Abstract
Photosynthesis is not only essential for plants, but it also sustains life on Earth. Phytohormones play crucial roles in developmental processes, from organ initiation to senescence, due to their role as growth and developmental regulators, as well as their central role in the regulation of photosynthesis. Furthermore, phytohormones play a major role in photoprotection of the photosynthetic apparatus under stress conditions. Here, in addition to discussing our current knowledge on the role of the phytohormones auxin, cytokinins, gibberellins, and strigolactones in promoting photosynthesis, we will also highlight the role of abscisic acid beyond stomatal closure in modulating photosynthesis and photoprotection under various stress conditions through crosstalk with ethylene, salicylates, jasmonates, and brassinosteroids. Furthermore, the role of phytohormones in controlling the production and scavenging of photosynthesis-derived reactive oxygen species, the duration and extent of photo-oxidative stress and redox signaling under stress conditions will be discussed in detail. Hormones have a significant impact on the regulation of photosynthetic processes in plants under both optimal and stress conditions, with hormonal interactions, complementation, and crosstalk being important in the spatiotemporal and integrative regulation of photosynthetic processes during organ development at the whole-plant level.
In addition to mediating stoma-induced reductions in photosynthesis during stress, phytohormones modulate the spatiotemporal and integrative regulation of photosynthetic and photoprotection processes.
Advances
Hormones strongly impact photosynthesis, both indirectly and directly.
Not only CKs, but also auxin, GAs, and SLs are essential to modulate photosynthetic rates under optimal conditions at the whole-plant level.
ABA, JAs, SA, and ethylene play a major role in the regulation of photosynthesis under various stress conditions.
An integrated hormonal response at the whole-plant level allows the most adequate photosynthetic response to every developmental and stress situation.
Introduction
In a seminal paper that started this series of Topical Reviews in Plant Physiology, Evans (2013) nicely discussed photosynthesis as the basis for promoting plant growth, urging the scientific community to gain further knowledge on the regulation of photosynthesis that will help contribute toward greater food security in the coming decades. Since then, several technological breakthroughs to improve photosynthesis have been made, including the incorporation of the C4 pathway into C3 rice (Oryza sativa) through genetic engineering, an approach that has already achieved some success in overexpressing transcription factors in the cytokinin (CK) signaling network that influence chloroplast volume (Ermakova et al., 2020). Limitations of this approach include a greater ATP requirement and the need for further improvements in the vasculature to support this increased energy requirement, which can be facilitated by further knowledge of the impact of phytohormones on the regulation of photosynthesis. Knowledge on hormonal impact on photosynthesis and its regulation is essential for improving photosynthetic rates in C3 plants and shifting plants from C3 to C4 photosynthesis to increase yields, which can solve future problems of food security around the globe. Furthermore, this can lead to a better understanding of source–sink relationships and plant stress responses at the whole-plant level, which are currently hot topics in the application of agrobiotechnology in the agri-food sector (Sengar and Singh, 2018; Smith et al., 2018; Rodrigues et al., 2019; Paul et al., 2020). Therefore, a better knowledge of photosynthetic processes is nowadays a major challenge for science and society.
Almost a century ago (1928), Fritz W. Went isolated auxin, which diffused out from the tips of oat (Avena sativa) coleoptiles in a gelatine block. At that time, the term phytohormone was synonymous with auxin, although it was anticipated that other phytohormones (then called cell division factors) would be discovered (Masuda and Kamisaka, 2000). Thereafter, ethylene (1934), gibberellins (GAs; 1938), CKs (1955), and abscisic acid (ABA; 1965) were identified, joining auxin as phytohormones. Together, they were regarded as the “classical five” by the end of the last century (Kende and Zeevart, 1997), although other hormonal groups had already been discovered at that time, such as salicylates, jasmonates (JAs), and brassinosteroids (BRs; Raskin, 1992; Creelman and Mullet, 1997; Peres et al., 2019). Nowadays, strigolactones (SLs) and peptide hormones are also considered plant hormones (also called phytohormones) based on their physiological function in growth and development, as well as their hormonal action in short- and long-distance signaling in plants, which are being progressively elucidated in more detail (Borghi, 2016; Hirakawa and Sawa, 2019). Among these hormonal groups (Box 1), we will focus on the role of auxin, CKs, and GAs in promoting photosynthesis, as well as that of ABA beyond stomatal closure in modulating photosynthesis and photoprotection under various stress conditions through crosstalk with ethylene, salicylates, JAs, and BRs. The possible role of SLs and peptide hormones will also be discussed in relation to that of the other phytohormones.
Box 1 Hormonal chemistry and basic concepts related to hormonal regulation of photosynthesis in vascular plants
Hormonal action: physiological function exerted by a hormone as a result of recognition of a given hormone by its receptor and the subsequent activation of a signaling pathway leading to the activation or repression of gene expression.
Hormonal complementation: additive or synergistic effects of the action of two or more hormones at the functional level.
Hormonal crosstalk: interaction between two or more hormones or hormone pathways in cell signaling at the molecular level.
Hormonal interplay: hormonal interaction in the regulation of a physiological function (may or may not imply a hormonal crosstalk, and it can lead to either a positive or negative regulation).
Photoinhibitory damage: injuries caused by excess light in chloroplasts that are reversible or not (depending on the severity and duration of excess light).
Photo-oxidative stress: oxidative stress caused by excess light in chloroplasts.
Photo-oxidative damage: photo-inhibitory damage associated with oxidative stress.
Photosynthesis: production of organic molecules using inorganic compounds and solar energy in plants.
Phytohormone: organic compound found at nano- or micromolar concentrations that exerts a hormonal action at short and/or long distances in plants.
Redox signaling: cell signaling based on reduction/oxidation processes.
Sink strength: capacity of a plant organ to import photoassimilates (sugars and other compounds) from other organs.
Water use efficiency: net carbon or dry matter assimilated in relation to water consumed.
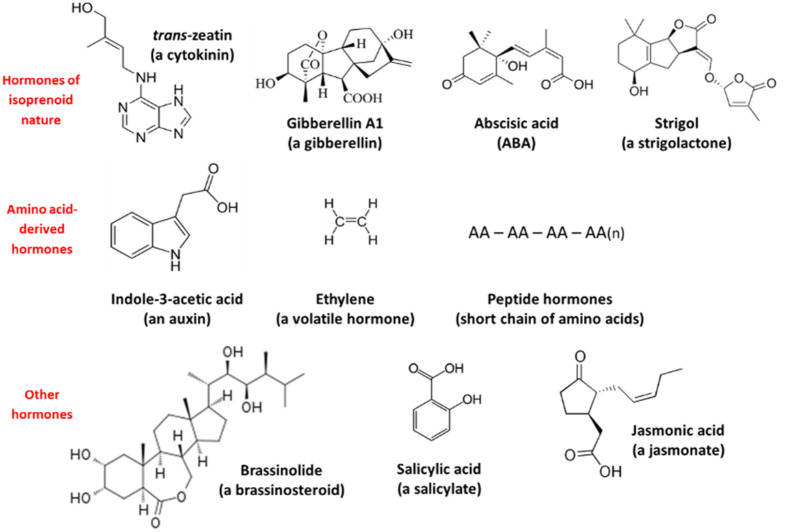
Chemical structure of phytohormones described in plants. While CKs, GAs, ABA and SLs share an isoprenoid biosynthetic origin, auxin, ethylene and peptide hormones come from amino acid metabolism. Other hormones, such as BRs, salicylates and JAs, come from sterol, shikimic acid and fatty acid metabolism, respectively.
Photosynthesis under optimal conditions is a key (but not the only) determinant of vegetative growth and plant yield. At suboptimal conditions for plant growth, CO2 assimilation rates decrease due to stomatal, mesophyll, and biochemical limitations, with the biochemical limitations generally associated with reduced photosynthetic electron transport and, subsequently, photoinhibition (Takahashi and Badger, 2011). Excessive levels of light in chloroplasts, caused by drought, salinity, extreme temperatures, high solar radiation, or a combination of these factors, lead to photoinhibition and photo-oxidative stress, thus potentially causing photoinhibitory damage to the photosynthetic apparatus unless effective photoprotection is activated (Demmig-Adams and Adams, 2002; Takahashi and Badger, 2011; Muñoz and Munné-Bosch, 2018). Much progress has recently been made on elucidating the crosstalk between redox and hormonal signaling in the modulation of photosynthesis, as well as on the role of phytohormones in regulating the production and scavenging of photosynthesis-derived reactive oxygen species (ROS), which is essential to understand the role of phytohormones in the modulation of photosynthesis and photoprotection and will be discussed here in detail. For example, what types of phytohormones beyond ABA in stomatal closure are responsible for modulating ROS production and elimination in chloroplasts? Can phytohormones play a role in photoprotection and redox signaling in chloroplasts?
Recent reviews have already discussed the impact of (i) ABA on stomatal development and regulation (Chater et al., 2014); (ii) auxin on chloroplast development (Salazar-Iribe and De-la-Peña, 2020), (iii) CKs and ethylene on leaf senescence and photosynthesis (Cortleven and Schmülling, 2015; Ceusters and Van de Poel, 2018; Hönig et al., 2018); (iv) various hormones separately on the protection from photosystem II (PSII) damage (Gururani et al., 2015); and (v) salicylic acid (SA; Janda et al., 2014) and BRs (Siddiqui et al., 2018) on photosynthesis. However, there is no comparative analysis of the role of phytohormones on photosynthesis and photoprotection based on spatiotemporal and integrative processes at the whole-plant level as well as their crosstalk. Here, we aim to go beyond a descriptive analysis of the impact of phytohormones on photosynthesis by providing a new conceptual framework for phytohormones as central players in the regulation of photosynthesis both under optimal and stress conditions at the whole-plant level. This review will first discuss recent advances in research in photosynthesis, focusing on key spatiotemporal processes that determine specific responses in each organ, not only in leaves but also in fruits. Furthermore, the coordinated role of hormones in the regulation of stress responses, with a focus on high light and drought, will be discussed in detail from the angle of photosynthetic machinery. Finally, this knowledge will be integrated to provide new insights into the interplay, complementation, and crosstalk of phytohormones at the whole-plant level. This might have important applications in agronomy and agri-food biotechnology to improve yields and the quality of the produce.
Hormonal impact on photosynthesis under optimal conditions
Phytohormones promote plant growth and development under optimal conditions through their effects on cell division, embryogenesis, organ size, reproductive development, and photosynthesis (Figure 1). Many phytohormones are partially synthesized in chloroplasts. Thus, it is not surprising that they play a central role in the direct and indirect regulation of photosynthesis. Leaves are the main location where photosynthesis occurs, but they are not the only plant tissue where this process takes place. Petioles, stems, seeds, fruits, ears of wheat (Triticum aestivum), and the husks of maize (Zea mays) have been reported to photosynthesize (Simkin et al., 2020). For instance, 4% of the total photosynthetic activity is generated by the stems in tomato (Solanum lycopersicum; Hetherington et al., 1998). In some plants, such as Justicia california, the stems photosynthesize during flower and fruit development, which occurs in the absence of leaves (Tinoco-Ojanguren, 2008). Generally, chloroplasts develop from proplastids, which are the progenitors of all plastids (Pribil et al., 2014). However, the process depends on whether it takes place under the light (proplastids directly develop into chloroplasts) or in the dark (proplastids first form etioplasts and then develop into chloroplasts in the light). In contrast to cotyledons, chloroplast development in true leaves occurs in both the shoot apical meristem (SAM) and the primordia of leaves, with the subsequent multiplication driven by chloroplast division instead of de novo assembly (Adam et al., 2011). Cell layers of SAM (L1 and L3) and leaf primordia contain plastids with a developed thylakoid network and photosynthetically active proteins, while the L2 layer is not photosynthetically active due to the proplastid-like structures (Charuvi et al., 2012). Mature chloroplasts can turn into chromoplasts and in some cases, this process is reversible, and chloroplasts can develop from chromoplasts due to regreening (Thomson et al., 1967). Under illumination, chloroplasts develop immediately via the process of photomorphogenesis, in which phytohormones are the most important regulators (Figure 1; Wang et al., 2012; Han et al., 2014). The interaction between light and phytohormones plays important roles during chloroplast development in photoautotrophic plants, such as in the modulation of organelle size, thylakoid membrane organization, pigment accumulation, organelle division, and chloroplast genome copy number (Stern et al., 2004). Moreover, the photosynthetic apparatus is composed of nuclear- and chloroplast-encoded components (Pfannschmidt et al., 2001), with the majority of the proteins required for chloroplast function being encoded in the nucleus (Barkan and Goldschmidt-Clermont, 2000; Liere et al., 2011).
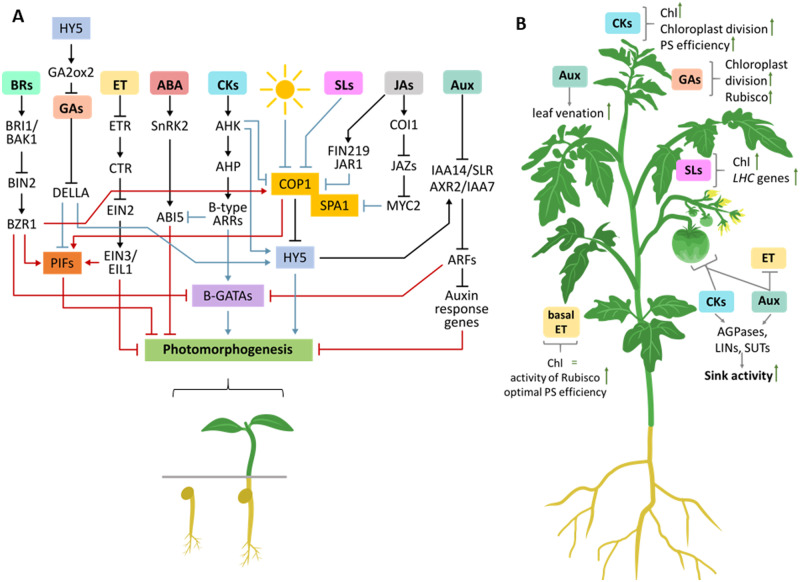
Figure 1.
Impact of phytohormones regulating photomorphogenesis and photosynthesis at the whole-plant level under optimal growth conditions. A, Hormonal regulation of photomorphogenesis in plants. B, Positive (blue) and negative (red) interactions are represented by arrows or inhibition lines, respectively. A, Indirect and direct hormonal impacts of photosynthesis at the whole-plant level. For further information, see text. ABI5, ABA INSENSTIVE5; AGPases, ADP-glucose pyrophosphorylase; AHK, ARABIDOPSIS HISTIDINE KINASE; AHP, ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEINS; ARFs, auxin response factors; ARRs, Arabidopsis response regulators; Aux, auxins; BRI1/BAK1, BR INSENSITIVE1/BRI1-ASSOCIATED RECEPTOR KINASE1; BIN2, BR INSENSITIVE2; BZR1, BRASSINAZOLE-RESISTANT1; COI1, CORONATINE-INSENSITIVE1; COP1, CONSTITUTIVE PHOTOMORPHOGENIC1; CTR, CONSTITUTIVE TRIPLE RESPONSE1; EIN2, ETHYLENE INSENSITIVE2; EIL1, ETHYLENE INSENSITIVE-LIKE PROTEIN1; ET, ethylene; ETR, ethylene receptor; FIN219, FAR-RED INSENSITIVE 219; GA2ox2, GA A2-OXIDASE2; HY5, ELONGATED HYPOCOTYL 5; IAA14/SLR, INDOLE-3-ACETIC ACID/SOLITARY ROOT; JAR1, JA RESISTANT1; JAZ, JA ZIM-domain family proteins; LHC, light harvesting complex; LINs, apoplastic invertases; MYC2, helix–loop–helix JA transcription factor; PIF, phytochrome interacting factor; PS, photosynthesis; SnRK2, SNF1-RELATED PROTEIN KINASE2; SPA1, SUPPRESSOR OF PHYA-105 1; and SUTs, sucrose transport proteins.
