Abstract
Understanding the regulation mechanisms of photosynthesis is key to improving its efficiency and, ultimately, crop yield. In this study, we report that DEEP GREEN PANICLE1 (DGP1) is involved in photosynthesis regulation in rice (Oryza sativa L.). We identified the dgp1 mutant, which has increased chlorophyll content in glumes. The mutated gene was isolated by map-based cloning. Knockout plants, generated using a gene editing approach, mimic the phenotype of dgp1. Overexpression of DGP1 leads to chlorotic leaves and glumes. DGP1 is a plant-specific protein with a conserved TIGR01589 domain. The expression of DGP1 was detected in green tissues and is induced by light. Moreover, genes involved in key steps of chlorophyll synthesis are upregulated in the glumes of dgp1. Importantly, we found that DGP1 interacts with the rice proteins GOLDEN2-LIKE1 (OsGLK1) and GOLDEN2-LIKE2 (OsGLK2), the two transcription factors involved in the regulation of photosynthesis. Transactivation assays showed that DGP1 represses the activation activity of OsGLK1 on its target genes. Our results demonstrate that DGP1 is a repressor of OsGLK activity and thus photosynthesis in rice. Manipulation of this gene and its homologs in other crops may provide new approaches for high photosynthetic efficiency breeding.
A plant-specific protein directly regulates chlorophyll biosynthesis-related transcription factors and, thus, is a negative regulator of photosynthesis in rice glumes.
Introduction
Photosynthesis converts light energy into chemical energy and its efficiency is a major target for the improvement of crop yield potential (Long et al., 2015). Chlorophyll, a green pigment found in phototrophic organisms, is essential for light harvesting and energy transduction in photosynthesis (Fromme et al., 2003). The chlorophyll biosynthesis pathway is complex and is mediated by various biosynthetic enzymes (Tanaka and Tanaka, 2007). Mutants of some of these genes negatively affect chlorophyll biosynthesis and show color changes. In rice (Oryza sativa L.), for example, mutants of Mg-chelatase D subunit (OsCHLD) and Mg-chelatase I subunit (OsCHLI), encoding two subunits of Mg-chelatase, have yellowish-green leaves (Zhang et al., 2006). The substitution of a conserved amino acid in magnesium-protoporphyrin IX monomethyl ester cyclase leads to a chlorosis phenotype with abnormal chloroplast development in young leaves (Kong et al., 2016). FADED GREEN LEAF encodes protochlorophyllide oxidoreductase B (OsPORB), one of the two rice protochlorophyllide oxidoreductase (Sakuraba et al., 2013). Disruption of rice chlorophyllide a oxygenase1 (OsCAO1), which catalyzes the synthesis of chlorophyll b (Chlb) from chlorophyll a (Chla), leads to pale-green leaves (Lee et al., 2005).
GOLDEN2-LIKE proteins exist as pairs (GLK1 and GLK2) and are GOLDEN2, ARR, and Psr1 ( GARP) transcription factors that regulate chloroplast development in diverse plant species. Double mutants of these two genes in Arabidopsis (Arabidopsis thaliana) are pale green with reduced expression of genes involved in light harvesting and chlorophyll biosynthesis (Fitter et al., 2002). Chromatin immunoprecipitation (ChIP) assays demonstrated that AtGLK directly regulates the expression of downstream genes by binding to their promoters (Waters et al., 2009). Similarly, Osglk1 Osglk2 of rice, generated by introducing an RNAi construct of OsGLK1 into T-DNA insertion line of OsGLK2, are phenotypically pale and the overexpression of OsGLK1 induces the expression of genes related to chloroplast function (Nakamura et al., 2009; Wang et al., 2013). OsGLK also binds to the promoters of OsPORB, OsCAO1, and to the genes encoding the light-harvesting complex (Sakuraba et al., 2017). Constitutive expression of maize GLK genes in rice enhances biomass and grain yields, which makes the GLK promising targets for crop breeding (Li et al., 2020).
The expression of AtGLK1 is regulated by light (Fitter et al., 2002). Phytochrome-interacting factor (PIF) 4 binds with the promoters of both AtGLK1 and AtGLK2 and represses their expression (Oh et al., 2012; Song et al., 2014). OsGLK1 and OsGLK2 are directly upregulated by phytochrome-interacting factor-like1 (OsPIL1), one of the six PIF proteins in rice (Sakuraba et al., 2017). In addition, plastid-to-nucleus retrograde signals (RSs), emitted by dysfunctional chloroplasts, decrease the expression of AtGLK1 and AtGLK2 (Waters et al., 2009). Phytochrome and RS pathways converge antagonistically to regulate the expression of AtGLK1 (Martin et al., 2016). ORESARA1 (ORE1), a key senescence-control transcription factor, interacts with AtGLK1 and AtGLK2 and antagonizes their transcriptional activity (Rauf et al., 2013). Interestingly, Turnip Yellow Mosaic Virus P69 can interact with AtGLK and interferes with the binding of AtGLK proteins to the promoters of their target genes (Ni et al., 2017). Furthermore, the ubiquitin–proteasome system participates in the degradation of Arabidopsis GLK1 in response to plastid signals (Tokumaru et al., 2017). Thus, both the expressions of GLK genes and activity of GLK proteins are genetically regulated.
