Abstract
Plants possess a systemic signaling system whereby local stimuli can lead to rapid, plant-wide responses. In addition to the redistribution of chemical messengers that range from RNAs and peptides to hormones and metabolites, a communication system acting through the transmission of electrical, Ca2+, reactive oxygen species and potentially even hydraulic signals has also been discovered. This latter system can propagate signals across many cells each second and researchers are now beginning to uncover the molecular machineries behind this rapid communications network. Thus, elements such as the reactive oxygen species producing NAPDH oxidases and ion channels of the two pore channel, glutamate receptor-like and cyclic nucleotide gated families are all required for the rapid propagation of these signals. Upon arrival at their distant targets, these changes trigger responses ranging from the production of hormones, to changes in the levels of primary metabolites and shifts in patterns of gene expression. These systemic responses occur within seconds to minutes of perception of the initial, local signal, allowing for the rapid deployment of plant-wide responses. For example, an insect starting to chew on just a single leaf triggers preemptive antiherbivore defenses throughout the plant well before it has a chance to move on to the next leaf on its menu.
Introduction
Plants live in a complex and dynamic environment and thus have evolved systems that monitor multiple stimuli and then integrate this information into an appropriate plant-wide response. To accomplish this feat, plants require signaling systems driven by local stimulus perception and response combined with a communication network to broadcast this information throughout the plant body. In this way, responses to even highly localized stimuli, such as an insect chewing on one leaf, can play out in distant tissues. There are numerous chemical signaling molecules used to perform this systemic signaling ranging from mobile hormones and peptides to metabolites and RNAs. Additionally, evidence for a parallel informational system built around changes in ion fluxes, reactive oxygen species (ROS), and electrical signaling has begun to emerge. In this update, we will briefly introduce some recent ideas about the multiple, systemic communication pathways operating over different scales and timeframes within the plant. In particular, for the rapid ionic/ROS/electrical systemic signaling system, key progress has now been made in defining the molecular identities of some of the channels, pumps, and enzymes involved in driving signal propagation.
Advances
Confirmation of the extensive use of mobile peptide signaling in plants and identification of the protein kinases that act as their receptors adds important new context for understanding plant systemic signaling.
Discovery that GLRs and CNGCs act as critical elements of the systemic wound VP/Ca2+ signal provides a key anchor point from which to start building a mechanistic understanding of how systemic signaling is triggered and propagated.
Defining the involvement of ROS as a central regulator of systemic signaling highlights a potentially central integrator of systemic responses.
Development of technologies to visualize the ROS wave and Ca2+ changes have provided a clearer understanding of the speed and patterning of systemic signaling within the plant.
Cell-to-cell and long-range chemical signaling in plants: slow and steady
Plants have long been known to integrate their physiology and development through mobile chemical signals. The hormone auxin is a classic example, where the coordinated activity of membrane transporters such as the AUX/LAX, PIN, and ABCBs move auxin in a polar fashion from cell to adjacent cell (Grones and Friml, 2015). The auxin flux then provides both temporal and spatial information to the plant, e.g. driving local patterning within the developing vegetative meristem (Galvan-Ampudia et al., 2020). In addition to hormones, various other regulators are also now known to similarly integrate development and physiology by moving from cell to adjacent cell. For example, in the root, the SHORTROOT protein must move from where it is made in the root xylem, procambium, and xylem-adjacent pericycle to other nearby cells, such as the phloem poles and quiescent center, where it then acts to, e.g. control phloem development (Kim et al., 2020).
In addition to such systems providing relatively “local” communication between nearby cells and tissues, there are also a host of mobile signaling molecules that integrate activities over much larger distances within the plant, such as between organs. The polar transport of auxin once again provides an example of one kind of machinery for this long-range exchange of information. Thus, the movement of auxin across many cells within the shoot system allows the apical meristem to integrate its activities with axillary meristems through the process of apical dominance (Barbier et al., 2019), or enables the shoot to communicate with roots about its CO2 environment (Zhou et al., 2019). In other cases, such long-distance communication systems are thought to use the mass flow within the vascular system to support their more rapid transport throughout the plant. Thus, movement of RNA, proteins, peptides, hormones, metabolites, and even nutrients within the xylem or phloem allows one part of the plant to communicate and coordinate its activities with distant organs (reviewed in Ham and Lucas, 2017; Winter and Kragler, 2018). For example, Ca2+ moving up in the transpiration stream may couple stomatal responses in the leaf to Ca2+ supply from the roots (Han et al., 2003). This mobile Ca2+ signal acts through a plastid-based receptor system in the guard cells that itself may further integrate these responses with, e.g. photoacclimation (Cutolo et al., 2019).
The potential scope of the vasculature as an information highway for chemical messengers is exemplified by studies on the RNAs found to be mobile in the phloem. In addition to mRNAs, micro RNAs, silencing-induced RNAs, transfer RNAs, and even ribosomal RNAs are also found moving in phloem sap (Kehr and Kragler, 2018), with estimates of approximately 20% of the protein-coding RNAs found to move systemically. The presence of many of these RNAs in the translocation stream may reflect non-specific leakage of companion cell contents into sieve tubes (Calderwood et al., 2016) or remnants from sieve tube differentiation (Knoblauch et al., 2018). Even so, motifs such as transfer RNA-like sequences can tag RNAs for selective transport in the translocation stream (Zhang et al., 2016), and there does appear to be selectivity in the macromolecules found to be moving within the phloem (Garg and Kühn, 2020).
Of note in systemic signals moving in the xylem and phloem is the rising profile of mobile peptides as a theme in how plants integrate their activities between organs. Systemin was discovered as the first plant (wound) signaling peptide several decades ago (Pearce et al., 1991), and now small secreted peptides are known to underlie a host of developmental and physiological responses ranging from guard cell differentiation and meristem function to gravitropism (reviewed in Matsubayashi, 2014; Blackburn et al., 2020). This recent characterization of the extent of such mobile peptide signaling by the plant, along with defining the array of plasma membrane receptor-like kinases that underlie many of their perception machineries (reviewed in Chen et al., 2020), represents an important and exciting recent step forward in our understanding of plant systemic signals.
For example, an array of peptides released to the vascular system in the root are now known to carry information such as drought (CLAVATA3/ESR-related 25 peptide; Takahashi et al., 2018) or nitrate status (C-terminally encoded peptides; CEPs; Tabata et al., 2014) to the aerial parts of the plant. In the case of nitrogen signaling, CEP DOWNSTREAM peptides (Ohkubo et al., 2017) appear to complete the circuit, carrying information from the shoot to the roots through the phloem. Although CEP and CEP DOWNSTREAM are integral to coordinating plant-wide nitrate responses, plants likely use multiple parallel signals in these long-range communication systems. For example, the plant hormone cytokinin is known to operate alongside these peptide signals in the systemic nitrogen status signaling network (Ruffel et al., 2016; Poitout et al., 2018).
Undoubtedly, such chemical exchanges of information between adjacent cells and through the vascular highways explain many key components of the systemic signaling occurring within the plant. However, the speed seen in the plant’s internal communications suggests additional signaling networks must be operating. Auxin moves through the plant at rates of only micrometers per second (Kramer et al., 2011) and small molecules passing through plasmodesmata likely travel at a similar speed (e.g. Rutschow et al., 2011). Although mass transport in the xylem or phloem is much faster, typically in the 200–400 µm/s range (e.g. Peuke et al., 2001; Savage et al., 2013; Knoblauch et al., 2016), systemic signals to, e.g. wounding or high light stress can move at rates in excess of 1,000 µm/s (Table 1 and see below). Systems using self-reinforcing waves of ROS, Ca2+, electrical, and potentially even hydraulic signals are thought to support this much more rapid propagation of systemic information.
Table 1.