Direct and indirect impact of the “classical five” on photosynthesis in leaves
CKs are present in all plant tissues and regulate numerous developmental processes, such as the cell cycle, shoot and root meristem size, plant growth, leaf senescence, and acclimation to environmental stresses (Li et al., 2016). Since their discovery, CKs have been reported to also play a central role in the development and function of chloroplasts and in chlorophyll biosynthesis (reviewed by Cortleven and Schmülling, 2015). For instance, CKs regulate more than 100 genes associated with photosynthesis, including the most widely documented genes encoding the light-harvesting chlorophyll a/b-binding proteins (Chla/bBP) of PSII and the small and large subunits of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco, RBCS, RBCL, Brenner and Schmülling, 2012). Danilova et al. (2017), using CK-insensitive mutants, demonstrated that CKs positively regulate the nuclear-encoded sigma factor genes SIG2 and SIG6, but negatively modulate SIG4 and SIG5 (for a detailed description of genes described in this review, see Supplemental Table S1). Sigma factors are required for the transcription of plastid-encoded RNA polymerase (PEP), which in turn is required for the transcription of photosynthesis-related genes. Thus, sigma factors may be early targets of CK-controlled plastid gene expression, depending on developmental and environmental cues. Yang et al. (2018) reported that exogenous 6-benzylaminopurine (BA) elevates endogenous zeatin levels in wheat, which increases the relative and maximum quantum yield of PSII (ΦPSII and Fv/Fm, respectively) and the electron transport rate (ETR). Moreover, BA treatment improves the electron transfer capability of the donor and acceptor sides at the PSII reaction center, and the application of lovastatin, an inhibitor of CK synthesis, produces the opposite effects, suggesting that decreased zeatin levels might promote the inactivation of the PSII reaction center.
GAs are growth phytohormones that stimulate, for instance, the growth of most organs through cell elongation and help also promote cell division (Colebrook et al., 2014). During light-induced de-etiolation, GA concentrations decrease through the activities of catabolic enzyme GA A2-OXIDASE2 (GA2OX2), while DELLA proteins accumulate (Ait-Ali et al., 1999). DELLA proteins inhibit the activity of PHYTOCHROME INTERACTING FACTOR3 (PIF3, a transcription factor that promotes skotomorphogenesis), increase the levels of ELONGATED HYPOCOTYL5 (HY5), and trigger the biosynthesis of chlorophylls and carotenoids, the latter being essential to protect plastids from photo-oxidative damage during light-induced de-etiolation (Alabadi et al., 2008; Cheminant et al., 2011). JAs delay GA-mediated DELLA degradation, while the expression of Arabidopsis (Arabidopsis thaliana) AtJAZ9 (JA ZIM-domain [JAZ] family proteins, which suppress the transcription of JA-responsive genes) inhibits the interaction between DELLA and PIF3 (Yang et al., 2012), suggesting that photomorphogenesis is modulated by crosstalk between the JA and GA signaling pathways. Moreover, the GATA, NITRATE‐INDUCIBLE, CARBON METABOLISM‐INVOLVED (GNC), and GNC-LIKE (GNL) transcription factors suppress GA signaling downstream of DELLA and PIFs and regulate protochlorophyllide oxidoreductase (POR) levels and, consequently, chlorophyll biosynthesis (Richter et al., 2010). GAs also stimulate Rubisco activity (Nath and Mishra, 1990; Yuan and Xu, 2001), although early studies based on exogenous applications of GA3 suggest an inhibitory effect (Huber and Sankhla, 1974). Jiang et al. (2012) revealed that GA-deficient mutants of Arabidopsis (ga1-3) and rice (d18-AD) show a reduced expression of genes associated with chloroplast division. The authors also revealed that the DELLA proteins REPRESSOR OF GA1-3 (RGA) and GIBBERELLIC ACID INSENSITIVE (GAI) are the main repressors in the regulation of GA-induced chloroplast division, which agrees with a recent report by Shanmugabalaji et al. (2018) showing that the interaction between DELLA and TRANSLOCON OF THE OUTER ENVELOPE OF CHLOROPLASTS159TOC159 is essential for chloroplast division. Therefore, GAs directly and indirectly promote photosynthesis, as it occurs with CKs.
Auxins are involved in the regulation of almost all parts of plant growth and development, including chloroplast development (reviewed by Salazar-Iribe and De-la-Peña, 2020). Furthermore, auxin can indirectly influence photosynthetic capacity through the regulation of leaf stomata formation and leaf venation. Excessive auxin levels through exogenous application or overexpression reduce the number of stomata, while a loss-of-function mutation in either auxin biosynthesis or signaling causes a stomatal cluster (Balcerowicz et al., 2014; Zhang et al., 2014). The inhibitory effect on stomatal formation could be mediated by the auxin-responsive transcription factor MONOPTEROS (MP), which inhibits the stomatal pathway by repressing STOMAGEN expression (Qi and Torii, 2018). Furthermore, mutations at the Crispoid (Crd) locus in pea (Pisum sativum), a member of the YUCCA gene family encoding key enzymes in auxin biosynthesis, reduce leaf vein density and impair the formation of free-ending veinlets, leading to reduced leaf hydraulic conductance and CO2 assimilation (McAdam et al., 2017). Thus, compelling evidence suggests that auxin plays a major indirect role in photosynthesis through the modulation of chloroplast development, stomata patterning, and leaf venation.
ABA is a key stress phytohormone and participates in various physiological processes during the plant life cycle (Dong et al., 2015). In seedlings grown in the dark, ABA inhibits the greening of etioplasts. However, under red or blue light, the level of ABA decreases to a minimum through the downregulation of 9-CIS- EPOXYCAROTENOID DIOXYGENASE2 and 4 (NCED2 and NCED4), which are involved in ABA biosynthesis (Weatherwax et al., 1996; Guan et al., 2014; Humplík et al., 2015). Recently, Zhu et al. (2020) revealed that in transgenic potato lines overexpressing ABA INSENSITIVE5 (ABI5), most genes involved in photosynthesis were downregulated, particularly those associated with chlorophyll a/b-binding proteins (Chla/bBPs), photosystem I (PSI), and PSII. CKs, however, have been reported to antagonize ABA-mediated inhibitory effects through the degradation of the transcription factor ABI5, with this pathway involving the CK receptor CRE1/AHK4; the phosphotransfer proteins AHP2, AHP3, and AHP5; and the B-type transcription factor ARR12 (Guan et al., 2014). In fully developed chloroplasts, exogenously applied ABA represses the transcription of almost all the chloroplast genes (Yamburenko et al., 2013), providing further evidence of its negative role. In addition, ABA regulates chloroplast genes by stimulating SIG5 expression and inducing the transcription of the chloroplast psbd gene (Yamburenko et al., 2015). Thus, sigma factors may be early targets of both ABA and CK in the regulation of plastid gene expression. In mature leaves, HY5 has recently been linked to ABA signaling (Ortiz-Alcaide et al., 2019). As nodes of the chloroplast-modulated network, both HY5 and ABA (via ABA INSENSITIVE 3 [ABI3] and ABI4) repress growth under a low R/FR to achieve optimal photosynthesis rates under changing light conditions (Ortiz-Alcaide et al., 2019).
The gaseous phytohormone ethylene is a simple two-carbon atom molecule with roles in fruit ripening, senescence, and responses to various stresses (Arraes et al., 2015). In buried seedlings, Liu et al. (2017) reported that ethylene signaling is involved in halting etioplast–chloroplast differentiation through the interdependent module ETHYLENE-INSENITIVE3 (EIN3)–PIF3 and the repression of LIGHT HARVESTING COMPLEX (LHC) genes until the seedlings have emerged from the soil. While the removal of mechanical soil pressure largely decreases ethylene production and EIN3 accumulation, light-activated phytochrome B promotes the degradation of both the EIN3 and PIF3 proteins and initiates the prolamellar body (PLB)-to-chloroplast transition (Cortleven and Schmülling, 2015; Liu et al., 2017). Moreover, ethylene represses the accumulation of dark-induced protochlorophyllide (Pchlide) through the transcription factors EIN3/EIN3-like 1 (EIL1). Pchlide overaccumulation induces phototoxicity in plants; therefore, appropriate levels are crucial in the transition from dark to light conditions. Thus, ethylene signaling via EIN3/EIL1 and in cooperation with PIF3 appears to be involved in the protection of seedlings from photo-oxidative damage during de-etiolation (Zhong et al., 2009, 2010). Several studies comparing the young and mature leaves of plants with loss-of-function mutations in genes encoding for ethylene biosynthesis enzymes (such as 1-aminocyclopropane-1-carboxylic acid [ACC] oxidase [ACO] and ACC synthase [ACS]) or in receptor or signaling genes (such as ETHYLENE RESISTANT1 [ETR1] and ETHYLENE INSENSITIVE2 [EIN2]) have shown reduced chlorophyll levels in non-senescing leaves. By contrast, high chlorophyll concentrations have been found in mature leaves susceptible to senescence, indicating that basal ethylene levels are required to ensure normal chlorophyll levels in non-senescing leaves and promote chlorophyll degradation in mature leaves (Grbic and Bleecker, 1995; Oh et al., 1997; Young et al., 2004; Yang et al., 2008; Monteiro et al., 2011). Moreover, ethylene-insensitive mutants (etr1-1) show reduced PSII activity (Kim et al., 2017), with reduced Rubisco activity in young leaves but higher activity in the older leaves of Arabidopsis mutants compared with wild-type plants (Grbic and Bleecker, 1995). These findings suggest that ethylene sensitivity at relatively low levels is required for the optimal photochemical efficiency of PSII and the maximum activity of Rubisco.
Impact of other phytohormones on photosynthesis in leaves
BRs modulate plant growth and development through regulating cell division and elongation, photomorphogenesis, senescence, and the responses to biotic and abiotic stresses (Chen et al., 2017). Many studies in recent years have reported on the role of BRs in regulating photosynthesis, as recently reviewed by Siddiqui et al. (2018). Although BRs are not affected by light, BR signaling via the BRASSINAZOLE-RESISTANT1 (BZR1) transcription factor regulates light signaling components, for example, through the direct interaction with PIF4 to promote etiolation (Lau and Deng, 2012; Oh et al., 2012). Qi and Torii (2018) hypothesized that discrepancies in reports on the stimulating or repressing effects of BRs on stomatal development could be partly due to the different ligand–receptor pairs in cotyledons and hypocotyls. While BRs in cotyledons and leaves repress stomatal development through BR INSENSITIVE2 (BIN2; Kim et al., 2012), BIN2 mediates the phosphorylation of SPEECHLESS (SPCH) and promotes stomatal formation in hypocotyls (Gudesblat et al., 2012; Serna, 2013).
Jasmonic acid (JA) and its derivatives, collectively known as JAs, are defense-related phytohormones that also play key roles in several developmental and growth-related processes. JAs are associated with several photomorphogenesis-related genes (Chen et al., 2013) and inhibit the expression of a group of light-inducible photosynthesis-related genes (Zhai et al., 2007). The helix–loop–helix JA transcription factor MYC2 binds to the promoter region of SUPPRESSOR OF PHYA-105 1 (SPA1), a negative regulator of photomorphogenesis (Gangappa et al., 2010). Wang et al. (2011) revealed a link between the role of FAR-RED INSENSITIVE219 (FIN219) in the integration of light and JA signaling. They showed that FIN219/JA RESISTANT1 (JAR1) negatively regulates CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) by modulating the subcellular location of COP1, with FIN219 required for the stability of the HY5 protein. JA amino acid synthetase (JAR1) mediates the conjugation of JA with amino acids such as isoleucine (Staswick and Tiryaki, 2004). Thus, FINS219/JAR1 seems to act as a positive regulator of phyA-mediated far-red light signaling by controlling COP1 levels and HY5 stability in the regulation of photomorphogenesis in Arabidopsis (Wang et al., 2011). Moreover, exogenous MeJA upregulates the expression of photosynthesis-related genes, such as PSII subunits Q-1 and Q-2, which encode the PsbQ subunit of the PSII oxygen-evolving complex, in opr3-1 mutants lacking endogenous JAs (Qi et al., 2020). Lin et al. (2016) indicated an interaction between digalactosyldiacylglycerol (DGDG) and JAs, showing that DIGALACTOSYLDIACYLGLYCEROL SYNTHASE1 (Dgd1) mutants had reduced DGDG levels (90%), decreased photosynthesis, and an altered chloroplast morphology that were accompanied by highly upregulated JA-responsive genes.
Salicylic acid (SA) is a stress-related phytohormone that influences photosynthesis under stress conditions. However, several studies using exogenously applied SA have noted that the effects of SA on photosynthesis under optimal conditions depend on the SA dose and the plant species (reviewed by Rivas-San Vicente and Plasencia [2011] and Janda et al. [2014]). Indeed, the molecular regulation of photosynthesis by SA remains to be elucidated. To our knowledge, only Danilova et al. (2018) reported that exogenously applied SA caused a decrease in the transcript levels of nuclear genes encoding most of the proteins of the PEP-associated complex, which is required for the transcription of photosynthesis-related genes.
SLs are carotenoid-derived terpenoid lactones that were recently identified as phytohormones due to their role in suppressing shoot branching (Gomez-Roldan et al., 2008). However, the focus has quickly shifted to other roles in plant development such as photomorphogenesis. Like the other phytohormones, SLs regulate COP1 and, thus, HY5 activity indirectly in Arabidopsis seedlings (Tsuchiya et al., 2010). Continuously high levels of SLs (over a 24-h period) inhibit COP1 by relocating COP1 away from the nucleus or by promoting its nuclear degradation, consequently leading to HY5 accumulation in the nucleus and providing a link between SLs and photomorphogenesis. A short-term increase in SLs through the MORE AXILLARY GROWTH LOCUS 2 (MAX2) F-box protein positively modulates light-related growth responses (Tsuchiya et al., 2010). Moreover, the max2 f-box Arabidopsis mutant shows clear photomorphogenic phenotypes such as long hypocotyls under light (Tsuchiya et al., 2010; Jia et al., 2014). However, several studies have reported conflicting results on the interaction between the MAX2 F-box protein and SLs, indicating that the MAX2 F-box protein is not necessarily linked to SL signaling. For instance, the MAX2 F-box protein plays a role in growth regulation under light conditions through interactions with auxin signaling and independently of SLs (Shen et al., 2012; Waters and Smith, 2013). The exogenous application of the synthetic analog of SLs (GR24) induces the expression of light-related genes in Arabidopsis, with these genes suppressed in SL-deficient mutants (Mashiguchi et al., 2009). In addition, mutants with impaired SL biosynthesis (SI-ORT1) show reduced chlorophyll levels and a decreased expression of LHC genes compared with wild-type tomato plants, with G24 treatment increasing chlorophyll levels and inducing the expression of LHC genes to increase photosynthetic activity (Mayzlish-Gati et al., 2010). Together with SLs, peptides are another important group of phytohormones that require further research on their effects on photosynthesis.