Here, we isolated the light-regulated gene DGP1 from a rice mutant with dark-green panicles. Our results indicate that DGP1 participates in the regulation of photosynthesis genes by repressing the activities of OsGLK in rice.
Results
Characterization of a mutant with dark-green panicles
Through mutagenesis, we obtained the dark-green panicle1 (dgp1). This mutant exhibited similar plant architecture to wild-type but had greener panicles (Figure 1, A and B). This phenotype lasted from the heading stage to the mature stage. We compared the content of Chla and Chlb, the green pigment in plants, between wild-type and dgp1. The glumes of dgp1 had significantly more Chla (0.374 ± 0.015 mg·g−1 in wild-type and 0.731 ± 0.018 mg·g−1 in dgp1) and Chlb (0.209 ± 0.007 mg·g−1 in wild-type and 0.436 ± 0.008 mg·g−1 in dgp1) than the wild-type (Figure 1C). However, no difference in leaf color before senescence was observed between wild-type and dgp1, in accordance with their comparable chlorophyll content (Supplemental Figure S1).
Figure 1.

Characterization of the dgp1 mutant. (A) Comparison of the plants of wild-type and dgp1. Bar=10 cm. (B) Comparison of the panicles of wild-type and dgp1. Bar=5 cm. (C) Chlorophyll content including Chla and Chlb in glumes of wild-type and dgp1. Mean and sd values were obtained from three biological replicates. Asterisks indicate significant differences according to Student’s t test (**P <0.01).
Map-based cloning of DGP1
The mutated gene was mapped to a 594-kb interval on chromosome 1 by map-based cloning (Figure 2A). Through high-throughput sequencing, a 196-kb deletion was detected in dgp1 and no other mutations were found in the mapped region. There are 32 genes in this deleted sequence according to “Rice Genome Annotation Project”. Among these genes, LOC_Os01g62060 attracted our attention because the overexpression of its homolog in Arabidopsis, At3g55240, cause pale-green phenotype (Ichikawa et al., 2006). This gene consists of three exons (Figure 2B). The open reading frame (ORF) is 309 bp in length, and the deduced protein contains 102 amino acids.
Figure 2.

Map-based cloning and knock-out of DGP1. (A) Map-based cloning of DGP1. The deletion region is shown by dashed line. The numbers of recombinants are listed under the corresponding markers. Cent, centromere. Rec, the number of recombinants. The location of DGP1 in the deleted region is shown by triangle. (B) Gene structure of DGP1. Coding regions are shown as black boxes. 5′- and 3′-untranslated regions are shown as grey boxes. Introns are shown as black lines. The triangle indicates the position of target sequence for CRISPR-Cas9. Details of sequence modification are listed below. (C) Comparison of the panicles of wild-type and dgp1-cas9-1. (D) Chlorophyll content including Chla and Chlb in glumes of wild-type and dgp1-cas9-1. Mean and sd values were obtained from three biological replicates. Asterisks indicate significant differences according to Student’s t test (**P <0.01).
To test whether deletion of this gene was responsible for the observed panicle phenotype, we knocked it out in wild-type by clustered regularly interspaced short palindromic repeats/CRISPR-associated protein9 (CRISPR-Cas9) gene editing approach. Two mutant lines derived from the same target sequence, dgp1-cas9-1 and dgp1-cas9-2, were obtained. A 5-bp deletion was detected in dgp1-cas9-1 (Figure 2B). There is no significant difference in plant morphology between wild-type and dgp1-cas9-1 (Supplemental Figure S2). Similar to dgp1, dgp1-cas9-1 presented dark-green panicle phenotype and contained significantly more chlorophyll than the wild-type in glumes (Figure 2, C and D). A 3-bp deletion was detected in dgp1-cas9-2, which leads to the loss of Ile18. This mutant also showed dark-green glumes phenotype, which indicated the necessity of Ile18 for the protein’s function (Supplemental Figure S3). These results confirmed that the phenotype of dgp1 is indeed caused by LOC_Os01g62060 dysfunction and this gene was accordingly designated as DGP1.
Overexpression of DGP1 leads to chlorotic leaves and panicles
We also overexpressed DGP1 in wild-type plants to test its biological function. Two representative transgenic lines, OE-1 and OE-2, were selected for further study. There is no significant difference in plant architecture between wild-type and OE-1 and the plant height of OE-2 is slightly lower than the wild-type (Figure 3A). We measured the expression levels of DGP1 in glumes and leaves from wild-type and overexpression plants. The glumes of OE-1 and OE-2 showed 3.35-fold and 6.56-fold increase on the DGP1 expression level, respectively. Similarly, the leaves of OE-1 and OE-2 showed 5.46- and 8.58-fold increase on the DGP1 expression level, respectively (Figure 3B). This indicated that DGP1 is successfully overexpressed in both OE-1 and OE-2, with OE-2 at a higher extent.
Figure 3.