Some examples of the speeds of systemic propagation of signals described in the text
| Organism | Stimulus | Signal | Speed | Number of 50-µm cells/s | Reference |
|---|---|---|---|---|---|
| Venus flytrap | Touch | AP | 54–115 mm/s | 1,080–2,300 | (Sibaoka, 1966) |
| M. pudica (rachilla) | Touch | AP | 3–7 mm/s | 60–140 | (Sibaoka, 1966) |
| M. pudica (petiole) | Touch | AP | 6–44 mm/s | 120–880 | (Sibaoka, 1966) |
| M. pudica (petiole) | Wound | VP | 1–4 mm/s | 20–80 | (Houwink, 1935) |
| Arabidopsis | Wound | VP vasculature | 1–2 mm/s | 20–40 | (Mousavi et al., 2013; Nguyen et al., 2018) |
| VP mesophyll | 0.4 mm/s | 8 | (Mousavi et al., 2013) | ||
| Ca2+ wave vasculature | 1–2 mm/s | 20–40 | (Nguyen et al., 2018; Toyota et al., 2018) | ||
| Ca2+ wave mesophyll | 0.4 mm/s | 8 | (Toyota et al., 2018) | ||
| ROS wave vasculature | ∼1 mm/s | 20 | (Miller et al., 2009; Lew et al., 2020) | ||
| ROS wave mesophyll | 0.4 mm/s | 8 | (Lew et al., 2020) | ||
| Arabidopsis | NaCl | Ca2+ wave root | 0.4 mm/s | 8 | (Choi et al., 2014) |
For context, the speed has also been converted to the number of 50-µm plant cells that the signal would be able to traverse in a single second.
Rapid systemic signaling: the need for speed
The rapid systemic signaling and response in leaf movements of the Venus flytrap (Dionaea muscipula) and sensitive plant (Mimosa pudica) provide a dramatic illustration of how fast plant systemic signals can travel. The Venus flytrap is a carnivorous plant that traps insects between two modified leaves that are joined at the base by a hinge that allows them to snap together to form a cage. The adaxial surface of each leaf possesses exquisitely sensitive sensory hairs that, when mechanically stimulated, trigger the trap to close (Scherzer et al., 2019). The hairs activate distant motor cells in the hinge region that levers the two halves of the trap together in approximately 100 ms (Forterre et al., 2005). This speed is required by the plant in order to catch fast moving insects, but how is it achieved? A combination of the elastic structure of the lobes of the trap and rapid turgor-driven changes in motor cell volume drive the physical movements of the trap lobes (Forterre et al., 2005), but what mediates the rapid communication from the sensory hair to the motor cells? The signal is electrical, i.e. mechanical stimulation of the sensory hair generates a transient depolarization of the membrane potential (an action potential, AP) in the cells at its base that propagates throughout the leaf at the rate of 54–115 mm/s. It is the arrival of this AP at the lobe that triggers the leaf closure (Sibaoka, 1966).
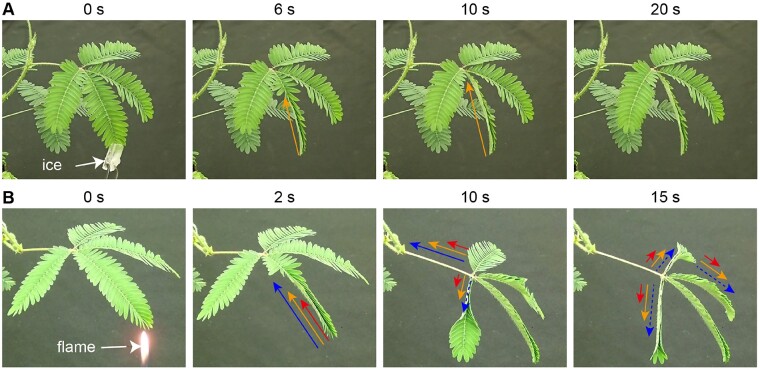
Similarly, the sensitive plant M. pudica folds its leaflets and moves its petioles quickly (∼1 s) in response to a variety of mechanical and other physical stimuli (Hagihara and Toyota, 2020). Again, the movements are driven by a turgor-powered motor, in this case an organ called the pulvinus, and the rapid signaling appears linked to propagating electrical signals. Non-damaging stimuli (e.g. cold shock or touch) generate an AP that transmits through the plant and then induces the leaflets to move upon its arrival at the tertiary pulvinus (Figure 1, A;Sibaoka, 1966). The AP propagates at 3–7 mm/s in the rachilla (the shaft to which the leaflets are attached) and 6–44 mm/s in the petiole at the base of the whole compound leaf structure (Sibaoka, 1966). These electrical signals are likely moving through the sieve tubes and the parenchyma cells in the protoxylem and phloem (Sibaoka, 1962; Samejima and Sibaoka, 1983; Fromm and Eschrich, 1988). By contrast, damaging stimuli (e.g. cutting or burning leaflets) generates both the AP and an additional electrical signal called a variation potential (VP, also known as a slow wave potential; Figure 1, B;Houwink, 1935). The VP appears to be related to the flow of stimulating chemicals in the xylem vessels and propagates at the rate of 1–4 mm/s in the petiole (Houwink, 1935), i.e. more slowly than that of the AP. Moreover, the existence of a non-electrical (possibly hydraulic), even faster signal is also implied (Houwink, 1935; Malone, 1994). Thus, when the entire organ is strongly damaged (e.g. pruning off of a collection of leaflets or pinna), the primary pulvinus and petiole move earlier than the arrival of either the APs or VPs (Houwink, 1935). In addition, when leaflets are extensively damaged by burning, the thickness of distant leaves is almost immediately changed by a signal moving in excess of 15 mm/s (Figure 1, B; Malone, 1994), possibly linked to wound-related pressure changes propagating through the vasculature. Thus, physical damage that breaks the integrity of the xylem is expected to release the tension in these vessels and due to the relatively incompressible nature of water, this pressure change will be almost instantly transmitted through the vasculature. Indeed, such a hydraulic wave has been proposed to mediate wounding events in a range of plants, where it is thought to propagate through the xylem and potentially phloem vessels. The pressure wave may act to either disperse a stimulating chemical from the wounded tissues or directly trigger mechanically responsive signaling systems within the systemic tissues (e.g. Boari and Malone, 1993; Farmer et al., 2014; Evans and Morris, 2017). However, such hydraulic effects are thought likely to move at speeds up to 100,000-fold faster than the observed propagation of wound-induced electrical and Ca2+ signals, making it complex to relate their kinetics to these other systemic signals (Evans and Morris, 2017). The action of hydraulic signals transmitted through the plant vasculature represents a rapid signaling mechanism seemingly well-tailored to plant anatomy. However, definitively showing such a mechanism in action has been, and remains, a significant challenge in experimental design. For excellent discussions of this area, see Evans and Morris (2017) and Farmer et al. (2020).
Figure 1.
Rapid signaling and response in Mimosa. A, Non-wounding stimulus (ice, 0 s) causes rapid movement of leaflets driven by turgor changes in the pulvini at their bases. Cold shock generates only an AP (orange arrows) that cannot propagate past the secondary pulvinus at the base of a pinna (20 s), leading to a local response of the stimulated pinna only. B, Damage (flame, 0 s) triggers rapid closing of a leaf. Such damage causes a putative hydraulic wave (blue arrows), a rapidly propagating AP (orange arrows), and a slower moving VP (red arrows). Once these signals arrive at the pulvinus, movements of ions and water cause changes in pulvinar cell volume that in turn causes the leaf to move.
Channels, pumps, and systemic signals
Just as for the potential hydraulic signal, the electrical signals that appear central to the rapid systemic signaling in Dionaea and Mimosa are thought to play similar roles in fast, long-distance communication within many plants. For example, an insect chewing on a leaf causes both APs and VPs propagating from the damaged leaf toward distal sites in many species (e.g. Mousavi et al., 2013; Salvador-Recatalà et al., 2014). Importantly, being able to study these phenomena in Arabidopsis has allowed the field to begin to define the machineries driving these changes with molecular precision.
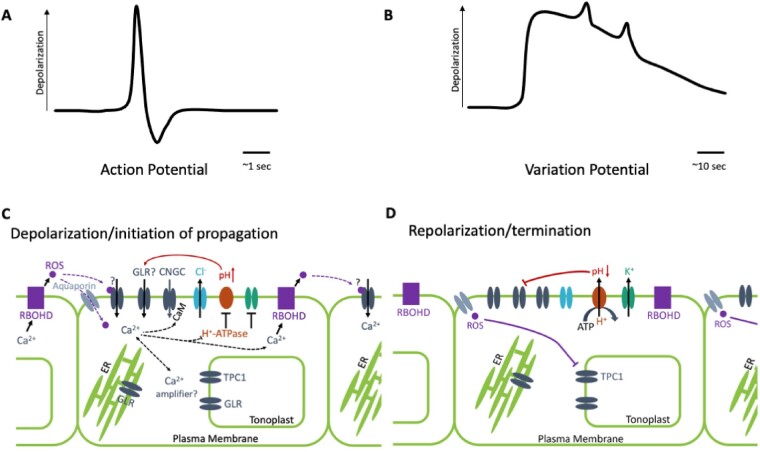
Plants maintain an approximately −200 mV potential across their plasma membranes, largely through the action of ion channels, pumps, and transporters (Tester, 1990). Propagating changes in this potential, as seen in Mimosa and Dionaea, may act as signals to rapidly carry information throughout many plants. APs are transient depolarization and repolarization events with set amplitude, velocity, and durations that last on the scale of seconds (Figure 2, A; e.g. Favre et al., 2001; Cuin et al., 2018). By contrast, VPs are characterized by variable initial amplitude and a much slower repolarization (sometimes with superimposed spikes similar to APs) that last for minutes (Figure 2, B). VPs also differ from APs in that the shape of their change in potential over time, and their velocity are dependent on the intensity of the triggering stimulus and decrease with distance from the location of their initiation (Fromm and Lautner, 2007; Sukhov et al., 2013; Vodeneev et al., 2016). Thus, VPs have the potential to encode information about the strength and distance of the stimulus that triggered them, whereas APs have the characteristics of an all-or-nothing, rapid, long-range signal. Both VPs and APs reflect the fluxes of ions that cause both depolarization and successive repolarization of the plasma membrane. Therefore, such electrical signaling is intimately related to the regulation of membrane ion channels, pumps, and other transporters. The recent elucidation of the molecular identities of some of these components has proven a key step forward in our understanding how systemic signals may be propagating.
