Abstract
Background
Supplemental oxygen is frequently administered to patients with acute respiratory distress syndrome (ARDS), including ARDS secondary to viral illness such as coronavirus disease 19 (COVID‐19). An up‐to‐date understanding of how best to target this therapy (e.g. arterial partial pressure of oxygen (PaO2) or peripheral oxygen saturation (SpO2) aim) in these patients is urgently required.
Objectives
To address how oxygen therapy should be targeted in adults with ARDS (particularly ARDS secondary to COVID‐19 or other respiratory viruses) and requiring mechanical ventilation in an intensive care unit, and the impact oxygen therapy has on mortality, days ventilated, days of catecholamine use, requirement for renal replacement therapy, and quality of life.
Search methods
We searched the Cochrane COVID‐19 Study Register, CENTRAL, MEDLINE, and Embase from inception to 15 May 2020 for ongoing or completed randomized controlled trials (RCTs).
Selection criteria
Two review authors independently assessed all records in accordance with standard Cochrane methodology for study selection.
We included RCTs comparing supplemental oxygen administration (i.e. different target PaO2 or SpO2 ranges) in adults with ARDS and receiving mechanical ventilation in an intensive care setting. We excluded studies exploring oxygen administration in patients with different underlying diagnoses or those receiving non‐invasive ventilation, high‐flow nasal oxygen, or oxygen via facemask.
Data collection and analysis
One review author performed data extraction, which a second review author checked. We assessed risk of bias in included studies using the Cochrane 'Risk of bias' tool. We used the GRADE approach to judge the certainty of the evidence for the following outcomes; mortality at longest follow‐up, days ventilated, days of catecholamine use, and requirement for renal replacement therapy.
Main results
We identified one completed RCT evaluating oxygen targets in patients with ARDS receiving mechanical ventilation in an intensive care setting. The study randomized 205 mechanically ventilated patients with ARDS to either conservative (PaO2 55 to 70 mmHg, or SpO2 88% to 92%) or liberal (PaO2 90 to 105 mmHg, or SpO2≥ 96%) oxygen therapy for seven days.
Overall risk of bias was high (due to lack of blinding, small numbers of participants, and the trial stopping prematurely), and we assessed the certainty of the evidence as very low. The available data suggested that mortality at 90 days may be higher in those participants receiving a lower oxygen target (odds ratio (OR) 1.83, 95% confidence interval (CI) 1.03 to 3.27). There was no evidence of a difference between the lower and higher target groups in mean number of days ventilated (14.0, 95% CI 10.0 to 18.0 versus 14.5, 95% CI 11.8 to 17.1); number of days of catecholamine use (8.0, 95% CI 5.5 to 10.5 versus 7.2, 95% CI 5.9 to 8.4); or participants receiving renal replacement therapy (13.7%, 95% CI 5.8% to 21.6% versus 12.0%, 95% CI 5.0% to 19.1%). Quality of life was not reported.
Authors' conclusions
We are very uncertain as to whether a higher or lower oxygen target is more beneficial in patients with ARDS and receiving mechanical ventilation in an intensive care setting. We identified only one RCT with a total of 205 participants exploring this question, and rated the risk of bias as high and the certainty of the findings as very low. Further well‐conducted studies are urgently needed to increase the certainty of the findings reported here. This review should be updated when more evidence is available.
Plain language summary
Approaches to guiding oxygen therapy in adult intensive care patients with acute respiratory distress syndrome
Background
Acute respiratory distress syndrome (ARDS) is a very severe breathing problem with a high mortality rate (chance of dying). It has many potential causes, including viral infections such as COVID‐19, and there are no specific treatments for it except for giving patients oxygen via a ventilator (artificial breathing machine) on an intensive care unit, often for long periods of time. However, large amounts of oxygen (either a high concentration of oxygen or oxygen administered for a long period of time) are associated with increased harm due to other illnesses (e.g. heart attack or stroke).
What did we want to find out?
We wanted to know whether patients with severe lung problems (ARDS) would do better (including less chance of dying) if they received higher or lower amounts of oxygen whilst they were on a ventilator in intensive care.
Methods
We searched major medical databases up to 15 May 2020 for clinical trials studying oxygen use in adult patients with ARDS in intensive care units. We only searched for studies with the sickest patients, that is those who needed help with their breathing through a breathing tube that was connected to an artificial breathing machine. We did not restrict the search by language of publication.
In addition to extracting and analysing the data from any studies that met these criteria, we also assessed the risk of bias (fairness) and the certainty (confidence) of the findings.
Results
We included only one study (205 participants) in the review. Patients with ARDS and receiving oxygen through a breathing tube in an intensive care unit may have a higher chance of death if they receive lower amounts of oxygen compared to receiving much higher amounts of oxygen, but the evidence is very uncertain.
Certainty of evidence
Our certainty (confidence) in these findings is very low as data were only available from one study that had only a small number of participants, and was stopped earlier than anticipated because of safety concerns. We are therefore unable to definitively say whether giving more or less oxygen to ARDS patients is helpful.
Summary of findings
Summary of findings 1. Oxygen targets in the intensive care unit during mechanical ventilation for acute respiratory distress syndrome.
| Patients or population: adult (≥ 18 years of age) patients receiving mechanical ventilation (via either an endotracheal tube or a tracheostomy) for acute respiratory distress syndrome (ARDS) (Berlin definition), including ARDS secondary to COVID‐19 or other viruses Settings: intensive care units in: France Intervention: ‘conservative’ oxygen target: PaO2 55 ‐ 70 mmHg or SpO2 88 ‐ 92% Comparison: ‘liberal’ oxygen target: PaO2 90 ‐ 105 mmHg or SpO2 ≥96% | |||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participant (studies) | Certainty of the evidence (GRADE) | |
| Liberal oxygen target | Conservative oxygen target | ||||
| Mortality at longest follow‐up (follow‐up: 90 days) | 304 per 1,000 | 444 per 1000 (310 to 588) | OR 1.83 (1.03 to 3.27) | 201 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b |
| Number of days ventilated | Mean number of days ventilated was 14.5 days | MD 0.5 days fewer (0.98 fewer to 0.02 fewer) | ‐ | 201 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b |
| Requirement for inotropic support (scale from 0 to 28, days of catecholamine use) | Mean duration of catecholamine use was 7.2 days | MD 0.8 days more (0.52 more to 1.08 more) | ‐ | 201 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b |
| Requirement for renal replacement therapy (follow‐up: 6 days) | 98 per 1,000 | 101 per 1,000 (43 to 220) | OR 1.03 (0.41 to 2.60) | 201 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b |
| Quality of life | ‐ | ‐ | ‐ | 0 | ‐ |
| CI: confidence interval; MD: mean difference; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded due to serious concerns about risk of bias. High risk of selection bias (open‐label and unblinded) and early stopping bias (stopped prematurely). bDowngraded two levels due to very serious concerns about imprecision. Only one study with a low overall total number of participants included in the review.