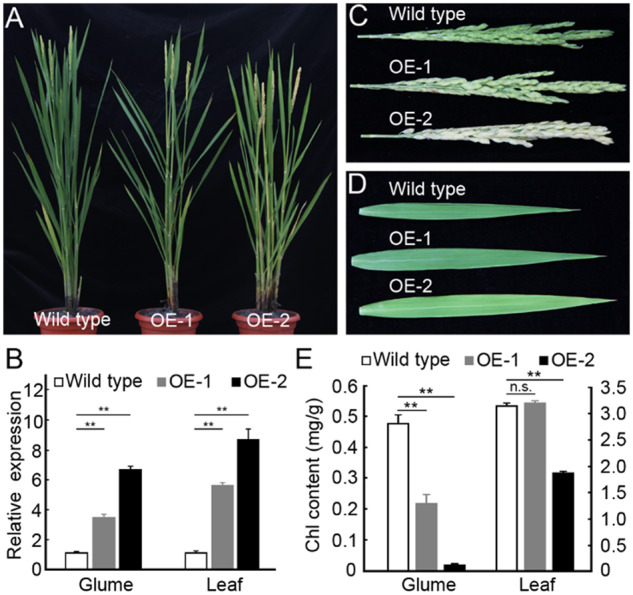
Characterization of overexpression plants of DGP1. (A) Comparison of the plants of wild-type, OE-1, and OE-2. (B) Relative expression levels of DGP1 in glumes and leaves from wild-type, OE-1, and OE-2. Ubiquitin is used as endogenous control. Error bars represent sd (n =3). (C) Comparison of the panicles from wild-type, OE-1, and OE-2. (D) Comparison of the flag leaves from wild-type, OE-1, and OE-2. (E) Chlorophyll content in glumes and leaves of wild-type, OE-1, and OE-2. Mean and sd values were obtained from three biological replicates. Asterisks indicate significant differences according to Student’s t test (**P<0.01). n.s., no significance.
Compared with the green panicles in wild-type, the panicles of OE-1 are yellow green and the panicles of OE-2 are white (Figure 3C). Accordingly, the chlorophyll content of glumes in OE-1 and OE-2 decreased to 45.44% and 3.92% of wild-type, respectively (0.480 ± 0.0253 mg·g−1 in wild-type, 0.218 ± 0.029 mg·g−1 in OE-1, 0.0188 ± 0.00276 mg·g−1 in OE-2; Figure 3E). Interestingly, despite the increase in the DGP1 expression, the leaves of OE-1 are indistinguishable from those of the wild-type (Figure 3D). The chlorophyll content in leaves is comparable between wild-type and OE-1 (Figure 3E). The leaves of OE-2 are yellow green, in accordance with its significantly decreased chlorophyll content (Figure 3, D and E). These results indicated that the overexpression of DGP1 indeed reduces the chlorophyll content in rice.
DGP1 is a plant-specific protein
To examine the subcellular localization of DGP1, we fused DGP1 with green fluorescent protein (GFP) and performed a transient expression assay in rice protoplasts. The OsbZIP52-RFP fusion protein was used as a nuclear localization marker in the assay (Liu et al., 2012). The result showed that the DGP1-GFP chimeric protein, like the free GFP control, displayed a ubiquitous distribution pattern throughout the cell (Figure 4A).
Figure 4.
Characterization of DGP1. (A) Subcellular localization of DGP1 in rice protoplasts. The fused protein OsbZIP52-RFP (red fluorescent protein) is used as the nuclear marker. BF, bright field. (B) Schematic representation of the TIGR01589 domain in the DGP1 protein. (C) Phylogenetic tree derived from the full-length amino acid sequences of DGP1 and its homologs in other plant species. (D) Multiple sequence alignment of TIGR01589 domain in DGP1 homologs. Identical amino acids are shaded in red. Os, Oryza sativa; Zm, Zea mays; At, Arabidopsis thaliana; Sl, Solanum lycopersicum.
The DGP1 protein contains a plant-specific domain, TIGR01589, with unknown function (Figure 4B). We performed a BLASTP search against the National Center for Biotechnology Information (NCBI) nonredundant protein database using DGP1 protein sequence as the query. Protein sequences with considerable similarity (percent similarity >70%) to DGP1 in O. sativa, Zea mays, A. thaliana, and Solanum lycopersicum were obtained and used for the construction of phylogenetic tree. These proteins were classified into two major clades and DGP1 grouped with its homologs in Z. mays (Figure 4C). Multiple sequence alignment revealed that the TIGR01589 domain is conserved among DGP1 homologs (Figure 4D).
Expression of DGP1 is specifically detected in green tissues and induced by light
The expression profile of DGP1 in different organs was determined by reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis (Figure 5A). DGP1 transcripts were detected in leaf, leaf sheath, emerging florets, and florets before flowering. However, the expression levels of DGP1 in roots and florets before heading were low. This result indicates that DGP1 might only be expressed in green tissues. In accordance with this hypothesis, the expression level of DGP1 in green internodes (above the leaf sheath) is much higher than in white internodes (enclosed by leaf sheath; Figure 5A). Moreover, DGP1 transcript levels increased dramatically when dark-grown etiolated seedlings were exposed to light for 12 h (Figure 5B).
Figure 5.
