The Striga, particularly S. hermonthica, problem has become a major threat to food security, exacerbating hunger and poverty in many African countries. A number of Striga control strategies have been proposed and tested during the past decade, however, further research efforts are still needed to provide sustainable and effective solutions to the Striga problem. In this paper, we provide an update on the recent progress and the approaches used in Striga management, and highlight emerging opportunities for developing new technologies to control this enigmatic parasite.
Recent progress and the approaches used in Striga management are summarized.
Abstract
The Striga, particularly S. he rmonthica, problem has become a major threat to food security, exacerbating hunger and poverty in many African countries. A number of Striga control strategies have been proposed and tested during the past decade, however, further research efforts are still needed to provide sustainable and effective solutions to the Striga problem. In this paper, we provide an update on the recent progress and the approaches used in Striga management, and highlight emerging opportunities for developing new technologies to control this enigmatic parasite.
Advances
The recently established Striga control technologies, such as push-pull, toothpick, and imidazolinone seed dressing have opened up new opportunities for smallholder farmers to overcome this parasite.
The development of low-cost and efficient germination stimulants together with an application protocol for rain-fed agriculture has made the suicidal germination strategy a realistic approach.
Molecular elucidation of strigolactone biosynthesis and perception has led to the development of new chemicals that disrupt the communication between Striga and its hosts.
Background
Striga species of the Orobanchaceae family are obligate root parasites that infest staple crops in sub-Saharan Africa (SSA), Middle East, and parts of Asia (Tank et al., 2006; Parker, 2012). Striga hermonthica, Striga asiatica, and Striga gesnerioides are the most economically important parasitic plants among the 42 known Striga species. The three species differ in their host specificity. Striga hermonthica and S asiatica parasitize on cereals and sugarcane (Saccharum officinarum; Parker, 2009; Pennisi, 2010), while cowpea (Vigna unguiculata) is the main host of S. gesnerioides (Ohlson and Timko, 2020). The Striga, particularly S. hermonthica, problem has become a major threat to food security, exacerbating hunger, and poverty in many African countries (Pennisi, 2010; Khan et al., 2014). Although consequences are difficult to measure, a few estimates have indicated that Striga is affecting the life of more than 300 million people in Africa and causing enormous yield losses with a value ranging from 7 to 10 billion US$ annually (Emechebe et al., 2004; Gressel et al., 2004; Ejeta, 2007; Scholes and Press, 2008; Rodenburg et al., 2010). In heavily infested regions, farmers have been forced to abandon cereal cultivation and to switch to other less important crops (Atera et al., 2012a). The severity of Striga depends upon degree of infestation, seed viability, ecotypes, virulence, host crop susceptibility, climatic/edaphic factors, and cultural practices (Rodenburg et al., 2016).
Striga species are among the hardest parasitic plants to control (Berner et al., 1995; Nickrent and Musselman, 2004). Adaptability of Striga to a wide range of hosts and environmental conditions has made it one of the most widespread and successful parasitic plants (Mohamed et al., 2006). In addition, long-term management of Striga is hampered by the tremendous number and longevity of seeds, vast genetic variability, complex life cycle, and subterranean nature of damage (Joel, 2000; Huang et al., 2012a, b). Indeed, it is estimated that about 900,000 S. hermonthica plants can emerge from one hectare of infested sorghum field, which can add about 4.5 × 1010 seeds in one growing cycle (Bebawi et al., 1984). Very tiny (0.3 nm × 0.15 nm) and light (4–7 μg) Striga seeds are easily dispersible in nearby fields through wind, animals, and agricultural tools, thereby gradually enriching seed reserve in the soil (Ejeta, 2007). The seeds remain dormant for a long period and germinate only after exposure to hot and humid conditions followed by perception of host derived germination stimulants, mainly strigolactones (SLs; Yoneyama et al., 2010; Joel and Bar, 2013; Al-Babili and Bouwmeester, 2015). Following germination, Striga radicle grows toward host roots. The perception of host-derived haustorium-inducing factors (HIFs), such as 2,6-dimethoxy-1,4-benzoquinone, prevents further growth of the radicle and induces cell expansion and division, and proliferation of hair cells at its tip, forming a haustorium, a special invasive organ that penetrates host roots to enable siphoning off water, minerals, and nutrients. The vital role of the haustorium provides a rarely exploited option for controlling Striga by breeding varieties with low HIF release or developing compounds that specifically inhibit haustorium formation (Cechin and Press, 1993; Shen et al., 2006; Yoshida and Shirasu, 2012; Yoshida et al., 2016; Goyet et al., 2019).
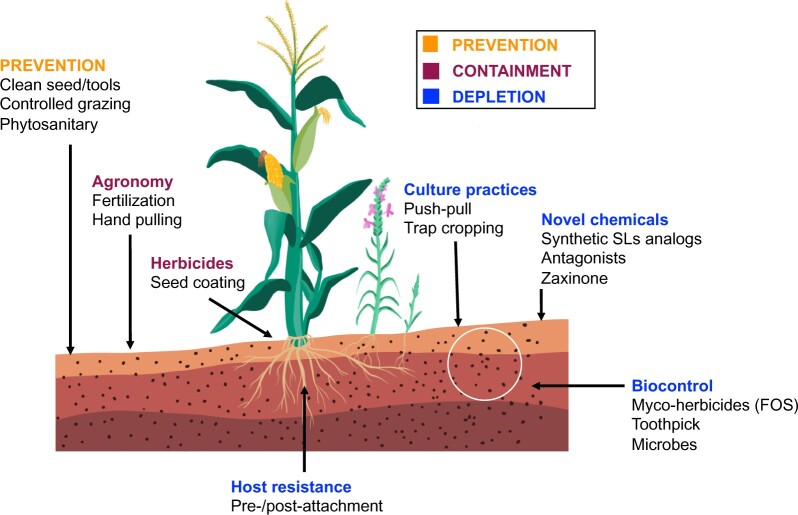
Due to the persistence and severity of the Striga problem, there has been an extensive effort to develop simple, easy, and effective control strategies that can be employed either alone or integrated with existing approaches (Figure 1). In the past decade, intensive research on the interaction of Striga with its host at molecular level has opened up opportunities to develop new management strategies. For sustainable Striga management, any control method should target at least one of the following goals (Figure 1): (1) PREVENTION: avoiding seed dispersal, for instance, by using clean crop seeds, tools, and fodder, controlling animal grazing or applying phytosanitary/quarantine measures; (2) CONTAINMENT: limiting new seed production by planting resistant (pre- and post-attachment, see below) varieties, using fertilizers, applying herbicides, employing a number of agronomic practices, such as hand weeding, deep sowing, burning, fallowing and soil solarization, and the application of chemicals that reduce the release of germination stimulants by the host; and (3) REDUCTION: reducing Striga seed bank accumulated in infested soils by employing cultural practices, such as trap cropping, inter cropping, and crop rotations, employing microbial agents that impact Striga and/or Striga/host interaction, applying synthetic germination stimulants in host’s absence, and by developing specific inhibitors that block germination of preconditioned seeds and decrease their viability. Reduction of seed bank can be also achieved by planting resistant varieties that stimulate Striga seed germination, but withstand the parasitic attack (post-attachment resistance, see below). In this article, we provide an update on the recent progress and the approaches used in Striga management, and highlight emerging opportunities for developing new technologies to control this enigmatic parasite.
Figure 1.