Background
Brief description of the condition/issue under consideration
COVID‐19, an acute respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), can cause acute respiratory failure with persistent hypoxaemia. A surge in demand for mechanical ventilation has resulted from the international spread of this novel virus, which has stretched and exceeded national critical care capacity in some countries (Grasselli 2020). Research on oxygen therapy in critically unwell ventilated patients is limited and conflicting. A previous Cochrane Review concluded that considerable uncertainty remains about how oxygen therapy should be targeted in all patients admitted to intensive care units; however, this review looked at all methods of oxygen therapy (including non‐invasive ventilation) for any cause of critical illness, including traumatic brain injury, chronic obstructive pulmonary disease (COPD), and out‐of‐hospital cardiac arrest (Barbateskovic 2019). Such a diverse and varied group of pathologies are likely to respond best to a variety of different ventilation and oxygenation strategies; COVID‐19‐induced lung disease is itself a novel and unusual cause of respiratory failure and one that has already been noted to behave differently to previously considered critical illness clinical syndromes (Gattinoni 2020; Roberts 2020). Acute respiratory distress syndrome (ARDS, the new term for severe acute lung injury), which can be secondary to viral illnesses such as COVID‐19 or other non‐viral causes, is associated with very high morbidity and mortality. A number of therapeutic agents have been trialled without successfully improving long‐term outcomes, including aerosolised prostacyclins (Afshari 2017), inhaled nitric oxide (Afshari 2010), corticosteroids and surfactants (Lewis 2019), and no specific treatments are currently available or in widespread clinical use. Furthermore, these patients often require prolonged durations of mechanical ventilation and oxygen therapy, which also both carry their own risks (Andersen 2016). Despite previous reviews studying oxygen use in the critical care unit, it remains unclear how best to target oxygen administration in mechanically ventilated patients with ARDS, or in the subgroup of these patients with viral‐induced lung injury (such as COVID‐19).
Description of the intervention
Patients with respiratory failure severe enough to necessitate mechanical ventilation also require supplementary oxygen to treat their hypoxaemia. The amount of oxygen administered may be described either as a percentage or as a fraction of the inspired gas mixture, for example a patient being administered normal room air would receive approximately 21% oxygen or FiO2 (fraction of inspired oxygen = 0.21). The clinical team will titrate the FiO2 being administered to achieve a particular level of oxygenation in the patient’s blood, usually by targeting either continuously monitored peripheral oxygen saturations (SpO2) or intermittent measurement of arterial partial pressures of oxygen (PaO2), which can both be easily monitored in the intensive care unit.
How the intervention might work
The body has no way of storing oxygen, so even short periods of hypoxaemia can rapidly cause irreversible harm, including organ failure (e.g. hypoxic brain injury, stroke, myocardial infarction, acute kidney injury) or death. Clinicians have historically tended towards administering more oxygen to avoid these risks (Leach 2002). However, hyperoxaemia is also increasingly recognized as being associated with more complications (particularly pulmonary complications) and worse outcomes in a number of other clinical contexts, including critical illness, cardiac disease (e.g. myocardial infarction), neonatal resuscitation, and stroke (Martin 2013). As well as its direct effects on the lung (e.g. absorption atelectasis or fibrosis), hyperoxaemia is thought to increase systemic production of reactive oxygen species (ROS) and pro‐inflammatory cytokines, possibly worsening outcomes in pro‐inflammatory conditions such as viral illnesses that are thought to induce a ‘cytokine storm’ (including COVID‐19). This is also one potential mechanism by which coronavirus‐induced ARDS might respond differently to other forms of ARDS. Although supplemental oxygen administration is now recognized as being a double‐edged sword in critically ill patients with ARDS, the optimal oxygenation target (i.e. maximizing benefits whilst minimizing the harms of both hypoxia and hyperoxia) in these patients is unknown. It is also unknown whether the optimal oxygen regimen depends on the underlying aetiology of the ARDS.
Objectives
To address how oxygen therapy should be targeted in adults with ARDS (particularly ARDS secondary to COVID‐19 or other respiratory viruses) and requiring mechanical ventilation in an intensive care unit, and the impact oxygen therapy has on mortality, days ventilated, days of catecholamine use, requirement for renal replacement therapy, and quality of life.
Methods
Criteria for considering studies for this review
Prespecified eligibility criteria were as follows.
Study design
Randomized controlled trials, including cluster‐randomized and cross‐over trials.
Minimum study duration
There was no minimum study duration.
Population
We included studies looking at adult (≥ 18 years of age) patients admitted to an intensive care unit or other level 3 area and receiving mechanical ventilation (via either an endotracheal tube or a tracheostomy) for ARDS (Berlin definition), including ARDS secondary to COVID‐19 or other viruses.
We excluded studies where participants received non‐invasive ventilation, high‐flow nasal oxygen, hyperbaric oxygen, cardiac bypass, or extra‐corporeal membrane oxygenation (ECMO).
Intervention
‘Liberal’ oxygen, defined as relative to a comparable ‘conservative’ or control group to maximize inclusion of studies. This could consist of targeting higher SpO2, PaO2, or any combination of these where the study intention was to compare groups receiving different amounts of oxygen, in the opinion of the review authors.
Comparator(s)
‘Conservative’ oxygen, that is the study intention (in the opinion of the review authors) was to compare giving a lower amount of oxygen to this group than the intervention arm in the same trial. This may have been targeting mild hyperoxaemia (but relatively less hyperoxaemia than the interventional group in a particular study), normoxaemia, or hypoxaemia.
Outcome(s)
Critical outcome measures
Mortality at longest available follow‐up
Number of days ventilated
Requirement for inotropic support
Requirement for renal replacement therapy
Quality of life (any recognized scale as reported by the trialists)
We included studies in the review irrespective of whether measured outcome data were reported in a ‘useable’ way.
Search methods for identification of studies
We adhered to the following methods prespecified in the protocol (see Appendix 1): an Information Specialist (Janne Vendt) designed and conducted all searches, which were informed by the authors as content experts and independently peer reviewed by a second Information Specialist (Robin Featherstone).
Electronic databases
We searched the Cochrane COVID‐19 Study Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase from inception to 15 May 2020 (Appendix 2).
Other searches
We handsearched bibliographic references from the included studies without any language restrictions.
Screening
Two review authors (AC, AO) with expertise in systematic reviewing independently screened all titles and abstracts for potential eligibility and reviewed the full texts for inclusion in the review. Any discrepancies were resolved by consensus. A third review author was available for consultation if consensus could not be reached, but this was not required.