Impact of phytohormones on photosynthesis in fruits
The indirect and direct impact of phytohormones on photosynthesis under optimal conditions also occurs in fruits. Fruit photosynthesis has recently been gaining increasing interest, as several studies have revealed important links between photosynthesis in the developing fruit, which undergoes a shift from green photosynthetic to fully heterotrophic metabolism in many species, and the quality of the ripe fruit (Lytovchenko et al., 2011; Pan et al., 2013; Sagar et al., 2013). Tomato fruit development, the most studied model system for fleshy fruit ripening as it is one of most consumed fruits worldwide (Klee and Giovannoni, 2011), includes three stages: (i) an increase in cell number and starch accumulation; (ii) cell enlargement with starch degradation and soluble sugar accumulation; and (iii) the accumulation of carotenoids, organic acids, soluble sugars, and volatile organic compounds (Schaffer and Petreikov, 1997; Klee and Giovannoni, 2011). Fruit photosynthesis is restricted to the green phase of fruit development, so that once the chloroplast irreversibly develops into a chromoplast, there is a loss of chlorophyll, the thylakoid membranes are degraded, the levels of photosynthesis-associated transcripts and proteins are significantly decreased, and there is an increase in carotenoid accumulation (Barsan et al., 2012). The processes that fruits undergo during development are controlled by an intricate interplay between multiple phytohormones that influence the overall fruit quality (Sagar et al., 2013). Moreover, chlorophyll accumulation and photosynthetic activity in green fruits enhance fruit quality, leading to increased levels of nutritional components and flavor of the ripening tomato fruit (Nadakuduti et al., 2014). Yuan et al. (2019) revealed that auxin mediates photosynthesis through SIARF6A. ARFs have been reported to act either as an activator or a repressor of the transcription of auxin-responsive genes (Zouine et al., 2014). SlARF6A positively regulates the expression of the SlGLK1, CAB, and RbcS genes by binding directly to their promoters. Thus, SIARF6A overexpression increases chlorophyll levels, chloroplast size, the photosynthesis rate, starch accumulation, and soluble sugar concentrations, while SlARF6A knockdown results in the opposite phenotype in tomato fruits. Moreover, SlARF6A binds directly to the S-ADENOSYLMETHIONINE SYNTHETASE1 (SAMS1) promoter and negatively regulates SAMS1 expression. SAMS catalyses the reaction between ATP and methionine, which forms S-adenosyl-l-methionine (SAM) in the ethylene biosynthetic pathway (Roje, 2006). In tomato fruits overexpressing SlARF6A, ethylene synthesis is inhibited, which consequently suppresses fruit ripening (Yuan et al., 2019). These results suggest that auxin delays fruit ripening by mediating fruit photosynthesis and ethylene biosynthesis via SIARF6A.
Delaying fruit ripening before harvest could enhance postharvest life and improve the size and organoleptic properties of fruits by inducing higher photoassimilate production during the green stages and activating redox mechanisms to increase antioxidant levels, as has been shown for some fruits (Cocaliadis et al., 2014; Rademacher, 2015). SIARF10 interacts with auxin signaling during fruit development, as it positively regulates chlorophyll synthesis via the direct activation of SIGLK1 expression (Yuan et al., 2018). By contrast, auxin represses SIGKL2 expression through SIARF4, a negative regulator of auxin signaling, while the effects of CKs are upregulated in the presence of SIGLK2 (Lupi et al., 2019). These findings suggest that auxin promotes photosynthesis in tomato fruits when SIARF4 is downregulated. Fruits with high chlorophyll contents can also be obtained after the exogenous application of CKs (Mustilli et al., 1999).
A link between phytochromes, auxin, and CKs during the early development of tomato fruits has been reported (Bianchetti et al., 2017). Phytochromobilin deficiency leads to the increased expression of AUXIN/INDOLE-3-ACETIC ACID3 (Aux/IAA3) and the downregulation of ARF and type-A TOMATO RESPONSE REGULATOR (TRR) in both the columella and pericarp tissues, thereby negatively affecting auxin and CK signaling in both tissues in immature green fruits. The reduced auxin and CK signaling in columella cells downregulates the genes encoding key enzymes involved in starch synthesis [such as ADP-glucose pyrophosphorylase (AGPases)] and in sink activity [such as apoplastic invertases (LINs) and sucrose transport proteins (SUTs)] (Bianchetti et al., 2017). The downregulation of these enzymes associated with carbohydrate metabolism, which contribute significantly to sink activity (Zanor et al., 2009; Albacete et al., 2014), reduces the import of sugar into the developing fruits. In pericarp cells, reduced auxin and CK signaling also inhibits chloroplast formation in addition to starch biosynthesis, suggesting a restriction in photoassimilate production through fruit photosynthesis (Bianchetti et al., 2017). These findings suggest that the interactions between phytochromes, CKs, and auxin are involved in the spatiotemporal regulation of fruit development, mediating sugar metabolism and photosynthesis.
All these examples illustrate the impact of phytohormones on photosynthesis in the control of fruit quality, an open avenue for future research. However, our understanding of fruit chloroplast formation and the role of photosynthesis in fruit metabolism and development is still incomplete, very far from our current knowledge on the regulation of photosynthesis by phytohormones in leaves, and very basic questions are still open. For instance, although green fruits are photosynthetically active, it is not clear to what extent. In tomato fruits, CO2 is exclusively liberated from the mitochondria and apparently not “newly” assimilated, since tomato fruits lack stomata (Vogg et al., 2004; Lemaire-Chamley et al., 2005). Furthermore, a reduction in fruit photosynthesis appears to be compensated by the upregulation of photosynthesis in the leaves (Araújo et al., 2011), thus suggesting an important role for the regulation of source–sink relations at the whole-plant level by CK action.
Hormonal impact on photosynthesis and photoprotection under stress
Being sessile organisms, plants need to adapt to several abiotic and biotic stresses during their life cycle. Unfavorable environmental conditions such as high light, water deficit, hypoxia, or anoxia due to waterlogging, extreme temperatures, salinity, and pollutants, among other abiotic factors, may reduce photosynthesis and lead to excess excitation energy in chloroplasts (Asada, 1999, 2006; Demmig-Adams and Adams, 2002). However, plants have several inbuilt photoprotection mechanisms to respond to these stresses and save their photosynthetic apparatus, including xanthophyll cycle-dependent excess energy dissipation (Demmig-Adams and Adams, 2002), activation of the water–water cycle (Asada, 1999), production and elimination of singlet oxygen (Triantaphylidès and Havaux, 2009), and enhanced photorespiration. The tolerance of plants to environmental stress and the way these photoprotection mechanisms are activated varies from species to species, but a general hierarchy of responses generally occurs in plants, some of them modulated by phytohormones. Indeed, a better understanding of the hormonal impact on photosynthesis and photoprotection under abiotic stresses is not only important for better understanding basic biological processes, but it could also help to develop new crop plants that have more robust photosynthetic machinery. This would be particularly useful given that the economic yield of plants is predicted to decrease significantly due to climate change, which is a major threat to food security. To illustrate the impact of phytohormones on photosynthesis and photoprotection in plants exposed to stress, we will highlight here the effects of phytohormones on photosynthesis under high light stress (Figure 2 and Table 1) and drought (Figure 3 and Table 1).
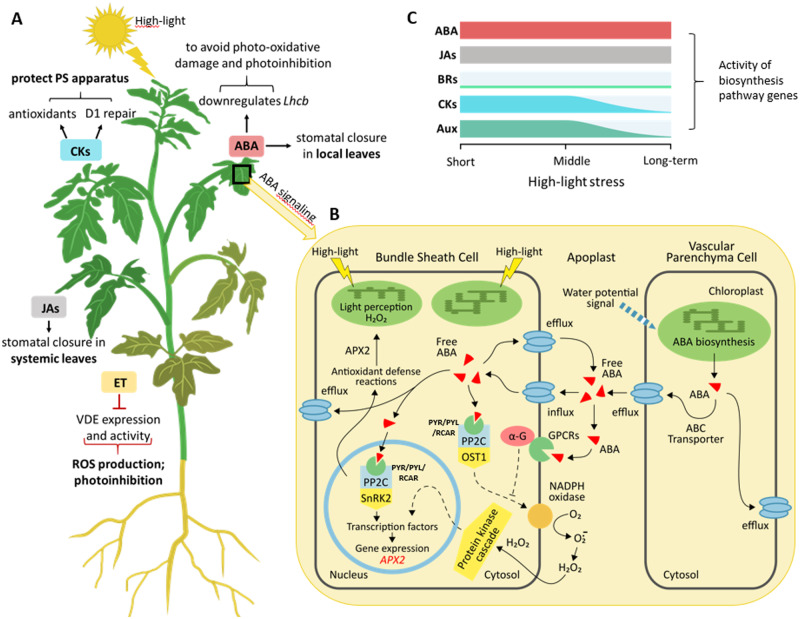
Figure 2.
Impact of phytohormones regulating photosynthesis and photoprotection under excess light conditions. A, Examples of phytohormone responses to high-light stress at whole-plant level. B, Proposed model of ABA signaling in response to high-light exposure. A rapid change in water potential triggers ABA biosynthesis in vascular parenchyma cells. Free ABA can be transported via ABC efflux transporter to (i) vascular tissue to relay systemic acclimation at the whole plant-level, (ii) guard cells triggering stomatal closure, and/or (iii) bundle sheath cells inducing the antioxidant gene APX2. PYR/PYL/RCAR, PP2C, and SnRK2/OST1 form a core signaling complex, which regulates ABA-responsive gene expression (including APX2) and activity of NADPH oxidase in bundle sheath cells. Meanwhile, high light provokes H2O2 accumulation in chloroplast initiating retrograde signaling that feeds into the ABA regulatory network and speeding it up. High light-mediated induction of APX2 requires in addition to ABA, linear electron flux, redox signals from reduced glutathione, and extracellular H2O2. C, Activity of phytohormone biosynthesis pathway genes during high-light exposure. Aux, auxins.
Table 1.
Examples of direct and indirect hormonal effects on photosynthesis and photoprotection in plants exposed to drought or high light stress described during the last 5 years
| Hormone | Species | Stress | Plant material | Treatment | Effects | Reference |
|---|---|---|---|---|---|---|
| ABA | Lycium chinense | Drought | 15-week-old plants with 35–40 leaves | 15% soil moisture level for 4 weeks | ABA modulates NPQ and ϕPSII through the regulation of VDE expression | Guan et al. (2015) |
| ABA | Arabidopsis thaliana | High light | 18–21-d-old wild type and mutants (Ler, aba1, abi1, abi4-102, gun1-9) | 1,000 µmol m−2 s−1 high light for (i) 0, 20, and 60 s; (ii) 0, 10, 60, and 300 s | 20% of transcript accumulation in plants responds to ABA in an ultra-fast response within seconds | Suzuki et al. (2015) |
| ABA | Arabidopsis thaliana | High light | 30–45-d-old wild type and mutants (Ler, rbohD, aba1-1 aba2-1; aba3-1, slac1, lox1, abi1-1, ghr1) | 2000 µmol m−2 s−1 high light (0, 1, 5, and 10 min) on a circular area (0.75 cm in diameter) at the tip of a mature leaf | ABA is involved in local and systemic signaling, regulates stomatal closure in local leaves, and is required for H2O2 and SA accumulation in local and systemic leaves | Devireddy et al. (2018) |
| ABA | Spring barley cvs. “Sebastian” | Drought | 4-d-old seedlings | Liquid MS medium with 200 µM ABA for 2 d; on day 15 severe drought stress application with 1.5% volumetric water content for 10 d | High-dose ABA application negatively affects the electron fate between photosynthetic antenna absorption and QA at the acceptor side of PSII | Daszkowska-Golec et al. (2019) |
| ABA | Arabidopsis thaliana | High light | 4-d-old wild type and mutants (NCED and pifq) | 1,200 µmol m−2 s−1 high light for 72 h | ABA regulates middle- and long-term high-light response; upregulation of ABA biosynthetic genes (NCED3, NCED5, and NCED9 within 0.5 h and NCED2 within 24 h); induction of NCED3/5 by high light was independent of PIFs | Huang et al. (2019) |
| Auxin | Arabidopsis thaliana | High light | 4-d-old seedlings | 1,200 µmol m−2 s−1 high light for 72 h | Auxin biosynthesis is repressed after long-term high light exposure | Huang et al. (2019) |
| Auxin | Clover | Drought | Seedlings at the two-leaf stage | 1 µM IAA about 7 d; 15% PEG-6000 for 14 d. | Exogenous IAA enhanced chlorophyll content accompanied with increased ABA and JA contents | Zhang et al. (2020) |
| BRs | Cowpea | Drought | 6-d-old seedlings | Semi-hydroponic conditions: 100 nM EBR spayed at 6-d interval until day 18; water deficit: days 18–20 solution was removed completely | BRs increased ϕPSII, qP, and ETR, antioxidant enzymes (SOD, CAT, APX, and POX) and total chlorophyll contents | Lima and Lobato (2017) |
| BRs | Maize | Drought | 30-d-old seedlings (drought-sensitive 2023 and drought-tolerant CE704) with three fully developed leaves | Cessation of watering for 14 d and final 3% soil water content. | Three-fold higher BRs (especially typhasterol and 28-norbrassinolide) contents in drought-tolerant genotype accompanied with higher chlorophyll and carotenoids contents compared with drought-sensitive genotype | Tůmová et al. (2018) |
| BRs | Arabidopsis thaliana | High light | 4-d-old seedlings | 1,200 µmol m−2 s−1 high light for 72 h | Biosynthesis genes were down regulated, suggesting a negative role | Huang et al. (2019) |
| CKs | Arabidopsis thaliana | High light | 5-d-old wild type and mutants (ahk2ahk3, ahk4, and ahk3ahk4) | 400 µmol m−2 s−1 light (8-h light/16-h dark) for 6 d on detached leaves | Mutants with insufficient CK signaling showed better PSII function than wild-type plants | Janečková et al. (2018) |
| CKs | Arabidopsis thaliana | High light | 4-d-old seedlings | 1,200 µmol m−2 s−1 high light for 72 h | CK biosynthesis was repressed after long-term high light exposure | Huang et al. (2019) |
| Ethylene | Arabidopsis thaliana | High light | 3-week-old wild type and mutants (eto1-1 and crt1-3) | 1,300 and 1900 PFD high light | Ethylene repressed the expression and activation of VDE and increased ROS | Chen and Gallie (2015) |
| JAs | Arabidopsis thaliana | High light |
5–8-week-old wild type and oxi1 null mutants deficient in the OXI1 kinase |
1,500 µmol m−2 s−1 PFD, 7°C/14°C day/night temperature, and 380 ppm CO2 for 26 h |
oxi mutants showed a down-regulation of the JA pathway genes in leaves, suggesting a link between OXI1 protein and JAs in PCD under high light stress; OPDA appeared to antagonize PCD, while JA and JA-Ile promoted 1O2-induced PCD | Shumbe et al. (2016) |
| JAs | Arabidopsis thaliana | High light | 30–45-d-old wild type and mutants (Ler, rbohD, aba1-1 aba2-1; aba3-1, slac1, lox1, abi1-1, ghr1) | 2,000 µmol m−2 s−1 high light (0, 1, 5, and 10 min) on a circular area (0.75 cm in diameter) at the tip of a mature leaf | JAs were involved in systemic signaling (stomatal closure of systemic leaves) | Devireddy et al. (2018) |
| JAs | Arabidopsis thaliana | High light | 30-d-old wild type and mutants (aos, sid2) | 650 µmol m−2 s−1 light + 42°C for 7 h | JAs regulated unique transcriptional responses under combined high light and heat stress and promoted APX1 and APX2 expression | Balfagón et al. (2019) |
| JAs | Arabidopsis thaliana | High light | 4-d-old seedlings | 1,200 µmol m−2 s−1 high light for 72 h | Upregulation of JA biosynthetic genes during long-term high-light treatment | Huang et al. (2019) |
| SA | Wheat | Drought | 45-d-old seedlings | 0.5 mM SA application with 15 d intervals from day 45 till harvesting; water stress levels were maintained based on RWC (50% and 75%) | SA increased Rubisco abundance, Rubisco activators, Rubp regenerating enzymes, SBPase, FBPase, OEE1, OEE2, FNR, Chla/bBP, TLP, and ATP synthase | Sharma et al. (2017) |
| SA | Soybean | Drought | 45-d-old seedlings | Seed priming (0.5 mM SA solution for 6 h); water stress levels were maintained based on RWC of leaf (50% and 75%) | SA increased Rubisco, Rubisco activase, Chla/bBP, OEE1, OEE2, FNR, Chla/bBP, PSII stability proteins, and antioxidant enzymes (SOD, APX, GR, CAT) | Sharma et al. (2018) |
ATP, adenosine triphosphate; Chla/bBP, chlorophyll a/b binding protein; EBR, 24-epibrassinolide; FBPase, fructose-1,6-bisphosphatase; IAA, índole-3-acetic acid; JA-Ile, jasmonoyl-isoleucine; MS, Murashige and Skoog; NCED, 9-cis-epoxycarotenoid dioxygenase; OEE, oxygen evolving enhancer protein; OXI1, OXIDATIVE SIGNA-INDUCIBLE1; PIFs, phytochrome interacting factors; PN, net photosynthesis rate; qP, photochemical quenching; ϕPSII, quantum yield of PSII; RWC, relative leaf wáter content; Rubisco, ribulose-1,5-bisphosphate carboxylase oxygenase; Rubp, ribulose-1,5-bisphosphate; SBPase, sedoheptulose-1,7-bisphosphatase; TLP, thaumatin like protein.