List of complementary approaches for Striga control. Methods depicted are used either for prevention and containment of Striga infestation, or for depletion of accumulated seed banks.
Host resistance
Deployment of resistant varieties is generally considered as the most economical, practical, and suitable long-term approach for controlling Striga (Hearne, 2009; Mandumbu et al., 2019). There are two main types of host resistance, that is, pre-attachment resistance resulting from SL profiles with low seed germinating activity, and post-attachment resistance that is based on hypersensitive response (HR)/incompatible response (IR). The recent identification of genes mediating the synthesis of different SLs opens up the possibility of modifying the SL profile of host plants by using genetic engineering and gene editing tools and, hence, increasing their resistance (Wakabayashi et al., 2019, 2020). Genetic resistance can be employed independently or as a central element of an integrated Striga management (ISM). Here, we present the latest knowledge on Striga resistance in maize (Zea mays), sorghum (Sorghum bicolor), pearl millet (Pennisetum glaucum), rice (Oryza sativa), and cowpea (V. unguiculata).
Maize has surpassed the traditional cereals in SSA, with the highest cultivation area of about 39 million ha in 2018 (FAOSTAT, 2018). Considering the importance of this crop, significant progress has been achieved in identifying a number of Striga resistant maize varieties and hybrids with different types of resistance (Akaogu et al., 2013, 2019; Badu-Apraku et al., 2019; Menkir and Meseka, 2019). For instance, Amusan et al. (2008) identified ZD05 a resistant maize inbred line that exhibits very low Striga attachments and high mortality of attached parasites, compared with the susceptible inbred line 5057. This resistance in ZD05 has been attributed to multilevel post-attachment barriers, particularly physiological or biochemical incompatibility to parasite growth and development. It is likely that this resistance is controlled by different genes that can be employed for durable and stable resistance. Similarly, Nyakurwa et al. (2018) compared quality protein maize (QPM) with non-QPM genotypes for S. asiatica resistance/tolerance under field/pot and agar gel assay conditions. They found several QPM genotypes with considerable levels of tolerance (i.e. no impairment of growth and yield despite of infestation), although they showed susceptibility similar to that of other genotypes in agar gel assays. However, the mechanisms underlying the tolerance of QPM genotypes remain elusive. In a similar study, Midega et al. (2016) evaluated six landraces for Striga infection under pot and natural field infestation and showed low Striga emergence, relative to hybrids.
In addition to investigating resistance in the field, a number of pre-attachment resistance studies based on SL analysis have been conducted in the past few years. For instance, Karaya et al. (2012) screened a collection of 420 maize landraces, populations, and inbred lines and identified several landraces with low Striga germinating activity and reduced release of the highly active strigol, however, without further investigation of responsible genetic factors. Later, the effect of SL composition on Striga/maize interaction was further demonstrated by comparing the two cultivars Pioneer 3253 (Striga-susceptible) and KSTP94 (resistant), which showed that 5-deoxystrigol was exclusively released by the susceptible variety, while sorgomol was the main SL in root exudates of the resistant one (Yoneyama et al., 2015). Interestingly, the two varieties were indistinguishable with respect to arbuscular mycorrhizal (AM) symbiosis, indicating that the effect of SL composition on the interaction with AM fungi is different from that on Striga attack (Yoneyama et al., 2015). Furthermore, a study of S. hermonthica infestation using rhizotron unraveled the presence of post-attachment resistance in the aforementioned cultivar KSTP94, demonstrated by lower number of Striga attachments and biomass in comparison to the susceptible maize inbred line CML144 (Mutinda et al., 2018). Similarly, Gasura et al. (2019) evaluated 30 maize inbred lines for S. asiatica resistance in pots and using agar gel assays, and identified seven inbred lines with low germination stimulant production and decreased root attachment and emergence under pot conditions, while four inbred lines exhibited low Striga attachment.
Recently, a genome-wide association study for S. hermonthica resistance in maize identified significant loci on chromosomes 3, 9, and 10, which are related to plant defense. A total of 24 single nucleotide polymorphisms (SNPs; under Striga infested conditions) and 11 SNPs (under Striga-free conditions) showed significant association with grain yield and number of ears per plant. The identified loci and candidate genes (GRMZM2G060216, GRMZM2G057243, and GRMZM2G164743) could be excellent breeding source for the development of Striga-resistant maize genotypes through marker-assisted selection (MAS) in SSA (Adewale et al., 2020). Moreover, as mentioned above, the ability of cultivars, such as KSTP94, to resist Striga infestation at pre- and post-attachment stages makes them a very suitable genetic source for resistance breeding. In addition, the identification of genetic factors underlying the resistance will allow stacking them by using genetic engineering/genome editing technologies to generate highly resistant varieties to Striga infestation.
Sorghum is the second most important cereal crop after maize in SSA. It was grown on about 30 million ha in 2018 (FAOSTAT, 2018). In the past two decades, significant progress has been made with the identification and characterization of quantitative trait loci (QTLs) associated with Striga resistance in this cereal. Initially, Haussmann et al. (2004) detected QTLs in recombinant inbred lines derived from the cross between IS9830 and N13. By deploying MAS techniques, some QTLs have been transferred into elite sorghum varieties, leading to the development of sorghum cultivars with improved resistance to Striga (Ejeta, 2007). Moreover, by using 328 recombinant inbred lines, derived from a cross between SRN39 (low germination stimulant) and Shanqui Red (high germination stimulant) sorghum, Satish et al. (2012) fine mapped LOW GERMINATION STIMULANT 1 (LGS1) locus to a 400-kb region on chromosome 5. Interestingly, it was later demonstrated that mutations in LGS1 (lgs1-1 to lgs1-5 mutants) lead to a change in the stereochemistry of released SLs, replacing the dominant SL 5-deoxystrigol by the less active orobanchol (Gobena et al., 2017). These findings were confirmed by Mohemed et al. (2018) who showed that sorghum genotypes with high release of 5-deoxystrigol are more susceptible to Striga, compared to orobanchol releasing genotypes. More importantly, LGS1-mediated (loss-of-function) resistance was further characterized in various sorghum landraces with respect to S. hermonthica diversity and geographic distribution (Bellis et al., 2020). These authors reported that LGS1 loss-of-function mutations are adaptive and widely distributed among African landraces across a large region of highly Striga infestation in Africa. Using gene-edited sorghum lines, it has been further shown that the degree of LGS1-mediated resistance depends on parasite genotype and abiotic environment (Bellis et al., 2020). In addition to the well-characterized pre-attachment mechanism, few reports documented the presence of strong HR or incompatibility-based post-attachment resistance (Mohamed et al., 2010a, 2010b; Mbuvi et al., 2017).
