Abstract
Purpose
To analyze the association between glucosamine (GlcN) use and the risk of age-related macular degeneration (AMD) using claims data from the National Health Insurance Research Database (NHIRD).
Methods
A retrospective, population-based study was conducted with NHIRD data from a 14-year period (2000–2013). Chi-squared and Student’s t-tests were used to evaluate differences between the study and comparison cohorts for categorical and continuous variables, respectively. Risk factors for disease development were examined by the adjusted hazard ratio (aHR) with 95% confidence interval. Kaplan-Meier analysis was performed to compare the cumulative risk of AMD between the two cohorts.
Results
In total, 1,344 patients with GlcN treatment were enrolled in the study cohort and 5,376 patients without GlcN use were enrolled in the comparison cohort. The incidence rate of AMD was lower with GlcN use (3.65%) than without GlcN use (5.26%) (P = 0.014). GlcN use was associated with a lower risk of developing AMD among patients with hyperlipidemia, coronary artery disease, chronic obstructive pulmonary disease, stroke, other neurological disorders, or degenerative arthritis. Although the incidence of wet type AMD did not significantly differ (P = 0.91), the incidence of dry type AMD was lower in patients with GlcN use (2.9%) than those without GlcN use (4.84%) (P = 0.003). Kaplan-Meier analysis similarly revealed a lower rate of dry type AMD in patients with GlcN use compared to those without GlcN use (log-rank P = 0.004).
Conclusions
GlcN treatment can decrease the risk of developing dry type AMD. Further prospective controlled studies are needed to determine the effectiveness of GlcN treatment in patients with AMD and the associated mechanism.
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible visual impairment in the developed world, especially among those older than 50 years [1]. In terms of the worldwide prevalence, the predicted number of people with AMD will increase from 196 million in 2020 to 288 million in 2040 [2]. AMD places a heavy burden on patients, caregivers, and physicians [3]. In Taiwan, AMD is a common eye disease among the elderly, and age is the most significant factor associated with AMD [4].
Clinically, AMD is classified into two types: dry (non-neovascular) and wet (neovascular). The dry type is predominant, accounting for 85–90% of all patients with AMD. The early stage of dry AMD causes mild vision loss, and drusen may appear in the macula under fundus examination [5]. Subsequently, it may progress to geographic atrophy (GA), causing irreversible visual impairment [6]. The wet type affects approximately 10–15% of all patients with AMD, but accounts for 90% of cases of vision loss among patients with AMD [7].
Glucosamine (GlcN), a naturally occurring amino monosaccharide, is the most commonly taken dietary supplement; its long-term administration is considered safe in humans [8]. GlcN has been widely used as an alternative regimen for rheumatoid arthritis and osteoarthritis [9, 10]. Our previous in vitro and in vivo studies have shown that GlcN plays many important roles in anti-inflammatory effects [11–13]. In addition, GlcN modulates oxidative stress-induced senescence of retinal pigment epithelium (RPE) cells [14]. GlcN also attenuates native photoreceptor outer segment (POS)-derived lipofuscin-like autofluorescence (LLAF) in RPE cells in vitro [15]. In Taiwan, GlcN is one of the prescription drugs for the alleviation of degenerative arthritis pain insured by the health administration. According to our literature review, no previous studies have investigated the association between GlcN use and AMD. Thus, the purpose of this study was to evaluate the impact of GlcN use on the risk of developing AMD in Taiwan by using claims data from the National Health Insurance Research Database (NHIRD).
Materials and methods
Data sources
We utilized outpatient data from the Longitudinal Health Insurance Database (LHID) (2000–2013) of the NHIRD to investigate the association between GlcN treatment and the subsequent development of AMD in Taiwan over a 14-year period. The claims data includes medical information of all those insured, with a coverage rate of more than 99% of the 23 million people in Taiwan. In this study, the medical diagnoses were determined according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
Study design and sampled participants
We conducted a retrospective matched-cohort study. The study cohort comprised patients who first received GlcN therapy between January 2000 and December 2013. The index date was defined as the date that the patient first received GlcN therapy. The exclusion criteria were as follows: received GlcN treatment before 2000, diagnosed with AMD before receiving GlcN treatment, diagnosed with central serous chorioretinopathy (ICD-9-CM code 362.41) and pathologic myopia (ICD-9-CM code 360.21), received Bilimycin, aged <50 years, and unknown gender. To construct a comparison cohort, 4-fold propensity score matching was applied, randomly selecting patients without GlcN usage who matched those who received GlcN according to gender, age, and index year (under the same exclusion criteria). The tracking endpoint was defined as the date of AMD onset or the end of the study period. AMD was identified by the following ICD-9-CM codes: 362.50, 362.51, 362.52, and 362.57. AMD types were identified as follows: wet type AMD: ICD-9-CM codes 362.52; dry type AMD: ICD-9-CM codes 362.50, 362.51, and 362.57.
Covariates
The evaluated covariates included gender, age, diabetes (ICD-9-CM codes 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM codes 272), coronary artery disease (CAD; ICD-9-CM codes 410–414), asthma (ICD-9-CM codes 493), chronic obstructive pulmonary disease (COPD; ICD-9-CM codes 491, 492, and 496), stroke (ICD-9-CM codes 430–438), tobacco dependency (ICD-9-CM code 305.1), heart failure (HF; ICD-9-CM codes 428), dementia (ICD-9-CM codes 290), other neurological disorders (ICD-9-CM codes 344 and 342), degenerative arthritis (ICD-9-CM codes 715) and the Charlson Comorbidity Index (CCI). The number of ophthalmic outpatient visits was adjusted to minimize the surveillance bias.
Statistical analysis
All analyses were performed using SPSS software version 22 (SPSS Inc., Chicago, Illinois, USA). Chi-squared and t-tests were used to evaluate differences between the study and comparison cohort for categorical and continuous variables, respectively. Multivariable Cox proportional hazards regression analysis was used to determine the risk of AMD, and the results are presented as the adjusted hazard ratio (aHR) with 95% confidence interval (CI). The difference in the cumulative risk of AMD between the study and comparison cohorts was investigated using the Kaplan-Meier method with the log-rank test. A two-tailed p value <0.05 was considered statistically significant.
Ethics
This study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Patient consent was not required to access the data in the NHIRD. The Institutional Review Board of the Tri-Service General Hospital approved this study (TSGHIRB: B-109-42) and waived the need for individual written informed consent.