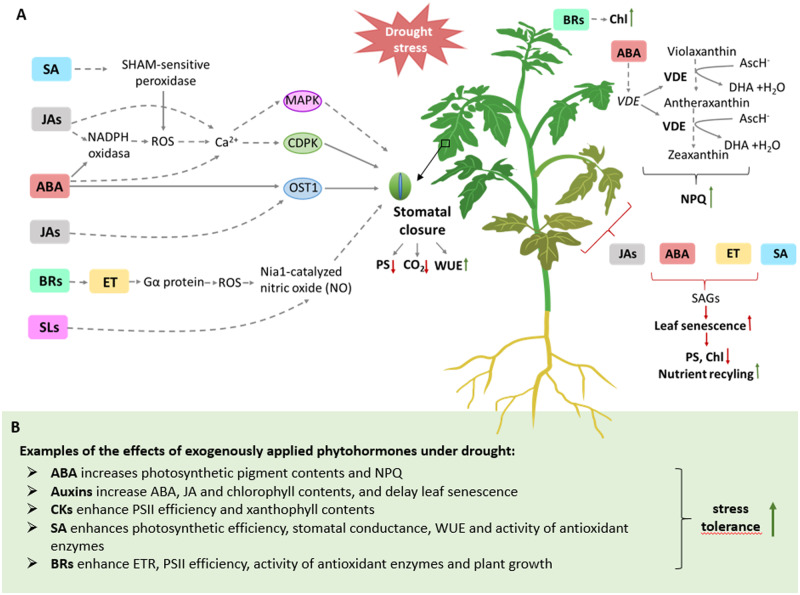
Figure 3.
Impact of phytohormones regulating photosynthesis and photoprotection under drought. A, Examples of phytohormone responses to drought at whole-plant level. B, Examples of the effects of exogenously applied phytohormones on photosynthesis under drought. Aux, auxins; Chl, chlorophyll; ET, ethylene; NPQ, non-photochemical quenching; OST1, open stomata 1; PS, photosynthesis; SAGs, senescence-associated genes; and WUE, water use efficiency.
Impact of phytohormones on photosynthesis under high light stress
Light is the most rapidly changing and variable environmental factor. Plants encounter high light intensity that exceeds their photosynthetic capacity on most days throughout their life cycle. Excessive light energy causes a range of stress responses in plants, with the interactions between phytohormones and ROS playing a pivotal role in plant stress acclimation. In leaves, the excessive energy in chloroplasts can be dissipated as thermal energy in non-photochemical quenching (NPQ), which serves as a first line of defence to protect the PSII reaction centers against photodamage, despite leading to a (generally reversible, depending on the species and stress intensity) photoinhibition of photosynthesis. NPQ requires de-epoxidized pigments from the xanthophyll cycle, in which light induces the de-epoxidation of violaxanthin (V) into antheraxanthin (A) and zeaxanthin (Z) through the activity of violaxanthin de-epoxidase (VDE). The xanthophyll cycle is not only involved in the protection of PSII, but also in the protection of the photosynthetic membranes against photo-oxidation (Havaux et al., 2000). Excessive light energy, if not quenched, causes the accumulation of multiple ROS, including singlet oxygen (1O2), superoxide (), and hydrogen peroxide (H2O2; Asada, 2006; Pintó-Marijuan and Munné-Bosch, 2014; Dietz et al., 2016). ROS have harmful effects on lipid peroxidation and on the oxidation of carbohydrates, proteins, and DNA, leading to programmed cell death (PCD) during ROS overproduction (Gill and Tuteja, 2010). Chen and Gallie (2015) demonstrated that ethylene, under high light stress, affects the xanthophyll cycle by repressing the expression and activity of VDE, which leads to increased ROS production, photoinhibition, and growth impairment. However, under redox homeostasis, there is a balance between ROS production and its detoxification by both enzymatic and non-enzymatic antioxidants (Foyer and Noctor, 2005a, 2005b; Juvany et al., 2013). Antioxidants, such as carotenoids and tocopherols (Muñoz and Munné-Bosch, 2019), determine the duration and location of specific ROS, thus eliciting different signaling responses. 1O2 is a short-lasting ROS with a lifetime of approximately 4 µs and can diffuse over relatively short distances (can only travel around 200 nm, but chloroplasts are around 5–10 µm wide; Gill and Tuteja, 2010; Ogilby, 2010). In contrast to 1O2, H2O2 can be produced in different compartments in plant cells and is a more stable ROS, with greater diffusion distances of around 1 µm (Vestergaard et al., 2012). Thus, H2O2 can diffuse from chloroplasts to the nuclei to modulate gene expression (Exposito-Rodriguez et al., 2017). When H2O2 accumulates in chloroplasts, a retrograde signaling network involving ABA signaling induces the expression of the antioxidant gene ASCORBATE PEROXIDASE 2 (APX2; Fryer et al., 2003; Ball et al., 2004). The APX2 protein is densely distributed around chloroplasts, with key roles in H2O2 homeostasis and chloroplast protection. Ascorbate peroxidases (APXs) catalyze the conversion of H2O2 into H2O in the so-called ascorbate–glutathione cycle, which is part of the water–water cycle (Asada, 2006; Miret and Müller, 2018). High light-mediated induction of APX2 requires a linear electron flux, redox signals from reduced glutathione, ABA, and extracellular H2O2 (Fryer et al., 2003; Chang et al., 2004; Galvez-Valdivieso et al., 2009; Gorecka et al., 2014).
Phytochromes promote the expression of the genes encoding the light-harvesting Chla/bBPs of PSII (Lhcb). However, excessive light energy reduces Lhcb expression, which has been hypothesized to be regulated by an interaction between H2O2 and ABA signaling. In addition, exogenously applied ABA reduces the activity of an Lhcb promoter, while a mutation in the Lhcb promoter that abolishes the ABA response reduces the impact of high light stress (Staneloni et al., 2008). Light stress triggers an ultra-fast transcription response within seconds. Through RNA sequencing, it has been found that 20% of the transcripts accumulating in plants are H2O2- and ABA-response transcripts (Suzuki et al., 2015). Several studies have reported that high light stress is associated with induced ABA signaling and stomatal closure, which leads to low CO2 availability (Merilo et al., 2001; Raven, 2014). A reduction in the antenna size can re-establish the balance between photochemical and carbon-fixation reactions (Staneloni et al., 2008). Thus, the ABA-mediated downregulation of Lhcb expression could be of functional importance to avoid photo-oxidative damage and photoinhibition in chloroplasts.
Systemic signaling may play a role in acclimation to high light stress. Devireddy et al. (2018) found that the application of light stress to a single rosette leaf in Arabidopsis plants resulted in a plant-wide acclimation response through coordinated stomatal closure. In systemic leaves that are not exposed to direct light, signals triggered by light stress led to closed stomata, which could be an adaptation mechanism against exposure to excessive light energy. Although ABA appears to play a key role in triggering light stress-induced systemic stomatal closure responses, such as H2O2 production, it has been suggested that ABA is not directly involved in the signaling for stomatal closure in systemic leaves. It has been proposed that ROS accumulation and the activation of respiratory burst oxidase homolog D (RBOHD)-dependent ROS production in local leaves are ABA-dependent, which could trigger a SLOW ANION CHANNEL-ASSOCIATED1 (SLAC1)-dependent or ABA-INSENSITIVE2 (ABI2)-induced stomatal closure through GUARD CELL HYDROGEN PEROXIDE RESISTANT1 (GHR1) in response to light stress. In systemic leaves, light stress induces SLAC1-dependent stomatal closure through GHR1 and probably JA (Devireddy et al., 2018). These findings reveal a complex spatiotemporal network of phytohormone signaling that coordinates and optimizes plant responses to excessive light energy at the whole-plant level.
Hormonal crosstalk may also be involved in the regulation of photosynthesis and photoprotection under high light stress, in some cases combined with other abiotic stress factors. On the one hand, Balfagón et al. (2019) found increased JA, JA-Ile, and OPDA concentrations in plants subjected to a combination of high light and heat stress. Furthermore, mutants with impaired JA biosynthesis (allene oxide synthase; aos) showed increased sensitivity and reduced APX1 and APX2 expression compared with wild-type plants in response to the combination of high light and heat stress. On the other hand, Huang et al. (2019) performed a genome-wide and dynamic transcriptome analysis of high-intensity light-driven stress responses over 72 h in 7-d-old Arabidopsis seedlings. While the genes associated with the ABA and JA biosynthetic pathways showed increased expression at all time points, those involved in the biosynthesis of other phytohormones, such as CKs, auxin, SA, and GAs, showed changes in their expression only at one or a few time points. Expression of nine of the genes involved in ABA biosynthesis, which included NCED3, NCED5, and NCED9, were upregulated within 0.5 h, with NCED2 expression increasing after 24 h of high light exposure. Interestingly, endogenous ABA levels increased slightly after 0.5 h but rose more than two-fold after 6 h of exposure to high light stress. Furthermore, the genes associated with the ABA signaling pathway were also upregulated under high light conditions (Huang et al., 2019). All these results together suggest that ABA plays an important role in the middle- and long-term responses to high light stress. In contrast to ABA, the genes involved with BR biosynthesis were downregulated, suggesting their negative role in the response to high light as well as an antagonistic interaction between ABA and BRs (Huang et al., 2019). This would be in line with the findings of Zhou et al. (2014), who studied responses to heat and oxidative stress. Furthermore, it has been hypothesized that plants start their acclimation response by activating ABA and JA biosynthesis after a short-term exposure to high light, while repressing BR biosynthesis and nucleotide metabolic processes. After middle-term exposure to high light, anthocyanin accumulation increases and the photosynthesis-related genes are repressed.
The biosynthesis of growth-related phytohormones, such as auxins and CKs, is suppressed after long-term exposure to high light, which leads to the inhibition of plant growth (Huang et al., 2019). However, CKs play a photoprotective role in plants exposed to light stress for 24 h. Mutant 4-week-old Arabidopsis plants with reduced endogenous CK levels show strongly reduced D1 protein levels and antioxidant levels (enzymatic and non-enzymatic antioxidants) in response to light stress (Cortleven et al., 2014). The D1 protein, a key subunit of PSII, is highly photosensitive, with photoinhibition occurring when the ROS-mediated damage to D1 exceeds the repair capacity (Barber et al., 1997). Thus, CKs appear to promote the activities of both enzymatic and non-enzymatic antioxidants for ROS scavenging as well as D1 repair. This response to light stress is mediated by the AHKS2 and AHKS3 receptors and the type B response regulators ARR1 and ARRR12 (Cortleven et al., 2014). However, PSII functions better in double mutants with insufficient CK signaling (ahk2ahk3 and ahk3ahk4) compared with wild-type plants in response to high light, with a drastic decrease in the levels of 2-isopentenyl adenine (iP; Janečková et al., 2018). These findings suggest that plants exposed to high light can compensate for the impaired CK signaling through other ways to protect the photosynthetic apparatus. However, 1O2 overproduction due to a persistent exposure to excessive light leads to PCD. Shumbe et al. (2016) revealed that Arabidopsis oxi mutants show downregulated expression of the genes associated with the JA pathways in leaves, suggesting a link between the OXIDATIVE SIGNAL-INDUCIBLE 1 (OXI1) protein and JAs in PCD during exposure to high light stress. However, 12-oxo-phytodienoic acid (OPDA), the precursor of JA, appears to antagonise PCD, while JA and jasmonoyl-isoleucine (JA-Ile) promote 1O2-induced PCD (Shumbe et al., 2016).