Pearl millet was cultivated on around 22 million ha during 2018, making it the third most important cereal after maize and sorghum in SSA (FAOSTAT, 2018). However, with paucity of reliable and adapted resistance donor sources, research and breeding for Striga resistance in pearl millet is still challenging, compared with other cereals (Wilson et al., 2000, 2004; Kountche et al., 2013; Sattler et al., 2018). Although several studies have been conducted to characterize mechanisms and inheritance of resistance to Striga in other cereals (Amusan et al., 2008; Cissoko et al., 2011; Jamil et al, 2011a; Satish et al., 2012), knowledge on individual resistance mechanisms, their genetic and physiological basis are still lacking in pearl millet. To fill this gap and by deploying a genotyping-by-sequencing approach, a genetic map of SNP markers together with single sequence repeats was constructed using a segregating population derived from a cross between a wild relative, resistant to Striga (Wilson et al., 2004) and a cultivated-susceptible pearl millet parent (Moumouni et al., 2015). The availability of such genomic resources will help in identifying and mapping QTLs associated with Striga resistance, which can be deployed in a MAS. Also, using conventional field based breeding, significant progress has been made with the identification of six varieties (M141, M239, M029, M197, M017, and KBH), showing higher yield and less Striga susceptibility (Kountche et al., 2013). The response to five cycles of phenotypic recurrent selection for Striga resistance was evaluated in a diversified pearl millet gene pool developed from these varieties (Kountche et al., 2013). The authors also reported the development of the first Striga-resistant experimental varieties (Kountche et al., 2013).
Rice was grown in 2018 on about 14 million ha in SSA (FAOSTAT, 2018). Both, pre- and post-attachment resistances were detected in this cereal. The Nipponbare rice cultivar shows strong post-attachment resistance to S. hermonthica, likely because of its incapability to build xylem–xylem connections with the parasite (Gurney et al., 2006). To shed light on the genes underlying this resistance, the response of Nipponbare and the susceptible cultivar IAC 165 was investigated using gene expression profiling. This study unraveled an association of the induction of defense genes with resistance, and of that of genes involved in nutrient transport, amino acid metabolism and abiotic stress response with susceptibility (Swarbrick et al., 2008). Genes induced in the resistant cultivar include three with unknown function, which are localized within a major resistance QTL on chromosome 12 and might have a significant contribution to the resistance (Swarbrick et al., 2008). In another study, three Striga resistance QTLs were detected in Koshihikari–Kasalath backcross inbred lines. A QTL of major effect was verified and narrowed down and could be, therefore, a good target for MAS (Swarbrick et al., 2009). Striga attack on the resistant rice cultivar Nipponbare is accompanied by an accumulation of lignin, guaiacyl, and syringyl at the site of Striga infection and by induction of phenylpropanoid pathway genes. The role of lignification in Nipponbare post-attachment resistance was demonstrated through manipulation of genes regulating lignin composition, which led to Striga susceptibility in this cultivar (Mutuku et al., 2019). Besides this structural barrier, post-attachment resistance also depends on a change in the level of defense hormones. Transcriptome analysis of infested roots indicated an involvement of the plant hormones jasmonic acid (JA) and salicylic acid (SA) in Striga post-attachment resistance. Mutant analysis confirmed the role of JA, but not that of salicylic acid. However, WRKY45 knockdown—a regulator of the SA/benzothiadiazole-mediated defense response—can lead to Striga susceptibility. This phenotype (susceptibility) could be rescued by exogenous JA application, indicating that WRKY45 contributes to Striga defense by modulating the interaction between JA and SA and positively regulating SA/benzothiadiazole and JA pathways (Mutuku et al., 2015).
A screen for pre-attachment resistance unraveled NEw RICe for Africa (NERICA) cultivars, such as NERICA1, and their parent CG14, which release low amounts of SLs (Jamil et al., 2011a). In another study, rice high tillering cultivars, for example, Super Basmati or TN1, showed low SL production and Striga infection, in contrast to low tillering rice varieties, for example, IAC-165 or IAC-1246 (Jamil et al., 2012a). Interestingly, Cissoko et al. (2011) reported that the above-mentioned NERICA cultivars also exhibited post-attachment resistance to S. hermonthica and S. asiatica, caused by incompatibility response or lack of xylem–xylem connections to rice endodermis. Both resistance studies were further validated and confirmed under field conditions (Atera et al., 2012b; Rodenburg et al., 2015, 2017). Similarly, the upland rice variety Umgar was characterized by pre- and post-attachment resistance to S. hermonthica under lab, pot, and field conditions (Samejima et al., 2016a).
Cowpea is an important vegetable and food legume in many African countries but its yield is severely affected by Striga gesnerioides (Parker, 2009). About seven distinct races of S. gesnerioides (SG1–SG6 and SG4z) were classified based on their genetics and parasitism on cowpea in West Africa (Botanga and Timko, 2005; Botanga and Timko 2007). Cowpea resistance to S. gesnerioides is conferred by single dominant genes in a race-specific manner (Timko and Singh, 2008; Timko et al., 2012). In addition, many cowpea landraces and local accessions possess post-attachment resistance that is based on HR at the site of attachment (Timko and Singh, 2008). Indeed, Li and Timko (2009) identified and characterized RSG3‐301 from the cowpea cultivar B301, which is involved in S. gesnerioides resistance. Silencing of RSG3-301 in B301 plants caused susceptibility to S. gesnerioides race SG3 due to reduced HR. These findings led to the conclusion that the race‐specific Striga resistance in cowpea is likely an effector‐triggered immunity that activates intracellular NLR proteins (RSG3-301) upon the recognition of pathogen/parasite effectors. Supporting the race-specific interactions in S. gesneriodes–cowpea associations, the cultivar B301 was, however, susceptible to the S. gesnerioides race SG4z producing a small soluble effector protein at high amounts in haustoria, which is transferred to the host root (Huang et al., 2012a, b). This protein can suppress the host innate immunity by binding to a host BTB‐BACK domain‐containing ubiquitin E3 ligase homolog POB1 (POZ/BTB containing protein 1). Overexpression of VuPOB1 led to a reduction of SG4z parasitism due to increased HR, while its silencing caused susceptibility, suggesting that VuPOB1 might be a positive regulator of the HR response (Su et al., 2020). As mentioned above, S. gesnerioides races and their distribution are dynamic systems, influenced by genetic drift and gene flow. Ohlson and Timko (2020) recently investigated S. gesnerioides diversity and known sources of resistance in cowpea. They collected 58 unique S. gesnerioides populations from 9 West African countries and screened 7 cowpea lines for resistance. Results obtained showed that none of the cowpea lines was resistant to all S. gesnerioides populations and that there is no S. gesnerioides population that can overcome the resistance of all seven cowpea lines. Analysis of single sequence repeats of the Striga populations unraveled high differentiation and suggested that genetic relatedness is generally a result of geographic proximity rather than of host compatibility. This study indicates that generating a broad-spectrum and durable S. gesnerioides cowpea-resistant lines requires stacking of multiple resistance genes (Ohlson and Timko, 2020).
In a field study in Nigeria, Muranaka et al. (2011) evaluated the susceptibility of different cultivars toward S. gesnerioides race SG3, which confirmed the resistance of the cultivars B301, IT97K-499-35, and IT98K-205-8. In a further field study performed in Burkina Faso, Tignegre et al. (2013) identified 11 cowpea genotypes with resistance to several S. gesnerioides races. Similarly, Omoigui et al. (2017) identified two high-resistant varieties, that is, UAM09 1046-6-1 and UAM09 1046-6-2, in the dry savanna agro-system in Nigeria, by phenotypic screening and using biplot analysis.
