-negative vs pre-COVID group: P = 0.0052; COVID-positive vs pre-COVID: P => 0.8602) (Table 1).
Six of the 20 COVID-positive patients and 4 of the 31 COVID-negative patients required admission to ICU. Zero of the 37 patients in the pre-COVID period required admission to ICU. Five patients (1/31 COVID-negative and 4/20 COVID-positive) died during the admission. The COVID-negative patient had T1DM and all four COVID-positive patients who died had T2DM. Of the four T2DM COVID-positive patients, only one had been admitted to ICU. In patients with T2DM, all of the deaths occurred in the COVID-positive group. No deaths were noted in the cohort of patients from the pre-COVID period.
Discussion
Our result did not identify any effect of COVID-19 on the overall presentation or duration of DKA. However, differences emerged when T1DM and T2DM subtypes were analysed individually. Compared to those who were COVID-negative, COVID-positive patients with T1DM presented with significantly higher blood glucose. This perhaps reflects delayed presentation and access to healthcare services, a notion discussed by Beliard et al. and Kamrath et al. (9, 10). They also tended to have a longer recovery time compared to the two other groups. Such differences were not apparent in those with T2DM. However, COVID-positive patients with T2DM were more likely to need ICU with higher mortality rates. There was also a notable increase in DKA in the T2DM patient group – not seen in either of the COVID-negative groups.
A particular strength of our study is that we have compared the presentation and outcomes in COVID-negative patients and also those from pre-COVID period providing a realistic measure of the impact of COVID-19 on the natural history of DKA. Further, we only included those meeting the criteria for DKA and excluded those with hyperosmolar hyperglycaemic state (HHS), mixed HHS and DKA, and acidosis due to other causes. Our study has several limitations. Given that the data represent admissions with DKA over a short period, our sample size was small. Retrospective nature of the study and missing data further limited our ability to undertake extended analyses and explore other associations. The false negative rates in the SARS-coronavirus-PCR test could have further impacted the results of our study (14).
The findings of our study need to be interpreted in the context of available evidences (15, 16, 17, 18). COVID-19 as a trigger for DKA has been reported in a number of studies (19, 20, 21). In contrast, there are very few studies that have reported on the severity or outcomes in patients presenting with DKA. Studies on patients who presented with DKA and COVID-19 have reported significantly prolonged ketosis and duration of DKA for up to 35 h (22, 23). The duration of ketosis in our study was much shorter (15–17 h) and similar in all three groups suggesting the duration of DKA is not impacted by COVID-19 status. The heterogeneity of these results could be due to the differences in the sample sizes and the characteristics of comparator groups. Other factors such as severity of the COVID-19 itself and associated co-morbidities may also have an effect. Moreover, as observed in our cohort, differences may exist between those with T1DM and T2DM and this differentiation needs to be studied further.
Consistent with the observations of Armeni et al. (22), there was an over presentation of T2DM in our cohort. This may be because patients with T2DM are likely to be older and at a greater risk of severe disease with COVID-19. Additionally, it would also reflect the demographics of population served. Of these, six (n => 6/15) of COVID-positive and one (n => 1/2) COVID-negative were on SGLT2-i. The risk of DKA in patients on SGLT2-i is well recognised (24, 25). Current guidance recommends discontinuation of these agents in the setting of acute illness and highly pertinent in COVID-positive patients (26).
As was observed in other studies, proportionately more COVID-positive patients were admitted to ICU with higher mortality compared to the COVID-negative group, particularly in those with T2DM. In comparison with the data from Armeni et al. (22), our mortality rate was higher (n => 4/20 vs n => 1/11). However, it is important to note that these data might not represent the true mortality rate for patients with DKA and COVID-19 given the small sample sizes. Moreover, in our study population, those who were COVID-positive had a median age of 59.8 (43.0–69.0), significantly younger than those people who were most likely to die as demonstrated by Barron et al. (2). Larger studies with bigger cohort sizes are needed to give a better estimate on this rate. Our results also suggest that the higher mortality rate in patients with T1DM infected with COVID-19 is not attributable to DKA.