Results
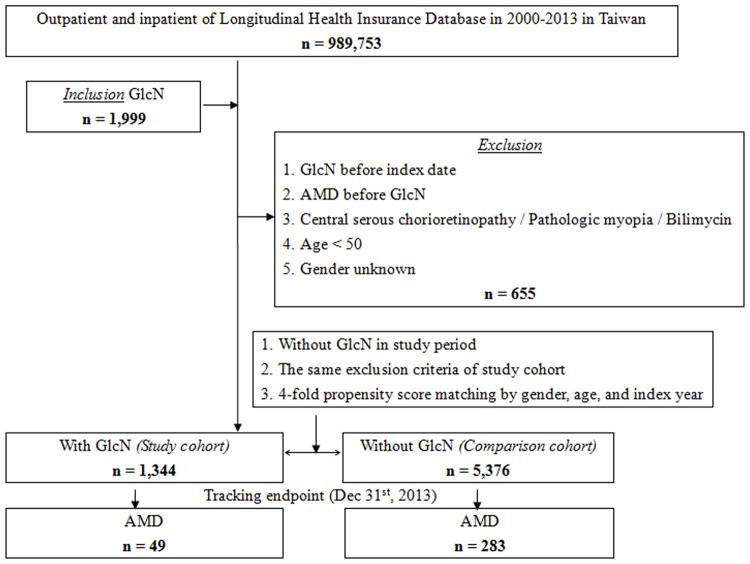
Among a total of 989,753 patients in the LHID during the study period, 1999 patients received GlcN treatment. Among these, 655 patients were excluded based on the exclusion criteria; thus, 1344 patients were enrolled in the study cohort. After 1:4 matching, 5376 patients without GlcN treatment were enrolled in the comparison cohort. The mean age at baseline was 72.25±16.89 years and 72.31±16.97 years for the study and comparison cohorts, respectively. There were no significant differences in the age at baseline and the gender distribution between the study and comparison cohorts. The study flowchart is depicted in Fig 1.
Fig 1. Flowchart of patient selection from the National Health Insurance Research Database in Taiwan.
Patient characteristics
As shown in Table 1, at the tracking endpoint, the incidence of newly developed AMD was significantly lower with GlcN use (3.65%, 49/1344 patients) than without GlcN use (5.26%, 283/5376 patients) (P = 0.014). Furthermore, the incidence of wet type AMD did not significantly differ between the two cohorts (P = 0.91), whereas the incidence of dry type AMD was significantly lower in patients with GlcN use (2.9%) than those without GlcN use (4.84%) (P = 0.003).
Table 1. Patient characteristics at tracking endpoint.
| GlcN | Total | With | Without | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 6,720 | 1,344 | 20.00 | 5,376 | 80.00 | ||
| AMD | 0.014 | ||||||
| Without | 6,388 | 95.06 | 1,295 | 96.35 | 5,093 | 94.74 | |
| With | 332 | 4.94 | 49 | 3.65 | 283 | 5.26 | |
| AMD subgroup | 0.011 | ||||||
| Without | 6,388 | 95.06 | 1,295 | 96.35 | 5,093 | 94.74 | 0.017 |
| Wet type | 33 | 0.49 | 10 | 0.74 | 23 | 0.43 | 0.910 |
| Dry type | 299 | 4.45 | 39 | 2.90 | 260 | 4.84 | 0.003 |
| Gender | 0.999 | ||||||
| Male | 3,125 | 46.50 | 625 | 46.50 | 2,500 | 46.50 | |
| Female | 3,595 | 53.50 | 719 | 53.50 | 2,876 | 53.50 | |
| Age (yrs) | 75.08 ± 9.34 | 78.14 ± 7.17 | 74.31 ± 9.66 | <0.001 | |||
| Age group (yrs) | <0.001 | ||||||
| 50–59 | 203 | 3.02 | 10 | 0.74 | 193 | 3.59 | 0.155 |
| 60–69 | 1,148 | 17.08 | 183 | 13.62 | 965 | 17.95 | 0.036 |
| 70–79 | 2,490 | 37.05 | 572 | 42.56 | 1,918 | 35.68 | 0.003 |
| ≧80 | 2,879 | 42.84 | 579 | 43.08 | 2,300 | 42.78 | 0.897 |
| DM | <0.001 | ||||||
| Without | 4,569 | 67.99 | 705 | 52.46 | 3,864 | 71.88 | |
| With | 2,151 | 32.01 | 639 | 47.54 | 1,512 | 28.13 | |
| HTN | 0.961 | ||||||
| Without | 3,414 | 50.80 | 682 | 50.74 | 2,732 | 50.82 | |
| With | 3,306 | 49.20 | 662 | 49.26 | 2,644 | 49.18 | |
| Hyperlipidemia | <0.001 | ||||||
| Without | 4,600 | 68.45 | 675 | 50.22 | 3,925 | 73.01 | |
| With | 2,120 | 31.55 | 669 | 49.78 | 1,451 | 26.99 | |
| CAD | <0.001 | ||||||
| Without | 4,690 | 69.79 | 760 | 56.55 | 3,930 | 73.10 | |
| With | 2,030 | 30.21 | 584 | 43.45 | 1,446 | 26.90 | |
| Asthma | <0.001 | ||||||
| Without | 5,857 | 87.16 | 1,056 | 78.57 | 4,801 | 89.30 | |
| With | 863 | 12.84 | 288 | 21.43 | 575 | 10.70 | |
| COPD | <0.001 | ||||||
| Without | 4,603 | 68.50 | 677 | 50.37 | 3,926 | 73.03 | |
| With | 2,117 | 31.50 | 667 | 49.63 | 1,450 | 26.97 | |
| Stroke | <0.001 | ||||||
| Without | 5,012 | 74.58 | 823 | 61.24 | 4,189 | 77.92 | |
| With | 1,708 | 25.42 | 521 | 38.76 | 1,187 | 22.08 | |
| Tobacco dependency | 0.097 | ||||||
| Without | 6,672 | 99.29 | 1,326 | 98.66 | 5,346 | 99.44 | |
| With | 48 | 0.71 | 18 | 1.34 | 30 | 0.56 | |
| HF | <0.001 | ||||||
| Without | 5,861 | 87.22 | 1,059 | 78.79 | 4,802 | 89.32 | |
| With | 859 | 12.78 | 285 | 21.21 | 574 | 10.68 | |
| Dementia | 0.003 | ||||||
| Without | 6,066 | 90.27 | 1,141 | 84.90 | 4,925 | 91.61 | |
| With | 654 | 9.73 | 203 | 15.10 | 451 | 8.39 | |
| Other neurological disorders | 0.794 | ||||||
| Without | 6,544 | 97.38 | 1,300 | 96.73 | 5,244 | 97.54 | |
| With | 176 | 2.62 | 44 | 3.27 | 132 | 2.46 | |
| Degenerative arthritis | <0.001 | ||||||
| Without | 5,377 | 80.01 | 199 | 14.81 | 5,178 | 96.32 | |
| With | 1,343 | 19.99 | 1,145 | 85.19 | 198 | 3.68 | |
| CCI_R | 0.19 ± 0.77 | 0.13 ± 0.64 | 0.20 ± 0.80 | 0.003 | |||
| Number of NHI claims for ophthalmic outpatient visits | 7.86 ± 8.02 | 8.02 ± 8.11 | 7.85 ± 8.01 | 0.443 | |||
P: Chi-square / Fisher exact test on category variables, t-test on continue variables, and proportional test for percentage. GlcN: glucosamine; AMD: age-related macular degeneration; DM: diabetes mellitus; HTN: hypertension; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; HF: heart failure; CCI: Charlson comorbidity index.