Expression pattern of DGP1. (A) Real-time PCR analysis of DGP1 in different tissues. R, root; WI, white internode; GI, green internode; L, leaf; Sh, leaf sheath; F1, ∼3 mm florets; F2, ∼5 mm florets; F3, ∼7.5 mm florets; F4, ∼8 mm florets; F5, ∼9 mm florets. F1–F3 are florets before heading. F4 are florets during heading. F5 are florets just before flowering. (B) Relative expression of DGP1 in dark-grown etiolated seedlings (dark) and seedlings moved from dark to light (light). (C) Time-course of the DGP1 expression in response to light and dark conditions. Leaves of about 2-week-old seedlings, harvested every 2 h in both dark treatment and light treatment, were used for expression analysis. Ubiquitin is used as endogenous control. Error bars represent sd (n =3).
We examined the effects of light- and dark-growing conditions on the expression of DGP1 using green rice seedlings (Figure 5C). The expression level achieved a three-fold increase when the treatment was switched from dark to light for 2 h. This increase continued in the following 2 h. However, the expression level decreased after 6–12 h light exposure. This indicates that expression of DGP1 is induced by light.
The chlorophyll synthesis pathway is upregulated in glumes of dgp1
Given that the chlorophyll content is increased in glumes of dgp1, we compared the expression levels of genes in chlorophyll synthesis pathway in glumes of wild-type and dgp1 by RT-qPCR. Nineteen genes, which function in 15 steps, were examined. The results showed that, except for OsHEMB, OsHEMD, and OsHEME1, all genes were upregulated in glumes of dgp1 (Figure 6). Interestingly, the increase in the expression of genes involved in 5-aminolevulinic acid synthesis from glutamyl-tRNA (OsHEMA1 and OsHEML) and the late steps from protoporphyrin IX to chlorophyll seems more pronounced. Thus, the flux through the chlorophyll synthesis pathway is increased in the absence of DGP1.
Figure 6.
Expression analysis of genes involved in chlorophyll synthesis in wild-type and dgp1 glumes. Ubiquitin is used as endogenous control. Error bars represent sd (n =3). Asterisks indicate significant differences according to Student’s t test (*P <0.05, **P <0.01).
DGP1 interacts with OsGLK1 and OsGLK2
As mutation of DGP1 generally increases the expression of chlorophyll synthesis genes and DGP1 lacks a typical transcription regulation domain, we assumed that DGP1 may exert its function by interacting with transcription regulators to enhance or repress their activities. GLK1 and GLK2 are promising candidates as they coordinate expression of transcripts for antenna proteins and chlorophyll biosynthetic enzymes (Fitter et al., 2002; Waters et al., 2009). Moreover, induction of AtGLK increased the expression of HEMA1 and the synthesis branch from protoporphyrin IX to chlorophyll, corresponding to the genes that were differentially expressed in dgp1. In addition, the expression of OsLHCB2 and OsLHCB4, which are directly activated by OsGLK (Sakuraba et al., 2017), were increased in dgp1 (Supplemental Figure S4). We therefore investigated whether DGP1 can interact with OsGLK1 and OsGLK2 by yeast two-hybrid (Y2H) assay. As transcription factors, OsGLK1 and OsGLK2 can autonomously activate the reporter gene in the absence of a prey protein (Supplemental Figure S5). Hence, ORFs of OsGLK1 and OsGLK2 were cloned into the Gal4 activation domain (AD) vector and ORF of DGP1 was cloned into the Gal4 DNA-binding domain (BD) vector. Corresponding transformants grew on quadruple dropout medium, confirming the interactions between them (Figure 7A).
Figure 7.
DGP1 interacts with OsGLK and represses the transcriptional activity of OsGLK1. (A) DGP1 interacts with OsGLK1 and OsGLK2 in yeast two-hybrid assays. DDO, double dropout medium. QDO, quadruple dropout medium. (B) DGP1 interacts with OsGLK1 and OsGLK2 in BiFC assay. CFP, cyan fluorescent protein. BF, bright field. (C) Schematic diagram of construct used in transactivation activity assays. Promoters are shown as white boxes and CDS are shown as green boxes. (D, E), Analysis of the effect of DGP1 on the transcription activities of OsGLK1 on promoter of OsHEMA1 (D) and OsLHCB4 (E). Error bars represent sd (n =3). Asterisks indicate significant differences according to Student’s t test (**P <0.01).
We also verified the interactions between DGP1 and OsGLK using the bimolecular fluorescence complementation (BiFC) assay. Co-transformation of DGP1 and OsGLK fused with split CFP reconstituted the cyan fluorescence in rice protoplasts (Figure 7B). No cyan fluorescence was observed in transformations with DGP1 or OsGLK alone (Supplemental Figure S6). These results validated the interactions between DGP1 and OsGLK.
DGP1 suppresses OsGLK-mediated transcription
Our finding that DGP1 and OsGLK functions oppositely on the expression of photosynthesis genes and that DGP1 can interact with OsGLK prompted us to investigate whether DGP1 can downregulate the target genes of OsGLK at the transcriptional level. To this end, we fused the promoters of the OsGLK target genes, OsHEMA1 (chlorophyll biosynthetic gene) and OsLHCB4 (antenna protein gene), to the dual luciferase reporter and performed luciferase-based transactivation assays using rice protoplasts (Figure 7C).