Careful monitoring of potassium due to the risk of hypokalaemia was also highlighted (23). We did not observe increased rates of kalaemic complications in our DKA and COVID-positive group. On the contrary, COVID-negative cohort had higher rates of hyper- and hypokalaemia compared to COVID-positive and pre-COVID groups. Although we did not observe a significant difference for the insulin requirements during DKA between the three groups, we still feel this is the most plausible explanation for kalaemic changes during DKA and we hope this can be answered in future larger cohorts.
Our study is an important contribution in an area where there is a paucity of information and while the findings of our study are indicative, they are not definitive. On the other hand, our findings highlight a greater need for studies involving larger cohorts and structured data collection. Also, factors such as comorbidity, pre-admission medications and disease control need to be factored in the assess their impact of severity and morbidity associated with DKA. Considering rates of COVID-19 can vary greatly, and therefore the likelihood of DKA presentations also variable, a collaborative approach involving multiple centres is required.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contribution statement
P K conceptualised and coordinated all aspects of the study. E M, L W, L T, D Z, C H, A J K and M A K collected the data. A J N analysed the data. P N, S B supervised the study. All authors contributed towards the design of the study, preparation of first draft and approval of the final draft of the paper as per ICMJE criteria. P K, E M, P N and S B contributed equally to this work.
Acknowledgements
The authors thank all the staff of the Department of Diabetes and Endocrinology, University Hospitals Birmingham NHS Foundation Trust for their help and support for this study. The authors particularly thank Prof Wasim Hanif and Dr Sandip Ghosh for their help and support for the study.
References
- 1.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A.Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetology and Metabolic Syndrome 2020. 14 535–545. ( 10.1016/j.dsx.2020.04.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar Net al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet: Diabetes and Endocrinology 2020. 8 813–822. ( 10.1016/S2213-8587(2030272-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JA, Courtney CH, Hilton L, Dyer PH, Hamersley MS.Joint British Diabetes Societies. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabetic Medicine 2011. 28 508–515. ( 10.1111/j.1464-5491.2011.03246.x) [DOI] [PubMed] [Google Scholar]
- 4.Braatvedt G, Kwan A, Dransfield W, McNamara C, Schauer C, Miller S, Khanolkar M.Differing protocols of managing adult diabetic ketoacidosis outside of the intensive care unit make no difference to the rate of resolution of hyperglycaemia and acidosis. New Zealand Medical Journal 2019. 132 13–23. [PubMed] [Google Scholar]
- 5.Karajgikar ND, Manroa P, Acharya R, Codario RA, Reider JA, Donihi AC, Salata RA, Korytkowski MT.Addressing pitfalls in management of diabetic ketoacidosis with a standardized protocol. Endocrine Practice 2019. 25 407–412. ( 10.4158/EP-2018-0398) [DOI] [PubMed] [Google Scholar]
- 6.Lin SF, Der Lin JD, Huang YY.Diabetic ketoacidosis: comparisons of patient characteristics, clinical presentations and outcomes today and 20 years ago. Chang Gung Medical Journal 2005. 28 24–30. [PubMed] [Google Scholar]
- 7.Alkundi A, Mahmoud I, Musa A, Naveed S, Alshawwaf M.Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in the United Kingdom: a retrospective single centre study. Diabetes Research and Clinical Practice 2020. 165 108263. ( 10.1016/j.diabres.2020.108263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamorro-Pareja N, Parthasarathy S, Annam J, Hoffman J, Coyle C, Kishore P.Letter to the Editor: Unexpected high mortality in COVID-19 and diabetic ketoacidosis. Metabolism: Clinical and Experimental 2020. 110 154301. ( 10.1016/j.metabol.2020.154301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beliard K, Ebekozien O, Demeterco-Berggren C, Alonso GT, Gallagher MP, Clements M, Rapaport R.Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. Journal of Diabetes 2021. 13 270–272. ( 10.1111/1753-0407.13141) [DOI] [PubMed] [Google Scholar]