Additionally, the mean age at the tracking endpoint was higher among patients with GlcN use (78.14±7.17 years) than those without GlcN use (74.31±9.66 years) (P<0.001). The percentage of patients in the age groups of 50–59 years and ≧80 years did not significantly differ between the two cohorts, whereas that for the age groups of 60–69 years and 70–79 years significantly differed between the two cohorts (P = 0.036 and P = 0.003, respectively). In addition, the rates of comorbid DM, hyperlipidemia, CAD, Asthma, COPD, stroke, HF, dementia, and degenerative arthritis, were significantly higher in patients with GlcN use than those without GlcN use. The CCI scores were lower in patients with GlcN use than patients without GlcN use (P = 0.003). The number of NHI claims for ophthalmic outpatient visits was 8.02 ± 8.11 in patients with GlcN use and 7.85 ± 8.01 in those without GlcN use. There was no significant difference between two cohorts (P = 0.443).
Cumulative risk of AMD by Kaplan-Meier analysis
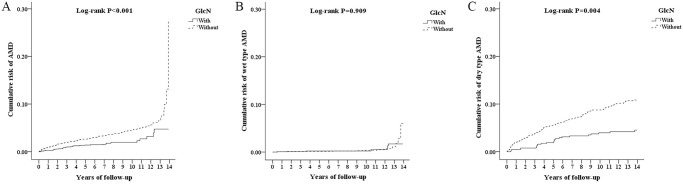
As shown in Fig 2A, the cumulative risk of AMD at the end of the 14-year follow-up period was significantly lower in patients with GlcN use than those without GlcN use (log-rank P <0.001). The result of the cumulative risk in AMD subtypes showed that wet type AMD did not significantly differ between the two cohorts (Fig 2B, log-rank P = 0.909), but dry type AMD was significantly lower in patients with GlcN use than those without GlcN use (Fig 2C, log-rank P = 0.004).
Fig 2. Cumulative risk of AMD in patients stratified by GlcN use, calculated by the Kaplan-Meier method with log-rank test.
(A) all AMD; (B) Wet type AMD; (C) Dry type AMD. AMD, Age-related macular degeneration; GlcN, Glucosamine.
Factors associated with the development of AMD by univariate and multivariate analyses
The results of univariate and multivariate analyses for the risk factors associated with the development of AMD are shown in Table 2. After adjustment for age, gender, comorbidities, and the number of ophthalmic outpatient visits, patients with GlcN use showed a decreased risk for developing AMD compared to that for those without GlcN use (aHR = 0.756; p = 0.009).
Table 2. Factors associated with the development of AMD as evaluated by Cox regression.
| Variables | Crude HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| GlcN | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.507 | 0.374 | 0.688 | <0.001 | 0.756 | 0.581 | 0.919 | 0.009 |
| Gender | ||||||||
| Male | 1.709 | 0.869 | 1.338 | 0.491 | 1.149 | 0.909 | 1.464 | 0.286 |
| Female | Reference | Reference | ||||||
| Age group (yrs) | ||||||||
| 50–59 | Reference | Reference | ||||||
| 60–69 | 1.428 | 1.099 | 1.856 | 0.008 | 1.320 | 0.953 | 1.708 | 0.129 |
| 70–79 | 1.446 | 1.005 | 1.831 | 0.045 | 1.336 | 0.984 | 1.741 | 0.063 |
| ≧80 | 3.039 | 2.090 | 4.418 | <0.001 | 1.889 | 1.349 | 2.025 | 0.007 |
| DM | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.553 | 1.410 | 1.694 | <0.001 | 1.768 | 0.591 | 2.006 | 0.063 |
| HTN | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.646 | 1.360 | 1.598 | <0.001 | 1.847 | 0.633 | 2.096 | 0.287 |
| Hyperlipidemia | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.530 | 0.408 | 0.688 | <0.001 | 0.687 | 0.530 | 0.901 | 0.003 |
| CAD | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.356 | 0.269 | 0.471 | <0.001 | 0.576 | 0.427 | 0.787 | <0.001 |
| Asthma | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.413 | 0.275 | 1.260 | 0.297 | 1.122 | 0.754 | 1.894 | 0.529 |
| COPD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.301 | 1.244 | 1.402 | <0.001 | 1.403 | 1.285 | 1.528 | <0.001 |
| Stroke | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.306 | 1.221 | 1.425 | <0.001 | 1.535 | 1.383 | 1.751 | <0.001 |
| Tobacco dependency | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.688 | 0.172 | 2.746 | 0.588 | 1.032 | 0.255 | 4.224 | 0.925 |
| HF | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.792 | 0.197 | 1.488 | 0.245 | 1.172 | 1.003 | 1.930 | 0.047 |
| Dementia | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.862 | 0.089 | 2.314 | 0.358 | 1.269 | 1.135 | 1.555 | <0.001 |
| Other neurological disorders | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.288 | 0.097 | 0.897 | 0.034 | 0.487 | 0.182 | 1.451 | 0.198 |
| Degenerative arthritis | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.986 | 0.876 | 2.897 | 0.702 | 1.865 | 0.732 | 2.786 | 0.711 |
| CCI_R | 1.323 | 0.975 | 2.633 | 0.314 | 1.059 | 0.450 | 1.778 | 0.882 |
| Number of NHI claims for ophthalmic outpatient visits | 1.009 | 1.001 | 1.018 | 0.046 | 1.004 | 0.993 | 1.010 | 0.055 |
HR = hazard ratio, CI = confidence interval, Adjusted HR: Adjusted variables listed in the table. GlcN: glucosamine; AMD: age-related macular degeneration; DM: diabetes mellitus; HTN: hypertension; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; HF: heart failure; CCI: Charlson comorbidity index.