As expected, OsGLK1 significantly activated the promoters of OsHEMA1 and OsLHCB4 (Figure 7, D and E). The relative luciferase expression increased by 4 times for OsHEMA1 promoter and near 60 times for OsLHCB4 promoter when co-transfected with a 35S::OsGLK1 effector. More importantly, this transcriptional activation was significantly decreased when the 35S::DGP1 effector was co-transfected with 35S::OsGLK1 (Figure 7, D and E). This observation clearly indicates that DGP1 represses the transcriptional activity of OsGLK1.
Discussion
dgp1 was initially identified as a mutant with color change in panicles. Actually, this mutant is sterile and only produces abortive pollen grains (Supplemental Figure S7, A and B). We checked the genes within the deleted region of dgp1 and found OsABCG3 (LOC_Os01g61940). Osabcg3 is completely sterile due to the defective pollen wall formation (Chang et al., 2018). Moreover, knock-out mutant dgp1-cas9-1, which cause frame shift of DGP1 and is deemed to be a null allele, is fertile (Supplemental Figure S7, C and D). Thus, we concluded that DGP1 functions specifically on photosynthesis and has no effect on rice fertility.
DGP1 is a negative regulator of OsGLK activity
In most characterized plant genomes, including Arabidopsis, maize, rice, sorghum, and Physcomitrella patens, GLK genes exist as pairs and function redundantly in the regulation of chloroplast development (Rossini et al., 2001; Fitter et al., 2002; Yasumura et al., 2005; Chen et al., 2016). A ChIP assay in Arabidopsis revealed that AtGLK directly bind to the promoters of genes involved in light harvesting and chlorophyll synthesis via the “CCAATC” motif and G-box element (CACGTG; Waters et al., 2009). The G-box element is also overrepresented in the promoters of HY5 target genes. Interestingly, the promoter of DGP1 contains one G-box element (−289 bp) and At3g55240, one homolog of DGP1 in Arabidopsis, was identified as a direct target of HY5 (Lee et al., 2007).
ORE1, one of the key transcriptional factors regulating senescence in Arabidopsis, was identified as a negative regulator of GLK activity (Rauf et al., 2013). Overexpression of ORE1 significantly decreased the transcriptional activation of AtGLK2 on its target genes. P69, virulence protein of turnip yellow mosaic virus, suppresses GLK transcription factors to cause pale-green symptoms in Arabidopsis (Ni et al., 2017). P69 exerts its negative regulation likely through reducing the binding of AtGLK to their target promoters. In this study, we identified DGP1 as a negative regulator of GLK activation activity in rice. DGP1 interacts with OsGLK1 and OsGLK2 (Figure 7, A and B). Overexpression of DGP1 reduced the activation activity of OsGLK1 on OsHEMA1 and OsLHCB4 (Figure 7, D and E). In dgp1, this repressing effect is released and chlorophyll synthesis genes, targets of OsGLK, are upregulated in glumes (Figure 6). This leads to the production of more chlorophyll and a dark-green phenotype in the mutants.
Besides functions in chloroplast development and chlorophyll synthesis, GLKs are also involved in senescence, biotic stress defense, and abscisic acid response (Ahmad et al., 2019). As mentioned above, ORE1 antagonizes GLK transcriptional activity, shifting the balance from chloroplast maintenance towards deterioration (Rauf et al., 2013). Overexpression of AtGLK1 enhances the expression of disease defense-related genes (Savitch et al., 2007). Further studies are needed to verify whether DGP1, a negative regulator of OsGLK, also participated in these biological processes.
The phenotype of dgp1 was exclusively observed in panicles
Most mutants of photosynthesis-related genes display their phenotypes in leaves. For example, the double mutants of GLK in both Arabidopsis and rice have pale-green leaves (Fitter et al., 2002; Wang et al., 2013). However, the phenotype of dgp1 was exclusively observed in panicles. The color and chlorophyll content of leaves are comparable between wild-type and dgp1 (Supplemental Figure S1). Interestingly, the expression of DGP1 is detected in most photosynthetic tissues, including leaves and leaf sheath. Thus, the specificity of phenotype is not caused by tissue specificity of the DGP1 expression.
As DGP1 directly interacts with OsGLK, it seems also unlikely that other factors are needed in leaves to mediate the repression effects on OsGLK. Actually, the DGP1 overexpression plants showed yellow-green leaves (Figure 3D). The transactivation activity assays, which demonstrated the repression effect on OsGLK1 by DGP1, were performed in protoplasts extracted from rice young seedlings. These data indicate that DGP1, at least in excess amounts, can repress the activity of OsGLK in leaves.
We speculate that the sensibilities of rice leaves and glumes to the change of DGP1 quantity are different. In OE-2 leaves, the expression of DGP1 increased 8.58-fold and chlorophyll content decreased to 60% of that in wild-type (Figure 3, B and E). In OE-2 glume, the expression of DGP1 increased 6.56-fold and chlorophyll content decreased sharply to 3.92% of that in wild-type. Moreover, OE-1, which showed relatively moderate change of the DGP1 expression, contains similar chlorophyll content in leaf with wild-type. However, the chlorophyll content of OE-1 glume decreased significantly compare with wild-type (Figure 3E). This difference between leaf and glume is most likely due to the relative abundance of DGP1 and OsGLK in these two tissues. In support of this, the expression level of DGP1 in florets is higher than that of leaves (Figure 5A). In addition, we measured the expression levels of OsGLK1 and OsGLK2 in leaves at different developmental stages and glumes. The results indicated that the expression levels of OsGLK1 and OsGLK2 in leaves are significantly higher than that of glumes (Supplemental Figure S8). Thus, we assume that, in wild-type leaves, the repressive effect of DGP1 on chlorophyll synthesis is diluted by relatively higher content of OsGLK to a level below the threshold that can cause obvious phenotype.