- 10.Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, Holl RW.Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA 2020. 324 801–804. ( 10.1001/jama.2020.13445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsadhan I, Alruwashid S, Alhamad M, Alajmi S, Alshehri S, Alfadhli E, Ekhzaimy A.Diabetic ketoacidosis precipitated by coronavirus disease 2019 infection: case series. Current Therapeutic Research, Clinical and Experimental 2020. 93 100609. ( 10.1016/j.curtheres.2020.100609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy PK, Kuchay MS, Mehta Y, Mishra SK.Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetology and Metabolic Syndrome 2020. 14 1459–1462. ( 10.1016/j.dsx.2020.07.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft A, Bucca A, Jansen JH, Motzkus C, Herbert A, Wang A, Hunter BR.First-time diabetic ketoacidosis in Type 2 diabetics with Covid-19 infection: a novel case series. Journal of Emergency Medicine 2020. 59 e193–e197. ( 10.1016/j.jemermed.2020.07.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson J, Whiting PF, Brush JE.Interpreting a covid-19 test result. BMJ 2020. 369 m1808. ( 10.1136/bmj.m1808) [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du Ket al. Diabetes is a risk factor for the progression and prognosis of COVID -19. Diabetes/Metabolism Research and Reviews 2020. 36 e3319. ( 10.1002/dmrr.3319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang Met al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Medicine 2020. 8 475–481. ( 10.1016/S2213-2600(2030079-5). Erratum in: Lancet Respiratory Medicine 2020. 8 e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayman G, Lumb A, Kennon B, Cottrell C, Nagi D, Page E, Voigt D, Courtney H, Atkins H, Platts Jet al. Guidance on the management of diabetic ketoacidosis in the exceptional circumstances of the COVID-19 pandemic. Diabetic Medicine 2020. 37 1214–1216. ( 10.1111/dme.14328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppell KJ, Hall RM, Downie M, Fraser SK, Garrett M, Jefferies CA, Kenealy TW, Milne RE, Orr-Walker BJ, Paul RGet al. Diabetes and COVID-19-the meeting of two pandemics: what are the concerns? New Zealand Medical Journal 2020. 133 85–87. [PubMed] [Google Scholar]
- 19.Kim NY, Ha E, Moon JS, Lee YH, Choi EY.Acute hyperglycemic crises with coronavirus disease-19: case reports. Diabetes and Metabolism Journal 2020. 44 349–353. ( 10.4093/dmj.2020.0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A.COVID-19 infection may cause ketosis and ketoacidosis. Diabetes, Obesity and Metabolism 2020. 22 1935–1941. ( 10.1111/dom.14057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma WX, Ran XW.The management of blood glucose should be emphasized in the treatment of COVID-19. Sichuan Da Xue Xue Bao Yi Xue Ban 2020. 51 146–150. ( 10.12182/20200360606) [DOI] [PubMed] [Google Scholar]
- 22.Armeni E, Aziz U, Qamar S, Nasir S, Nethaji C, Negus R, Murch N, Beynon HC, Bouloux P, Rosenthal Met al. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet: Diabetes and Endocrinology 2020. 8 660–663. ( 10.1016/S2213-8587(2030221-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chee YJ, Ng SJH, Yeoh E.Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Research and Clinical Practice 2020. 164 108166. ( 10.1016/j.diabres.2020.108166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Zhang S, Zhang L.Newer perspectives of mechanisms for euglycemic diabetic ketoacidosis. International Journal of Endocrinology 2018. 2018 7074868. ( 10.1155/2018/7074868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration. FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood (accessed 6 Nov 2019), 2015. (available at: https://www.fda.gov/media/92185/download) [Google Scholar]
- 26.Diabetes and COVID-19 – NHS Leeds Clinical Commissioning Group (accessed 18 Aug 2020), 2020. (available at: https://www.leedsccg.nhs.uk/about/covid-19-primary-care/resources-for-professionals/medicines-information/diabetes-and-covid-19/) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.



 This work is licensed under a
This work is licensed under a