We recorded the reasons for exclusion for all studies excluded after full‐text review (see Characteristics of excluded studies).
Inclusion of non‐English language studies
We considered abstracts and full texts from published articles in any language for inclusion.
We planned for the methods of all potentially eligible non‐English language abstracts to be translated for screening, and for the full texts of any abstracts that progressed to full‐text review to be translated; however, this was not necessary.
Data collection and analysis
We adhered to the following methods as prespecified in the protocol (see Appendix 1).
Data management
One review author (AC) extracted data from the included studies into Review Manager (RevMan) Version 5.3, which another review author (AO) independently checked for accuracy.
Data extraction
We extracted the following information.
Study design (including methods, location, sites, groups)
Setting
Participant characteristics (age, gender, and disease severity using an appropriate critical illness score, e.g. Sequential Organ Failure Assessment (SOFA), which is a widely validated score that has been developed to assess the acute morbidity of critical illness) (Lambden 2019)
Intervention characteristics (PaO2, SpO2 or FiO2 used in each study to set interventional group targets)
Comparator characteristics
Outcomes assessed
Numerical data for outcomes of interest
'Risk of bias' assessment
Two review authors (AC, AO) used the Cochrane 'Risk of bias' tool to independently assess risk of bias of the included studies based on the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants, personnel, and outcome assessors (performance and detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
Other potential sources of bias
Any discrepancies would have been resolved by discussion or by involving a third review author where required.
Contacting study authors
It was not necessary to contact study authors for missing data, as all data required to complete the review were available within the published trial reports and supplementary files.
Measures of treatment effect
We assessed continuous outcomes as mean difference (MD) with 95% confidence intervals (CI). If continuous outcome data were reported using different scales, we assessed these outcomes as standardized mean differences (SMD) and 95% CI. We assessed dichotomous outcomes as odds ratios (ORs) with 95% CIs.
Assessment of heterogeneity
We had planned to inspect forest plots and use the I2 statistic to quantify possible heterogeneity (I2 statistic > 50% to signify substantial heterogeneity), but this was not possible due to the small number of included studies.
Assessment of reporting biases
We did not formally investigate assessment of reporting bias due to the small number of included studies.
Data synthesis
We had planned to combine studies with a random‐effects model; however, this could not be performed due to the lack of available data.
Subgroup analyses
We did not perform subgroup analysis because we found insufficient studies to do so. If appropriate data had been available (i.e. multiple included studies with at least one relevant to the appropriate comparison group), we would have performed a subgroup analysis for any studies specifically reporting on viral‐induced ARDS.
Sensitivity analyses
A sensitivity analysis was not planned for this review.
GRADE
We employed the GRADE approach to interpret findings, and used GRADEpro GDT to create 'Summary of findings' tables as suggested in the Cochrane Handbook for Systematic Reviews of Interventions when results of randomized controlled trials are available (Schünemann 2019). These tables provide outcome‐specific information concerning the overall quality of evidence from the included study. We used this approach to assess the certainty of all reported outcomes.
Results
Description of studies
Results of the search
The initial searches yielded a total of 5125 results after removal of 405 duplicates. Following title and abstract review, three potentially eligible records were identified. On reviewing the full texts of these records, two were excluded for not meeting the inclusion criteria (see Characteristics of excluded studies). Consequently, only one study was eligible for inclusion in the review (see Characteristics of included studies). The study flow diagram is shown in Figure 1.
1.
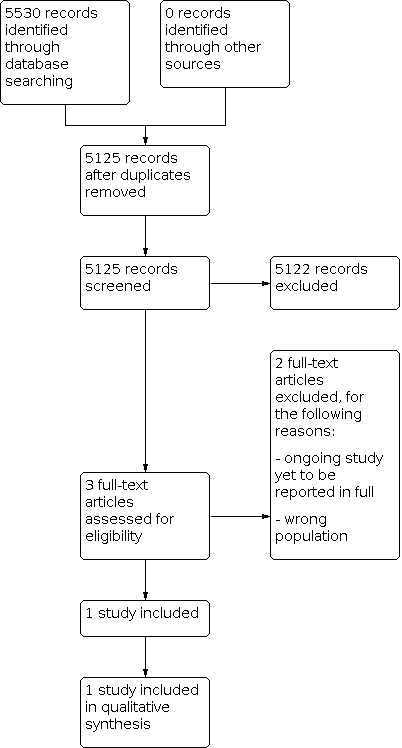
Study flow diagram.
Included studies
Only one study met all of our inclusion criteria (Barrot 2020). This prospectively registered randomized controlled trial, conducted across 13 intensive care units in France, assigned participants with ARDS (Berlin definition) who had been mechanically ventilated (for less than 12 hours by the time of enrolment) to receive either conservative oxygen therapy (to target either PaO2 55 to 70 mmHg, or SpO2 88% to 92%) or liberal oxygen therapy (target PaO2 90 to 105 mmHg, or SpO2≥ 96%) for 7 days. The trial was stopped prematurely by the data and safety monitoring board due to safety concerns after the enrolment of 205 out of the planned 850 participants. The conservative oxygen group (n = 99) had a mean age of 63, mean SOFA score of 9.3, and were 66% male; the liberal oxygen group (n = 102) had a mean age of 63.5, mean SOFA of 8.9, and were 62.7% male.
Although the initial ventilation strategy was the same in both groups except for the amount of oxygen administered (volume‐assist control mode with tidal volumes of 6 mL per kg of predicted body weight), the settings for mechanical ventilation including positive end‐expiratory pressure (PEEP) (and also timing of prone positioning and use of neuromuscular blockade) were coupled to the PF ratio (PaO2/FiO2) in each group by the protocol. The FiO2 could also be altered at the clinician’s discretion for procedures or transfers, but oxygenation was not systematically adjusted for routine tracheal suctioning.
We found no completed studies specifically looking at viral‐induced ARDS, including ARDS associated with COVID‐19.
Excluded studies
On reviewing the full texts, two records were excluded for not meeting the inclusion criteria. Both were ongoing studies that were not specifically investigating oxygen targets in ARDS patients undergoing invasive mechanical ventilation in an intensive care setting (i.e. the wrong population) (ChiCTR2000032456; NCT03174002).
Risk of bias in included studies
The overall risk of bias for the one included study was high (see Figure 2 for individual domain judgements), as the trial was open‐label and also stopped early after a pre‐planned trial safety committee meeting (criteria for stopping were not specified).
2.

Risk of bias summary
Effects of interventions
Mortality at longest available follow‐up
The longest reported follow‐up for mortality was at 90 days: 44 of the 99 participants in the lower‐target group died compared to 31 of the 102 in the higher‐target group (OR 1.83, 95% confidence interval (CI) 1.03 to 3.27).