Figure 2.
Plant electrical/ionic systemic signaling. A, Change in electrical potential of an AP is characterized by a short depolarization followed by a repolarization. B, Change in electrical potential of a VP is typified by a sharp depolarization and slow repolarization often with AP-like spikes superimposed. The magnitude of the VP diminishes with distance from the originating stimulus. C, Model for possible series of events related to wound-induced VP and Ca2+ wave. Dashed lines represent speculative regulatory activities. Cation channel activation (through ROS and/or ligand binding) triggers Ca2+ influx with contributions from CNGCs and potentially from plasma membrane-localized GLRs. The molecular identity of the proposed ROS-activated Ca2+-permeable plasma membrane channel in this system remains unknown. GLRs on internal membranes, along with TPC1 on the tonoplast, may be operating in an amplification system that reinforces the cytosolic Ca2+ change. When coupled with Cl− efflux and inactivation of H+-ATPases, these events lead to depolarization of the plasma membrane. Ca2+-dependent processes would be likely candidates to coordinate these activities but precise molecular machineries await to be defined. The accompanying rise in apoplastic pH from inhibition of the H+-ATPase further activates putative GLR Ca2+ conductances at the plasma membrane. The resultant cytoplasmic Ca2+ elevation should then lead to activation of RBOHs and further apoplastic ROS production, and thus to a feed-forward loop supporting cell-to-cell propagation of the signal. D, Slow influx of ROS into the cytosol via aquaporins could then inactivate TPC1, inhibiting the cytosolic Ca2+ amplifier. Repolarization would then be driven by H+-ATPase reactivation and K+ release, restoring the membrane potential. A reduction in apoplastic pH would inhibit GLR activity, further contributing to the resetting of the system.
The glutamate receptor-like channels
Whereas it has long been known that fluxes of Ca2+, K+, Cl−, and H+ are all contributing to the production of APs and VPs (Figure 2, C and D; Vodeneev et al., 2016), the specific channels and pumps involved have only just begun to be defined. We currently have perhaps the most detailed model for channel involvement in the systemic wound response in Arabidopsis, where cation channels of the GLUTAMATE RECEPTOR-LIKE (GLR) family have been confirmed to be integral to generating VPs. The GLRs are ligand- (amino acid-) gated, non-selective (Na+ and Ca2+ permeable; e.g. Wudick et al., 2018; Shao et al., 2020) cation channels, with 20 isoforms in Arabidopsis. Mutants in GLRs 3.1, 3.2, 3.3, and 3.6 disrupt the duration of the wound-related VP in Arabidopsis (Mousavi et al., 2013). The associated systemic cytosolic Ca2+ signal (Nguyen et al., 2018; Toyota et al., 2018; Shao et al., 2020), spread by a systemic increase in ROS (Lew et al., 2020; and see below) and electrical signaling in the phloem (Salvador-Recatalà et al., 2014), are also disrupted by mutations in GLR3.3 and GLR3.6 (Figure 3, A), suggesting a role for GLRs in the cation influx during the depolarization phase of the wound-triggered VP. Wound-induced APs and local wound-triggered Ca2+ increases are still generated in the directly damaged leaves in these mutants; however, they fail to propagate throughout the plant (Salvador-Recatalà et al., 2014; Hedrich et al., 2016; Toyota et al., 2018), implying their role in events specifically related to VP propagation. In contrast, GLR3.5 is thought to act to limit the extent of electrical signaling, with mutants in this channel showing much more extensive spread of damage-induced APs (Salvador-Recatalà, 2016). Similarly, GLR3.1 may play a role in determining the extent of the spread of systemic Ca2+ increases across distal leaves (Nguyen et al., 2018). This role for GLRs in systemic signaling does not appear limited to Arabidopsis. For example, a CRISPR/Cas9-induced mutation that prematurely stops translation of the AtGLR 3.6 ortholog in tomato (Solanum-lycopersicum; SlGLR3.5) blocks wound-induced systemic VPs and increases in ROS (Wang et al., 2019). Mutations in eight other Arabidopsis GLRs tested by Mousavi et al. (2013) did not have a significant effect on the dynamics of wound-triggered VPs, suggesting specificity within the GLRs for activities in the systemic electrical signaling system. However, as a note of caution, it takes a double mutant in both GLR3.3 and 3.6 to effectively ablate the wound-induced Ca2+ wave and VP. Therefore, we must await a systematic analysis of the role(s) of the GLRs alone, and in combination, to robustly define how much of the temporal and spatial dynamics of systemic signaling can be explained by the actions of this channel family.
Figure 3.
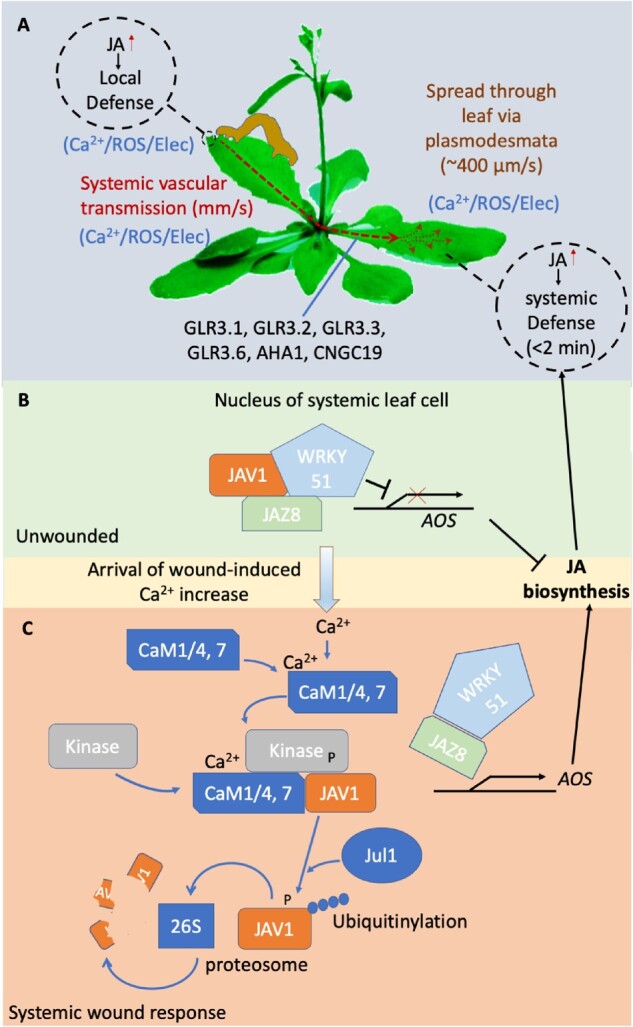
Model for systemic wound signaling in Arabidopsis. A, Local wounding rapidly triggers production of JA in the directly damaged tissues and also in distant tissues. JA signaling then triggers both local and systemic anti-herbivory defenses. Damage at the wound site (putatively signaled by release of glutamate from damaged cells; Toyota et al., 2018) triggers VP propagation and a wave of increased ROS and Ca2+ that moves through the vasculature to distal leaves where it spreads through plasmodesmatal connections to trigger systemic defenses (Toyota et al., 2018). Transmission is dependent on the action of the GLR ion channels and the plasma membrane H+ pump AHA1. B and C, In the systemic leaves, the Ca2+ rise in the nucleus of target cells triggers phosphorylation of the JAV1 transcriptional inhibitor in a calmodulin-dependent manner. This change then targets JAV1 for ubiquitinylation by the JUL1 E3 ubiquitin ligase and degradation by the 26S proteasome. Loss of JAV1 disrupts the inhibitory complex it forms with WRKY51/JAZ8, relieving transcriptional repression of AOS, which is an enzyme integral to JA biosynthesis. Arabidopsis image: modified from Eric Belfield, Eurekalert.org.