Cultural and agronomic practices
Trap cropping, sowing of false host such as cowpea, groundnut, sesame, and cotton to stimulate suicidal germination and to improve soil fertility were reported to be an effective way of seed bank depletion (Atera et al., 2013; Goldwasser and Rodenburg, 2013). Combining trap crops and nitrogen fertilizers was also reported to significantly decrease Striga seed bank (Tadesse, 2018). Cover cropping, sowing crops for the protection and enrichment of the soil showed Striga suppression directly through mulching, induction of suicidal germination, or its shading effect (Pickett et al., 2010; Goldwasser and Rodenburg, 2013; Randrianjafizanaka et al., 2018). Intercropping of cereals with legumes or a trap crop such as Desmodium spp. (Push–Pull) reduced Striga emergence by improving soil fertility, organic matter, and soil moisture content and releasing allelochemicals, such as C-glycosylflavonoids, isoflavanones, isoschaftoside, phenolics, 3,4-dihydroxybenzoic acid, which might impact Striga germination, growth, or development (Makoi and Ndakidemi, 2012; Khan et al., 2014; Pickett et al., 2014; Hooper et al., 2015; Midega et al., 2017; Hailu et al., 2018). A combination of herbicide-resistant maize varieties intercropped with legumes appeared more effective against Striga (Kanampiu et al., 2018).
Fallow and crop rotation were found not only to improve soil fertility and crop yield, but also to lower Striga infestation in Cameroon (Ayongwa et al., 2010). In a similar study, a reduction in Striga seed bank through fallowing was reported in Mali and Niger (Van Mourik et al., 2011). Rotation of a nonhost legume crop can considerably reduce the Striga seedbank in infested fields, leading to decreased infestation and significantly enhanced yield, compared with continuous cereal cultivation (Franke et al., 2018). Recently, Kountche et al. (2019) suggested field partitioning into two sections where suicidal germination agents (see below) and existing integrated Striga and soil management practices can be rotated to sustain Striga seed bank reduction. Hand pulling, uprooting Striga by hand or hand tools, is still considered as the cheapest traditional Striga control method (Goldwasser and Rodenburg, 2013). It is recommended to apply this method before Striga flowering, to prevent further seed setting and seed bank accumulation (Ayongwa et al., 2010; Sibhatu, 2016). However, hand weeding is laborious, time-consuming and less effective in reducing damage to standing crop (Mahuku et al., 2017). Fertilizer application and organic amendments showed a negative impact on Striga emergence (Ayongwa et al., 2011). The reduction of Striga infestation of rice upon fertilizer application is likely caused by a decrease in SL exudation (Jamil et al., 2011b). Similarly, nitrogen–phosphate–potassium fertilizer, micro-dosing of di-ammonium phosphate, and phosphate-based seed priming have been shown to reduce SL release and Striga parasitism in sorghum, pearl millet, and rice (Jamil et al., 2012b, 2014a, 2014b; Isah et al., 2013,).
Recently, combining conservation agriculture practices, such as cover cropping and fertilizer applications, with Striga-resistant varieties was found to alleviate Striga impact on rice and maize (Abdallah et al., 2015; Rodenburg et al., 2020). Although most of the cultural practices are less expensive and helpful in reducing parasitic seed bank and improving soil fertility and soil texture, they are constrained by low farmer acceptance, the need for introducing additional crops, labor forces, and financial resources (Murage et al., 2011).
Biocontrol by microbiome
Myco-herbicides developed from the fungus Fusarium oxysporum showed Striga inhibition by reducing its attachment to cereals and decreasing seed bank in infested soils (Rebeka et al., 2013; Zimmermann et al., 2016; Bàrberi, 2019). Some F. oxysporum strains produce high amounts of the amino acids l-leucine and l-tyrosin which are toxic to Striga—but not to maize—as they disrupt the tightly regulated free amino acid homeostasis. In addition, methionine released by F. oxysporum strains can be converted by soil microbes into the germination stimulant ethylene, causing suicidal germination of Striga seeds (Nzioki et al., 2016; Rubiales et al., 2018). Seed coating with F. oxysporum (FOXY2) was proposed as an effective way to deal with Striga under field conditions (Elzein et al., 2010; Ndambi et al., 2011, 2012; Rebeka et al., 2013; Watson, 2013). Later PSM197 and FOXY2 were encapsulated in a granular formulation (PESTA) for easy application/longevity, and a reduction of 75% in Striga emergence was observed in maize and sorghum crops (Schaub et al., 2006). FOXY2 was further classified as F. oxysporum f. sp. strigae (FOS) based on its highly selective inhibition of S. asiatica and S. hermonthica emergence (Mrema et al., 2018; 2020; Shayanowako et al., 2020). The characteristics that make mycoherbicides striking bioagents against Striga include host specificity, high aggressiveness, easy mass production, genetic diversity, and long storage life (Rebeka et al., 2013; Nzioki et al., 2016). Fungal delivery of primary inoculum on toothpick, multiplication of secondary field inoculum and farmers training are key components of this approach (Nzioki et al., 2016). The application of FOXY T14 led to an increase of 42%–56% in crop yield and reduction of 80% in Striga infestation under experimental conditions. However, F. oxysporum has not been extensively used in real fields, which might be due to a low effectiveness (Nzioki et al., 2016). Moreover, F. oxysporum might cause diseases, such as Fusarium wilt and dieback, in solanaceous crops (Zarafi et al., 2015).
Soil microbes like plant growth-promoting bacteria (PGPR), employment of AM fungi and some bacterial strains caused considerable reduction in Striga germination, attachment, and emergence (Lendzemo et al., 2009; Babalola, 2010; Hassan and Babiker, 2011; Mazaheri-Naeini et al., 2015). The potential of AM fungi in alleviating Striga infection has been indicated in a number of studies (Akiyama et al., 2005; Lendzemo et al., 2006; Bouwmeester et al., 2007; Xie et al., 2010). AM fungi not only enhance cereal growth and performance to withstand Striga damage but also facilitate host plant’s uptake of water, phosphorus (P), and micronutrients from the soil through the wide net of extraradical fungal hyphae (Bonfante and Genre, 2010). Increased uptake of P through symbiotic interaction by AM fungi could ultimately reduce SLs exudation by the host in the soil, thereby lowering Striga infection (Lendzemo et al., 2007; López-Ráez et al., 2011).
In a screening study, four PGPR suppressed Striga infestation in sorghum. Moreover, application of the strain B. subtilis GBO3 led to the death of 35%–59% of emerging Striga tubercles and to a 23% reduction in Striga attachment (Mounde et al., 2015). Striga seed germinating activity of sorghum root exudates decreased significantly upon treatment with Pseudomonas bacterial suspensions, which might be due to degradation of SLs (Ali et al., 2013). As shown for isolates of Bacillus, Streptomyces, and Rhizobium genera, the production of compounds with antibiotic activity and of extracellular enzymes, such as xylanases, pectinases, and amylases, can directly cause Striga seed decay (Neondo et al., 2017). In addition, soil microbes could impact Striga by releasing amino acids, such as tyrosine, leucine, and/or methionine (Vurro et al., 2009) or by producing secondary metabolites, such as anthranilic acid, β-lactone derivatives sesquiterpenoids, tricothecenes, which might interfere with SL perception (Tyc et al., 2017). Similarly, there is some evidence for SL degradation by fungi, which might lead to reduction in Striga germinating activity of released exudates (Boari et al., 2016). The germinating activity of root exudates and, hence, infestation by Striga can be also affected by soil microbes that modulate root architecture and growth (Huang et al., 2014).