Impact of phytohormones on water stress tolerance
Changes in the intensity, frequency, and severity of drought are predicted to pose major threats to future global agricultural productivity (Nadeem et al., 2019). The response to drought stress largely depends on the duration of the stress, the developmental stage of the plant, its genetic potential, and the surrounding environment. The prime response of plants to drought stress is stomatal closure, which leads to a reduction in the net CO2 assimilation and decreases the potential activity of the Calvin cycle enzymes, particularly Rubisco (Yang et al., 2017). This in turn triggers changes in the partitioning of photoassimilates at the whole-plant level and generally corresponds to an ABA-mediated increase in the root:shoot ratio (Wilkinson and Davies, 2010). ABA is synthesized either in the leaves or in the roots and is considered to be the most important phytohormone in the regulation of stomatal closure and, consequently, carbon uptake during water scarcity (summarized in Munemasa et al., 2015; Saradadevi et al., 2017). In addition to ABA, however, JAs, SA, BRs, SLs, and ethylene are also involved in the stomatal response. Like ABA, JAs are positive regulators of stomatal closure, leading to increased drought tolerance in plants. Methyl JA (MeJA) induces ROS formation and increases the influx of Ca2+ into the cytoplasm, which activates Ca2+-dependent protein kinase 6 (CDPK6) and, subsequently, SLAC1 (Suhita et al., 2004). Furthermore, it has been reported that the Ca2+-independent protein kinase Open Stomata1 (OST1) also participates in MeJA signaling (Yin et al., 2016). However, exogenous MeJA treatment in ABA-deficient mutants (aba2-2) only results in stomatal closure in the presence of ABA (Hossain et al., 2011). Interestingly, the exogenous application of the JA precursor OPDA induces stomatal closure both independently and together with ABA (Savchenko et al., 2014). SA accumulation has also been linked to the ABA signaling pathway for stomatal closure and tolerance to drought stress (Miura and Tada, 2014). Prodhan et al. (2018) revealed that SA triggers SHAM-sensitive peroxidase-mediated ROS signaling, leading to the activation of CDPK1 and CDPK6, which activate SLAC1. In contrast to ABA and MeJA, SA mediates stomatal movement only through the CDPKs and not through OST1. MAPKs (MPK9 and MPK12) are also involved in SA signaling in guard cells (Khokon et al., 2017). However, it remains to be elucidated whether there is an interdependent mechanism of the CDPK and MAPK signaling pathways. The role of ethylene in the regulation of stomatal movement is controversial because it has been linked to both stomatal opening and closure (Levitt et al., 1987; Morgan et al., 1990; Merritt et al., 2001). The severity of the stress may play a key role in the link between ethylene production and stomatal movement. Ethylene inhibits ABA- and Me-JA-induced stomatal closure by targeting S-type anion channels and ROS production in guard cells, therefore ensuring a minimum supply of carbon dioxide for photosynthesis by keeping the stomata half opened (Tanaka et al., 2005; Munemasa et al., 2019). OST1 and K+ channels are not affected by ACC pre-treatment (Munemasa et al., 2019). Elevated ABA levels usually limit the production of ethylene, indicating that severe drought stress decreases ethylene levels (Sharp, 2002). Furthermore, Shi et al. (2015) showed that BRs induce ethylene synthesis and, thus, activate the Gα protein, which then promotes AtrbohF-dependent H2O2 production and, subsequently, an accumulation of Nia1-catalyzed nitric oxide (NO), leading to stomatal closure. Lv et al. (2017) revealed that SLs mediate stomatal closure through H2O2 and NO accumulation and SLAC1 activation, independently of ABA. However, the molecular mechanism by which SLs mediate stomatal closure remains to be elucidated. Auxins and CKs are typically known for their positive roles in stomatal opening. CKs affect stomatal opening by scavenging H2O2 in guard cells, while auxins limit H2O2 production (Song et al., 2005). Moreover, auxins stimulate the activity of plasma membrane H(+)-ATPase in guard cells. Therefore, low auxin levels activate the inward K+ channels, which lead to stomatal opening. However, high auxin concentrations can inhibit stomatal opening and positively modulate the lateral root architecture, suggesting that auxins might participate positively in the regulation of drought stress tolerance (Lohse and Hedrich, 1992; Shi et al., 2014). By contrast, drought stress inhibits CK biosynthesis in the roots through ABA, which downregulates the ADENOSINE PHOSPHATE-ISOPENTENYLTRANSFERASE (IPT) genes, eliciting stomatal closure (Pospíšilová, 2003; Nishiyama et al., 2011). On the other hand, tobacco (Nicotiana tabacum) transgenic lines (PSARK::IPT, for Senescence-Associated Receptor Kinase::Isopentenyl Transferase) that overproduce CKs show an induced protection of photosynthesis through enhanced photorespiration without effects on stomatal limitations under drought stress (Rivero et al., 2009). The authors suggest that the contribution of the CK-dependent photorespiration to the drought tolerance was linked to increased transcripts coding for Rubisco, phosphoglycolate phosphatase (PGPase), glycolate oxidase (GO), glycine decarboxylase complex (GDC), serine hydroxymethyltranserase (SHMT), and glycerate kinase (GK). An increasing body of evidence indicates that GAs have a negative role in drought stress tolerance. Mutants that overaccumulate GAs show increased sensitivity to water deficiency, while GA-deficient mutants with increased DELLA accumulation exhibit decreased stomatal aperture in response to exogenously applied ABA and increased drought tolerance (Nir et al., 2014; Sukiran et al., 2020; Wang et al., 2020). Thus, there is crosstalk between ABA and GAs through the DELLA proteins in the stomatal response to drought stress.
ABA and photoprotection by the xanthophyll cycle are highly interconnected under drought stress. Guan et al. (2015) linked drought stress and VDE activity through an ABA-dependent signaling pathway. They cloned a VDE gene (LcVDE) from Chinese boxthorn (Lycium chinense) and observed that in response to drought stress, LcVDE expression, the de-epoxidation ratio, and NPQ all increased significantly, enhancing drought stress tolerance. However, when a potent ABA inhibitor was applied, LcVDE expression and the de-epoxidation ratio of the xanthophyll cycle were markedly reduced. These results suggest that endogenous ABA is involved in the regulation of VDE gene expression, thereby indirectly modulating the extent of the impaired photosynthesis caused by drought stress. These findings are in agreement with a previous study that revealed that increasing xanthophylls and ABA synthesis in rice elicited drought tolerance (Du et al., 2010). Moreover, the role of exogenously applied ABA in water stress tolerance has been reported in many species such as barley (Hordeum vulgare), wheat, bean (Phaseolus vulgaris), sugar beet (Beta vulgaris), tobacco, and maize (Mizrahi et al., 1974; Agarwal et al., 2005; Haisel et al., 2006). Generally, ABA treatment decreases the degradation of photosynthetic pigments, with ABA mediating a higher pool of pigments from the xanthophyll cycle during water stress, thus increasing the NPQ. The detection and transduction of ABA signals are also closely linked to redox signaling, which in turn may disrupt the electron transport chain in chloroplasts. In the study of Daszkowska-Golec et al. (2019), 200 µM of ABA negatively affected the electron fate between the photosynthetic antenna absorption and the QA at the acceptor side of PSII, while 10 µM did not have any harmful effects during drought stress. These data suggest that the concentration of ABA is critical in determining whether it acts as an inhibitor or a stimulator during water stress.
Auxin may also play a role in the modulation of photosynthesis under drought stress, although its possible role in photoprotection is still unknown. Zhang et al. (2020) recently revealed that exogenously applied IAA significantly increased chlorophyll concentrations and elevated ABA and JA levels in clover, thereby increasing drought tolerance. A potent IAA inhibitor significantly reduced chlorophyll, ABA, and JA levels under drought conditions. The elevated chlorophyll levels might increase the photosynthetic efficiency mediated by hormonal crosstalk. Moreover, IAA treatment inhibited the transcription of the SAG101 and SAG102 genes, which regulate leaf senescence through chlorophyll degradation. This suggests that exogenous IAA inhibits leaf senescence and, thus, chlorophyll degradation and photoinhibition (Zhang et al., 2020).
Endogenous BRs appear to affect photosynthetic efficiency and photoprotection, and they may play a crucial role in drought tolerance. Tůmová et al. (2018) observed that endogenous BR levels varied between drought-sensitive and drought-tolerant maize genotypes, the latter showing three-fold higher total BR levels (particularly those of typhasterol and 28-norbrassinolide) as well as significantly higher chlorophyll and carotenoid concentrations. BR levels were maintained, while the chlorophyll concentration increased after 14 d without watering in the drought-tolerant genotype. Moreover, drought reduced plant growth in the drought-sensitive maize plants but not in the drought-tolerant plants. However, the net CO2 assimilation rate showed no significant differences among the genotypes during drought stress, although the drought-sensitive plants showed a slightly more pronounced decline in the reduction rate of electron acceptors (Tůmová et al., 2018). Exogenously applied BR (24-epibrassinolide, EBR) can improve photosynthetic efficiency and, thus, plant growth during water deficiency. These positive effects on photosynthesis include, for example, increased PSII efficiency, higher ETR values, increased activities of the antioxidant enzymes [superoxide dismutase (SOD), APX, catalase (CAT), and guaiacol peroxidase (POX)] as well as elevated chlorophyll and carotenoid concentrations, thus favoring photoprotection (Yu et al., 2004; Hu et al., 2013; Lima and Lobato, 2017).
CK levels generally decrease during drought stress. However, the exogenous application of CKs elicits a transient rise in fluorescence and an increase in the electron donation capacity of PSII in maize (Shao et al., 2010). In bean, tobacco, sugar beet, and maize, BA treatments increase xanthophyll cycle pigment contents, thus enhancing photoprotection (Haisel et al., 2006). SA treatment also exerts positive effects on photosynthesis in wheat and soybean (Gycine max) during drought stress, improving drought tolerance and productivity (Sharma et al., 2017, 2018). For instance, the exogenous application of SA in soybean maintains physiological efficiency during water deficiency through increased levels of Rubisco activase, Rubisco subunits, Chla/bBPs, oxygen-evolving enhancer protein 1 (OEE1) and OEE2, ferredoxin NADP reductase (FNR), as well as proteins associated with photosynthesis and PSII stability. Moreover, SA treatment improves photosynthesis by increasing the rate of photosynthesis, stomatal conductance, and WUE during water stress. Increased activities of the antioxidant enzymes SOD, CAT, APX, and glutathione reductase (GR) have been also observed in SA-treated soybean plants under stress conditions (Sharma et al., 2018). Hayat et al. (2008) reported a positive role of exogenous SA treatment in photosynthesis in tomato plants through the maintenance of photosynthetic parameters, leaf water potential, carbonic anhydrase (CA) activity, and chlorophyll levels. These results also explain the growing interest in the agricultural sector in the use of phytohormones to improve plant productivity and quality both under optimal and suboptimal growth conditions.
Hormonal interplay, complementation, and cross-talk in the regulation of photosynthesis at the whole-plant level
To understand the impact of phytohormones on photosynthesis at the whole-plant level, it is relevant to consider this on an integrated physiological scale, since root–shoot and shoot–root communication strongly depends on their interconnection. In this context, a crucial process in photosynthesis is the need to continuously remove triose phosphates from the Calvin cycle through starch formation and transient storage in the chloroplasts or to export them to the cytosol to fuel sucrose synthesis and supplement the non-photosynthetic parts of the plants. Thus, plant tissues are generally characterized as source tissues, such as mature leaves, or sink tissues, such as seeds, flowers, roots, fruits, and tubers, depending on whether they export or import photosynthetically produced assimilates. Plants have an intricate network for carbohydrate partitioning that interrelates sugar and phytohormone signaling, which often leads to the increased sink strength required for growth and development. CKs and GAs are thought to be key regulators in the translocation of photosynthate from the source organ to the developing sink organ. GAs and CKs mediate the translocation by increasing the activities of extracellular invertase and hexose-uptake carriers, which enhance the sink strength (Balibrea Lara et al., 2004; Iqbal et al., 2011). Extracellular invertase is essential for apoplastic phloem unloading as it catalyses the hydrolytic cleavage of the transport sugar sucrose into glucose and fructose (Wu et al., 1993). Furthermore, CKs and auxins induce sink activity in tomato fruits by activating enzymes such as AGPases, LINs, and SUTs in columella cells (Bianchetti et al., 2017). Auxins have also recently been linked to sucrose synthase (SUS; Goren et al., 2017), which cleaves sucrose into fructose and UDP-glucose and might be important for sink strength (Stein and Granot, 2019). Dominguez et al. (2013) found an interaction among glucose, GAs, and ABA through the transcription factor ABA STRESS RIPENING 1 (ASR1), which is present in many species, but not in Arabidopsis. ASR1 regulates the expression of hexose transporters. Transgenic lines of tobacco with reduced ASR1 protein levels show impaired glucose metabolism, increased ABA and GA concentrations, and reduced CO2 assimilation (Dominguez et al., 2013). These findings suggest that the interactions between sugar, ABA, and GAs affect photosynthetic activity as well as carbon partitioning at the whole-plant level.
Ethylene and BRs also modulate photosynthesis in a cross-talk with ABA, carbohydrates, and the circadian clock. The synergistic effects between sugar and ABA through ASR1 involve many photosynthesis-related genes, such as the repression of photosynthesis‐associated nuclear genes (PhANGs; Acevedo-Hernández et al., 2005). Furthermore, plants with mutations in EIN2 and SIS1/CTR1 (associated with a constitutive response to ethylene) present greater disruption in their sugar-induced tolerance to atrazine, which is an inhibitor of electron transport in PSII (Sulmon et al., 2007). Thus, ethylene signaling appears to be required in the sucrose-dependent protective mechanism of PSII against atrazine. Moreover, ethylene accelerates the circadian oscillator by shortening the circadian period, which can be reversed by exogenously applying sucrose (Haydon et al., 2017). These results suggest crosstalk between ethylene and the pool of sucrose that affects circadian rhythms and, thus, plant photosynthetic activity. High glucose levels repress the CONSTITUTIVE PHOTOMORPHOGENESIS AND DWARFISM (CPD) gene, which is involved in the biosynthesis of BRs (Smeekens, 1998). CPD-antisense Arabidopsis lines with phenotypic changes that are characteristic of BR-deficient plants show a marked reduction in starch levels and CO2 assimilation capacity (Schlüter et al., 2002), suggesting an interaction between glucose and BRs in the regulation of photosynthesis in leaves. Interestingly, sugar (as a key signaling molecule) and phytohormones interact both antagonistically and/or agonistically in many growth and developmental processes, depending on the concentrations of both the sugar and the hormone (Zhou et al., 1998; León and Sheen, 2003; Moore et al., 2003; Kushwah and Laxmi, 2014; Gupta et al., 2015). For instance, mutants (eto) overexpressing ethylene have decreased sugar sensitivity. By contrast, plants with loss-of-function mutations (etr1, ein2, and ein3) show hypersensitivity to glucose (Zhou et al., 1998; León and Sheen, 2003). Glucose stimulates EIN3 degradation, which reduces ethylene sensitivity and causes a feedback mechanism that amplifies glucose sensitivity (Yanagisawa et al., 2003). In summary, plants possess a spatiotemporal network in which carbohydrate partitioning is a crucial connecting point for photosynthesis and phytohormone signaling to allow for plant growth and development as well as responses to environmental changes at the whole-plant level.