GLRs 3.1, 3.3, and 3.6 have been reported in a range of subcellular sites in plants, including the ER, tonoplast, and plasma membrane (e.g. Singh et al., 2016; Nguyen et al., 2018; Wudick et al., 2018), suggesting a potentially complex interaction of organellar signaling machineries and the plasma membrane ion fluxes behind VPs. These channels are also expressed in different cell types in aerial tissues, with e.g. GLR3.6 (and GLR3.1) being predominantly produced in xylem contact cells (xylem parenchyma cells adjacent to the conducting vessels) and GLR3.3 in the phloem sieve tubes (Nguyen et al., 2018; Toyota et al., 2018). One explanation for the observations that mutating both GLR3.3 and 3.6 is required to fully ablate systemic wound signals, even though they are in different cell types, is the presence of a putative electro-osmotic signal that couples hydraulic changes in the xylem and phloem during propagation of the systemic wound signal (Farmer et al., 2020). Alternatively, two independent systemic wound signaling pathways may be operating in the vascular tissues: one in the xylem mediated by GLR3.6 and one in the phloem using GLR3.3, where each is sufficient to trigger a systemic wound response on its own.
Other candidate channels in systemic signaling networks
In addition to the GLRs, there are a host of other promising candidates for systemic signaling-related channel activities such as the Ca2+ permeable CYCLIC NUCLEOTIDE GATED CHANNELs (CNGCs). CNGCs are a family of 20 cation channels reported to be found on both the plasma and internal membranes. CNGCs have already been linked to responses to stimuli that are known to trigger systemic signaling, such as heat stress, wounding, or pathogen attack (e.g. Ali et al., 2007; Meena et al., 2019; Tian et al., 2019; Jogawat et al., 2020). Indeed, CNGC19 has been shown to be a Ca2+-permeable, plasma membrane- (Meena et al., 2019) and possibly vacuolar-localized (Yuen and Christopher, 2013) channel required for propagation of the systemic wound-induced Ca2+ signal across the wounded leaf (Meena et al., 2019). Thus, mutants in this gene show a failure to efficiently transmit the wound-triggered Ca2+ wave within the damaged leaf, and are also compromised in plant-wide defenses against insect herbivory (Meena et al., 2019). Whether the CNGC family as a whole plays as integral a role in these systemic events as the GLRs appear to do remains to be investigated.
K+ channels are also thought to be integral elements of electrical signal propagation (Figure 2, C and D), but again, we have only just scratched the surface as far as analyses of their potential role(s) in systemic signal propagation. Mutants in the ubiquitously expressed GUARD CELL OUTWARD RECTIFYING K+ channel (GORK) do have altered kinetics of membrane depolarization and repolarization and generate altered dynamics of APs (Cuin et al., 2018). By comparison, although mutants in the ubiquitously expressed ARABIDOPSIS K+ TRANSPORTER 2 (AKT2) do not alter AP characteristics, they do reduce the proportion of plants that produce stimulus-evoked APs (Cuin et al., 2018). By contrast, the STELAR OUTWARD RECTIFYING K+ channel (SKOR), a close relative of GORK that is expressed in the vasculature (Garcia-Mata et al., 2010), does not appear to play a role in wound-related VPs (Mousavi et al., 2013).
Similar to this limited knowledge of systemic signaling-related cation channels, the channels and transporters moving anions such as Cl− across the plasma membrane during systemic electrical signal propagation (Figure 2, C and D) also remain to be fully explored. Thus, although the anion channels CLC-b (tonoplast; von der Fecht-Bartenbach et al., 2010) and CLC-e (thylakoid; Herdean et al., 2016) do not appear to be involved in wound-induced systemic VPs (Mousavi et al., 2013), the roles of other plant anion channels in systemic signaling are yet to be characterized.
H+ pumps
H+-ATPases have long been known to be inactivated during the depolarization phase and then reactivated during the repolarization phase of electrical signal propagation (Vodeneev et al., 2016). However, the specific H+-ATPases involved have only recently begun to be defined. In Arabidopsis, there are 12 H+-ATPases (AHAs) with AHA1-5 and -11 showing widespread expression throughout the plant. AHA1 (predominately shoot) and -2 (predominately root) are especially highly expressed (Gaxiola et al., 2007). Mutants in AHA1, -2, and -3 were not originally thought to play roles in wound-induced VPs (Mousavi et al., 2013). However, recent analysis has shown that AHA1 is involved in repolarization of the plasma membrane following a wound-induced VP (Kumari et al., 2019). Additionally, in aha1, the duration of the wound-triggered systemic Ca2+ signal is extended, providing further mechanistic links between the Ca2+ wave, ion fluxes, and VP propagation. The GLRs themselves are regulated by pH (Shao et al., 2020), providing a further link between H+ pumping and Ca2+ signal. Direct wounding is accompanied by cytosolic acidification, suggesting a likely coupled apoplastic alkalinization (Behera et al., 2018) that would be predicted to activate plasma membrane-localized GLRs (Shao et al., 2020). However, precisely how cytosolic and apoplastic pH change as systemic signals propagate and how this might relate to the activity of GLRs potentially acting in organelles as well as at the plasma membrane remains to be defined.
aha2 mutants do not show equivalent disruption of the wound VP, suggesting that only specific H+-ATPases may be involved in supporting these kinds of electrical signals (Kumari et al., 2019). The AHAs are known to controlled by a complex network of elements, including interaction with lipids, ions, protein kinases, and regulators such as 14-3-3 proteins (Falhof et al., 2016). Considering its central role in wound VP dynamics, defining how the factors controlling AHA1 activity relate to the generation of VPs could offer important insight into the underlying regulatory networks driving propagation of systemic signals.
Thus, there are some tantalizing clues as to how channels and pumps may make contributions to different aspects of systemic signals, such as to their thresholds or dynamics of propagation. However, we must await a much more comprehensive exploration of the role(s) of the large number of candidate channels, pumps, and exchangers to build a picture of the network of elements that are supporting the fluxes of ions that underlie the systemic signaling network.
ROS: The great integrator?
In addition to Ca2+ waves and electrical signals, plants additionally employ waves of ROS (see Box 1) as part of their systemic signaling apparatus. These waves are likely being maintained through a ROS-induced ROS release machinery (reviewed in Zandalinas and Mittler, 2018). As seen for systemic electrical signals and the Ca2+ wave, the ROS wave can propagate through both vascular and nonvascular tissues, depending on the stimulus (Fichman et al., 2019; Lew et al., 2020). For example, a wound-induced wave of ROS accumulation and response has been seen to move at speeds in excess of 1 mm/s throughout the vasculature in aerial parts of Arabidopsis (Miller et al., 2009; Fichman et al., 2019; Lew et al., 2020), or at ∼400 µm/s in non-vascular tissues of the root (Evans et al., 2016) and leaves (Lew et al., 2020; see Table 1 and Choi et al. [2016] for summary of propagation speeds). In Arabidopsis, these propagating ROS signals appear dependent on RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) and RBOHF (Zandalinas et al., 2020), both plasma membrane, ROS-producing NADPH oxidases known to be central to a range of signaling systems from pathogen defense to drought (Kadota et al., 2015). The ROS wave is detected as both ROS production in the apoplast (Evans et al., 2016; Lew et al., 2020)—as expected for the product of RBOH action—and in the cytoplasm (Miller et al., 2009; Fichman et al., 2019; Zandalinas et al., 2020). The observation of cytoplasmic changes implies either RBOH-derived apoplastic ROS subsequently enter the cell across the plasma membrane, likely through aquaporins (Bienert et al., 2007; Dynowski et al., 2008), or that another, cytoplasmic ROS producing system is also being engaged by the systemic signaling network.
Box 1.