The usage of soil microbes (AM fungi, PGPR, and other bacterial strains) is now considered as a promising, cost-effective, and environmentally safe approach for combating Striga (Watson, 2013; Samejima and Sugimoto, 2018). However, a number of biotic and abiotic factors can affect the efficacy of this approach, especially under field conditions. Finding a suitable inoculum medium, its mass production, suitable formulation, storage, shelf-life, consistency, and compatibility of applied microbes with the host, and the maintenance of their activity in infested soils must be taken into consideration. The validation and further development of this microbes-based biocontrol approach still requires intensive research and field testing under varying climatic and edaphic conditions (Müller‐Stöver et al., 2016; Mohammadi, 2019).
Use of herbicides and suicidal agents
Seed coating of imazapyr-resistant maize with imidazolinone herbicides, such as imazapyr, imazapic, pyrithiobac, and imazaquin, caused a reduction in Striga emergence throughout the planting season and led to a three- to four-fold increase in maize yield (Menkir et al., 2010; Chikoye et al., 2011; Habimana et al., 2014; Makumbi et al., 2015). Similarly, treatment of cowpea seeds with imazaquin at 0.24 kg a.i. ha−1 significantly decreased Striga infection in different cowpea genotypes (Lado et al., 2018). Albeit promising results, availability of IR resistance seeds to the farmers, application technology, the risk of generating resistance in Striga itself, and the impact on environment are important issues that need to be considered (Ransom et al., 2012).
Application of synthetic germination stimulants to deplete Striga seed bank in infested soils by inducing suicidal germination in the absence of host has recently gained a lot of attention (Samejima et al., 2016b; Zwanenburg et al., 2016; Kountche et al., 2019). Several SL analogs (Fig. 2) have been developed and evaluated (Xie et al., 2010; Mwakaboko and Zwanenburg, 2011; Kgosi et al., 2012; Boyer et al., 2014; Screpanti et al., 2016; Vurro et al., 2016; Jamil et al., 2018, 2020; Uraguchi et al., 2018; Prandi and McErlean, 2019). Functional tests revealed large variation in the efficiency of these analogs in exerting different SL functions, including the induction of Striga seed germination under lab conditions. Generally, SL analogs/mimics are quite variable in their structure but have in common the D-ring and ether bridge characteristic for natural SLs (Zwanenburg and Mwakaboko, 2011; Fukui et al., 2013, 2017; Cala et al., 2016; Takahashi et al., 2016; Oancea et al., 2017; Jamil et al., 2020). Structure–activity relationship studies have shown that minor structure modification can lead to significant changes in the biological activity (Cohen et al., 2013; Zwanenburg et al., 2013; Jamil et al., 2018, 2019, 2020). Following the discovery of the central SL biosynthesis intermediate carlactone (Alder et al., 2012), a carlactone-based SL analog, nitro-phenlactone was developed. Nitro-phenlactone showed high parasitic seed germinating activity in Phelipanche ramosa, which was at least comparable to GR24, but not in Striga (Jia et al., 2016). Subsequently, a series of carlactonoic acid-based analogs, called methyl-phenlactonoates (MPs), were developed and tested (Jamil et al., 2018, 2020). The three analogs MP1, MP3 and MP16 showed high suicidal germination activity on S. hermonthica in lab, greenhouse and field trials performed in Burkina Faso (Jamil et al., 2018, 2020; Kountche et al., 2019). Similarly, a carbamate-derived SL analog T-010 was applied in an infested field in Sudan, which led to a significant reduction in Striga emergence and better growth of sorghum (Samejima et al., 2016b). Recently, a chemical screen for SL agonists, followed by structural modifications, led to a highly active compound that triggers Striga seed germination under lab conditions at femtomolar range concentrations (Uraguchi et al., 2018). However, the question about the activity of this analog in field is still open.
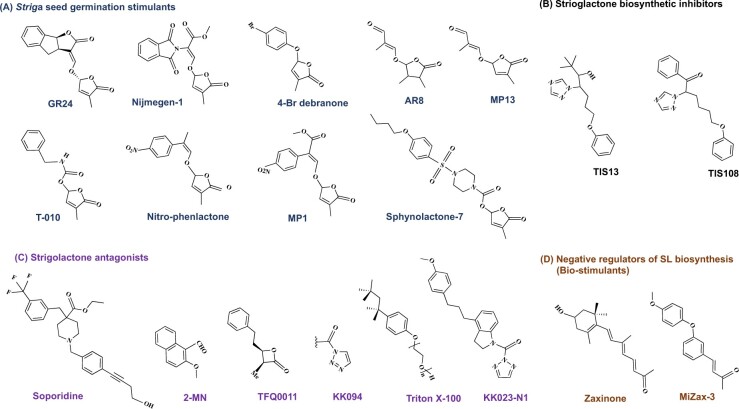
Figure 2.
Structure of germination stimulants and further chemicals used in Striga control. A, Structure of the SL analogs GR24, Nijmegen-1, 4-Br debranone, AR8, MP13, Nitro-phenlactone, MP1, and Sphynolactone-7. B, Structure of TIS13, TIS108, two inhibitors of SL biosynthesis. C, Structure of the SL antagonists Soporidine, 2-methoxy-1-naphthaldehyde, TFQ0011, KK094, Triton X-100, and KK023-N1. D, Structure of zaxinone and its mimic MiZax3, which act as growth-promoting compounds (biostimulants) and negative regulator of SL biosynthesis at transcript level in rice.
Although a large number of synthetic stimulants have been developed and proposed for usage as suicidal germination agents, there are only very few studies that assess the practicability and success of the suicidal germination approach and the activity of these stimulants in infested fields (Samejima et al., 2016b; Zwanenburg et al., 2016; Kountche et al., 2019). Indeed, the evaluation of the synthetic-stimulant-based suicidal germination strategy requires an application protocol suitable for infested regions in Africa, in addition to efficient and easy-to-synthesize SL analogs. Such a protocol needs to include an appropriate formulation and to account for local conditions, including less developed infrastructure. Given the water scarcity in the Sahel zone, the realization of the suicidal germination concept in the arid and semi-arid Striga-infested regions in SSA requires also a method that does not consume large amounts of water. Recently, Kountche et al. (2019) established a pipeline for assessing the germinating activity of SL analogs, which includes tests under lab conditions and in greenhouse, and mini and large field trials in Africa. For application in field, they developed a protocol for rain-fed agriculture, which requires a minimum of water and exploits rainfall for diluting and distributing of sprayed SL analogs. Following this protocol, the application of three SL analogs, that is, MP1, MP3, Nijmegen-1, at a final concentration below one micromole led to an up to 55% and 65% reduction in Striga emergence in an infested sorghum and pearl millet field, respectively (Kountche et al., 2019). These results and the study of Samejima et al. (2016b) demonstrate that the suicidal germination is a practicable and promising strategy to deplete Striga seed banks in infested African fields.
Development of novel chemicals for Striga control
Recent knowledge on SL biosynthesis and perception, and the availability of sequenced genomes have paved the way for developing new alternatives to combat root parasitic plants by chemicals, which decrease host SL release by inhibiting biosynthetic enzymes, down-regulating related transcripts, or blocking SL perception in the parasite.