Additionally patients aged ≧80 years showed an increased risk for developing AMD (aHR = 1.889; p = 0.007) compared to those aged 50–59 years. Interestingly, patients with hyperlipidemia or CAD showed a decreased risk of developing AMD, whereas those with COPD, stroke, HF, or dementia showed an increased risk of developing AMD compared to those without these comorbidities. In addition, the risk of developing AMD was affected in Male or patients with comorbidities (HTN, Asthma, tobacco dependency, other neurological disorders, or degenerative arthritis), but there was no statistical significance.
Stratified analyses comparing the risk of developing AMD between the two cohorts according to background factors
Table 3 provides the results of stratified analyses comparing the risk of developing AMD between patients with and without GlcN use according to each evaluated variable. The risk of developing AMD was lower in patients with GlcN use than those without GlcN use for both gender and for 60–69 and 70–79 age groups; however, the aHR increased with increasing age. Furthermore, the risk of developing AMD was lower in patients with GlcN use than patients without GlcN use among those with comorbidities (hyperlipidemia and CAD), or without comorbidities (DM, HTN, asthma, tobacco dependency, HF, and dementia). Besides, regardless of patients with or without comorbidities (COPD, stroke, other neurological disorders, and degenerative arthritis), patients with GlcN use could decrease the risk of developing AMD compared to those without GlcN use.
Table 3. Stratified Cox regression analyses comparing the risk of developing AMD between the two cohorts according to background factors.
| GlcN | With | Without | With vs. Without (Reference) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Events | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | Ratio | Adjusted HR | 95% CI | 95% CI | P |
| Total | 49 | 15,375.87 | 318.68 | 283 | 39,259.07 | 720.85 | 0.442 | 0.756 | 0.581 | 0.919 | 0.009 |
| Gender | |||||||||||
| Male | 26 | 5,631.56 | 461.68 | 152 | 17,298.14 | 878.71 | 0.525 | 0.899 | 0.690 | 1.092 | 0.062 |
| Female | 23 | 9,744.31 | 236.04 | 131 | 21,960.93 | 596.51 | 0.396 | 0.676 | 0.520 | 0.822 | <0.001 |
| Age group (years) | |||||||||||
| 50–59 | 0 | 1,233.08 | 0.00 | 39 | 1,783.28 | 2,186.98 | 0.000 | 0.000 | - | - | 0.997 |
| 60–69 | 6 | 2,759.74 | 217.41 | 60 | 6,258.46 | 958.70 | 0.227 | 0.388 | 0.298 | 0.471 | <0.001 |
| 70–79 | 19 | 7,164.46 | 265.20 | 107 | 16,620.06 | 643.80 | 0.412 | 0.704 | 0.541 | 0.856 | 0.006 |
| ≧80 | 24 | 4,218.59 | 568.91 | 77 | 14,597.27 | 527.50 | 1.079 | 1.844 | 0.940 | 2.241 | 0.285 |
| DM | |||||||||||
| Without | 33 | 7,522.00 | 438.71 | 224 | 22,376.23 | 1,001.06 | 0.438 | 0.749 | 0.576 | 0.911 | 0.002 |
| With | 16 | 7,853.87 | 203.72 | 59 | 16,882.83 | 349.47 | 0.583 | 0.998 | 0.765 | 1.211 | 0.156 |
| HTN | |||||||||||
| Without | 17 | 5,991.86 | 283.72 | 155 | 9,778.49 | 1,585.11 | 0.179 | 0.306 | 0.235 | 0.372 | <0.001 |
| With | 32 | 9,384.01 | 341.01 | 128 | 29,480.58 | 434.18 | 0.785 | 1.344 | 0.936 | 1.632 | 0.271 |
| Hyperlipidemia | |||||||||||
| Without | 31 | 5,817.22 | 532.90 | 213 | 21,911.45 | 972.09 | 0.548 | 0.938 | 0.720 | 1.139 | 0.125 |
| With | 18 | 9,558.65 | 188.31 | 70 | 17,347.62 | 403.51 | 0.467 | 0.798 | 0.612 | 0.970 | <0.001 |
| CAD | |||||||||||
| Without | 31 | 6,948.29 | 446.15 | 200 | 21,714.05 | 921.06 | 0.484 | 0.829 | 0.636 | 1.007 | 0.053 |
| With | 18 | 8,427.58 | 213.58 | 83 | 17,545.02 | 473.07 | 0.451 | 0.772 | 0.593 | 0.939 | 0.001 |
| Asthma | |||||||||||
| Without | 41 | 11,752.59 | 348.86 | 263 | 32,644.55 | 805.65 | 0.433 | 0.740 | 0.569 | 0.900 | <0.001 |
| With | 8 | 3,623.28 | 220.79 | 20 | 6,614.51 | 302.37 | 0.730 | 1.249 | 0.959 | 1.517 | 0.355 |
| COPD | |||||||||||
| Without | 28 | 6,409.00 | 436.89 | 202 | 21,381.41 | 944.75 | 0.462 | 0.791 | 0.607 | 0.961 | <0.001 |
| With | 21 | 8,966.87 | 234.20 | 81 | 17,877.66 | 453.08 | 0.517 | 0.884 | 0.678 | 0.995 | 0.048 |
| Stroke | |||||||||||
| Without | 38 | 9,373.10 | 405.42 | 228 | 24,946.32 | 913.96 | 0.444 | 0.759 | 0.583 | 0.922 | <0.001 |
| With | 11 | 6,002.77 | 183.25 | 55 | 14,312.74 | 384.27 | 0.477 | 0.816 | 0.626 | 0.991 | 0.042 |
| Tobacco dependency | |||||||||||
| Without | 49 | 14,938.77 | 328.01 | 281 | 38,920.91 | 721.98 | 0.454 | 0.777 | 0.597 | 0.944 | 0.001 |
| With | 0 | 437.10 | 0.00 | 2 | 338.16 | 591.44 | 0.000 | 0.000 | - | - | 0.897 |
| HF | |||||||||||
| Without | 39 | 11,953.40 | 326.27 | 238 | 30,849.79 | 771.48 | 0.423 | 0.723 | 0.556 | 0.879 | <0.001 |