Materials and methods
Plant materials and growth conditions
The dgp1 mutant was isolated from the rice (Oryza sativa L.) variety Wuyujing 8 induced by 60Co γ-ray irradiation. Wuyujing 8 was used as wild-type in the related experiments. All plants were grown in paddy fields in Yangzhou (Jiangsu Province, China) or Sanya (Hainan Province, China) during the natural growing season.
Chlorophyll content analyses
Chlorophyll was extracted from leaves and glumes of wild-type, dgp1, and dgp1-cas9-1. 0.1 g fresh tissue was cut and soaked in a solution of 1:1 ethanol/acetone (v/v). After 12 h of treatment in darkness, the absorbance values at 649, and 665-nm wavelength were measured using a spectrophotometer. The chlorophyll contents were calculated based on the absorbance values.
Map-based cloning of DGP1
For map-based cloning of DGP1, mutant plants of dgp1 were crossed with the indica variety Xieqingzao, to generate the mapping populations. Recessive individuals in the F2 and F3 were used to screen recombinants. InDel markers were developed based on sequence differences between indica variety 9311 and japonica variety Nipponbare, according to the data published in NCBI (http://www.ncbi.nlm.nih.gov). Polymorphic markers for mapping are listed in Supplemental Table S1. DNA of wild-type and dgp1 leaves was extracted for high-throughput sequencing by illumina HiSeq.
Knock out of DGP1 by CRISPR-Cas9 gene editing approach
For the CRISPR-Cas9 targeting of DGP1, the reported CRISPR-Cas9 binary vector pC1300-Cas9 and the intermediate vector SK-gRNA were used in this study (Wang et al., 2015). The target sequence of DGP1 was “GCCGGCTTCGTACATCAGAT”. The construct was introduced into Agrobacterium tumefaciens strain EHA105 and then transformed into the japonica variety Yandao 8. Homozygous mutants were obtained by sequencing the transgenic plants.
Overexpression of DGP1
To generate transgenic rice plants overexpressing DGP1, its coding region was amplified from first-strand cDNA. The PCR product was then inserted into pCAMBIA23A vector with rice actin promoter. The construct was transformed into the japonica variety Wuyujing 8. T0 plants were used for the measurement of expression levels and chlorophyll content. The primers used for vector construction are listed in Supplemental Table S1.
Subcellular localization of DGP1
The coding sequence of DGP1 was cloned into pJIT163-GFP vector in frame with GFP. The coding sequence of OsbZIP52 was cloned into pSAT6-RFP vector in frame with RFP. The two plasmids were co-transfected into rice protoplasts. Empty pJIT163-GFP vector was used as the control. After incubation in the dark at 28°C for 20 h, fluorescence signals were observed with a confocal laser scanning microscope (Carl Zeiss LSM 710). GFP and RFP were excited at 488 and 561 nm, and emissions were collected at 500–540 nm and 600–650 nm, respectively.
RT-qPCR assay
Total RNA was extracted from rice tissues using TRIzol reagent. Reverse transcription was performed using HiScript® III RT SuperMix for RT-qPCR (+gDNA wiper; Vazyme). Real-time PCR analysis was performed using ChamQTM SYBR® qPCR Master Mix (Vazyme) and the Bio-Rad CFX96 real-time PCR instrument. All reactions were performed in triplicate, with Ubiquitin as the normalized reference gene for all comparisons. The primers for RT-qPCR are listed in Supplemental Table S1.
Y2H
The coding sequence (CDS) of DGP1 and OsGLK were amplified and cloned into the pGADT7 and pGBKT7 vectors. The transformations were conducted using Frozen-EZ Yeast Transformation II kit according to the manufacturer’s instructions. Double dropout medium (SD/-Leu-Trp) was used to verify the co-transformation and quadruple dropout medium (SD/-Leu-Trp-His-Ade) was used to test the interactions. The yeast strain used in Y2H assays was Y2HGOLD. Primers used are listed in Supplemental Table S1.
BiFC assay
The BiFC assay was performed as previously described (Waadt et al., 2008). The CDS of DGP1 and OsGLK were amplified and cloned into the pSCYNE(R) and pSCYCE(R) vectors, respectively. The plasmid pairs were co-transfected into protoplasts of young rice seedling by 40% (w/v) polyethylene glycol (PEG). Transfected protoplasts were incubated in the dark at 28°C overnight, and observed using a laser scanning confocal microscopy (Carl Zeiss LSM 710). CFP were excited at 405 nm, and emissions were collected at 300–483 nm. Primers used are listed in Supplemental Table S1.