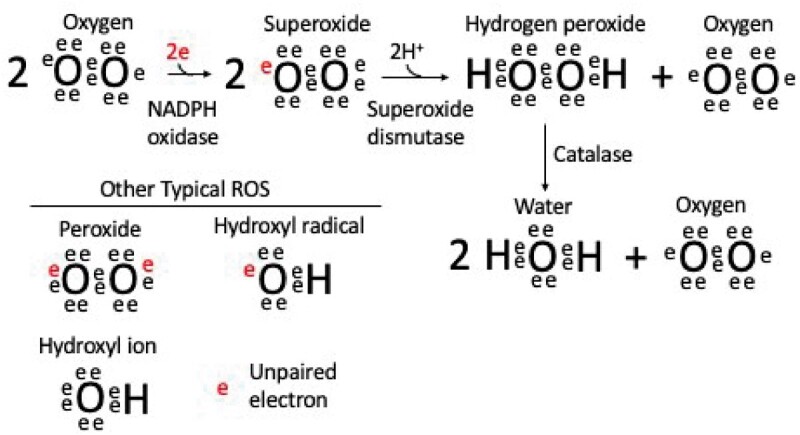
What are the RBOHs and ROS?
The Respiratory Burst Oxidase Homologs (RBOHs) are a family of NADPH oxidases. Their enzymatic activity is to take an electron from cytosolic NADPH and transfer it across the plasma membrane to molecular oxygen to produce the superoxide radical (see Box 1 figure). Superoxide dismutases then act on this highly chemically reactive species to produce the much more stable H2O2 that can be further processed by catalase to produce water and oxygen (Smirnoff and Arnaud, 2019). Superoxide and H2O2 are both forms of ROS, reactive chemical species that contain oxygen. Other ROS include the hydroxyl radical, peroxides, and singlet oxygen. ROS are produced during reactions within the cell and can cause damage to biological macromolecules such as proteins, lipids, and nucleic acids due to their highly reactive nature. However, ROS are also used by biology as ubiquitous signaling agents. For example, in Arabidopsis, HYDROGEN-PEROXIDE INDUCED Ca2+ INCREASE 1 (HPCA1) is a plasma membrane leucine-rich receptor kinase that was recently discovered to link H2O2 perception in the apoplast to Ca2+ signaling in the cytoplasm (Wu et al., 2020).
The Arabidopsis RBOH family shows regulation by a host of modulators such as a wide range of kinases (including members of the calcium-dependent protein kinase [CDPK] and Calcineurin B-Like/CBL interacting protein kinase [CBL/CIPK] families), small GTPases, and the lipid-based signal phosphatidic acid (e.g. Kadota et al., 2015; Qu et al., 2017; Lee et al., 2020). Of note, the CDPKs, the CBL/CIPKs, and the RBOHs themselves are all directly regulated by Ca2+. Further, the stability of the RBOHD regulating BOTRYTUS INDUCED KINASE 1 (BIK1) is modulated through Ca2+-dependent regulation of CDPK action (Monaghan et al., 2014). Thus, RBOH-based ROS production should be inextricably linked with Ca2+ dynamics. Indeed, mathematical modeling by Evans et al. (2016) supports the idea that it is the interaction of ROS-induced Ca2+ influx at the plasma membrane with the direct and indirect Ca2+ regulation of the RBOHs that sustains cell-to-cell propagation of both the Ca2+ and ROS waves.
A direct mechanistic relationship of ROS to the propagating systemic electrical signal remains less clear. In addition to ROS production, RBOHs should alter both apoplastic pH and membrane potential as they move an electron from cytosolic NADPH to the apoplast in order to generate the superoxide radical (see Box 1; Segal, 2016). However, whether the magnitude of these effects is at all significant relative to the activity of channels and pumps driving electrical changes at the plasma membrane is unclear. There are also a number of identified channels and electrophysiologically measured membrane conductances where ROS regulation has either been inferred or directly monitored, including SKOR, GORK, the annexins, TWO PORE CHANNEL 1 (TPC1; see below), and the CNGCs (reviewed in Demidchik, 2018), providing hints at how ROS could modulate electrical signaling phenomena. RBOHD function is known to be required for the systemic propagation of high light- or heat stress-induced VPs (Suzuki et al., 2013); however, a mutant in RBOHD did not alter wound-triggered VP characteristics in Arabidopsis (Mousavi et al., 2013). Nonetheless, a RBOH mutant in tomato does abolish wound-induced, root-to-shoot VP transmission (Wang et al., 2019). Recent experiments driving tissue-specific RBOHD expression in the rbohD knockout background suggests that systemic signaling to local high-light stress requires this enzyme to be located in the xylem parenchyma or phloem (Zandalinas et al., 2020). These are the same tissues where GLR3.3 and GLR3.6 are expressed and are required for systemic wound signaling via VPs and Ca2+ (Nguyen et al., 2018; Toyota et al., 2018). Such observations reinforce the idea that plants are likely employing complex and multifaceted propagation mechanisms that may vary from organ to organ or stimulus to stimulus.
ROS, RBOHD, and systemic signaling: it’s complicated
The RBOHs are multigene families in plants. For example, in Arabidopsis, there are eight other RBOH isoforms in addition to RBOHs D and F. Similarly, although the NADPH oxidases have received the most attention as components of this ROS-based systemic signaling network, ROS dynamics are in reality modulated by a much more complex interplay of ROS producing, degrading, and scavenging processes, including respiratory and photosynthetic electron transport and photorespiration, enzymatic systems involving superoxide dismutases, ascorbate peroxidases, catalases, glutathione peroxidases, and peroxiredoxins, as well as the action of non-enzymatic scavengers such as glutathione, ascorbic acid, and flavonoids (Mittler et al., 2004; Smirnoff and Arnaud, 2019). Further, specific receptors for ROS, such as HYDROGEN PEROXIDE-INCUCED Ca2+ INCREASE 1 (HPCA1) are beginning to be defined (Wu et al., 2020). Dissecting the potential roles of these other ROS-related elements in systemic signaling systems remains an important goal for future research as it offers the possibility to provide some of the missing linkages between Ca2+, ROS, and electrical responses.
Propagation of systemic signals also implies a directionality to their transmission and here ROS regulation of tonoplast channels could play a role. Thus, the systemic spread of both the wound- (Kiep et al., 2015) and NaCl- (Choi et al., 2014) driven systemic Ca2+ increases is known to involve the action of a tonoplast channel, TPC1. Activation of RBOHs produces apoplastic ROS that are then expected to trigger ion fluxes (such as Ca2+ influx) at the plasma membrane by gating currently unidentified channels (Demidchik, 2018). TPC1 is then thought to act either directly or indirectly to facilitate Ca2+ release from the vacuole, providing an amplifier for the initial cytosolic Ca2+ increases (Figure 2). The resulting elevated cytosolic Ca2+ could then further trigger RBOH activation, and so cause propagation of the signal from cell to cell (Choi et al., 2014; Evans et al., 2016). TPC1 is inhibited by cytosolic ROS (Pottosin et al., 2009), suggesting a mechanism whereby the initial ROS produced by RBOH activation could enter the cell to inhibit TPC1, leading to a refractory period for the calcium-induced calcium release amplifier. The time delay between activation of the RBOH/TPC1 system and ROS-dependent TPC1 inactivation would then lead to the propagating, wave front-like character to the transmission events. However, although mutants in TPC1 disrupt systemic wound defense responses, local wound-induced Ca2+ increases are not altered (Kiep et al., 2015; Lenglet et al., 2017). In addition, although experimental addition of ROS does trigger Ca2+ increases, the resulting elevated Ca2+ and ROS levels do not propagate systemically (Miller et al., 2009; Evans et al., 2016), suggesting the model described above is likely missing some important elements. For example, TPC1 appears to act in concert with TANDEM PORE K+ Channel 1 (TPK1) and -3 to mediate electrical excitability of the tonoplast (Jaślan et al., 2019). However, TPK1 and -3 are not thought to play roles in wound-induced VP dynamics (Mousavi et al., 2013). Clearly, we are just at the beginning of unraveling the complexities and capabilities of the vacuole as a signaling hub.
Systemic responses: what happens at the finish line?
Following the propagation of a signal, the response in the distal tissues ranges from the physiological, such as rapid systemic stomatal responses (e.g. Devireddy et al., 2020; Ehonen et al., 2020), alterations in photosynthetic activity (Guo et al., 2016), and leaf movements (Kurenda et al., 2019), to the molecular, with shifts in metabolites (Suzuki et al., 2013; Choudhury et al., 2018), hormone levels (e.g. Glauser et al., 2009; Koo et al., 2009; Toyota et al., 2018), and gene expression levels (Suzuki et al., 2015; Zandalinas et al., 2019). For example, in Arabidopsis, local high-light exposure to a single Arabidopsis leaf triggers a systemic change in metabolites such as glucose, sucrose, and glycine within minutes, and a suite of transcriptional changes within seconds (Suzuki et al., 2013; Suzuki et al., 2015; Choudhury et al., 2018; Zandalinas et al., 2019). Both these metabolic and transcriptional phenomena are dependent on the action of RBOHD, again suggesting an integral role for ROS in the signaling system. However, precisely where the ROS are operating in the circuitry of signal generation, propagation, and rapid response remains to be fully defined.