| With | 10 | 3,422.47 | 292.19 | 45 | 8,409.28 | 535.12 | 0.546 | 0.934 | 0.717 | 1.135 | 0.496 |
| Dementia | |||||||||||
| Without | 49 | 13,943.65 | 351.41 | 280 | 34,034.57 | 822.69 | 0.427 | 0.730 | 0.561 | 0.888 | <0.001 |
| With | 0 | 1,432.23 | 0.00 | 3 | 5,224.50 | 57.42 | 0.000 | 0.000 | - | - | 0.916 |
| Other neurological disorders | |||||||||||
| Without | 47 | 13,964.03 | 336.58 | 277 | 37,532.02 | 738.04 | 0.456 | 0.780 | 0.599 | 0.948 | 0.026 |
| With | 2 | 1,411.84 | 141.66 | 6 | 1,727.05 | 347.41 | 0.408 | 0.697 | 0.536 | 0.847 | <0.001 |
| Degenerative arthritis | |||||||||||
| Without | 13 | 2,078.37 | 625.49 | 279 | 37,929.67 | 735.57 | 0.850 | 0.724 | 0.488 | 0.801 | <0.001 |
| With | 36 | 13,297.50 | 270.73 | 4 | 1,329.40 | 300.89 | 0.900 | 0.867 | 0.596 | 0.948 | 0.011 |
PYs: Person-years; Adjusted HR: Adjusted Hazard ratio: Adjusted for the variables listed in Table 2; CI: confidence interval; GlcN: glucosamine; AMD: age-related macular degeneration; DM: diabetes mellitus; HTN: hypertension; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; HF: heart failure.
Stratified analyses according to AMD type
The risk of developing wet type AMD did not significantly differ between two cohorts (aHR = 0.987; P = 0.186), whereas the risk of developing dry type AMD was 0.663-fold less in patients with GlcN use than those without GlcN use (P = 0.009) (Table 4).
Table 4. Stratified Cox regression analyses according to AMD type.
| GlcN | With | Without (Reference) | With vs. Without (Reference) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMD subgroup | Events | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | Ratio | Adjusted HR | 95% CI | 95% CI | P |
| All type | 49 | 15,375.87 | 318.68 | 283 | 39,259.07 | 720.85 | 0.442 | 0.756 | 0.581 | 0.919 | 0.009 |
| Wet type | 10 | 15,375.87 | 65.04 | 23 | 39,259.07 | 58.59 | 1.110 | 0.987 | 0.746 | 1.485 | 0.186 |
| Dry type | 39 | 15,375.87 | 253.64 | 260 | 39,259.07 | 662.27 | 0.383 | 0.663 | 0.482 | 0.810 | 0.009 |
AMD: age-related macular degeneration; PYs: Person-years; Adjusted HR: Adjusted Hazard ratio: Adjusted for the variables listed in Table 2; CI: confidence interval.
Association between the duration of GlcN use and the development of AMD
As shown in Table 5, after adjustment for covariates, the duration of GlcN use impacted the development of AMD (all type), but only patient with GlcN use≧3 years has statistical significance (aHR = 0.456; p = 0.011). Furthermore, we analyzed this relationship in the wet and dry type AMD. The result shown that there was no significant association between the druation of GlcN use and the development of wet type AMD. Interestingly, the development of dry type AMD was related to the duration of GlcN use, especially those with GlcN use ≧1 year but <3 year (aHR = 0.742; P = 0.043) and GlcN use ≧3 years (aHR = 0.493; P = 0.003) subgroup. Taken together, this result demonstrated that the effect of GlcN on the reduced risk of developing dry type AMD was depended on the duration of GlcN use.
Table 5. Association between the duration of GlcN use and the development of AMD.
| AMD subgroup | GlcN usage | Events | PYs | Rate (per 105 PYs) | Crude HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All type | 0 (Without) | 283 | 39,259.07 | 720.85 | Reference | Reference | ||||||
| <1 year | 33 | 10,218.30 | 322.95 | 0.537 | 0.309 | 0.984 | 0.019 | 0.779 | 0.433 | 1.420 | 0.416 | |
| ≧1 year, <3 years | 11 | 3,220.81 | 341.53 | 0.508 | 0.927 | 0.929 | 0.027 | 0.713 | 0.389 | 1.345 | 0.271 | |
| ≧3 years | 5 | 1,936.76 | 258.16 | 0.302 | 0.125 | 0.732 | 0.009 | 0.456 | 0.183 | 0.896 | 0.011 | |
| Wet type | 0 (Without) | 23 | 39,259.07 | 58.59 | Reference | Reference | ||||||
| <1 year | 9 | 10,218.30 | 88.08 | 1.036 | 0.356 | 3.021 | 0.924 | 1.657 | 0.658 | 7.121 | 0.432 | |
| ≧1 year, <3 years | 1 | 3,220.81 | 31.05 | 0.289 | 0.036 | 2.099 | 0.922 | 0.593 | 0.077 | 4.625 | 0.621 | |
| ≧3 years | 0 | 1,936.76 | 0.00 | 0.000 | - | - | 0.960 | 0.000 | - | - | 0.989 | |
| Dry type | 0 (Without) | 260 | 39,259.07 | 662.27 | Reference | Reference | ||||||
| <1 year | 24 | 10,218.30 | 234.87 | 0.449 | 0.227 | 0.882 | 0.006 | 0.606 | 0.303 | 1.212 | 0.142 | |
| ≧1 year, <3 years | 10 | 3,220.81 | 310.48 | 0.543 | 0.289 | 0.954 | 0.036 | 0.742 | 0.389 | 0.986 | 0.043 | |
| ≧3 years | 5 | 1,936.76 | 258.16 | 0.360 | 0.149 | 0.702 | 0.001 | 0.493 | 0.199 | 0.775 | 0.003 |
AMD: age-related macular degeneration; PYs: Person-years; Adjusted HR: Adjusted Hazard ratio: Adjusted for the variables listed in Table 2; CI: confidence interval.