Transactivation activity assays
The 2-kb DNA fragments of the promoter regions of OsLHCB4 and OsHEMA1 were amplified and cloned into the pGreen II 0800-LUC vector, respectively (Hellens et al., 2005). The CDS of OsGLK1 was fused into the pUC19-35S-FLAG-RBS vector (Li et al., 2005). The construct for subcellular localization, pJIT163-DGP1-GFP, was used to induce the expression of DGP1. The different combinations of vectors were co-transfected into rice protoplasts by PEG-mediated transformation. After incubation in the dark at 28°C for 20 h, protoplasts were harvested and lysed for the detection of firefly luciferase and renilla luciferase activities. The ratios of firefly luciferase to renilla luciferase activity were calculated to define relative promoter activity. Data were obtained from three replicates. Primers used are listed in Supplemental Table S1.
Computational and database analysis
The phylogenetic tree was constructed using Mega7 based on the neighbor-joining method. Jones-Taylor-Thornton (JTT) substitution models and four-gamma rate categories plus invariant sites were used in distance analyses. The pairwise deletion option and 1,000 bootstrap replicates were applied. Multiple sequence alignment was conducted using MAFFT (https://toolkit.tuebingen.mpg.de/#/tools/mafft) and colored with ESPript (http://espript.ibcp.fr/ESPript/ESPript/). Data analyses were mainly conducted using GraphPad Prism 8 software.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: DGP1, XM_015773637.2; OsGLK1, XM_015788758.1; OsGLK2, XM_015760419.2; OsHEMA1, XM_015759215.2; OsHEML, XM_015795386.2; OsHEMB, XM_015787519.1; OsHEMC, XM_015771769.2; OsHEMD, XM_015775122.2; OsHEME1, XM_015756287.2; OsHEMF, XM_015778045.2; OsHEMG1, XM_015770568.2; OsCHLD, XM_015775598.2; OsCHLH, XM_026024474.1; OsCHLI, XM_015772495.1; OsGUN4, XM_015762532.2; OsCHLM, XM_015785870.2; OsCHL27, XM_015766623.2; OsPORA, XM_015778380.2; OsPORB, XM_015759459.1; OsDVR, XM_015776837.2; OsCHLG, XM_015782164.2; OsCAO1, XM_015758600.1.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Chlorophyll content in leaves of wild-type and dgp1.
Supplemental Figure S2. Comparison of plants of wild-type and dgp1-cas9-1.
Supplemental Figure S3. Genotype and phenotype of dgp1-cas9-2.
Supplemental Figure S4. Expression analysis of OsLHCB2 and OsLHCB4 in wild-type and dgp1 glumes.
Supplemental Figure S5. OsGLK1 and OsGLK2 can autonomously activate the reporter gene in yeast two-hybrid system.
Supplemental Figure S6. No interactions between DGP1, OsGLK, and corresponding empty vectors are observed in the BiFC assay.
Supplemental Figure S7. Pollen grains of wild-type, dgp1, and dgp1-cas9-1.
Supplemental Figure S8. Expression analysis of OsGLK1 and OsGLK2 in leaves and glumes.
Supplemental Table S1. Primers used in this study.
Funding
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (2016YFD0102001), the National Natural Science Foundation of China (31872859), the Natural Science Foundation of Jiangsu province (BK20200951), the China Postdoctoral Science Foundation (2020M671613), Taicang City Science and Technology Plan (TC2019JC10), and the Jiangsu Higher Education Institutions of China (PAPD).
Conflict of interest statement. The authors have no conflicts of interest to declare.
Supplementary Material
H.Y. and T.Z. conceived the project. C.Z. performed most of the experiments (mutagenesis, map-based cloning, most RT-qPCR assay, Y2H, BiFC, and transactivation assay). J.Z. and Y.T. conducted measurements of chlorophyll content, knock-out and overexpression of DGP1, and the subcellular localization assay. K.L., Y.L., and J.T. helped with map-based cloning. C.Z. wrote the article with the supervision of H.Y. and T.Z.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys) is: Hengxiu Yu (hxyu@yzu.edu.cn).