The flexible interplay, complementation, and crosstalk among the phytohormones in the regulation of photosynthesis during plant growth and development are necessary to optimize vigor and fitness in a changing environment (Figure 4). The development of chloroplasts depends on a sophisticated interplay among the phytohormones in which plants ensure that any possible photo-oxidative damage in chloroplasts is minimized during light-induced de-etiolation. For instance, CKs, SLs, JAs, and DELLA proteins promote photomorphogenesis directly or indirectly through HY5 (Tsuchiya et al., 2010; Wang et al., 2011; Yang et al., 2012). By contrast, ethylene, auxins, GAs, and BRs negatively regulate photomorphogenesis through PIFs or the suppression of B-GATAs (Lau and Deng, 2012; Yang et al., 2012; Liu et al., 2017). Furthermore, the crosstalk between JAs and GAs might mediate photomorphogenesis by delaying GA-mediated DELLA degradation through JAs. Moreover, JAZ proteins repress JA-responsive genes and the interaction between DELLA and PIFs (Yang et al., 2012). At the whole-plant level, the complementation of phytohormones is essential for the regulation of photosynthesis during plant growth and development, including that of different plant tissues and cell compartments. It is therefore not surprising that phytohormones regulate different or opposite physiological responses depending on their spatiotemporal position. CKs and SLs positively regulate photosynthesis through the expression of photosynthesis-related genes (Mayzlish-Gati et al., 2010; Brenner and Schmülling, 2012). By contrast, auxins negatively regulate photosynthesis through the inhibition of stoma formation but improve photosynthesis through leaf venation (McAdam et al., 2017; Qi and Torii, 2018). Moreover, the interplay between CKs and auxins regulates fruit photosynthesis and enhances fruit sink activity (Bianchetti et al., 2017), while the crosstalk between auxin and ethylene represses ethylene biosynthesis and leads to prolonged photosynthesis and delayed fruit ripening (Yuan et al., 2019).
Figure 4.
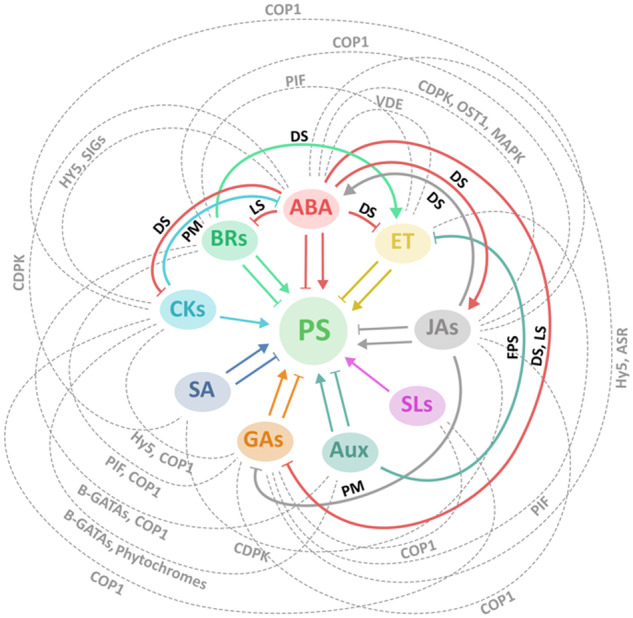
Network of phytohormone signaling regulating photosynthesis under optimal and stress conditions. Color lines connecting phytohormones indicating hormonal crosstalk. Lines with arrowheads present positive (promoting) effects. Blocked lines present negative (inhibitory) effects. Gray dash lines present hormonal interplay. ASR, Arabidopsis stress ripening 1; Aux, auxins; COP1, CONSTITUTIVE PHOTOMORPHOGENIC1; DS, drought stress; ET, ethylene; FPS, fruit photosynthesis; HY5, elongated hypocotyl 5; LS, excess light stress; PIF, PHYTOCHROME INTERACTING FACTOR; PM, photomorphogenesis; PS, photosynthesis; SIGs, sigma factor genes; VDE, violaxanthin de-epoxidase.
Conclusions and prospects
The recent findings discussed in this review reveal that understanding the impact of phytohormones on photosynthesis at the whole-plant level remains a complex challenge. Advances in recent years have helped to identify many different aspects in this network of processes. The interactions between light and phytohormones play an important role in chloroplast development and function. In this context, plants employ multiple layers of negative and positive regulators to avoid photo-oxidative damage under unfavorable conditions. For instance, CKs, JAs, and SLs promote photomorphogenesis, while ethylene, ABA, BRs, auxins, and GAs act as negative regulators. Ethylene signaling has recently been reported to halt etioplast–chloroplast differentiation through EIN3-PIF and the repression of LHC genes in seedlings. In addition to PIF, phytohormones have been linked to B-GATAs, HY5, COP1, CDPK, MAPK, and ASR. Although we have a much better understanding of hormonal signaling pathways, we are still far from understanding the complex network of hormonal interplay, complementation, and crosstalk that regulates photosynthesis. At the whole-plant level, CKs, GAs, SLs, auxins, and basal ethylene levels are essential in optimizing photosynthetic efficiency under optimal conditions. Moreover, exogenously applied SA has positive effects on photosynthesis under optimal conditions. However, target genetic approaches are required to identify the molecular regulation of photosynthesis by SA. Furthermore, the impact of phytohormones on photosynthesis is focused mainly at the leaf level, and our understanding of phytohormones regulating photosynthetic processes in other green tissues, such as fruits, is still incomplete. Further research is required to have a complete understanding of the impact of phytohormones on fruit photosynthesis and source/sink communication in the contribution to fruit quality, particularly under stress conditions. An interconnection node between ROS, antioxidants, and phytohormones coordinate a complex signaling network in response to environmental stress conditions, in which ABA and JAs appear to be the master regulators of photosynthesis. Moreover, an integrated hormonal response enables plants to regulate the most adequate photosynthetic response to every environmental condition (such as local and systemic stomatal closure, activation of antioxidants, downregulation of Lchb genes, and the induction of chlorophyll biosynthesis). It is noteworthy that almost all the phytohormones regulate photosynthesis both positively and negatively. The tolerance of plants to abiotic and biotic stresses, however, varies from species to species and between the developmental stages, leading to different adaptation responses. Thus, there are still many gaps in our knowledge on the hormonal regulation of photosynthesis and photoprotection, most particularly in non-model plants (see Outstanding Questions). New high-resolution technologies, refined analyses of transcriptomic data, and computer simulations could be of great help in shedding further light on how phytohormone signaling affects photosynthesis spatiotemporally. This is not only essential for a better understanding of basic biology, but it also has important economic implications in the agricultural, horticultural, and agri-food biotechnology sectors.
Supplemental data
Supplemental Table S1. List of selected genes of interest, their functions, and involvements in biological processes.
Funding
Parts of this work were supported by the Spanish Government [through the grants BFU2015-64001-P/MINECO/FEDER and PID2019-110335GB-I00/AEI/FEDER] and the Generalitat de Catalunya [ICREA Academia award to S.M.B. and grant 2017 SGR 980].
Conflict of interest statement. None declared.
Outstanding questions
What roles do COP1, HY5, PIFs, B-GATAs, CDPK, MAPK, or ASR play in hormonal crosstalk during retrograde signaling? Are they important nodes of hormonal crosstalk?
How do leaves and fruits communicate with each other through long-distance hormonal communication to modulate photosynthetic rates and source–sink relations under optimal growth or stress conditions? Are ABA and CKs the master regulators of this long-distance communication?
How do phytohormones regulate plant-wide stress acclimation to optimize photosynthesis? Do JAs regulate systemic stomatal closure through crosstalk with other phytohormones or independently?
How do leaves finely regulate the threshold of hormonal production to strictly regulate photosynthesis or photoprotective processes? Have these mechanisms been evolutionary conserved?
Supplementary Material
S.M.-B. conceived the idea and prepared the outline. M.M. wrote the manuscript with the help of S.M.-B. M.M. prepared the figures and tables, and S.M.-B. wrote the boxes. M.M. and S.M.-B. revised and edited the final manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Sergi Munné-Bosch (smunne@ub.edu).
References
- Acevedo-Hernández GJ, León P, Herrera-Estrella LR (2005) Sugar and ABA responsiveness of a minimal RBCS light-responsive unit is mediated by direct binding of ABI4. Plant J 43: 506–519 [DOI] [PubMed] [Google Scholar]
- Adam Z, Charuvi D, Tsabari O, Knopf RR,, Reich Z (2011) Biogenesis of thylakoid networks in angiosperms: knowns and unknowns. Plant Mol Biol 76: 221–234 [DOI] [PubMed] [Google Scholar]
- Agarwal S, Sairam RK, Srivastava GC, Meena RC (2005) Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol Plant 49: 541–550 [Google Scholar]
- Ait-Ali T, Frances S, Weller JL, Reid JB, Kendrick RE, Kamiya Y (1999) Regulation of gibberellin 20-oxidase and gibberellin 3 betahydroxylase transcript accumulation during de-etiolation of pea seedlings. Plant Physiol 121: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabadi D, Gallego-Bartolome J, Orlando L, Laura García-Cárcel L, Vicente Rubio V, Cristina Martínez C, Martín Frigerio M, Juan Manuel Iglesias-Pedraz JM, Ana Espinosa A, Xing Wang Deng X, et al. (2008) Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J 53: 324–335 [DOI] [PubMed] [Google Scholar]
- Albacete A,, Cantero-Navarro E, Balibrea ME, Großkinsky DK, de la Cruz González M, Martínez-Andújar C, Smigocki AC, Roitsch T, Pérez-Alfocea F (2014) Hormonal and metabolic regulation of tomato fruit sink activity and yield under salinity. J Exp Bot 65: 6081–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo WL, Nunes-Nesi A, Osorio S, Usadel B, Fuentes D, Nagy R, Balbo I, Lehmann M, Studart-Witkowski C, Tohge T, et al. (2011) Antisense inhibition of the iron–sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid-mediated effect on stomatal aperture. Plant Cell 23: 600–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arraes FB, Beneventi MA,, Lisei de Sa ME, Paixao JF, Albuquerque EV, Marin SR, Purgatto E, Nepomuceno AL, Grossi-de-Sa MF (2015) Implications of ethylene biosynthesis and signalling in soybean drought stress tolerance. BMC Plant Biol 15: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141: 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcerowicz M, Ranjan A, Rupprecht L, Fiene G, Hoecker U (2014) Auxin represses stomatal development in dark-grown seedlings via Aux/IAA proteins. Development 141: 3165–3176 [DOI] [PubMed] [Google Scholar]
- Balfagón D, Sengupta S, Gómez-Cadenas A, Fritschi FB, Azad RK, Mittler R, Zandalinas SI (2019) Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol 181: 1668–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balibrea Lara ME, Gonzalez Garcia MC, Fatima T, Ehness R, Lee TK, Proels R, Tanner W, Roitsch T (2004) Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16: 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, et al. (2004) Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16: 2448–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J, Nield J, Morris EP, Zheleva D, Hankamer B (1997) The structure, function, and dynamics of photosystem II. Physiol Plant 100: 817–827 [Google Scholar]
- Barkan A, Goldschmidt-Clermont M (2000) Participation of nuclear genes in chloroplast gene expression. Biochimie 82: 559–572 [DOI] [PubMed] [Google Scholar]
- Barsan C, Zouine M, Maza E, Bian W, Egea I, Rossignol M, Bouyssie D, Pichereaux C, Purgatto E, Bouzayen M, et al. (2012) Proteomic analysis of chloroplast-to-chromoplast transition in tomato reveals metabolic shifts coupled with disrupted thylakoid biogenesis machinery and elevated energy-production components. Plant Physiol 160: 708–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti RE, Cruz AB, Oliveira BS, Demarco D, Purgatto E, Peres LEP, Rossi M, Freschi L. (2017) Phytochromobilin deficiency impairs sugar metabolism through the regulation of cytokinin and auxin signaling in tomato fruits. Sci Rep 7: 7822–7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi L, Liu G-W, Emonet A, Kretzschmar T, Martinoia E (2016) The importance of strigolactone transport regulation for symbiotic signaling and shoot branching. Planta 243: 1351–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner WG, Schmülling T (2012) Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organ-specific responses. BMC Plant Biol 12: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceusters J, Van de Poel B (2018) Ethylene exerts species-specific and age-dependent control of photosynthesis. Plant Physiol 176: 2601–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CCC, Ball L, Fryer MJ, Baker NR, Karpinski S, Mullineaux PM (2004) Induction of ASCORBATE PEROXIDASE 2 expression in wounded Arabidopsis leaves does not involve known wound-signaling pathways but is associated with changes in photosynthesis. Plant J 38: 499–511 [DOI] [PubMed] [Google Scholar]
- Charuvi D, Kiss V, Nevo R, Shimoni E, Adam Z, Reich Z (2012) Gain and loss of photosynthetic membranes during plastid differentiation in the shoot apex of Arabidopsis. Plant Cell 24: 1143–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater CCC, Oliver J, Casson S, Gray JE (2014) Putting the brakes on: abscisic acid as a central environmental regulator of stomatal development. New Phytol 202: 376–391 [DOI] [PubMed] [Google Scholar]
- Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y (2017) Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 29: 1425–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sonobe K, Ogawa N, Masuda S, Nagatani A, Kobayashi Y, Ohta H (2013) Inhibition of Arabidopsis hypocotyl elongation by jasmonates is enhanced under red light in phytochrome B dependent manner. J Plant Res 126: 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR (2015) Ethylene regulates energy-dependent non-photochemical quenching in Arabidopsis through repression of the xanthophyll cycle. PLoS ONE 10: e0144209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheminant S, Wild M, Bouvier F, Pelletier S, Renou JP, Erhardt M, Hayes S, Terry MJ, Genschik P, Achard P (2011) DELLAs regulate chlorophyll and carotenoid biosynthesis to prevent photooxidative damage during seedling deetiolation in Arabidopsis. Plant Cell 23: 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocaliadis MF, Fernández-Muñoz R, Pons C, Orzaez D, Granell A (2014) Increasing tomato fruit quality by enhancing fruit chloroplast function. A double-edged sword? J Exp Bot 65: 4589–4598 [DOI] [PubMed] [Google Scholar]
- Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217: 67–75 [DOI] [PubMed] [Google Scholar]
- Cortleven A, Nitschke S, Klaumünzer M, Abdelgawad H, Asard H, Grimm B, Riefler M, Schmülling T (2014) A novel protective function for cytokinin in the light stress response is mediated by the Arabidopsis histidine kinase 2 and Arabidopsis histidine kinase 3 receptors. Plant Physiol 164: 1470–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortleven A, Schmülling T (2015) Regulation of chloroplast development and function by cytokinin. J Exp Bot 66: 4999–5013 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1997) Oligosaccharins, brassinolides, and jasmonates: nontraditional regulators of plant growth, development, and gene expression. Plant Cell 9: 1211–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova MN, Andreeva AA, Doroshenko AS, Kudryakova NV, Kuznetsov VIV, Kusnetsov VV (2018) Phytohormones regulate the expression of nuclear genes encoding the components of the plastid transcription apparatus. Biochem Biophys 478: 25–29 [DOI] [PubMed] [Google Scholar]
- Danilova MN, Doroshenko AS, Zabrodin DA, Kudryakova NV, Oelmüller R, Kusnetsov VV (2017) Cytokinin membrane receptors modulate transcript accumulation of plastid encoded genes. Russ J Plant Physiol 64: 301–309 [Google Scholar]
- Daszkowska-Golec A, Collin A, Sitko K, Janiak A, Kalaji HM, Szarejko I (2019) Genetic and physiological dissection of photosynthesis in barley exposed to drought stress. Int J Mol Sci 20: 6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW (2002) Antioxidants in photosynthesis and human nutrition. Science 298: 2149–2153 [DOI] [PubMed] [Google Scholar]
- Devireddy AR, Zandalinas SI, Gómez-Cadenas A, Blumwald E, Mittler R (2018) Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci Signal 20: 11eaam9514. [DOI] [PubMed] [Google Scholar]