Inhibitors of SL biosynthesis reported so far target either the formation of carotenoids, the precursor of SLs, or SL biosynthesis itself. Application of fluridone or norflurazon, which inhibit the desaturation of phytoene in carotenoid biosynthesis (Zheng et al., 2020), to rice plants decreased SL production and, hence, Striga germination (Jamil et al., 2010). However, treatment with carotenoid biosynthesis inhibitors may cause unwanted effects, given the diverse functions of carotenoids (Felemban et al., 2019; Zheng et al., 2020). Ito et al. (2011) developed the triazole-derivative TIS13 as inhibitor of SL biosynthesis and demonstrated its effect in decreasing rice SL release. On the basis of these results, a new set of structurally related SL biosynthesis inhibitor candidates were synthesized, including TIS108 that showed considerable reduction in the release of Striga germination stimulants without affecting the host (Ito et al., 2013). Further development of TIS108 led to KK05 that caused higher reduction of 4-deoxyorobanchol release in rice exudates (Kawada et al., 2019).
SL antagonists, which specifically inhibit Striga SL perception, are a promising tool that could be applied in the presence of the host, enabling Striga control throughout the cropping season and complementing the suicidal germination strategy employed in host’s absence. The development of specific germination inhibitors has become possible through the identification of Striga SL receptors, particularly the most sensitive one ShHTL7, which are involved in Striga seed germination and differ from the receptors mediating SL response in host plants (Toh et al., 2015). Striga SL receptors that regulate seed germination are supposed to have evolved from the homolog receptor KAI2 that perceives karrikins, smoke derived compounds mimicking a yet unidentified plant growth regulator inducing seed germination in nonparasitic plants (Toh et al., 2014; Waters et al., 2014; Conn and Nelson, 2016). Thus, the Striga ShHTL7 can replace KAI2 and mediate SL-dependent seed germination in Arabidopsis kai2 mutant (Holbrook-Smith et al., 2016). This capability allowed the establishment of a high-throughput screening for chemicals that block the Striga ShHTL7 receptor and act as SL antagonists, which led to the identification of soporidine (SOP; Holbrook-Smith et al., 2016). Evaluation of SOP activity on Striga seed germination indicated its capability to block this process (Tsuchiya et al., 2018). A serendipity discovery of a potent SL antagonist that specifically blocks Striga ShHTL7 was recently reported (Hameed et al., 2018). The authors aimed at elucidating the structure of ShHTL7 by crystallography and found the receptor tightly bound to the detergent Triton X-100 usually used in protein purification. Functional studies confirmed that Triton X-100 is a SL antagonist that specifically blocks the Striga ShHTL7, but not host SL receptors, and reduce Striga infestation in greenhouse (Hameed et al., 2018). SOP and Triton X-100 can be considered as lead compounds for the development of efficient Striga herbicides that act by inhibiting seed germination. An improvement of Triton X-100 efficiency might be achieved by modifying it to become a covalently binding SL antagonist. Similarly, 2-methoxy-1-naphthaldehyde as an SL antagonist (Mashita et al., 2016) or simple β-lactones (TFQ0011) that act as covalent and high-efficient inhibitors of SL receptors have been developed based on the general SL perception mechanism (Xiang et al., 2017). Triazole urea compounds, such as KK094, that covalently bind to nonparasitic plant SL receptors have been recently produced and shown to efficiently inhibit rice SL perception (Nakamura et al., 2019). A combination of structural elements of Triton X-100 and KK094 or β-lactone inhibitors might lead to highly efficient Striga-specific herbicides. The recently developed Triton X-100/KK094 hybrid structure KK023-N1 (Figure 2) is an example for such compounds (Randa et al., manuscript under review).
Zaxinone is a natural growth-regulating apocarotenoid metabolite that has been recently shown to be required for normal rice growth and development. In addition, application of this compound promotes rice root growth and downregulates SL biosynthesis at the transcript level (Wang et al., 2019). A greenhouse study demonstrated that zaxinone application can alleviate Striga infestation (Wang et al., 2019). These activities point to zaxinone as a suitable candidate for reducing Striga infestation by accelerating host growth improving its performance and decreasing parasitic seed germination. Very recently, a series of easy-to-synthesize Mimics of Zaxinone (MiZax) have been developed and tested in lab and greenhouse. This study unraveled MiZax3 and MiZax5 as potent zaxinone mimics that promote rice growth, reduce SL release and decrease Striga infestation (Wang et al., 2020). The simple synthesis protocol and high efficacy make MiZax very promising candidates for field application.
Integrated Striga management
Effective Striga management cannot be achieved by a single control method and requires the integration of different approaches (Figure 1; Ejeta, 2007; Sibhatu, 2016). For instance, complementing host resistance with the use of F. oxysporum caused effective Striga reduction (Mrema et al., 2020; Shayanowako et al., 2020). Similarly, the reduction in Striga infestation achieved through seed coating of imazapyr-resistant hybrid maize can be significantly further increased by exploiting maize Striga-resistance (Kamara et al., 2020). As a further example, a cereal-legume crop rotation can be combined with the application of synthetic germination stimulants to deplete accumulated seed bank in infested soils (Hailu et al., 2018; Kountche et al., 2019). It can be also anticipated that the integration of suicidal germination technology with Striga-specific herbicides and/or zaxinone analogs can be very effective in dealing with the Striga problem in African agriculture.
Concluding remarks and perspectives
A number of Striga control strategies have been proposed and tested during the past decade; however, further research efforts are still needed to provide sustainable and effective solutions to the Striga problem (see Outstanding Questions).
First, further understanding of the molecular and genetic basis of host resistance and host–parasite interaction is needed to breed crops with durable resistance. Use of genomic resources and modern tools, such as targeted gene editing or mutation breeding, can translate this knowledge into resistant crops. Second, rotation and/or intercropping with false host are important and cost-effective components of Striga management. This approach can be a vital element in existing ISM in SSA. Third, farmers willingness, commitment and planning, labor, capital, and input availability in particular cropping system are important factors for the effectiveness of push–pull, seed coating, or toothpick/FOS technologies. Compatibility to climatic and soil factors, famers awareness, dissemination of information, and transfer of technology to smallholders’ farmers are essential factors that need further attention. Fourth, the effectiveness of novel chemicals (SL analogs, antagonists, bio-stimulants) might depend upon formulation and method and time of application. A suitable formulation of efficient compounds, mass scale, low-cost synthesis, and practical field application for rain-fed African agriculture are crucial, particularly for seed bank depletion by suicidal agents. Moreover, impact of these chemicals on soil fauna and flora, soil structure, persistency, and residual effects on environment must be investigated prior to their release for on-farm application. Last, but not least, a smart package of technology integrating Striga-resistant cultivars with either fertilizers, mycoherbicides, herbicide-based seed coating, or new chemicals still needs to be worked out to achieve complete and robust control of Striga.
Acknowledgments
We highly appreciate and acknowledge the help of Justin Braguy, KAUST, in designing and improving Figure 1.
Funding
This study was supported by the Bill & Melinda Gates Foundation Grant (OPP1194472) and the King Abdullah University of Science and Technology (KAUST).
Conflict of interest statement. None declared.
Outstanding questions
What are the molecular factors underlying Striga resistance?
How can we efficiently mobilize the existing omics tools to breed durable Striga-resistant varieties?
What determines the stereochemistry of released strigolactones?
Can we design a microbiome community to suppress Striga impact and enhance host crop productivity in sub-Saharan Africa?
What is the impact of synthetic germination stimulants and strigolactone-related novel chemicals on soil fauna and flora?
M.J. contributed to the conception and proposal of the article, interpreted the relevant literature, and designed the figures. B.A.K. took part in the revision, particularly in the host resistance and suicidal part of the article. S.A.B. substantially contributed in the conception, editing, and structuring of the article and revised it critically to make the final version.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Author (https://academic.oup.com/plphys/pages/general-instructions) is: Salim Al-Babili (salim.babili@kaust.edu.sa).