Wound-induced JA production: integrating Ca2+ and wound signaling responses
The response to the arrival of the systemic VP and Ca2+ signal triggered by wounding provides one example of the molecular machinery whereby systemic signaling molecules may be fairly directly turned into the target cell response. In this case, the systemic effect is a rapid (within minutes) increase in the level of the wound hormone jasmonic acid (JA; Glauser et al., 2009; Koo et al., 2009; Toyota et al., 2018). Once the Ca2+ increase arrives at the systemic tissues, it activates the Ca2+-dependent regulatory protein calmodulin, which in turn activates a protein kinase that phosphorylates JASMONATE-ASSOCIATED VQ-MOTIF GENE1 (JAV1; Figure 3, B and C; Yan et al., 2018). JAV1 is part of a repressor complex that along with JAZ8 and WRKY51 inhibits expression of ALLENE OXIDE SYNTHASE (AOS; encoding a key enzyme in JA biosynthesis). The phosphorylation of JAV1 causes this complex to dissociate and triggers JAV1 ubiquitinylation via the E3-ubiquitin ligase JAV1-ASSOCIATED UBIQUITIN LIGASE 1 (JUL1), targeting JAV1 for degradation by the 26S proteasome. The disruption of the inhibitory complex removes repression of AOS transcription and so leads to JA production. There are seven calmodulin genes in Arabidopsis, which encode only four different proteins (CaM1/4, -2/3/5, -6, and -7). In planta JAV1 interacts strongly with CaM1/4 and CaM7, but not with the others (Yan et al., 2018). CaM7 differs from CaM6 and CaM2/3/5 by a single, but different, amino acid, suggesting an exquisitely tailored protein–protein interaction underlies response to the arrival of the systemic Ca2+ signal in this system. Further, as discussed above, CNGC19 plays a role in propagating the wound-induced Ca2+ wave. This channel is also thought to be regulated by CaM, but in this case the CaM2 isoform (Meena et al., 2019). Thus, different CaMs appear to be allowing the plant to both respond to the propagating Ca2+ signal by supporting its transmission and also to translate this same Ca2+ wave to the triggering of systemic JA-mediated defenses once it arrives at its target.
Conclusion
Plants have evolved a complex series of local and systemic signaling systems that allow the integration and coordination of their physiology and development. As early as the 1920s we knew that some of the most rapid of responses, such as the leaflet movements in Mimosa, are associated with electrical changes (Snow, 1923; Houwink, 1935). We are now finding similar kinds of rapid, systemic signaling systems in a wide variety of plants operating in response to stimuli ranging from the leaf wounding caused by insect herbivory to local high-light stress.
Although APs and VPs are intimately linked to plasma membrane-related events, reflecting as they do ion movements across this membrane, the important roles of the vacuolar ion channel TPC1 and the potential for GLRs located on intracellular membranes such as the tonoplast and ER in systemic signaling highlight the likely role of organelles in propagating these events. Both the ER and the vacuole are emerging as important signaling hubs in plants (Peiter, 2011; Schönknecht, 2013; Liu and Li, 2019) and we can expect that an intricate set of interactions between these organelles and the plasma membrane are going to be important in systemic signal propagation. Defining which channels are acting where, their orientations, and what ion fluxes they support are clearly key pieces of information still needed to understand the mechanisms underlying signal propagation. For example, a plasma membrane locale of the relevant GLRs fits well with the idea of the amino acid glutamate being a trigger for systemic wound responses (Toyota et al., 2018). In this model, cellular damage causes leakage of glutamate to the apoplast that then triggers GLR action and supports the Ca2+ fluxes involved in systemic signal propagation. Consistent with these ideas, in a GLR3.3- and GLR3.6-dependent manner, local application of glutamate can trigger both systemic spread of a Ca2+ increase and activation of JA defense responses throughout the plant (Toyota et al., 2018). How then do GLRs on the organelles play roles in this system? Where do the ligand binding sites face? Are there cytoplasmic amino acid ligands in operation or is some other gating mechanism at play? Despite some major advances in defining molecular mechanisms in the last few years, our models of these systemic signaling events are clearly still far from complete (see Outstanding Questions). Other major open questions are whether or not a simple Ca2+/RBOH feedforward loop is sufficient to explain rapid, systemic signal propagation or whether there are multiple signaling networks tuned to specific stimuli. How central is the vasculature as a highway for these signals and do such systems operate in concert with a rapid hydraulic communication network? We are only just beginning to dissect the molecular underpinnings of this electrical, ROS-based, ionic, and hydraulic systemic signaling system(s), but we do know one thing already, it is fast.
Outstanding Questions
What are the channels, pumps, co-transport systems, and ion exchangers forming the underlying molecular machineries that generate and propagate AP and VPs involved in systemic signaling?
What is the relative role for plasma membrane versus organellar membranes in supporting systemic transmission events and how do they communicate with each other?
Wherein lies the specific information in the systemic signal? Are there features of the Ca2+/ROS or electrical signals that encode specific information, or do these changes simply prime systemic tissues for actions that are dictated by other signaling molecules?
Do stress-induced hydraulic changes propagating through the vasculature act to carry specific systemic information?
Acknowledgments
The authors apologize to the many researchers whose work was not cited in this review solely due to length limitations. They thank Dr. Sarah Swanson for helpful discussions and critical reading of the manuscript.
Funding
This work was supported by grants from the National Science Foundation [IOS1557899 and MCB2016177], the National Aeronautics and Space Administration [NNX14AT25G, 80NSSC19K0126 to S.G.], and the Japan Society for the Promotion of Science [17H05007 and 18H05491 to M.T.].
Conflict of interest statement. None declared.
S.J., T.H., M.T., and S.G. wrote the manuscript, prepared the figures, and reviewed and edited the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys) is: Simon Gilroy (sgilroy@wisc.edu).