Number of days ventilated
The mean number of days ventilated in the lower‐target group was 14.0 (95% CI 10.0 to 18.0) compared to 14.5 (95% CI 11.8 to 17.1) in the higher‐target group. Although all participants were initially intubated and undergoing invasive ventilation, for follow‐up purposes, mechanical ventilation was defined as including non‐invasive ventilation and high‐flow nasal oxygen as well as invasive ventilation.
Requirement for inotropic support
The mean number of days of catecholamine use in the lower‐target group was 8.0 (95% CI 5.5 to 10.5) compared to 7.2 (95% CI 5.9 to 8.4) in the higher‐target group.
Requirement for renal replacement therapy
At day 6, 10 of the 99 participants in the lower‐target group were receiving renal replacement therapy (13.7%, 95% CI 5.8% to 21.6%) compared to 10 of 102 participants in the higher‐target group (12.0%, 95% CI 5.0% to 19.1%).
Quality of life
Quality of life was not reported in the included study.
Discussion
Summary of main findings
The aim of this review was to assess how oxygen therapy should be targeted in patients with ARDS (particularly ARDS secondary to COVID‐19) and requiring mechanical ventilation in an intensive care setting.
We included one randomized controlled trial in this review. The overall risk of bias was rated as high, and the certainty of the evidence was very low. Consequently, we are very uncertain as to whether higher or lower oxygen targets should be used to treat mechanically ventilated patients with ARDS.
Overall completeness and applicability of the evidence
Despite thorough searches, we were only able to identify one completed randomized controlled trial investigating how oxygen therapy is targeted and administered to patients with ARDS who are receiving invasive mechanical ventilation (Barrot 2020). This trial enrolled only 205 participants after which it was stopped prematurely, and only two oxygenation targets were compared: PaO2 55 to 70 mmHg or SpO2 88% to 92%, and PaO2 90 to 105 mmHg or SpO2≥ 96%. It is not clear whether these results are generalizeable to all patients with ARDS (including those with ARDS secondary to COVID‐19). Similarly, it is not clear how alternative oxygen targets might affect outcomes in patients with ARDS. For example, an intermediate target (e.g. SpO2 92% to 96%/PaO2 70 to 90 mmHg, or similar range not tested here) could also affect clinical outcomes in this group.
Although the oxygenation targets were well maintained in both groups, other factors could also have confounded the apparent mortality benefit in the liberal group. Firstly, prone positioning was more frequent in the liberal arm (51% versus 34%), and PEEP was higher because ventilation settings were linked to the PF ratio in the protocol. Secondly, even though there was no apparent difference in the duration of ventilation, because mechanical ventilation was defined for follow‐up purposes as including other supportive oxygen modalities (including non‐invasive ventilation and high‐flow nasal oxygen), one group could potentially still have been extubated earlier than the other. Apart from an excess of ischaemic events seen in the conservative group (5 versus 0), there is no evidence confirming that the excess deaths seen in the conservative oxygen group were related to differences in oxygenation.
The large ICU‐ROX study, which randomized 1000 patients receiving mechanical ventilation in the intensive care unit to either a conservative or usual oxygen therapy, also included a very large prespecified subgroup of participants with a PF ratio < 300 mmHg. Although these participants were not specified as having a diagnosis of ARDS in the ICU‐ROX trial, it is likely that many participants in this low PF ratio group would have fulfilled the Berlin criteria for ARDS. Conservative oxygen therapy did not significantly alter ventilator‐free days in ICU‐ROX either overall or in the low‐PF subgroup, and 28‐day survival was also unaltered overall. However, the definition of ‘conservative’ in ICU‐ROX (SpO2 < 97%) was much closer to the definition of ‘liberal’ oxygenation in Barrot 2020 (SpO2≥ 96%), which makes direct comparison difficult.
We also identified two ongoing studies (yet to report their results) that were not eligible for inclusion in this review due to including patients receiving other methods of oxygen administration to mechanical ventilation. The handling oxygenation targets in the intensive care unit (HOT‐ICU) trial is randomizing patients with acute respiratory failure to receive oxygen to target a PaO2 of either 8 kPa (60 mmHg) or 12 kPa (90 mmHg) (NCT03174002), and the second trial is investigating the effects of administering lower amounts of oxygen in patients with COVID‐19 infection (ChiCTR2000032456).
Certainty of the evidence
We downgraded the certainty of the evidence for all outcomes one level for risk of bias and two levels for imprecision. Overall, this means that the certainty of this evidence remains very low for all outcomes.
Potential biases in the review process
We deliberately set out to include the best available evidence for this review, and therefore limited the results to only randomized controlled trials. Consequently, this may have limited our findings, but has increased the reliability of this review.
We sought the support of two experienced Information Specialists to construct, review, and run a sensitive search strategy, and all relevant databases and trial registries were searched to identify all relevant trials, both those completed and still ongoing. Furthermore, and in contrast to the recommended methodology for rapid Cochrane Reviews, all steps of reviewing the searches were conducted independently in duplicate by two authors experienced at reviewing systematic review search results. Risk of bias was also assessed independently in duplicate.
Although the utility of the evidence is limited by the quantity of studies available for inclusion, we believe the review process itself is as robust as it could be.
Agreements and disagreements with other studies or reviews
The data from this review suggest that using a low oxygen target may be associated with worse outcomes in mechanically ventilated patients with ARDS. Although this is the best available evidence, these findings are notably based on one single study and contrast the findings of many recent relevant reviews. A previous Cochrane Review assessing the benefits and harms of using supplementary oxygen in all patients in an intensive care setting identified 10 trials (not including Barrot 2020) with a total of 1285 participants, and concluded that mortality may be higher in patients receiving high fractions of oxygen (risk ratio (RR) 1.18, 95% CI 1.01 to 1.37) (Barbateskovic 2019). However, as with our findings, their conclusions remained uncertain due to only being able to identify very low‐certainty evidence. Similarly, another recent systematic review that aimed to assess the safety and effectiveness of high‐flow nasal oxygen administration in intensive care also found insufficient evidence to do this (Corley 2017), further highlighting the urgent need for more certain evidence into how oxygen is used in critical illness.