References
- Abdallah B, Saha H, Tsanuo M (2015) Control of Striga asiatica through the integration of legume cover crops and Striga resistant maize. Int J Pure Appl Sci Technol 29: 42–53 [Google Scholar]
- Adewale SA, Badu-Apraku B, Akinwale RO, Paterne AA, Gedil M, Garcia-Oliveira AL (2020) Genome-wide association study of Striga resistance in early maturing white tropical maize inbred lines. BMC Plant Biol 20: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaogu IC, Badu-Apraku B, Adetimirin VO, Vroh-Bi I, Oyekunle M, Akinwale RO (2013) Genetic diversity assessment of extra-early maturing yellow maize inbreds and hybrid performance in Striga-infested and Striga-free environments. J Agric Sci 151: 519–537 [Google Scholar]
- Akaogu IC, Badu-Apraku B, Tongoona P, Ceballos H, Gracen V, Offei SK, Dzidzienyo D (2019) Inheritance of Striga hermonthica adaptive traits in an early-maturing white maize inbred line containing resistance genes from Zea diploperennis. Plant Breed 138: 546–552 [Google Scholar]
- Akiyama K, Matsuzaki K-i, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol 66: 161–186 [DOI] [PubMed] [Google Scholar]
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335: 1348–1351 [DOI] [PubMed] [Google Scholar]
- Ali HA, Elamin HB, Dirar HA (2013) Biological control of Striga hermonthica Del. Benth: Screening for bacteria scavenging Strigol. Univ Africa J Sci 1: 106–119 [Google Scholar]
- Amusan IO, Rich PJ, Menkir A, Housley T, Ejeta G (2008) Resistance to Striga hermonthica in a maize inbred line derived from Zea diploperennis. New Phytol 178: 157–166 [DOI] [PubMed] [Google Scholar]
- Atera EA, Itoh K, Azuma T, Ishii T (2012a) Farmers' perspectives on the biotic constraint of Striga hermonthica and its control in western Kenya. Weed Biol Manag 12: 53–62 [Google Scholar]
- Atera EA, Itoh K, Azuma T, Ishii T (2012b) Response of NERICA rice to Striga hermonthica infections in Western Kenya. Int J Agric Biol 14: 271–275 [Google Scholar]
- Atera EA, Ishii T, Onyango JC, Itoh K, Azuma T (2013) Striga infestation in Kenya: Status, distribution and management options. Sustain Agric Res 2: 99–108 [Google Scholar]
- Ayongwa G, Stomph T, Hoevers R, Ngoumou T, Kuyper T (2010) Striga infestation in northern Cameroon: Magnitude, dynamics and implications for management. NJAS-Wagen J Life Sci 57: 159–165 [Google Scholar]
- Ayongwa GC, Stomph TJ, Kuyper TW (2011) Host-parasite dynamics of Sorghum bicolor and Striga hermonthica—the influence of soil organic matter amendments of different C:N ratio. Crop Prot 30: 1613–1622 [Google Scholar]
- Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett 32: 1559–1570 [DOI] [PubMed] [Google Scholar]
- Badu-Apraku B, Talabi AO, Fakorede MAB, Fasanmade Y, Gedil M, Magorokosho C, Asiedu R (2019) Yield gains and associated changes in an early yellow bi-parental maize population following genomic selection for Striga resistance and drought tolerance. BMC Plant Biol 19: 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bàrberi P (2019) Ecological weed management in Sub-Saharan Africa: prospects and implications on other agroecosystem services. Adv Agron 156: 219–264 [Google Scholar]
- Bebawi FF, Eplee RE, Harris CE, Norris RS (1984) Longevity of witchweed (Striga asiatica) seed. Weed Sci 32: 494–497 [Google Scholar]
- Bellis ES, Kelly EA, Lorts CM, Gao HR, Deleo VL, Rouhan G,, Budden A, Bhaskara GB, Hu ZB, Muscarella R, et al. (2020) Genomics of sorghum local adaptation to a parasitic plant. Proc Natl Acad Sci USA 117: 4243–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner D, Kling J, Singh B (1995) Striga research and control. A perspective from Africa. Plant Dis 79: 652–660 [Google Scholar]
- Boari A, Ciasca B, Pineda‐Martos R, Lattanzio VM, Yoneyama K, Vurro M (2016) Parasitic weed management by using strigolactone‐degrading fungi. Pest Manag Sci 72: 2043–2047 [DOI] [PubMed] [Google Scholar]
- Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat Commun 1: 1–11 [DOI] [PubMed] [Google Scholar]
- Botanga CJ, Timko MP (2005) Genetic structure and analysis of host and nonhost interactions of Striga gesnerioides (witchweed) from central Florida. Phytopathology 95: 1166–1173 [DOI] [PubMed] [Google Scholar]
- Botanga CJ, Timko MP (2007) Phenetic relationships among different races of Striga gesnerioides (Willd.) Vatke from West Africa. Genome 1365: 1351–1365 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Becard G (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12: 224–230 [DOI] [PubMed] [Google Scholar]
- Boyer F-D, de Saint Germain A, Pouvreau J-B, Clavé G, Pillot J-P, Roux A, Rasmussen A, Depuydt S, Lauressergues D, dit Frey NF (2014) New strigolactone analogs as plant hormones with low activities in the rhizosphere. Mol Plant 7: 675–690 [DOI] [PubMed] [Google Scholar]
- Cala A, Ghooray K, Fernandez-Aparicio M, Molinillo JMG, Galindo JCG, Rubiales D, Macias FA (2016) Phthalimide-derived strigolactone mimics as germinating agents for seeds of parasitic weeds. Pest Manag Sci 72: 2069–2081 [DOI] [PubMed] [Google Scholar]
- Cechin I, Press M (1993) Nitrogen relations of the sorghum‐Striga hermonthica host‐parasite association: germination, attachment and early growth. New Phytol 124: 681–687 [DOI] [PubMed] [Google Scholar]
- Chikoye D, Fontem LA, Menkir A (2011) Seed coating herbicide tolerant maize hybrids with imazapyr for Striga hermonthica (Del.) Benth control in the West African savanna. J Food Agric Environ 9: 416–421 [Google Scholar]
- Cissoko M, Boisnard A, Rodenburg J, Press MC, Scholes JD (2011) New Rice for Africa (NERICA) cultivars exhibit different levels of post-attachment resistance against the parasitic weeds Striga hermonthica and Striga asiatica. New Phytol 192: 952–963 [DOI] [PubMed] [Google Scholar]
- Cohen M, Prandi C, Occhiato EG, Tabasso S, Wininger S, Resnick N, Steinberger Y, Koltai H, Kapulnik Y (2013) Structure–function relations of strigolactone analogs: activity as plant hormones and plant interactions. Mol Plant 6: 141–152 [DOI] [PubMed] [Google Scholar]
- Conn CE, Nelson DC (2016) Evidence that KARRIKIN-INSENSITIVE2 (KAI2) receptors may perceive an unknown signal that is not karrikin or strigolactone. Front Plant Sci 6: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejeta G (2007) Breeding for Striga resistance in sorghum: exploitation of an intricate host-parasite biology. Crop Sci 47: 216–227 [Google Scholar]
- Elzein A, Heller A, Ndambi B, De Mol M, Kroschel J, Cadisch G (2010) Cytological investigations on colonization of sorghum roots by the mycoherbicide Fusarium oxysporum f. sp strigae and its implications for Striga control using a seed treatment delivery system. Biol Control 53: 249–257 [Google Scholar]