References
- Ahmad R, Liu Y, Wang TJ, Meng Q, Yin H, Wang X, Wu Y, Nan N, Liu B, Xu ZY (2019) GOLDEN2-LIKE transcription factors regulate WRKY40 expression in response to abscisic acid. Plant Physiol 179:1844–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ZY, Jin MN, Yan W, Chen H, Qiu SJ, Fu S, Xia JX, Liu YC, Chen ZF, Wu JX, et al. (2018) The ATP-binding cassette (ABC) transporter OsABCG3 is essential for pollen development in rice. Rice 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Ji M, Wen B, Liu L, Li S, Chen X, Gao D, Li L (2016) GOLDEN 2-LIKE transcription factors of plants. Front Plant Sci 7:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitter DW, Martin DJ, Copley MJ, Scotland RW, Langdale JA (2002) GLK gene pairs regulate chloroplast development in diverse plant species. Plant J 31:713–727 [DOI] [PubMed] [Google Scholar]
- Fromme P, Melkozernov A, Jordan P, Krauss N (2003) Structure and function of photosystem I: interaction with its soluble electron carriers and external antenna systems. FEBS Lett 555:40–44 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Nakazawa M, Kawashima M, Iizumi H, Kuroda H, Kondou Y, Tsuhara Y, Suzuki K, Ishikawa A, Seki M, et al. (2006) The FOX hunting system: an alternative gain-of-function gene hunting technique. Plant J 48:974–985 [DOI] [PubMed] [Google Scholar]
- Kong W, Yu X, Chen H, Liu L, Xiao Y, Wang Y, Wang C, Lin Y, Yu Y, Wang C, et al. (2016) The catalytic subunit of magnesium-protoporphyrin IX monomethyl ester cyclase forms a chloroplast complex to regulate chlorophyll biosynthesis in rice. Plant Mol Biol 92:177–191 [DOI] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19:731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim JH, Yoo ES, Lee CH, Hirochika H, An G (2005) Differential regulation of chlorophyll a oxygenase genes in rice. Plant Mol Biol 57:805–818 [DOI] [PubMed] [Google Scholar]
- Li X, Wang P, Li J, Wei S, Yan Y, Yang J, Zhao M, Langdale JA, Zhou W (2020) Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun Biol 3:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Lin HQ, Zhang WG, Zou Y, Zhang J, Tang XY, Zhou JM (2005) Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc Natl Acad Sci USA 102:12990–12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CT, Wu YB, Wang XP (2012) bZIP transcription factor OsbZIP52/RISBZ5: a potential negative regulator of cold and drought stress response in rice. Planta 235:1157–1169 [DOI] [PubMed] [Google Scholar]
- Long SP, Marshall-Colon A, Zhu XG (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161:56–66 [DOI] [PubMed] [Google Scholar]
- Martin G, Leivar P, Ludevid D, Tepperman JM, Quail PH, Monte E (2016) Phytochrome and retrograde signalling pathways converge to antagonistically regulate a light-induced transcriptional network. Nat Commun 7:11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Muramatsu M, Hakata M, Ueno O, Nagamura Y, Hirochika H, Takano M, Ichikawa H (2009) Ectopic overexpression of the transcription factor OsGLK1 induces chloroplast development in non-green rice cells. Plant Cell Physiol 50:1933–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni F, Wu L, Wang Q, Hong J, Qi Y, Zhou X (2017) Turnip yellow mosaic virus P69 interacts with and suppresses GLK transcription factors to cause pale-green symptoms in Arabidopsis. Mol Plant 10:764–766 [DOI] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Wang ZY (2012) Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 14:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf M, Arif M, Dortay H, Matallana-Ramirez LP, Waters MT, Gil Nam H, Lim PO, Mueller-Roeber B, Balazadeh S (2013) ORE1 balances leaf senescence against maintenance by antagonizing G2-like-mediated transcription. EMBO Rep 14:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini L, Cribb L, Martin DJ, Langdale JA (2001) The maize golden2 gene defines a novel class of transcriptional regulators in plants. Plant Cell 13:1231–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Kim EY, Han SH, Piao W, An G, Todaka D, Yamaguchi-Shinozaki K, Paek NC (2017) Rice phytochrome-interacting factor-like1 (OsPIL1) is involved in the promotion of chlorophyll biosynthesis through feed-forward regulatory loops. J Exp Bot 68:4103–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Rahman ML, Cho SH, Kim YS, Koh HJ, Yoo SC, Paek NC (2013) The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J 74:122–133 [DOI] [PubMed] [Google Scholar]
- Savitch LV, Subramaniam R, Allard GC, Singh J (2007) The GLK1 ‘regulon’ encodes disease defense related proteins and confers resistance to Fusarium graminearum in Arabidopsis. Biochem Biophys Res Commun 359:234–238 [DOI] [PubMed] [Google Scholar]
- Song Y, Yang C, Gao S, Zhang W, Li L, Kuai B (2014) Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol Plant 7:1776–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58:321–346 [DOI] [PubMed] [Google Scholar]
- Tokumaru M, Adachi F, Toda M, Ito-Inaba Y, Yazu F, Hirosawa Y, Sakakibara Y, Suiko M, Kakizaki T, Inaba T (2017) Ubiquitin-proteasome dependent regulation of the GOLDEN2-LIKE 1 transcription factor in response to plastid signals. Plant Physiol 173:524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J (2008) Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 56:505–516 [DOI] [PubMed] [Google Scholar]
- Wang C, Shen L, Fu Y, Yan C, Wang K (2015) A simple CRISPR/Cas9 system for multiplex genome editing in rice. J Genet Genomics 42:703–706 [DOI] [PubMed] [Google Scholar]
- Wang P, Fouracre J, Kelly S, Karki S, Gowik U, Aubry S, Shaw MK, Westhoff P, Slamet-Loedin IH, Quick WP, et al. (2013) Evolution of GOLDEN2-LIKE gene function in C(3) and C (4) plants. Planta 237:481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA (2009) GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21:1109–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumura Y, Moylan EC, Langdale JA (2005) A conserved transcription factor mediates nuclear control of organelle biogenesis in anciently diverged land plants. Plant Cell 17:1894–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li J, Yoo JH, Yoo SC, Cho SH, Koh HJ, Seo HS, Paek NC (2006) Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol Biol 62:325–337 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