Furthermore, our findings also contradict a systematic review and meta‐analysis looking at liberal versus conservative oxygen use in over 16,000 acutely unwell patients, which demonstrated that liberal oxygen therapy (defined as maintaining SpO2 > 96%) significantly increased in‐hospital mortality (RR 1.21, 95% CI 1.03 to 1.43) and 30‐day mortality (RR 1.14, 95% CI 1.01 to 1.29) (Chu 2018). Retrospective data looking at associations between arterial hyperoxia and mortality in critically ill patients from 17 separate studies also suggest that hyperoxia is associated with increased mortality in patients following cardiac arrest (OR 1.42, 95% CI 1.04 to 1.92), stroke (OR 1.23, 95% CI 1.06 to 1.43), and traumatic brain injury (OR 1.41, 95% CI 1.03 to 1.94) (Damiani 2014). Importantly, this review also concluded that data from mechanically ventilated intensive care patients were too heterogenous to analyse, primarily due to design flaws and the inconsistent definition of hyperoxia.
As our results were limited to a single paper with high risk of bias, it is not appropriate to generalize these findings beyond the scope of the patients with ARDS and receiving mechanical ventilation. However, our findings are consistent with some international clinical recommendations towards higher levels of inspired oxygen in other clinical contexts, which stand in contrast to the intensive care and acute illness trial data summarized above. For example, the World Health Organization (WHO) recommends administering 80% oxygen (FiO2 0.8) to all patients undergoing surgery who require intubation and anaesthesia in order to reduce the risk of surgical site infection (Allegranzi 2016). Notably however, as well as applying to a very different group of patients to critically unwell patients with ARDS, this recommendation is for all patients to receive a specific inspiratory fraction/concentration of oxygen regardless of their individual degree of oxygenation rather than a target of oxygenation to aim for. This recommendation also remains controversial amongst the anaesthetic community following previous Cochrane Reviews and evidence from other perioperative trials (Meyhoff 2008; Myles 2019; Oldman 2019; Wetterslev 2015). Importantly, this recommendation also only considers evidence focused on reducing surgical site infections and does not consider how any other outcomes (including those more relevant in a critical care context, such as mortality) may be affected by oxygen administration.
This rapid review highlights the profound limitations in volume and quality of studies evaluating oxygen targets in mechanically ventilated patients with ARDS. There remains considerable controversy surrounding oxygen across perioperative, acute medical, and intensive care literature, and this review serves to highlight where future trial efforts need to be focused.
Authors' conclusions
The currently available evidence on targeting oxygen administration in patients with ARDS and receiving invasive mechanical ventilation is of very low certainty. Consequently, all conclusions drawn from these data are of limited value to clinicians and may change with further updates of this review as and when more evidence becomes available. The one study included in this review reported that participants receiving supplemental oxygen to target a PaO2 = 90 to 105 mmHg or SpO2≥ 96% were more likely to survive beyond 90 days than those who received oxygen targeted towards achieving either PaO2 = 55 to 70 mmHg or SpO2 = 88% to 92%, but the certainty of the evidence for this finding was very low. We are also very uncertain how oxygen targets affect duration of mechanical ventilation, catecholamine use, or use of renal replacement therapy in these patients. Further randomized controlled trials, including studies investigating different oxygen targets not tested here, are urgently needed to add more certainty to these findings and assess how best to target oxygen administration in mechanically ventilated patients with ARDS.
What's new
| Date | Event | Description |
|---|---|---|
| 12 October 2020 | Amended | Changed review type to 'Rapid (Flexible review)' |
History
Review first published: Issue 9, 2020
Acknowledgements
We would like to thank Arash Afshari (Content Editor), Jing Xie (Statistical Editor), Ana Hutchinson and Paul Young (Peer Reviewers), Brian Li (Consumer Referee), Janne Vendt (Information Specialist), Robin Featherstone (Information Specialist), Liz Bickerdike (Network Associate Editor), Teo Quay (Managing Editor), and Harald Herkner (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Protocol
Criteria for considering studies for this review
| Study and source eligibility | |
| Study design |
☒RCTs ☐ Quasi‐RCTs ☐ Non-RCTs ☐ Prospective cohort studies ☐ Retrospective cohort studies ☐ Case-control studies ☐ Cross-sectional studies ☐ Controlled before-and-after studies ☐ Modelling studies ☐ Other (please specify) |
| Minimum duration | No minimum duration |
| ‘PICO’ eligibility | |
| Population |
|
| Intervention(s) | Liberal’ oxygen (defined only as relative to conservative/control group to maximize inclusion of studies and may consist of targeting higher SpO2, PaO2, or any combination of these where the study intention is to compare groups receiving different amounts of oxygen (in the opinion of the authors) |
| Comparator(s) | ‘Conservative’ oxygen ‐ defined only that the study intention (in the opinion of the review authors) is to compare giving a lower amount of oxygen to this group than the intervention arm in the trial. This may be targeting mild hyperoxaemia (but still less than the intervention arm in the same study targeting a higher degree of hyperoxaemia), normoxaemia, or hypoxaemia. |
| Outcome(s) | The following outcomes will be examined.
|
Search methods for identification of studies
| Search methods | |||||
| Expertise | The searches will be verified by a content expert, conducted by an Information Specialist, and independently peer reviewed. | ||||
| Electronic databases | Database [minimum checked – please specify one other] ☒ MEDLINE ☒ CENTRAL ☒ Embase ☒ Other ‐ Cochrane COVID‐19 Study Register ☐ Clinical Trial Registry (please specify) |
From: Inception |
To: 01/05/2020 | ||
| Other searches | ☒ Systematic review references ☒ Reference lists of included studies ☐ Grey literature (please specify) ☐ Citation tracking ☐ Data from the pharmaceutical industry ☐ Contact experts for references ☐ Other (please specify) |
||||
| Approach to ongoing and unpublished studies | ☐ Include ongoing studies ☐ Unpublished studies ☐ Studies in press ☒ Exclude all studies that are ongoing, unpublished, or in press |
Through handsearching references and including ongoing trials across major databases | |||
| Methods for screening search results | |||||
| Expertise | Screening will be performed by AO/AC | ||||