References
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA (2007) Death don’t have no mercy and neither does calcium: Arabidopsis Cyclic Nucleotide Gated Channel2 and innate immunity. Plant Cell Online 19: 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA (2019) An update on the signals controlling shoot branching. Trends Plant Sci 24: 220–236 [DOI] [PubMed] [Google Scholar]
- Behera S, Xu Z, Luoni L, Bonza MC, Doccula FG, De Michelis MI, Morris RJ, Schwarzländer M, Costa A (2018) Cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell 30: 2704–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert GP, Møller ALB, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282: 1183–1192 [DOI] [PubMed] [Google Scholar]
- Blackburn MR, Haruta M, Moura DS (2020) Twenty years of progress in physiological and biochemical investigation of RALF peptides. Plant Physiol 182: 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boari F, Malone M (1993) Wound-induced hydraulic signals: survey of occurrence in a range of species. J Exp Bot 44: 741–746 [Google Scholar]
- Calderwood A, Kopriva S, Morris RJ (2016) Transcript abundance explains mRNA mobility data in Arabidopsis thaliana. Plant Cell 28: 610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Fan KT, Hung SC, Chen YR (2020) The role of peptides cleaved from protein precursors in eliciting plant stress reactions. New Phytol 225: 2267–2282 [DOI] [PubMed] [Google Scholar]
- Choi W-G, Hilleary R, Swanson SJ, Kim S-H, Gilroy S (2016) Rapid, long-distance electrical and calcium signaling in plants. Annu Rev Plant Biol 90: 698–707 [DOI] [PubMed] [Google Scholar]
- Choi W-G, Toyota M, Kim S-H, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci U S A 1: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury FK, Devireddy AR, Azad RK, Shulaev V, Mittler R (2018) Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol 178: 1461–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuin TA, Dreyer I, Michard E (2018) The role of potassium channels in Arabidopsis thaliana long distance electrical signalling: AKT2 modulates tissue excitability while GORK shapes action potentials. Int J Mol Sci 19: 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo E, Parvin N, Ruge H, Pirayesh N, Roustan V, Weckwerth W, Teige M, Grieco M, Larosa V, Vothknecht UC (2019) The high light response in Arabidopsis requires the calcium sensor protein CAS, a target of STN7- and STN8-mediated phosphorylation. Front Plant Sci 10: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V (2018) ROS-activated ion channels in plants: biophysical characteristics, physiological functions and molecular nature. Int J Mol Sci 19: 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy AR, Arbogast J, Mittler R (2020) Coordinated and rapid whole-plant systemic stomatal responses. New Phytol 225: 21–25 [DOI] [PubMed] [Google Scholar]
- Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414: 53–61 [DOI] [PubMed] [Google Scholar]
- Ehonen S, Hölttä T, Kangasjärvi J (2020) Systemic signaling in the regulation of stomatal conductance. Plant Physiol 182: 1829–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Choi W-G, Gilroy S, Morris RJ (2016) A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol 171: 1771–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Morris RJ (2017) Chemical agents transported by xylem mass flow propagate variation potentials. Plant J 91: 1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falhof J, Pedersen JT, Fuglsang AT, Palmgren M (2016) Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol Plant 9: 323–337 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Gao Y, Lenzoni G, Wolfender J, Wu Q (2020) Wound‐ and mechano‐stimulated electrical signals control hormone responses. New Phytol 227: 1037–1050 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Gasperini D, Acosta IF (2014) The squeeze cell hypothesis for the activation of jasmonate synthesis in response to wounding. J Physiol 204: 282–288 [DOI] [PubMed] [Google Scholar]
- Favre P, Greppin H, Degli Agosti R (2001) Repetitive action potentials induced in Arabidopsis thaliana leaves by wounding and potassium chloride application. Plant Physiol Biochem 39: 961–969 [Google Scholar]
- von der Fecht-Bartenbach J, Bogner M, Dynowski M, Ludewig U (2010) CLC-b-mediated NO3−/H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant Cell Physiol 51: 960–968 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Miller G, Mittler R (2019) Whole-plant live imaging of reactive oxygen species. Mol Plant 12: 1203–1210 [DOI] [PubMed] [Google Scholar]
- Forterre Y, Skotheim JM, Dumals J, Mahadevan L (2005) How the Venus flytrap snaps. Nature 433: 421–425 [DOI] [PubMed] [Google Scholar]
- Fromm J, Eschrich W (1988) Transport processes in stimulated and non-stimulated leaves of Mimosa pudica. Trees 2: 18–24 [Google Scholar]
- Fromm J, Lautner S (2007) Electrical signals and their physiological significance in plants. Plant Cell Environ 30: 249–257 [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, Cerutti G, Legrand J, Brunoud G, Martin-Arevalillo R, Bayle V, Moussu S, Wenzl C, Jaillais Y, Lohmann JU, et al. (2020) Temporal integration of auxin information for the regulation of patterning. Elife 9: e55832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Wang J, Gajdanowicz P, Gonzalez W, Hills A, Donald N, Riedelsberger J, Amtmann A, Dreyer I, Blatt MR (2010) A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J Biol Chem 285: 29286–29294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Kühn C (2020) What determines the composition of the phloem sap? Is there any selectivity filter for macromolecules entering the phloem sieve elements? Plant Physiol Biochem 151: 284–291 [DOI] [PubMed] [Google Scholar]
- Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581: 2204–2214 [DOI] [PubMed] [Google Scholar]
- Glauser G, Dubugnon L, Mousavi SAR, Rudaz S, Wolfender JL, Farmer EE (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284: 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grones P, Friml J (2015) Auxin transporters and binding proteins at a glance. J Cell Sci 128: 1–7 [DOI] [PubMed] [Google Scholar]
- Guo Z, Wang F, Xiang X, Ahammed GJ, Wang M, Onac E, Zhou J, Xia X, Shi K, Yin X, et al. (2016) Systemic induction of photosynthesis via illumination of the shoot apex is mediated sequentially by phytochrome B, auxin and hydrogen peroxide in tomato. Plant Physiol 172: 1259–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara T, Toyota M (2020) Mechanical signaling in the sensitive plant Mimosa pudica L. Plants 9: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham B-K, Lucas WJ (2017) Phloem-mobile RNAs as systemic signaling agents. Annu Rev Plant Biol 68: 173–195 [DOI] [PubMed] [Google Scholar]
- Han S, Tang R, Anderson LK, Woerner TE, Pei ZM (2003) A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature 425: 196–200 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Salvador-Recatalà V, Dreyer I (2016) Electrical wiring and long-distance plant communication. Trends Plant Sci 21: 376–387 [DOI] [PubMed] [Google Scholar]
- Herdean A, Nziengui H, Zsiros O, Solymosi K, Garab G, Lundin B, Spetea C (2016) The Arabidopsis thylakoid chloride channel AtCLCe functions in chloride homeostasis and regulation of photosynthetic electron transport. Front Plant Sci 7: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwink AK (1935) The conduction of excitation in Mimosa pudica. Recl Trav Bot Neerl 32: 51–91 [Google Scholar]
- Jaślan D, Dreyer I, Lu J, O’Malley R, Dindas J, Marten I, Hedrich R (2019) Voltage-dependent gating of SV channel TPC1 confers vacuole excitability. Nat Commun 10: 2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogawat A, Meena MK, Kundu A, Varma M, Vadassery J (2020) Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J Exp Bot 71: 2752–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Shirasu K, Zipfel C (2015) Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol 56: 1472–1480 [DOI] [PubMed] [Google Scholar]
- Kehr J, Kragler F (2018) Long distance RNA movement. New Phytol 218: 29–40 [DOI] [PubMed] [Google Scholar]
- Kiep V, Vadassery J, Lattke J, Maaß JP, Boland W, Peiter E, Mithöfer A (2015) Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol 207: 996–1004 [DOI] [PubMed] [Google Scholar]
- Kim H, Zhou J, Kumar D, Jang G, Ryu KH, Sebastian J, Miyashima S, Helariutta Y, Lee J-Y (2020) SHORTROOT-mediated intercellular signals coordinate phloem development in Arabidopsis roots. Plant Cell 32: 1519–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M, Knoblauch J, Mullendore DL, Savage JA, Babst BA, Beecher SD, Dodgen AC, Jensen KH, Holbrook NM (2016) Testing the Münch hypothesis of long distance phloem transport in plants. Elife 5: e15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M, Peters WS, Bell K, Ross-Elliott TJ, Oparka KJ (2018) Sieve-element differentiation and phloem sap contamination. Curr Opin Plant Biol 43: 43–39 [DOI] [PubMed] [Google Scholar]
- Koo AJK, Gao X, Jones AD, Howe GA (2009) A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J 59: 974–986 [DOI] [PubMed] [Google Scholar]
- Kramer EM, Rutschow HL, Mabie SS (2011) AuxV: a database of auxin transport velocities. Trends Plant Sci 16: 461–463 [DOI] [PubMed] [Google Scholar]