Discussion
The current study revealed that, after adjusting for covariates, patients with GlcN treatment had a lower risk of developing AMD compared to that in matched patients without GlcN treatment. Furthermore, stratified analyses according to AMD subtype showed that patients who used GlcN had a decreased risk of developing dry type AMD, but not wet type AMD, compared to those without GlcN use. Similarly, the Kaplan-Meier analysis revealed that patients with GlcN treatment had a significantly lower risk of dry type AMD than those without GlcN treatment. Interestingly, GlcN use was associated with a decreased risk of developing AMD in both gender, 60–69 and 70–79 age groups, and patients with comorbid hyperlipidemia, CAD, COPD, stroke, other neurological disorders, or degenerative arthritis. We also found that durations of GlcN treatment ≧1 year were associated with decreased risk of dry type AMD. This is the first population-based study to investigate and demonstrate a relationship between GlcN use and the risk of developing AMD, especially dry type AMD.
AMD is known for photoreceptor death and RPE degeneration, with chronic inflammation, whereas GlcN has been shown to decrease inflammation in cellular and animal studies [11–13]. One pathological hallmark of AMD is the degeneration of RPE cells due to an excessive accumulation of lipofuscin, which forms reactive oxygen species. In vitro studies have shown that GlcN can induce autophagy through the AMP-activated protein kinase-mammalian target of rapamycin pathway, thereby reducing the increase in LLAF in native POS-treated ARPE-19 cells [15]. Autophagy is a self-destructive process, in which autophagosomes containing unused or damaged intracellular components are delivered to the lysosome for degradation. The timing of autophagy is important. In the late stages of AMD, autophagy may exacerbate the disease if the RPE is damaged past a critical point. A similar concept was performed in an animal model of Alzheimer’s disease (AD) [16].
AMD is one of progressive chronic disease. It progresses slowly from early stage to intermediate stage and ultimately late stage, either neovascular AMD (wet AMD) or geographic atrophy (GA, late stage of dry AMD). The progression of AMD is related to the elevated oxidative stress and lipid peroxidation in RPEcells [17]. Our previous study has reported that GlcN reduced the native POS- induced LLAF in RPE cells, but did not influence lipid peroxidation (Malondialdehyde or 4-hydroxynonenal) -modified POS induced LLAF [15]. In present study, we also found that GlcN use only decreased the risk of developing of dry type AMD, not wet type AMD. This result may mean that GlcN treatment plays a role to slowdown the progression from aging retina to early stages of dry AMD, but not early to late stages of AMD (GA or wet AMD). However, further prospective studies are necessary to confirm this association between GlcN treatment and the subtypes of AMD.
AMD is a multifactorial disease, and includes age, smoking, genetic variants and environmental factors [18–20]. By far, age is the strongest risk factor for AMD [18, 19]. The prevalence of AMD has been reported as 0.2% for those aged 55–64 years and 13.1% for those aged greater than 85 years [18]. Previously, one regional study of Taiwan has reported that the risk of AMD was significantly higher in older populations, with age over 65 years, and the prevalence increased from 5% in those aged 65–69 years to 24.4% in those aged over 80 years [4]. Consistent with previous studies [4, 18, 19], our result also demonstrated that age is a significant risk factor for the development of AMD. In addition, we also found that patients with GlcN use were significantly older than those without GlcN use at the tracking endpoint. To explain this result, we further analyzed the tracking period (between the index date and the tracking endpoint) of the study and comparison cohort. As shown in S1 Table, we found that patients with GlcN use had a longer period to develop AMD (mean±SD = 4.84 ± 3.96 years) than patients without GlcN use (mean±SD = 3.28 ± 3.36). This may explain why patients in comparison cohort (who supposed were age-matched with patients who were prescribed GlcN) were younger than those treated with GlcN at the tracking endpoint. Furthermore, in age groups of 60–69 and 70–79 years, patients with GlcN treatment have a lower risk of AMD than those without GlcN treatment.
In addition, previous studies have shown a higher incidence of AMD in women [4, 18], whereas other studies reported a higher incidence of AMD in men [21, 22]. In this study, our results found that compared to women, men had a slight increase of risk for AMD, but there was no statistical difference (aHR = 1.149, P = 0.286). This result is not consistent to the Shihpai Eye study of Taiwan [4]. One possible explanation was that our study encompassed almost the entire population in Taiwan and the enrolled population was different in studies.
Smoking, one modifiable risk factor, has also been strongly associated with AMD in several population studies [18, 23]. It can increase oxidative stress and vascular endothelial growth factor expression, and activate the immune system, particularly the alternative pathway through the complement system [22, 24]. In our study, we observed an increased risk of AMD in patients with tobacco dependency, but there was no statistical significance (aHR = 1.032, P = 0.925). This might be due to that patients with tobacco dependency are those who are addicted to tobacco to the point of hindering the patient’s social functioning and health, not included regular smokers who may or may not be addicted. Being retrospective in nature, our study could not obtain data regarding the smoking habits of each patient. Furthermore, there was no tobacco dependency patient in our GlcN treatment cohort; hence, it is difficult to interpret the results regarding GlcN use and AMD risk in patients with tobacco dependency.
There are multiple systemic risk factors associated with AMD include obesity, cardiovascular disease, hypertension, asthma, emphysema, and dementia [19, 25, 26]. In present study, we also found that several comorbidities increased the risk of AMD, including COPD, stroke, HF, and dementia compared to patients without these comorbidities. Due to COPD included emphysema and chronic bronchitis, this might explain that COPD was associated with a significantly increased risk of AMD in present study. In addition, patients with GlcN use could decrease the risk of developing AMD in patients with asthma or COPD. One possible explanation was that GlcN possesses anti-inflammatory effect and the pathogenesis of AMD, asthma, and COPD are associated with chronic inflammation [27, 28].
GlcN use was associated with a decreased risk of AMD among patients with CAD, stroke, and hyperlipidemia. A recent prospective study using United Kingdom Biobank data from 466,039 participants similarly found a lower risk of cardiovascular events in GlcN users, including those with CAD (HR: 0.82) and stroke (HR: 0.91) [29]. Another study using United Kingdom Biobank data found that GlcN was also associated with lower mortality in patients with cancer and cardiovascular disease [30]. This could be explained by GlcN’s anti-inflammation effects, with antioxidative stress-induced senescence and induced autophagy as shown in in vitro studies [12, 14, 15].