| Screening methods | Dual; second reviewer checks all excluded records Dual; second reviewer checks [X%] of excluded records Dual; independent screen and cross check |
Abstract ☐ ☐ ☒ |
Full text ☐ ☐ ☒ |
||
| Discrepancy resolution | ☒ Consensus and/or third reviewer ☐Other (please specify) |
||||
| Excluded studies | All decisions taken during full‐text screening will be documented and outlined in the final report with a list of excluded studies. | ||||
| Inclusion of abstracts and conference proceedings | ☐ Exclude all ☒ Include if clearly eligible and have useable data ☐ Include if clearly eligible regardless of useable data ☐ Include if eligibility is unclear and add to section in report |
||||
| Inclusion of non‐English language studies | ☒ Include abstracts and full texts [in Chinese/any language] ☐ Include full texts only [in Chinese only/ language] ☐ Exclude |
||||
| ☒ All potentially relevant abstracts will progress to full‐text screen ☐ [Single/dual] title/abstract screen by foreign‐language speaker(s) ☒ [Abstract/methods/full text] will be translated for abstract/full text screen ☐ Listed as non‐English language and not assessed further | |||||
Data collection and analysis
| Data extraction | ||
| Expertise | Data extraction will be performed by AO and AC | |
| Software | Data will be extracted using pilot‐tested data extraction forms through the online resource: Rayyan Systematic Review software | |
| Data to be extracted | Study design: systematic review and meta‐analysis. RCTs |
|
| Data extraction methods | ☐ Single, no second reviewer ☒ Dual; second reviewer checks all data ☐ Dual; second reviewer checks [add proportion] ☐ Dual; independent screen and cross check |
|
| Risk of bias tool |
[specify for each study design] ☒ Cochrane 'Risk of bias' tool (RCTs) ☐ ROBINS‐I tool for non‐randomized studies ☐ Adapted‐hybrid of the RCT‐ROBINS‐I tools ☐ Newcastle‐Ottawa Scale ☐ Another tool [please specify] |
|
| Method of risk of bias assessment | ☐ Single, no second reviewer ☒ Dual; second reviewer checks all judgements ☐ Dual; second reviewer checks [add proportion] ☐ Dual; independent screen and cross check |
☐ All outcomes ☐ Primary only |
| Discrepancy resolution | ☒ Consensus and/or third reviewer ☐ Other (please specify) |
|
| Contacting study authors | ☐ Authors will be contacted for missing information and data ☒ Authors will be contacted for missing outcome data only ☐ Authors will not be contacted |
|
| Data management | ||
| Software | Review Manager 5 | |
| Standardization | N/A | |
| Resolving conflicts between sources | If there is a conflict between data reported across multiple sources for a single study (e.g. between a published article and a trial registry record), we will contact researchers directly for clarification. | |
| Data synthesis | |
| Measures of treatment effect | ☒ Continuous outcome: mean difference and 95% confidence intervals (CIs) ☒ Continuous outcome: standardized mean difference ☐ Dichotomous outcome: risk ratio/relative risk (RR) and 95% CIs ☒ Dichotomous outcome: odds ratio (OR) and 95% CIs ☐ Dichotomous outcome: risk difference (absolute risk reduction) ☐ Peto odds ratio method ☐ Other (please specify) [Any data processing required will be performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions] |
| Unit of analysis issues | Advice from a statistician will be sought to address issues relating to double counting, correlation or unit of analysis posed by the following. ☒ Cluster RCTs ☐ Cross‐over trials ☐ Body‐part randomized trials ☒ Episodes of disease ☒ Multi‐arm studies ☐ Other (please specify) |
| Assessment of heterogeneity | ☒ Inspecting forest plots ☐ Statistical test (Chi2) for heterogeneity [specify P value] ☒ I2 statistic [state how values of I2 will be interpreted] ☒ Explore potential sources of the heterogeneity amongst study results ☐ Sensitivity analysis by excluding outlying studies (THIS WILL REQUIRE CLARIFICATION WITH STATISTICIAN) |
| Assessment of reporting biases | ☒ Funnel plots ☒ Test for funnel plot asymmetry (e.g. Begg, Egger test) ☐ Trim and fill technique |
| Data synthesis | ☒ Forest plots ☒ Qualitative synthesis ☐ Synthesis without meta‐analysis [Specify data type and study designs, interventions to be pooled If non‐randomized and observational studies are included, describe how these studies will be analysed] |
| Model | ☐ Fixed‐effect meta‐analyses ☒ Random‐effects meta‐analyses (DerSimonian and Laird method) ☐ Other [please specify] |
| Subgroup analyses | The following subgroups will be explored.
|
| Sensitivity analysis | ☐ Excluding studies at high risk of bias ☐ Excluding studies with dubious eligibility ☐ Alternative analysis methods [specify] ☐ Other [please specify] Any post hoc sensitivity analyses that arise during the review process will be justified in the final report. |
| GRADE approach | ☒ GRADE will be used for [all outcomes/the primary outcome(s)] and results presented in a 'Summary of findings' table |
Appendix 2. Search strategies
Database: Ovid MEDLINE(R) ALL <1946 to May 14, 2020>
1 Respiratory Distress Syndrome, Adult/
2 exp Severe Acute Respiratory Syndrome/
3 (ards or sars).ti,ab,kf.
4 (acute respiratory adj2 (syndrome* or distress or failure*)).ti,ab,kf.
5 ((acute or adult or syndrome*) adj2 respiratory distress).ti,ab,kf.
6 exp coronavirus/
7 exp Coronavirus Infections/
8 (coronavirus* or corona virus* or Covid 19 or Covid19 or SARS CoV* or SARSCov* or ncov* or 19ncov*).ti,ab,kf.
9 6 or 7 or 8
10 9 and (201912* or 2020*).dt.
11 1 or 2 or 3 or 4 or 5 or 10
12 Oxygen/ad, sd, th
13 exp Oxygen‐Inhalation‐Therapy/
14 exp Hyperoxia/
15 Hypoxia/
16 (hyperoxia or hyperoxemia or hyperoxaemia or hypoxia or hypoxemia or hypoxaemia or anoxia or anoxemia or anoxaemia or high* oxygen or oxygenat* or blood gas* or pao2 or sao2 or spo2).ti,ab,kf.
17 ((inspir* or inhal* or fraction* or concentrat* or arterial* or saturation or level* or tension* or supply* or supplement* or supplie* or therap* or administr* or dosag* or dose* or dosing* or conservative or liberal or restrictive or partial pressure) adj3 oxygen).ti,ab,kf.
18 12 or 13 or 14 or 15 or 16 or 17
19 exp Respiration, Artificial/
20 (artificial* adj3 respirat*).ti,ab,kf.
21 ventilat*.ti,ab,kf.
22 19 or 20 or 21
23 11 and 18 and 22
24 ((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (exp animals/ not humans.sh.)
25 23 and 24
Database: Embase <1974 to 2020 May 14>
1 adult respiratory distress syndrome/
2 severe acute respiratory syndrome/
3 (ards or sars).ti,ab,kw.
4 (acute respiratory adj2 (syndrome* or distress or failure*)).ti,ab,kw.
5 ((acute or adult or syndrome*) adj2 respiratory distress).ti,ab,kw.
6 exp coronavirinae/
7 exp coronaviridae infection/
8 (coronavirus* or corona virus* or Covid 19 or Covid19 or SARS CoV* or SARSCov* or ncov* or 19ncov*).ti,ab,kw.
9 6 or 7 or 8
10 9 and (201912* or 2020*).dc.