- Kumari A, Chételat A, Nguyen CT, Farmer EE (2019) Arabidopsis H+-ATPase AHA1 controls slow wave potential duration and wound-response jasmonate pathway activation. Proc Natl Acad Sci U S A 116: 20226–20231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenda A, Nguyen CT, Chételat A, Stolz S, Farmer EE (2019) Insect-damaged Arabidopsis moves like wounded Mimosa pudica. Proc Natl Acad Sci U S A 116: 26066–26071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Lal NK, Lin Z-JD, Ma S, Liu J, Castro B, Toruño T, Dinesh-Kumar SP, Coaker G (2020) Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat Commun 11: 1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglet A, Jaślan D, Toyota M, Mueller M, Müller T, Schönknecht G, Marten I, Gilroy S, Hedrich R, Farmer EE (2017) Control of basal jasmonate signalling and defence through modulation of intracellular cation flux capacity. New Phytol 216: 1161–1169 [DOI] [PubMed] [Google Scholar]
- Lew TTS, Koman VB, Silmore KS, Seo JS, Gordiichuk P, Kwak S-Y, Park M,, Ang MC-Y, Khong DT, Lee MA, et al. (2020) Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nat Plants 6: 404–415 [DOI] [PubMed] [Google Scholar]
- Liu L, Li J (2019) Communications between the endoplasmic reticulum and other organelles during abiotic stress response in plants. Front Plant Sci 10: 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone M (1994) Wound‐induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol 128: 49. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y (2014) Posttranslationally modified small-peptide signals in plants. Annu Rev Plant Biol 65: 385–413 [DOI] [PubMed] [Google Scholar]
- Meena MK, Prajapati R, Krishna D, Divakaran K, Pandey Y, Reichelt M, Mathew MK, Boland W, Mithöfer A, Vadassery J (2019) The Ca2+ channel CNGC19 regulates Arabidopsis defense against Spodoptera herbivory. Plant Cell 31: 1539–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 1360–1385 [DOI] [PubMed] [Google Scholar]
- Monaghan J, Matschi S, Shorinola O, Rovenich H, Matei A, Segonzac C, Malinovsky FG, Rathjen JP, Maclean D, Romeis T, et al. (2014) The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 16: 605–615 [DOI] [PubMed] [Google Scholar]
- Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426 [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Kurenda A, Stolz S, Chételat A, Farmer EE (2018) Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc Natl Acad Sci U S A 115: 10178–10183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo Y, Tanaka M, Tabata R, Ogawa-Ohnishi M, Matsubayashi Y (2017) Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat Plants 3: 17029. [DOI] [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA (1991) A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253: 895–897 [DOI] [PubMed] [Google Scholar]
- Peiter E (2011) The plant vacuole: emitter and receiver of calcium signals. Cell Calcium 50: 120–128 [DOI] [PubMed] [Google Scholar]
- Peuke AD, Rokitta M, Zimmermann U, Schreiber L, Haase A (2001) Simultaneous measurement of water flow velocity and solute transport in xylem and phloem of adult plants of Ricinus communis over a daily time course by nuclear magnetic resonance spectrometry. Plant Cell Environ 24: 491–503 [Google Scholar]
- Poitout A, Crabos A, Petřík I, Novák O, Krouk G, Lacombe B, Ruffel S (2018) Responses to systemic nitrogen signaling in Arabidopsis roots involve trans-zeatin in shoots. Plant Cell 30: 1243–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin I, Wherrett T, Shabala S (2009) SV channels dominate the vacuolar Ca2+ release during intracellular signaling. FEBS Lett 583: 921–926 [DOI] [PubMed] [Google Scholar]
- Qu Y, Yan M, Zhang Q (2017) Functional regulation of plant NADPH oxidase and its role in signaling. Plant Signal Behav 12: e1356970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffel S, Poitout A, Krouk G, Coruzzi GM, Lacombe B (2016) Long-distance nitrate signaling displays cytokinin dependent and independent branches. J Integr Plant Biol 58: 226–229 [DOI] [PubMed] [Google Scholar]
- Rutschow HL, Baskin TI, Kramer EM (2011) Regulation of solute flux through plasmodesmata in the root meristem. Plant Physiol 155: 1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Recatalà V (2016) New roles for the GLUTAMATE RECEPTOR-LIKE 3.3, 3.5, and 3.6 genes as on/off switches of wound-induced systemic electrical signals. Plant Signal Behav 11: e1161879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Recatalà V, Tjallingii WF, Farmer EE (2014) Real-time, in vivo intracellular recordings of caterpillar-induced depolarization waves in sieve elements using aphid electrodes. New Phytol 203: 674–684 [DOI] [PubMed] [Google Scholar]
- Samejima M, Sibaoka T (1983) Identification of the excitable cells in the petiole of Mimosa pudica by intracellular injection of procion yellow. Plant Cell Physiol 24: 33–39 [Google Scholar]
- Savage JA, Zwieniecki MA, Michele Holbrook N (2013) Phloem transport velocity varies over time and among vascular bundles during early cucumber seedling development. Plant Physiol 163: 1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer S, Federle W, Al-Rasheid KAS, Hedrich R (2019) Venus flytrap trigger hairs are micronewton mechano-sensors that can detect small insect prey. Nat Plants 5: 670–675 [DOI] [PubMed] [Google Scholar]
- Schönknecht G (2013) Calcium signals from the vacuole. Plants (Basel, Switzerland) 2: 589–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal AW (2016) NADPH oxidases as electrochemical generators to produce ion fluxes and turgor in fungi, plants and humans. Open Biol 6: 160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Gao Q, Lhamo D, Zhang H, Luan S (2020) Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci Signal 13: 1453. [DOI] [PubMed] [Google Scholar]
- Sibaoka T (1966) Action potentials in plant organs. Symp Soc Exp Biol 20: 49–73 [PubMed] [Google Scholar]
- Sibaoka T (1962) Excitable cells in mimosa. Science 137: 226. [DOI] [PubMed] [Google Scholar]
- Singh SK, Te Chien C, Chang IF (2016) The Arabidopsis glutamate receptor-like gene GLR3.6 controls root development by repressing the Kip-related protein gene KRP4. J Exp Bot 67: 1853–1869 [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221: 1197–1214 [DOI] [PubMed] [Google Scholar]
- Snow R (1923) The conduction of excitation in Mimosa. Nature 111: 237 [Google Scholar]
- Sukhov V, Akinchits E, Katicheva L, Vodeneev V (2013) Simulation of variation potential in higher plant cells. J Membr Biol 246: 287–296 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Devireddy AR, Inupakutika MA, Baxter A, Miller G, Song L, Shulaev E, Azad RK, Shulaev V, Mittler R (2015) Ultra-fast alterations in mRNA levels uncover multiple players in light stress acclimation in plants. Plant J 84: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. (2013) Temporal–spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y (2014) Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346: 343–346 [DOI] [PubMed] [Google Scholar]
- Takahashi F, Suzuki T, Osakabe Y, Betsuyaku S, Kondo Y, Dohmae N, Fukuda H, Yamaguchi-Shinozaki K, Shinozaki K (2018) A small peptide modulates stomatal control via abscisic acid in long-distance signaling. Nature 556: 235–238 [DOI] [PubMed] [Google Scholar]
- Tester M (1990) Tansley Review No. 21. Plant ion channels: whole-cell and single channel studies. New Phytol 114: 305–340 [DOI] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Wang C, Zhao F, Dahlbeck D, Hu S, Zhang L, Niu Q, Li L, et al. (2019) A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 572: 131–135 [DOI] [PubMed] [Google Scholar]
- Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S (2018) Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Vodeneev VA, Katicheva LA, Sukhov VS (2016) Electrical signals in higher plants: mechanisms of generation and propagation. Biophysics (Oxf) 61: 505–512 [Google Scholar]
- Wang G, Hu C, Zhou J, Liu Y, Cai J, Pan C, Wang Y, Wu X, Shi K, Xia X, et al. (2019) Systemic root-shoot signaling drives jasmonate-based root defense against nematodes. Curr Biol 29: 3430–3438.e4 [DOI] [PubMed] [Google Scholar]
- Winter N, Kragler F (2018) Conceptual and methodological considerations on mRNA and proteins as intercellular and long-distance signals. Plant Cell Physiol 59: 1700–1713 [DOI] [PubMed] [Google Scholar]
- Wu F, Chi Y, Jiang Z, Xu Y, Xie L, Huang F, Wan D, Ni J, Yuan F, Wu X, et al. (2020) Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578: 577–581 [DOI] [PubMed] [Google Scholar]
- Wudick MM, Portes MT, Michard E, Rosas-Santiago P, Lizzio MA, Nunes CO, Campos C, Santa Cruz Damineli D, Carvalho JC, Lima PT, et al. (2018) CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science 360: 533–536 [DOI] [PubMed] [Google Scholar]
- Yan C, Fan M, Yang M, Zhao J, Zhang W, Su Y, Xiao L, Deng H, Xie D (2018) Injury activates Ca2+/calmodulin-dependent phosphorylation of JAV1-JAZ8-WRKY51 complex for jasmonate biosynthesis. Mol Cell 70: 136–149.e7 [DOI] [PubMed] [Google Scholar]
- Yuen CCY, Christopher DA (2013) The group IV-A cyclic nucleotide-gated channels, CNGC19 and CNGC20, localize to the vacuole membrane in Arabidopsis thaliana. AoB Plants 5: 1–14 [Google Scholar]
- Zandalinas SI, Fichman Y, Mittler R (2020) Vascular bundles mediate systemic reactive oxygen signaling during light stress in Arabidopsis. Plant Cell tpc.00453.2020 [DOI] [PMC free article] [PubMed]
- Zandalinas SI, Mittler R (2018) ROS-induced ROS release in plant and animal cells. Free Radic Biol Med 122: 21–27 [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Sengupta S, Burks D, Azad RK, Mittler R (2019) Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J 89: 126–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Thieme CJ, Kollwig G, Apelt F, Yang L, Winter N, Andresen N, Walther D, Kragler F (2016) tRNA-related sequences trigger systemic mRNA transport in plants. Plant Cell 28: 1237–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ge S, Jin L, Yao K, Wang Y, Wu X, Zhou J, Xia X, Shi K, Foyer CH, et al. (2019) A novel CO(2)-responsive systemic signaling pathway controlling plant mycorrhizal symbiosis. New Phytol 224: 106–116 [DOI] [PubMed] [Google Scholar]