A recent meta-analysis reported a significantly increased risk of AMD in patients with dementia or Alzheimer’s disease (AD), and AMD was associated with cognitive impairment [26]. AD is the most prevalent cause of dementia. The present study results suggest that there is a significantly increased risk of AMD in patients with dementia. Not only AMD and dementia share similar risk factors, but also their pathogeneses show similarities as well. AD is known for the deposition of amyloid β aggregates in the central nervous system and retina, and AMD is known for amyloid β deposits in drusens and the RPE from studies with donor human eyes [26, 31]. A cross-sectional study reported that GlcN is associated with beneficial cognitive function [32] and in in vivo studies, GlcN can increase the level of brain-derived neurotrophic factor, which is involved in memory consolidation and cognitive function [33]. With these numerous similarities, one can hypothesize that GlcN may be beneficial for patients with AMD as well; however, further research is needed. It would be interesting to ascertain the risk of AMD in patients with dementia taking a GlcN supplement; the current study did not have patients in this category.
In the present study, patients with GlcN use had a lower prevalence of AMD, with a statistical significance of p = 0.005. Due to its immunosuppressive activity, seen in vitro and in vivo studies, GlcN has been widely used as an alternative supplement in osteoarthritis and rheumatoid arthritis to prevent joint space narrowing and reduce osteoarthritis-related surgeries [34]. Indeed, the prescription of GlcN was covered and regulated by the National Health Insurance (NHI) in Taiwan before 2018 for patients that met the following criteria: age >60 years, Ahlback classification of severity of knee osteoarthritis <stage 3, osteoarthritis symptoms lasting >6 months, and a Lequesne’s severity index for knee osteoarthritis of ≥7 points. A maximum dose of 750 mg/day of GlcN, with 2 courses of a 3-month treatment each year, can be reimbursed by the NHI. Thus, this might been the reason that the rates of degenerative arthritis was significantly higher in patients with GlcN use than those without GlcN use. Moreover, despite patient with or without degenerative arthritis, the result of the stratified analysis demonstrated that patients with GlcN use decreased the risk of developing AMD compared to those without GlcN use. In addition, the duration of GlcN use also affected the risk of AMD. The present study showed that GlcN use for ≥1 year but <3 years, and especially GlcN use for ≥3 years, was associated with a decreased risk of developing dry type AMD. Taken together, these results demonstrate that GlcN use for ≥1 year decreased the risk of dry type AMD, and suggest that the longer glucosamine treatment, the lower the risk of AMD.
To the best of our knowledge, the current study is the first population-based study to evaluate the association of GlcN use with the risk of AMD. Since GlcN was insured by the NHI, we were able to track the duration of GlcN use in our study cohort, an advantage of the current study. We were also able to observe the effect of GlcN in each AMD subtype. Our sample size was large and had long-term follow up, which provided considerable statistical power, and may better reflect the real-world situation than city-based or hospital-based studies.
There are several limitations to our study. First, the NHI stopped funding GlcN prescriptions after October 2018; hence, data could only be obtained before 2018. Secondly, data regarding the dietary habits of each patient could not be obtained; thus, it is unknown whether patients used GlcN supplements by other means. However, it is reasonable to assume that a majority of the patients would not pay the extra cost for GlcN supplements if they could acquire GlcN through the NHI (due to the burden of healthcare costs). Finally, this study was retrospective in nature, and the database lacked imaging examination findings to confirm the diagnosis. Thus, misclassifications are possible. Nevertheless, this study comprised a real-world population study.
In conclusion, this retrospective, population-based cohort study revealed that patients with GlcN use had a lower risk of developing dry type AMD than those without GlcN use. Furthermore, age, COPD, stroke, HF, and dementia were associated with a higher risk of developing AMD; however, GlcN use decreased the risk of AMD in those with older age, hyperlipidemia, CAD, COPD, stroke, other neurologic disorders, or degenerative arthritis. Additionally, the decrease of the developing dry type AMD in patients with GlcN use was depended on the duration of GlcN treatment, especially those with GlcN use ≧1 year. Future well-designed clinical trials are needed to confirm this association between GlcN use and AMD risk.
Supporting information
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This research was supported in part by Grant MOST 107-2314-B-016 -031-MY3 from the Ministry of Science and Technology, Taiwan, Republic of China; Grant TSGH-D-110112, TSGH-D-110109, TSGH-B-110012 from the Tri-Service General Hospital, Taiwan, Republic of China; Grant MND-MAB-110-084, MAB-E-110001 from the Ministry of National Defense, Taiwan, Republic of China. All the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. The New England journal of medicine. 2008;358(24):2606–17. Epub 2008/06/14. 10.1056/NEJMra0801537 . [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global health. 2014;2(2):e106–16. Epub 2014/08/12. 10.1016/S2214-109X(13)70145-1 . [DOI] [PubMed] [Google Scholar]
- 3.Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease Burden in the Treatment of Age-Related Macular Degeneration: Findings From a Time-and-Motion Study. American journal of ophthalmology. 2015;160(4):725–31.e1. Epub 2015/07/06. 10.1016/j.ajo.2015.06.023 . [DOI] [PubMed] [Google Scholar]
- 4.Chen SJ, Cheng CY, Peng KL, Li AF, Hsu WM, Liu JH, et al. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Investigative ophthalmology & visual science. 2008;49(7):3126–33. Epub 2008/04/09. 10.1167/iovs.08-1803 . [DOI] [PubMed] [Google Scholar]
- 5.Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Survey of ophthalmology. 1995;39(5):367–74. Epub 1995/03/01. 10.1016/s0039-6257(05)80092-x . [DOI] [PubMed] [Google Scholar]