- Dietz K, Turkan I, Krieger-Liszkay A (2016) Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol 171: 1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T, Park Y, Hwang I (2015) Abscisic acid: biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem 58: 29–48 [DOI] [PubMed] [Google Scholar]
- Dominguez PG, Frankel N, Mazuch J, Balbo I, Iusem N, Fernie AR, Carrari F (2013) ASR1 mediates glucose-hormone cross talk by affecting sugar trafficking in tobacco plants. Plant Physiol 161: 1486–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Wang N, Cui F, Li X, Xiao J, Xiong L (2010) Characterization of the beta-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol 154: 1304–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova M, Danila FR, Furbank RT, von Caemmerer S (2020) On the road to C4 rice: advances and perspectives. Plant J 101: 940–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM (2017) Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nature Commun 8: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2005a) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2005b) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28: 1056–1071 [Google Scholar]
- Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33: 691–705 [DOI] [PubMed] [Google Scholar]
- Galvez-Valdivieso G, Fryer MJ, Lawson T, Slattery K, Truman W, Smirnoff N, Asami T, Davies WJ, Jones AM, Baker NR, et al. (2009) The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 21: 2143–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Prasad VB, Chattopadhyay S (2010) Functional interconnection of MYC2 and SPA1 in the photomorphogenic seedling development of Arabidopsis. Plant Physiol 154: 1210–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Gorecka M, Alvarez-Fernandez R, Slattery K, McAusland L, Davey PA, Karpinski S, Lawson T, Mullineaux PM (2014) Abscisic acid signalling determines susceptibility of bundle sheath cells to photoinhibition in high light-exposed Arabidopsis leaves. Phil Trans R Soc Lond B Biol Sci 69: 20130234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren S, Lugassi N, Stein O, Yeselson Y, Schaffer AA, David-Schwartz R, Granot D (2017) Suppression of sucrose synthase affects auxin signaling and leaf morphology in tomato. PLoS ONE 12: e0182334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbic V, Bleecker AB (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J 8: 595–602 [Google Scholar]
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48: 909–930 [DOI] [PubMed] [Google Scholar]
- Guan C, Ji J, Zhang X, Li X, Jin C, Guan W, Wang G (2015) Positive feedback regulation of a Lycium chinense-derived VDE gene by drought-induced endogenous ABA, and over-expression of this VDE gene improve drought-induced photo-damage in Arabidopsis. J Plant Physiol 175: 26–36 [DOI] [PubMed] [Google Scholar]
- Guan C, Wang X, Feng J, Hong S, Liang Y, Ren B, Zuo J (2014) Cytokinin antagonizes abscisic acid-mediated inhibition of cotyledon greening by promoting the degradation of ABSCISIC ACID INSENSITIVE5 protein in Arabidopsis. Plant Physiol 164: 1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Schneider-Pizón J, Betti C, Mayerhofer J, Vanhoutte I, van Dongen W, Boeren S, Zhiponova M, de Vries S, Jonak C, et al. (2012) SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nat Cell Biol 14: 548–554 [DOI] [PubMed] [Google Scholar]
- Gupta A, Singh M, Laxmi A (2015) Multiple interactions between glucose and brassinosteroid signal transduction pathways in Arabidopsis are uncovered by whole-genome transcriptional profiling. Plant Physiol 168: 1091–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururani MA, Mohanta TK, Bae H (2015) Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int J Mol Sci 16: 19055–19085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisel D, Pospíšilová J, Synková H, Schnablová R, Baťková P (2006) Effects of abscisic acid or benzyladenine on pigment contents, chlorophyll fluorescence, and chloroplast ultrastructure during water stress and after rehydration. Photosynthetica 44: 606–614 [Google Scholar]
- Han VP,, Song PS, Kim J (2014) Phytochrome-mediated photomorphogenesis in plants. J Plant Biol 50: 230–240 [Google Scholar]
- Havaux M, Bonfils JP, Lütz C, Niyogi KK (2000) Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol 124: 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat S, Hasan SA, Fariduddin Q, Ahmad A (2008) Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J Plant Interact 3: 297–304 [Google Scholar]
- Haydon MJ, Mielczarek O, Frank A, Román Á, Webb AAR (2017) Sucrose and ethylene signaling interact to modulate the circadian clock. Plant Physiol 175: 947–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington SE, Smilie RM, Davies WJ (1998) Photosynthetic activities of vegetative and fruiting tissues of tomato. J Exp Bot 49: 1173–1181 [Google Scholar]
- Hirakawa Y, Sawa S (2019) Diverse function of plant peptide hormones in local signaling and development. Curr Opin Plant Biol 51: 81–87 [DOI] [PubMed] [Google Scholar]
- Hönig M, Plíhalová L, Husičková A, Nisler J, Doležal K (2018) Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int J Mol Sci 19: 40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2011) Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol 156: 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WH, Yan XH, Xiao YA, Zeng JJ, Qi HJ,, Ogweno JO (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic 150: 232–237 [Google Scholar]
- Huang J, Zhao X, Chory J (2019) The Arabidopsis transcriptome responds specifically and dynamically to high light stress. Cell Rep 29: 4186–4199. e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W, Sankhla N (1974) Effect of gibberellic acid on the activities of photosynthetic enzymes and 14CO2 fixation products in leaves of Pennisetum typhoides seedlings. Z Pflanzenphysiol 71: 275–280 [Google Scholar]
- Humplík JF, Turečková V, Fellner M, Bergougnoux V (2015) Spatio-temporal changes in endogenous abscisic acid contents during etiolated growth and photomorphogenesis in tomato seedlings. Plant Signal Behav 10: e1039213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Nazar R, Khan MIR, Masood A, Khan NA (2011) Role of gibberellins in regulation of source–sink relations under optimal and limiting environmental conditions. Curr Sci 100: 7 [Google Scholar]
- Janda T, Gondor OK, Yordanova R, Szalai G, Pál M (2014) Salicylic acid and photosynthesis: signalling and effects. Acta Physiol Plant 36: 2537–2546 [Google Scholar]
- Janečková H, Husičková A, Ferretti U, Prčina M, Pilařová E, Plačková L, Pospíšil P, Doležal K, Špundová M (2018) The interplay between cytokinins and light during senescence in detached Arabidopsis leaves. Plant Cell Environ 41: 1870–1885 [DOI] [PubMed] [Google Scholar]
- Jia K-P, Luo Q, He S-B, Lu X-D, Yang H-Q (2014) Strigolactone-regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signalling pathways in Arabidopsis. Mol Plant 7: 528–540 [DOI] [PubMed] [Google Scholar]
- Jiang X, Li H, Wang T, Peng C, Wang H, Wu H, Wang X (2012) Gibberellin indirectly promotes chloroplast biogenesis as a means to maintain the chloroplast population of expanded cells. Plant J 72: 768–780 [DOI] [PubMed] [Google Scholar]
- Juvany M, Müller M, Munné-Bosch S (2013) Photo-oxidative stress in emerging and senescing leaves: a mirror image? J Exp Bot 64: 3087–3098 [DOI] [PubMed] [Google Scholar]
- Kende H, Zeevart JAD (1997) The five “classical” plant hormones. Plant Cell 9: 1197–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon MAR, Salam MA, Jammes F, Ye W, Hossain MA, Okuma E, Nakamura Y, Mori IC, Kwak JM, Murata Y (2017) MPK9 and MPK12 function in SA-induced stomatal closure in Arabidopsis thaliana. Biosci Biotechnol Biochem 81: 1394–1400 [DOI] [PubMed] [Google Scholar]
- Kim GD, Cho YH, Yoo SD (2017) Phytohormone ethylene-responsive Arabidopsis organ growth under light is in the fine regulation of photosystem II deficiency-inducible AKIN10 expression. Sci Rep 7: 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-W, Michniewicz M, Bergmann DC, Wang Z-Y (2012) Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482: 419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Gen 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Kushwah S, Laxmi A (2014) The interaction between glucose and cytokinin signal transduction pathway in Arabidopsis thaliana. Plant Cell Environ 37: 235–253 [DOI] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Lemaire-Chamley M, Petit J, Garcia V, Just D, Baldet P, Germain V, Fagard M, Mouassite M, Cheniclet C, Rothan C (2005) Changes in transcriptional profiles are associated with early fruit tissue specialization in tomato. Plant Physiol 139: 750–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Levitt LK, Stein DB, Rubinstein B (1987) Promotion of stomatal opening by indoleacetic acid and ethrel in epidermal strips of Vicia faba L. Plant Physiol 85: 318–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Herrera-Estrella L, Tran LSP (2016) The Yin–Yang of cytokinin homeostasis and drought acclimation/adaptation. Trends Plant Sci 21: 548–550 [DOI] [PubMed] [Google Scholar]
- Liere K, Weihe A, Börner T (2011) The transcription machineries of plant mitochondira and chloroplast: composition, function, and regulation. J Plant Physiol 168: 1345–1360 [DOI] [PubMed] [Google Scholar]
- Lima JV, Lobato AKS (2017) Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol Mol Biol Plants 23: 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-T, Chen L-J, Herrfurth C, Feussner I, Li H (2016) Reduced biosynthesis of digalactosyldiacylglycerol, a major chloroplast membrane lipid, leads to oxylipin overproduction and phloem cap lignification in Arabidopsis. Plant Cell 28: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu R, Li Y, Shen X, Zhong S, Shi H (2017) EIN3 and PIF3 form an interdependent module that represses chloroplast development in buried seedlings. Plant Cell 29: 3051–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse G, Hedrich R (1992) Characterization of the plasma-membrane H(+)-ATPase from Vicia faba guard cells: modulation by extracellular factors and seasonal changes. Planta 188: 206–214 [DOI] [PubMed] [Google Scholar]
- Lupi ACD, Lira BS, Gramegna G, Trench B, Alves FRR, Demarco D, Peres LEP, Purgatto E, Freschi L, Rossi M (2019) Solanum lycopersicum GOLDEN 2-LIKE 2 transcription factor affects fruit quality in a light- and auxin-dependent manner. PLoS ONE 14: e0212224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytovchenko A, Eickmeier I, Pons C, Osorio S, Szecowka M, Lehmberg K, Arrivault S, Tohge T, Pineda B, Anton MT, et al. (2011) Tomato fruit photosynthesis is seemingly unimportant in primary metabolism and ripening but plays a considerable role in seed development. Plant Physiol 157: 1650–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S, Zhang Y, Li C, Liu Z, Yang N, Pan L, Wu J, Wang J, Yang J, Lv Y, et al. (2017) Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol 217: 290–304 [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Sasaki E, Shimada Y, Nagae M, Ueno K, Nakano T, Yoneyama K, Suzuki Y, Asami T (2009) Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci Biotech Biochem 73: 2460–2465 [DOI] [PubMed] [Google Scholar]
- Masuda Y, Kamisaka K (2000) Discovery of auxin. In Kung S-D, Yang S-F, eds, Discoveries in Plant Biology, Vol. III, World Scientific, Singapore, pp. 43–57 [Google Scholar]
- Mayzlish-Gati E, LekKala SP, Resnick N, Wininger S, Bhattacharya C, Lemcoff JH, Kapulnik Y, Koltai H (2010) Strigolactones are positive regulators of light-harvesting genes in tomato. J Exp Bot 61: 3129–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Eléouët MP, Best M, Brodribb TJ, Murphy MC, Cook SD, Dalmais M, Dimitriou T, Gélinas-Marion A, Gill WM, et al. (2017) Linking auxin with photosynthetic rate via leaf venation. Plant Physiol 175: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Jalakas P, Kollist H, Brosché M (2001) The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Mol Plant 8: 657–659 [DOI] [PubMed] [Google Scholar]
- Merritt F, Kemper A, Tallman G (2001) Inhibitors of ethylene synthesis inhibit auxin-induced stomatal opening in epidermis detached from leaves of Vicia faba L. Plant Cell Physiol 42: 223–230 [DOI] [PubMed] [Google Scholar]
- Miret JA, Müller M (2018) AsA/DHA redox pair influencing plant growth and stress tolerance. In Hossain MA, Munné-Bosch S, Burrit DJ, Díaz-Vivancos P, Fujita M, Lorence A, eds, Ascorbic Acid in Plant Growth, Development and Stress Tolerance. Springer, Berlin, Germany, pp. 297–319 [Google Scholar]
- Miura K, Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi Y, Scherings SG, Arad SM, Richmond AE (1974) Aspects of the effects of ABA on the water status of barley and wheat seedlings. Physiol Plant 31: 44–50 [Google Scholar]
- Moore B, Zhou L, Hall Q Rolland F, Cheng W-H, Liu YX, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Monteiro CC,, Carvalho RF, Gratão PL, Carvalho G, Tezotto T, Medici LO, Peres LEP, Azevedo RA (2011) Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environ Exp Bot 71: 306–320 [Google Scholar]
- Morgan PW, He CJ, De Greef JA, De Proft MP (1990) Does water deficit stress promote ethylene synthesis by intact plants? Plant Physiol 94: 1616–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI (2015) Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol 28: 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hirao Y, Tanami K, Mimata Y, Nakamura Y, Murata Y (2019) Ethylene inhibits methyl jasmonate-induced stomatal closure by modulating guard cell slow-type anion channel activity via the OPEN STOMATA 1/SnRK2.6 kinase-independent pathway in Arabidopsis. Plant Cell Physiol 60: 2263–2271 [DOI] [PubMed] [Google Scholar]
- Muñoz P, Munné-Bosch S (2018) Photo-oxidative stress during leaf, flower and fruit development. Plant Physiol 176: 1004–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P, Munné-Bosch S (2019) Vitamin E inplants: biosynthesis, transport and function. Trends Plant Sci 24: 1040–1051 [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Fenzi F, Ciliento R, Alfano F, Bowler C (1999) Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homolog of DEETIOLATED1. Plant Cell 11: 145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadakuduti SS, Holdsworth WL, Klein CL, Barry CS (2014) KNOX genes influence a gradient of fruit chloroplast development through regulation of GOLDEN2-LIKE expression in tomato. Plant J 78: 1022–1033 [DOI] [PubMed] [Google Scholar]
- Nadeem M, Li J, Yahya M, Sher A, Ma C, Wang X, Qiu L (2019) Research progress and perspective on drought stress in legumes: a review. Int J Mol Sci 20: 2541–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath N, Mishra D (1990) Changes in RUBP case activity of developing and sensing barley leaves and its alteration by plant growth regulators. Indian J Exp Biol 28: 665–670 [Google Scholar]
- Nir I, Moshelion M, Weiss D (2014) The Arabidopsis gibberellin methyl transferase 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Environ 37: 113–123 [DOI] [PubMed] [Google Scholar]
- Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, et al. (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23: 2169–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilby PR (2010) Singlet oxygen: there is indeed something new under the sun. Chem Soc Rev 39: 3181–3209 [DOI] [PubMed] [Google Scholar]
- Oh SA, Park JH, Lee GI, Paek KH, Park SK, Nam HG (1997) Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J 12: 527–535 [DOI] [PubMed] [Google Scholar]