11 1 or 2 or 3 or 4 or 5 or 10
12 exp oxygen therapy/
13 hyperoxia/
14 hyperoxia‐induced lung injury/
15 exp hypoxemia/
16 (hyperoxia or hyperoxemia or hyperoxaemia or hypoxia or hypoxemia or hypoxaemia or anoxia or anoxemia or anoxaemia or high* oxygen or oxygenat* or blood gas* or pao2 or sao2 or spo2).ti,ab,kw.
17 ((inspir* or inhal* or fraction* or concentrat* or arterial* or saturation or level* or tension* or supply* or supplement* or supplie* or therap* or administr* or dosag* or dose* or dosing* or conservative or liberal or restrictive or partial pressure) adj3 oxygen).ti,ab,kw.
18 12 or 13 or 14 or 15 or 16 or 17
19 exp artificial ventilation/
20 (artificial* adj3 respirat*).ti,ab,kw.
21 ventilat*.ti,ab,kw.
22 19 or 20 or 21
23 (randomized controlled trial/ or controlled clinical study/ or random$.ti,ab. or randomization/ or intermethod comparison/ or placebo.ti,ab. or (compare or compared or comparison).ti. or ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. or (open adj label).ti,ab. or ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. or double blind procedure/ or parallel group$1.ti,ab. or (crossover or cross over).ti,ab. or ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. or (assigned or allocated).ti,ab. or (controlled adj7 (study or design or trial)).ti,ab. or (volunteer or volunteers).ti,ab. or human experiment/ or trial.ti.) not (((random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)) or (cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)) or (((case adj control$) and random$) not randomi?ed controlled).ti,ab. or (Systematic review not (trial or study)).ti. or (nonrandom$ not random$).ti,ab. or Random field$.ti,ab. or (random cluster adj3 sampl$).ti,ab. or ((review.ab. and review.pt.) not trial.ti.) or (we searched.ab. and (review.ti. or review.pt.)) or update review.ab. or (databases adj4 searched).ab. or ((rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/) or (Animal experiment/ not (human experiment/ or human/)))
24 11 and 18 and 22 and 23
Central Issue 5 of 12, May 2020
#1 MeSH descriptor: [Respiratory Distress Syndrome, Adult] explode all trees
#2 MeSH descriptor: [Severe Acute Respiratory Syndrome] explode all trees
#3 (ards or sars):ti,ab,kw
#4 ((acute next respiratory) near (syndrome* or distress or failure*)):ti,ab,kw
#5 ((acute or adult or syndrome*) near (respiratory next distress)):ti,ab,kw
#6 MeSH descriptor: [Coronavirus] explode all trees
#7 MeSH descriptor: [Coronavirus Infections] explode all trees
#8 (coronavirus* or (corona next virus*) or (Covid next 19) or Covid19 or (SARS next CoV*) or SARSCov* or ncov* or 19ncov*):ti,ab,kw
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 MeSH descriptor: [Oxygen] explode all trees
#11 MeSH descriptor: [Oxygen Inhalation Therapy] explode all trees
#12 MeSH descriptor: [Hyperoxia] explode all trees
#13 MeSH descriptor: [Hypoxia] explode all trees
#14 (hyperoxia or hyperoxemia or hyperoxaemia or hypoxia or hypoxemia or hypoxaemia or anoxia or anoxemia or anoxaemia or (high* next oxygen) or oxygenat* or (blood next gas*) or pao2 or sao2 or spo2):ti,ab,kw
#15 ((inspir* or inhal* or fraction* or concentrat* or arterial* or saturation or level* or tension* or supply* or supplement* or supplie* or therap* or administr* or dosag* or dose* or dosing* or conservative or liberal or restrictive or (partial next pressure)) near oxygen):ti,ab,kw
#16 #10 or #11 or #12 or #13 or #14 or #15
#17 MeSH descriptor: [Respiration, Artificial] explode all trees
#18 (artificial* near respirat*):ti,ab,kw
#19 ventilat*:ti,ab,kw
#20 #17 or #18 or #19
#21 #9 and #16 and #20
#22 #21 in Trials
Cochrane Covid‐19 study register
Filtered by:
oxygen or hyperoxia or hyperoxemia or hyperoxaemia or hypoxia or hypoxemia or hypoxaemia or anoxia or anoxemia or anoxaemia or oxygenat* or "blood gas" or pao2 or sao2 or spo2
AND
ventilat* or respirat*
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrot 2020.
| Study characteristics | ||
| Methods | Randomized controlled trial Multicentre |
|
| Participants |
Sample size: conservative, n = 99, liberal, n = 102 Sex (male): conservative 65.7%, liberal 62.7% Age (mean): conservative 63.0, liberal 63.5 Country: France Setting: adults who had undergone intubation and had been receiving mechanical ventilation for less than 12 hours for ARDS (according to the Berlin definition) Disease severity score: SAPS III median 67.5, SOFA median 9.1 Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental (conservative): a modulation of inspired fraction of oxygen will be performed with an objective of PaO2 between 55 to 70 mmHg that will be checked on arterial blood gases. Between these measurements, SpO2 will be kept between 88 and 92 per cent. Alarms will be set between 87 and 93 per cent for SpO2. Control (liberal): a modulation of inspired fraction of oxygen will be performed with an objective of PaO2 between 90 to 105 mmHg that will be checked on arterial blood gases. Between these measurements, SpO2 will be kept more or equal to 96 per cent. Alarms will be set at 95 per cent for SpO2. |
|
Primary outcome measures:
Secondary outcome measures:
|
||
| Notes | Trial was funded by public grants. | |
| Item | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Yes | Randomization was stratified according to center, age (<45 years, 45 to 65 years, or >65 years), and severity of respiratory failure evaluated according to the Pao2:Fio2 (≤150 mm Hg or >150 mm Hg), with a PEEP of 5 cm of water and a Fio2. Computer randomization was performed in blocks of four. |
| Allocation concealment (selection bias) | Unclear | Not specified. |
| Blinding of participants and personnel (performance bias) | No | This was an open‐label trial because of the impossibility of masking treatment assignments with the use of Spo2 and Pao2 monitoring in the ICU. |
| Blinding of outcome assessment (detection bias) | No | This was an open‐label trial because of the impossibility of masking treatment assignments with the use of Spo2 and Pao2 monitoring in the ICU. |
| Incomplete outcome data (attrition bias) | Yes | 4% in the experimental group and none of the control group were excluded from the analysis |
| Selective reporting (reporting bias) | Yes | The trial was registered prior to randomisation (NCT02713451) |
| Other bias | No | Early stopping bias: the trial was stopped after a pre‐planned trial safety committee meeting, criteria for stopping not specified |
ARDS: acute respiratory distress syndrome, cmH2O: centimetre of water, FiO2: fraction of inspired oxygen, ICU: intensive care unit, PaO2: arterial partial pressure of oxygen SAPS: simplifed acute physiology score, SOFA: sequential organ failure assessment, SpO2: peripheral oxygen saturation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ChiCTR2000032456 | Ongoing trial yet to be published; wrong study population |
| NCT03174002 | Ongoing trial yet to be published; wrong study population |
Differences between protocol and review
All differences to the original protocol (i.e. the inability to assess for heterogeneity or to perform a separate subgroup analysis for studies specifically in patients with ARDS secondary to COVID‐19 or other viruses due to only finding one eligible study) have already been detailed and explained within the main text of the review.