- 6.Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Quiroz-Mercado H, Mandava N. Current knowledge and trends in age-related macular degeneration: genetics, epidemiology, and prevention. Retina (Philadelphia, Pa). 2014;34(3):423–41. Epub 2013/11/29. 10.1097/iae.0000000000000036 . [DOI] [PubMed] [Google Scholar]
- 7.Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Archives of ophthalmology. 1984;102(11):1640–2. Epub 1984/11/01. 10.1001/archopht.1984.01040031330019 . [DOI] [PubMed] [Google Scholar]
- 8.Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2005;43(2):187–201. Epub 2004/12/29. 10.1016/j.fct.2004.11.006 . [DOI] [PubMed] [Google Scholar]
- 9.Gouze JN, Bianchi A, Bécuwe P, Dauça M, Netter P, Magdalou J, et al. Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-κB pathway. FEBS Letters. 2002;510(3):166–70. 10.1016/s0014-5793(01)03255-0 [DOI] [PubMed] [Google Scholar]
- 10.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. The Lancet. 2001;357(9252):251–6. 10.1016/S0140-6736(00)03610-2 [DOI] [PubMed] [Google Scholar]
- 11.Chen J-T, Liang J-B, Chou C-L, Chien M-W, Shyu R-C, Chou P-I, et al. Glucosamine Sulfate Inhibits TNF-α and IFN-γ-Induced Production of ICAM-1 in Human Retinal Pigment Epithelial Cells In Vitro. Investigative ophthalmology & visual science. 2006;47(2):664–72. 10.1167/iovs.05-1008 [DOI] [PubMed] [Google Scholar]
- 12.Chen C-L, Liang C-M, Chen Y-H, Tai M-C, Lu D-W, Chen J-T. Glucosamine Modulates TNF-α–Induced ICAM-1 Expression and Function Through O-Linked and N-Linked Glycosylation in Human Retinal Pigment Epithelial Cells. Investigative ophthalmology & visual science. 2012;53(4):2281–91. 10.1167/iovs.11-9291 [DOI] [PubMed] [Google Scholar]
- 13.Chang Y-H, Horng C-T, Chen Y-H, Chen P-L, Chen C-L, Liang C-M, et al. Inhibitory Effects of Glucosamine on Endotoxin-Induced Uveitis in Lewis Rats. Investigative ophthalmology & visual science. 2008;49(12):5441–9. 10.1167/iovs.08-1784 [DOI] [PubMed] [Google Scholar]
- 14.Chen C-L, Chen Y-H, Liang C-M, Tai M-C, Chen J-T. Glucosamine attenuates hydrogen peroxide-induced premature senescence in human retinal pigment epithelial cells in vitro. Journal of Medical Sciences. 2018;38(1):16–23. 10.4103/jmedsci.jmedsci_138_17 [DOI] [Google Scholar]
- 15.Chen CL, Chen YH, Liang CM, Tai MC, Lu DW, Chen JT. Glucosamine-Induced Autophagy through AMPK(-)mTOR Pathway Attenuates Lipofuscin-Like Autofluorescence in Human Retinal Pigment Epithelial Cells In Vitro. International journal of molecular sciences. 2018;19(5). Epub 2018/05/12. 10.3390/ijms19051416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6(9):e25416. Epub 2011/10/08. 10.1371/journal.pone.0025416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–18. Epub 2017/03/25. 10.1016/j.preteyeres.2017.03.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108(4):697–704. Epub 2001/04/12. 10.1016/s0161-6420(00)00580-7 . [DOI] [PubMed] [Google Scholar]
- 19.Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC ophthalmology. 2010;10:31. Epub 2010/12/15. 10.1186/1471-2415-10-31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147–59. Epub 2018/10/12. 10.1016/S0140-6736(18)31550-2 . [DOI] [PubMed] [Google Scholar]
- 21.Cheung CM, Tai ES, Kawasaki R, Tay WT, Lee JL, Hamzah H, et al. Prevalence of and risk factors for age-related macular degeneration in a multiethnic Asian cohort. Archives of ophthalmology. 2012;130(4):480–6. Epub 2011/12/14. 10.1001/archophthalmol.2011.376 . [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Cruickshanks KJ, Nash SD, Krantz EM, Nieto FJ, Huang GH, et al. The prevalence of age-related macular degeneration and associated risk factors. Archives of ophthalmology. 2010;128(6):750–8. Epub 2010/06/16. 10.1001/archophthalmol.2010.92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye (Lond). 2005;19(9):935–44. Epub 2005/09/10. 10.1038/sj.eye.6701978 . [DOI] [PubMed] [Google Scholar]
- 24.Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313–30. Epub 2017/09/02. 10.2147/CIA.S143508 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein R, Knudtson MD, Klein BE. Pulmonary disease and age-related macular degeneration: the Beaver Dam Eye Study. Archives of ophthalmology. 2008;126(6):840–6. Epub 2008/06/11. 10.1001/archopht.126.6.840 . [DOI] [PubMed] [Google Scholar]
- 26.Rong SS, Lee BY, Kuk AK, Yu XT, Li SS, Li J, et al. Comorbidity of dementia and age-related macular degeneration calls for clinical awareness: a meta-analysis. Br J Ophthalmol. 2019;103(12):1777–83. Epub 2019/04/20. 10.1136/bjophthalmol-2018-313277 . [DOI] [PubMed] [Google Scholar]
- 27.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73(9):1765–86. Epub 2016/02/08. 10.1007/s00018-016-2147-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond). 2017;131(13):1541–58. Epub 2017/07/01. 10.1042/CS20160487 . [DOI] [PubMed] [Google Scholar]
- 29.Ma H, Li X, Sun D, Zhou T, Ley SH, Gustat J, et al. Association of habitual glucosamine use with risk of cardiovascular disease: prospective study in UK Biobank. BMJ. 2019;365:l1628. Epub 2019/05/16. 10.1136/bmj.l1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li ZH, Gao X, Chung VC, Zhong WF, Fu Q, Lv YB, et al. Associations of regular glucosamine use with all-cause and cause-specific mortality: a large prospective cohort study. Ann Rheum Dis. 2020;79(6):829–36. Epub 2020/04/08. 10.1136/annrheumdis-2020-217176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isas JM, Luibl V, Johnson LV, Kayed R, Wetzel R, Glabe CG, et al. Soluble and mature amyloid fibrils in drusen deposits. Investigative ophthalmology & visual science. 2010;51(3):1304–10. Epub 2009/11/07. 10.1167/iovs.09-4207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nevado-Holgado AJ, Kim CH, Winchester L, Gallacher J, Lovestone S. Commonly prescribed drugs associate with cognitive function: a cross-sectional study in UK Biobank. BMJ Open. 2016;6(11):e012177. Epub 2016/12/03. 10.1136/bmjopen-2016-012177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou LY, Chao YM, Peng YC, Lin HC, Wu YL. Glucosamine Enhancement of BDNF Expression and Animal Cognitive Function. Molecules. 2020;25(16). Epub 2020/08/19. 10.3390/molecules25163667 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reginster JY, Neuprez A, Lecart MP, Sarlet N, Bruyere O. Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int. 2012;32(10):2959–67. Epub 2012/03/31. 10.1007/s00296-012-2416-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.