- Oh E, Zhu J-Y, Wang Z-Y (2012) Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 14: 802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Alcaide M, Llamas E, Gomez-Cadenas A, Nagatani A, Martínez-García JF, Rodríguez-Concepcióna M (2019) Chloroplasts modulate elongation responses to canopy shade by retrograde pathways involving HY5 and abscisic acid. Plant Cell 31: 384–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Bradley G, Pyke K, Ball G, Lu C, Fray R, Marshall A, Jayasuta S, Baxter C, van Wijk R, et al. (2013) Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol 161: 1476–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Watson A, Griffiths CA (2020) Linking fundamental science to crop improvement through understanding source and sink traits and their integration for yield enhancement. J Exp Bot 71: 2270–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres ALGL, Soares JS, Tavares RG, Righetto G, Zullo MAT, Mandava NB, Menossi M (2019) Brassinosteroids, the sixth class of phytohormones: a molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int J Mol Sci 20: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T, Schutze K, Brost M, Oelmuller R (2001) A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J Biol Chem 276: 36125–36130 [DOI] [PubMed] [Google Scholar]
- Pintó-Marijuan M, Munné-Bosch S (2014) Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: advantages and limitations. J Exp Bot 65: 3845–3857 [DOI] [PubMed] [Google Scholar]
- Pospíšilová J (2003) Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol Plant 46: 491–506 [Google Scholar]
- Pribil M, Labs M, Leister D (2014) Structure and dynamics of thylakoids in land plants. J Exp Bot 65: 1955–1972 [DOI] [PubMed] [Google Scholar]
- Prodhan MY, Munemasa S, Nahar MN, Nakamura Y, Murata Y (2018) Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol 178: 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Zhao X, Li Z (2020) iTRAQ-based quantitative proteomic analysis of the Arabidopsis mutant opr3-1 in response to exogenous MeJA. Int J Mol Sci 21: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Torii KU (2018) Hormonal and environmental signals guiding stomatal development. BMC Biol 16: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher W (2015) Plant growth regulators: backgrounds and uses in plant production. J Plant Growth Regul 34: 845–872 [Google Scholar]
- Raskin I (1992) Salicylate, a new plant hormone. Plant Physiol 99: 799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA (2014) Speedy small stomata? J Exp Bot 65: 1415–1424 [DOI] [PubMed] [Google Scholar]
- Richter R, Behringer C, Müller IK, Schwechheimer C (2010) The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Dev 24: 2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62: 3321–3338 [DOI] [PubMed] [Google Scholar]
- Rodrigues J, Inzé D, Nelissen H, Saibo NJM (2019) Source–sink regulation in crops under water deficit. Trends Plant Sci 24: 652–663 [DOI] [PubMed] [Google Scholar]
- Roje S (2006) S-adenosyl-l-methionine: beyond the universal methyl group donor. Phytochemistry 67: 1686–1698 [DOI] [PubMed] [Google Scholar]
- Rivero RM, Shulaev V, Blumwald E (2009) Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol 150: 1530–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar M, Chervin C, Mila I, Hao Y, Roustan JP, Benichou M, Gibon Y, Biais B, Maury P, Latché A, et al. (2013) SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol 161: 1362–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Iribe A, De-la-Peña C (2020) Auxins, the hidden player in chloroplast development. Plant Cell Rep 39: 1595–1608 [DOI] [PubMed] [Google Scholar]
- Saradadevi R, Palta JA, Siddique KHM (2017) ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front Plant Sci 8: 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko T, Kolla VA, Wang CQ, Nasafi Z, Hicks DR, Phadungchob B, Chehab WE, Brandizzi F, Froehlich J, Dehesh K (2014) Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol 164: 1151–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AA, Petreikov M (1997) Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol 113: 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter U, Köpke D, Altmann T, Müssig C (2002) Analysis of carbohydrate metabolism of CPD antisense plants and the brassinosteroid deficient cbb1 mutant. Plant Cell Environ 25: 783–791 [Google Scholar]
- Sengar RS, Singh A (2018) Eco-Friendly Agro-biological Techniques for Enhancing Crop Productivity, Springer, Singapore [Google Scholar]
- Serna L (2013) What causes opposing actions of brassinosteroids on stomatal development? Plant Physiol 162: 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Gupta SK, Majumder B, Maurya VK, Deeba F, Alam A, Pandey V (2017) Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J Proteomics 163: 28–51 [DOI] [PubMed] [Google Scholar]
- Sharma M, Gupta SK, Majumder B, Maurya VK, Deeba F, Alam A, Pandey V (2018) Proteomics unravel the regulating role of salicylic acid in soybean under yield limiting drought stress. Plant Physiol Biochem 130: 529–541 [DOI] [PubMed] [Google Scholar]
- Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25: 211–222 [DOI] [PubMed] [Google Scholar]
- Shanmugabalaji V, Chahtane H, Accossato S, Rahire M, Gouzerh G, Lopez-Molina L, Kessler F (2018) Chloroplast biogenesis controlled by DELLA-TOC159 interaction in early plant development. Curr Biol 28: 2616–2623 [DOI] [PubMed] [Google Scholar]
- Shao R, Wang K, Shangguan Z (2010) Cytokinin-induced photosynthetic adaptability of Zea mays L. to drought stress associated with nitric oxide signal: probed by ESR spectroscopy and fast OJIP fluorescence rise. J Plant Physiol 167: 472–499 [DOI] [PubMed] [Google Scholar]
- Shen H, Zhu L, Bu QY, Huq E (2012) MAX2 affects multiple hormones to promote photomorphogenesis. Mol Plant 5: 224–236 [DOI] [PubMed] [Google Scholar]
- Shi C, Qi C, Ren H, Huang A, Hei S, She X (2015) Ethylene mediates brassinosteroid-induced stomatal closure via Gα protein-activated hydrogen peroxide and nitric oxide production in Arabidopsis. Plant J 82: 280–301 [DOI] [PubMed] [Google Scholar]
- Shi H, Chen L, Ye T, Liu X, Ding K, Chan Z (2014) Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol Biochem 82: 209–217 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Faralli M, Ramamoorthy S, Lawson T (2020) Photosynthesis in non-foliar tissues: implications for yield. Plant J 101: 1001–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumbe L, Chevalier A, Legeret B, Taconnat L, Monnet F, Havaux M (2016) Singlet oxygen-induced cell death in Arabidopsis under high-light stress is controlled by OXI1 kinase. Plant Physiol 170: 1757–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui H, Hayat S, Bajguz A (2018) Regulation of photosynthesis by brassinosteroids in plants. Acta Physiol Plant 40: 59 [Google Scholar]
- Smeekens S (1998) Sugar regulation of gene expression in plants. Curr Opin Plant Biol 1: 230–234 [DOI] [PubMed] [Google Scholar]
- Smith MR, Rao IM, Merchant A (2018) Source–sink relationships in crop plants and their influence on yield development and nutritional quality. Front Plant Sci 9: 1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X-G, She X-P, He J-H, Huang C, Song T (2005) Cytokinin- and auxin-induced stomatal opening involves a decrease in levels of hydrogen peroxide in guard cells of Vicia faba. Funct Plant Biol 33: 573–583 [DOI] [PubMed] [Google Scholar]
- Staneloni RJ, Rodriguez-Batiller MJ, Casal JJ (2008) Abscisic acid, high-light, and oxidative stress down-regulate a photosynthetic gene via a promoter motif not involved in phytochrome-mediated transcriptional regulation. Mol Plant 1: 75–83 [DOI] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16: 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O, Granot D (2019) An overview of sucrose synthases in plants. Front Plant Sci 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DB, Hanson MR, Barkan A (2004) Genetics and genomics of chloroplast biogenesis: maize as a model system. Trends Plant Sci 9: 293–301 [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukiran NA, Steel PG, Knight MR (2020) Basal stomatal aperture is regulated by GA-DELLAs in Arabidopsis. J Plant Physiol 250: 153182. [DOI] [PubMed] [Google Scholar]
- Sulmon C, Gouesbet G, El Amrani A, Couée I (2007) Involvement of the ethylene-signalling pathway in sugar-induced tolerance to the herbicide atrazine in Arabidopsis thaliana seedlings. J Plant Physiol 164: 1083–1092 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Devireddy AR, Inupakutika MA, Baxter A, Miller G, Song L, Shulaev E, Azad RK, Shulaev V, Mittler R (2015) Ultra-fast alterations in mRNA levels uncover multiple players in light stress acclimation in plants. Plant J 84: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16: 53–60 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S (2005) Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 138: 2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WW, Lewis LN, Coggins CW (1967) The reversion of chromoplasts to chloroplasts in Valencia oranges. Cytologia 32: 117–124 [Google Scholar]
- Tinoco-Ojanguren C (2008) Diurnal and seasonal patterns of gas exchange and carbon gain contribution of leaves and stems of Justicia californica in the Sonoran Desert. J Arid Environ 72: 127–140 [Google Scholar]
- Triantaphylidès C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14: 219–228 [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, McCourt P (2010) A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol 6: 741–749 [DOI] [PubMed] [Google Scholar]
- Tůmová L, Tarkowská D, Řehořová K, Marková H, Kočová M, Rothová O, Čečetka P, Holá D (2018) Drought-tolerant and drought-sensitive genotypes of maize (Zea mays L.) differ in contents of endogenous brassinosteroids and their drought-induced changes. PLoS ONE 13: e0197870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard CL, Flybjerg H, Møller M (2012) Intracellular signalling by diffusion: can waves of hydrogen peroxide transmit intracellular information in plant cells? Front Plant Sci 3: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogg G, Fischer S, Leide J, Emmanuel E, Jetter R, Levy AA, Riederer M (2004) Tomato fruit cuticular waxes and their effects on transpiration barrier properties: functional characterization of a mutant deficient in a very-long-chain fatty acid beta-ketoacyl-CoA synthase. J Exp Bot 55: 1401–1410 [DOI] [PubMed] [Google Scholar]
- Wang JG, Chen CH, Chien CT, Hsieh HL (2011) FAR-RED INSENSITIVE219 modulates CONSTITUTIVE PHOTOMORPHOGENIC1 activity via physical interaction to regulate hypocotyl elongation in Arabidopsis. Plant Physiol 156: 631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Bai MY, Oh E, Zhu JY (2012) Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 46: 701–724 [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu L, Cheng C, Ren Z, Xu S, Li X (2020) GAI functions in the plant response to dehydration stress in Arabidopsis thaliana. Int J Mol Sci 21: 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Smith SM (2013) KAI2- and MAX2-mediated responses to karrikins and strigolactones are largely independent of HY5 in Arabidopsis seedlings. Mol Plant 6: 63–75 [DOI] [PubMed] [Google Scholar]
- Weatherwax SC, Ong MS, Degenhardt J, Bray EA, Tobin EM (1996) The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiol 111: 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ 33: 510–525 [DOI] [PubMed] [Google Scholar]
- Wu L, Mitchell JP, Cohn NS, Kaufman PB (1993) Gibberellin (GA3) enhances cell wall invertase activity and mRNA levels in elongating dwarf pea (Pisum sativum) shoots. Int J Plant Sci 154: 280–289 [DOI] [PubMed] [Google Scholar]
- Yamburenko MV, Zubo YO, Börner T (2015) Abscisic acid affects transcription of chloroplast genes via protein phosphatase 2C-dependent activation of nuclear genes: repression by guanosine-3′-5′-bisdiphosphate and activation by sigma factor 5. Plant J 82: 1030–1041 [DOI] [PubMed] [Google Scholar]
- Yamburenko MV, Zubo YO, Vanková R, Kusnetsov VV, Kulaeva ON, Börner T (2013) Abscisic acid represses the transcription of chloroplast genes. J Exp Bot 64: 4491–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Yang DL, Yao J, Mei CS, Tong XH, Zeng LJ, Li Q, Xiao LT, Sun TP, Li J, Deng XW, et al. (2012) Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci U S A 109: E1192–E1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DQ, Luo YL, Dong WH, Yin YP, Li Y, Wang ZL (2018) Response of photosystem II performance and antioxidant enzyme activities in stay-green wheat to cytokinin. Photosynthetica 56: 567–577 [Google Scholar]
- Yang TF, Gonzalez-Carranza ZH, Maunders MJ, Roberts JA (2008) Ethylene and the regulation of senescence processes in transgenic Nicotiana sylvestris plants. Ann Bot 101: 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhang Z, Zhang T, Fahad S, Cui K, Nie L, Peng S, Huang J (2017) The effect of season-long temperature increases on rice cultivars grown in the central and southern regions of China. Front Plant Sci 8: 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Adachi Y, Nakamura Y, Munemasa S, Mori IC, Murata Y (2016) Involvement of OST1 protein kinase and PYR/PYL/RCAR receptors in methyl jasmonate-induced stomatal closure in Arabidopsis guard cells. Plant Cell Physiol 57: 1779–1790 [DOI] [PubMed] [Google Scholar]
- Young TE, Meeley RB, Gallie DR (2004) ACC synthase expression regulates leaf performance and drought tolerance in maize. Plant J 40: 813–825 [DOI] [PubMed] [Google Scholar]
- Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55: 1135–1143 [DOI] [PubMed] [Google Scholar]
- Yuan L, Xu D-Q (2001) Stimulation effect of gibberellic acid short-term treatment on the photosynthesis related to the increase in rubisco content in broad bean and soybean. Photosyn Res 68: 39–47. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Xu X, Gong Z, Tang Y, Wu M, Yan F, Zhang X, Zhang Q, Yang F, Hu X, et al. (2019) Auxin response factor 6A regulates photosynthesis, sugar accumulation, and fruit development in tomato. Hortic Res 6: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Mei L, Wu M, Wei W, Shan W, Gong Z, Zhang Q, Yang F, Yan F, Zhang Q, et al. (2018) SlARF10, an auxin response factor, is involved in chlorophyll and sugar accumulation during tomato fruit development. J Exp Bot 69: 5507–5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanor MI, Osorio S, Nunes-Nesi A, Carrari F, Lohse M, Usadel B, Kühn C, Bleiss W, Giavalisco P, Willmitzer L, et al. (2009) RNA interference of LIN5 in tomato confirms its role in controlling Brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol 150: 1204–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q, Li CB, Zheng W, Wu X, Zhao J, Zhou G, Jiang H, Sun J, Lou Y, Li C (2007) Phytochrome chromophore deficiency leads to overproduction of jasmonic acid and elevated expression of jasmonate-responsive genes in Arabidopsis. Plant Cell Physiol 48: 1061–1071 [DOI] [PubMed] [Google Scholar]
- Zhang JY, He SB, Li L, Yang HQ (2014) Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proc Natl Acad Sci U S A 111: E3015–E3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li Y, Hassan MJ, Li Z, Peng Y (2020) Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol 20: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Shi H, Xi Y, Guo H (2010) Ethylene is crucial for cotyledon greening and seedling survival during de-etiolation. Plant Signal Behav 5: 739–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Zhao M, Shi T, Shi H, An F, Zhao Q, Guo H (2009) EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc Natl Acad Sci U S A 106: 21431–21436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci U S A 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ (2014) H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot 65: 4371–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Li L, Feng L, Ren AM (2020) StABI5 involved in the regulation of chloroplast development and photosynthesis in potato. Int J Mol Sci 21: 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouine M, Fu Y, Chateigner-Boutin AL, Mila I, Frasse P, Wang H, Audran C, Roustan JP, Bouzayen M (2014) Characterization of the tomato ARF gene family uncovers a multi-levels post-transcriptional regulation including alternative splicing. PLoS ONE 9: e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.