Contributions of authors
AC ‐ conception and writing of this review
AO ‐ writing of this review
AS ‐ clinical and methodological expertise
DM ‐ conception of the review and clinical expertise
MG ‐ conception of the review and clinical expertise
All authors reviewed, edited and approved the final version of this review
Declarations of interest
AC has received funding through the National Institute for Health Research (NIHR) as an Academic Clinical Fellow and Southampton NIHR Biomedical Research Centre (BRC) as a Clinical Research Fellow.
AO has no competing interests.
AS has no competing interests.
DM has received consultancy fees from Siemens Healthineers and Masimo and lecture honoraria from Edwards Lifesciences and Deltex Medical. He is also a Director of Oxygen Control Systems Ltd.
MPWG is a director of Oxygen Control Systems Ltd. He has received honoraria for speaking and/or travel expenses from BOC Medical (Linde Group), AstraZeneca, Edwards Lifesciences, and Cortex GmBH. He leads the Xtreme Everest Oxygen Research Consortium and the Fit‐4‐Surgery research collaboration. He serves as the UK NIHR Clinical Research Network national specialty group lead for Anaesthesia Perioperative Medicine and Pain, and is an elected council member and Vice President of the Royal College of Anaesthetists.
Edited (no change to conclusions)
References
References to studies included in this review
Barrot 2020 {published data only}
- Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. New England Journal of Medicine 2020;382:999-1008. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ChiCTR2000032456 {published data only}
- A Randomized Controlled Study of the Effects of Low-Oxygen Consumption Instruction on the Prognosis of Patients with Novel Coronavirus Disease. Chinese clinical trial registry. [http://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000032456]
NCT03174002 {published data only}
- Handling Oxygenation Targets in the Intensive Care Unit (HOT-ICU). ClinicalTrials.gov. [https://www.clinicaltrials.gov/ct2/show/NCT03174002] [DOI] [PubMed]
Additional references
Afshari 2010
- Afshari A, Brok J, Møller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database of Systematic Reviews 2010, Issue 7. Art. No: CD002787. [DOI: 10.1002/14651858.CD002787.pub2] [DOI] [PubMed] [Google Scholar]
Afshari 2017
- Afshari A, Bille AB, Allingstrup M. Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS). Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD007733. [DOI: 10.1002/14651858.CD007733.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allegranzi 2016
- Allegranzi B, Zayed B, Bischoff P, Kubilay N Z, Jonge S, de Vries Fl, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infectious Diseases 2016;16(12):e288-303. [DOI] [PubMed] [Google Scholar]
Andersen 2016
- Andersen LW, Berg KM, Chase M, Cocchi MN, Massaro J, Donnino MW. Acute respiratory compromise on inpatient wards in the United States: incidence, outcomes, and factors associated with in-hospital mortality. Resuscitation 2016;105:123-9. [DOI] [PubMed] [Google Scholar]
Barbateskovic 2019
- Barbateskovic M, Schjørring OL, Krauss SR, Jakobsen J C, Meyhoff CS, Dahl RM, et al. Higher versus lower fraction of inspired oxygen or targets of arterial oxygenation for adults admitted to the intensive care unit. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD012631. [DOI: 10.1002/14651858.CD012631.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2018
- Chu DK, Kim LHY, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 2018;391(10131):1693-705. [DOI] [PubMed] [Google Scholar]
Corley 2017
- Corley A, Rickard CM, Aitken LM, Johnston A, Barnett A, Fraser JF, et al. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD010172. [DOI: 10.1002/14651858.CD010172.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Damiani 2014
- Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Critical Care 2014;18(6):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gattinoni 2020
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2020;201(10):1299-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.), available from gradepro.org.
Grasselli 2020
- Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020;323(16):1545-6. [DOI] [PubMed] [Google Scholar]
Lambden 2019
- Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score - development, utility and challenges of accurate assessment in clinical trials. Critical Care 2019;23(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leach 2002
- Leach RM, Treacher DF. The pulmonary physician in critical care• 2: Oxygen delivery and consumption in the critically ill. Thorax 2002;57(2):170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2019
- Lewis SR, Pritchard MW, Thomas CM, Smith AF. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD004477. [DOI: 10.1002/14651858.CD004477.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2013
- Martin DS, Grocott MPW. III. Oxygen therapy in anaesthesia: the yin and yang of O2. Br J Anaesth December 01, 2013;111(6):P867-871. [DOI: ] [DOI] [PubMed] [Google Scholar]
Meyhoff 2008
- Meyhoff CS, Wetterslev J, Jorgensen LN, Henneberg SW, Simonsen I, Pulawska T, et al. Perioperative oxygen fraction - effect on surgical site infection and pulmonary complications after abdominal surgery: a randomized clinical trial. Rationale and design of the PROXI-Trial. Trials 2008;9(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myles 2019
- Myles PS, Carlisle JB, Scarr B. Evidence for compromised data integrity in studies of liberal peri‐operative inspired oxygen. Anaesthesia 2019;74(5):573-84. [DOI] [PubMed] [Google Scholar]
Oldman 2019
- Oldman AH, Cumpstey AF, Martin DS, Grocott MPW. Data integrity issues: catalyst for a more robust approach to research on perioperative oxygen therapy? Perioperative Medicine 2019;8(7). [DOI: 10.1186/s13741-019-0118-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager (RevMan) Version 5.3 [Computer program]
- Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2020
- Roberts CM, Levi M, McKee M, Schilling R, Lim WS, Grocott MP. COVID-19: a complex multi-system disorder. British Journal of Anaesthesia (in press). [DOI: 10.1016/j.bja.2020.06.013] [DOI] [PMC free article] [PubMed]
Schünemann 2019
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). The Cochrane Collaboration 2019. Available from www.training.cochrane.org/handbook. [URL: https://training.cochrane.org/handbook/current/chapter-14]
Wetterslev 2015
- Wetterslev J, Meyhoff CS, Jørgensen LN, Gluud C, Lindschou J, Rasmussen LS. The effects of high perioperative inspiratory oxygen fraction for adult surgical patients. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD008884. [DOI: 10.1002/14651858.CD008884.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