- Emechebe A, Ellis-Jones J, Schulz S, Chikoye D, Douthwaite B, Kureh I, Tarawali G, Hussaini M, Kormawa P, Sanni A (2004) Farmers' perception of the Striga problem and its control in Northern Nigeria. Exp Agric 40: 215–232 [Google Scholar]
- FAOSTAT (2018) Food and Agriculture Organization of the United Nations. http://www.fao.org/ (June, 2020)
- Felemban A, Braguy J, Zurbriggen MD, Al-Babili S (2019) Apocarotenoids involved in plant development and stress response. Front Plant Sci 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Van den Brand G, Vanlauwe B, Giller K (2018) Sustainable intensification through rotations with grain legumes in Sub-Saharan Africa: a review. Agric Ecosyst Environ 261: 172–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K, Ito S, Asami T (2013) Selective mimics of strigolactone actions and their potential use for controlling damage caused by root parasitic weeds. Mol Plant 6: 88–99 [DOI] [PubMed] [Google Scholar]
- Fukui K, Yamagami D, Ito S, Asami T (2017) A taylor-made design of phenoxyfuranone-type strigolactone mimic. Front Plant Sci 8: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasura E, Setimela P, Mabasa S, Rwafa R, Kageler S, Nyakurwa C (2019) Response of IITA maize inbred lines bred for Striga hermonthica resistance to Striga asiatica and associated resistance mechanisms in southern Africa. Euphytica 215: 1–15 [Google Scholar]
- Gobena D, Shimels M, Rich PJ, Ruyter-Spira C, Bouwmeester H, Kanuganti S, Mengiste T, Ejeta G (2017) Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proc Natl Acad Sci USA 114: 4471–4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwasser Y, Rodenburg J (2013) Integrated agronomic management of parasitic weed seed banks. InJoel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies. Springer; New York, pp 393–413 [Google Scholar]
- Gressel J, Hanafi A, Head G, Marasas W, Obilana AB, Ochanda J, Souissi T, Tzotzos G (2004) Major heretofore intractable biotic constraints to African food security that may be amenable to novel biotechnological solutions. Crop Prot 23: 661–689 [Google Scholar]
- Goyet V, Wada S, Cui S, Wakatake T, Shirasu K, Montiel G, Simier P, Yoshida S (2019) Haustorium inducing factors for parasitic Orobanchaceae. Front Plant Sci 10: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A, Slate J, Press M, Scholes J (2006) A novel form of resistance in rice to the angiosperm parasite Striga hermonthica. New Phytol 169: 199–208 [DOI] [PubMed] [Google Scholar]
- Habimana S, Nduwumuremyi A, Chinama R (2014) Management of Orobanche in field crops: a review. J Soil Sci Plant Nutr 14: 43–62 [Google Scholar]
- Hailu G, Niassy S, Zeyaur KR, Ochatum N, Subramanian S (2018) Maize-legume intercropping and Push-Pull for management of fall armyworm, stemborers, and Striga in Uganda. Agron J 110: 2513–2522 [Google Scholar]
- Hameed US, Haider I, Jamil M, Kountche BA, Guo XR, Zarban RA, Kim D, Al-Babili S, Arold ST (2018) Structural basis for specific inhibition of the highly sensitive ShHTL7 receptor. EMBO Rep 19: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MM, Babiker AGT (2011) Effects of bacterial strains and isolates on in situ germination, subsequent developmental stage of Striga hermonthica onto sorghum roots. Adv Environ Biol 5: 3263–3270 [Google Scholar]
- Haussmann B, Hess D, Omanya G, Folkertsma R, Reddy B, Kayentao M, Welz H, Geiger H (2004) Genomic regions influencing resistance to the parasitic weed Striga hermonthica in two recombinant inbred populations of sorghum. Theor Appl Genet 109: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Hearne SJ (2009) Control—the Striga conundrum. Pest Manag Sci 65: 603–614 [DOI] [PubMed] [Google Scholar]
- Holbrook-Smith D, Toh S, Tsuchiya Y, McCourt P (2016) Small-molecule antagonists of germination of the parasitic plant Striga hermonthica. Nat Chem Biol 12: 724–729 [DOI] [PubMed] [Google Scholar]
- Hooper AM, Caulfield JC, Hao B, Pickett JA, Midega CAO, Khan ZR (2015) Isolation and identification of Desmodium root exudates from drought tolerant species used as intercrops against Striga hermonthica. Phytochemistry 117: 380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Whitlock R, Press M, Scholes J (2012a) Variation for host range within and among populations of the parasitic plant Striga hermonthica. Heredity 108: 96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Mellor KE, Paul SN, Lawson MJ, Mackey AJ, Timko MP (2012b) Global changes in gene expression during compatible and incompatible interactions of cowpea (Vigna unguiculata L.) with the root parasitic angiosperm Striga gesnerioides. BMC Genomics 13: 402–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X-F, Chaparro JM, Reardon KF, Zhang R, Shen Q, Vivanco JM. ( 2014) Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany 92: 267–275 [Google Scholar]
- Isah K, Kumar N, Lagoke S, Atayese M (2013) Management of Striga hermonthica on sorghum (Sorghum bicolor) using arbuscular mycorrhizal fungi (Glomus mosae) and NPK fertilizer levels. Pak J Biol Sci 16: 1563–1568 [DOI] [PubMed] [Google Scholar]
- Ito S, Umehara M, Hanada A, Kitahata N, Hayase H, Yamaguchi S, Asami T (2011) Effects of triazole derivatives on strigolactone levels and growth retardation in rice. PLos One 6: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Umehara M, Hanada A, Yamaguchi S, Asami T (2013) Effects of strigolactone-biosynthesis inhibitor TIS108 on Arabidopsis. Plant Signal Behav 8: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil M, Charnikhova T, Verstappen F, Bouwmeester H (2010) Carotenoid inhibitors reduce strigolactone production and Striga hermonthica infection in rice. Arch Biochem Biophys 504: 123–131 [DOI] [PubMed] [Google Scholar]
- Jamil M, Rodenburg J, Charnikhova T, Bouwmeester HJ (2011a) Pre-attachment Striga hermonthica resistance of New Rice for Africa (NERICA) cultivars based on low strigolactone production. New Phytol 192: 964–975 [DOI] [PubMed] [Google Scholar]
- Jamil M, Charnikhova T, Cardoso C, Jamil T, Ueno K, Verstappen F, Asami T, Bouwmeester HJ (2011b) Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus. Weed Res 51: 373–385 [Google Scholar]
- Jamil M, Charnikhova T, Houshyani B, van Ast A, Bouwmeester HJ (2012a) Genetic variation in strigolactone production and tillering in rice and its effect on Striga hermonthica infection. Planta 235: 473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil M, Kanampiu FK, Karaya H, Charnikhova T, Bouwmeester HJ (2012b) Striga hermonthica parasitism in maize in response to N and P fertilizers. Field Crops Res 134: 1–10 [Google Scholar]
- Jamil M, Charnikhova T, Jamil T, Ali Z, Mohamed NEMA, Van Mourik T, Bouwmeester HJ (2014a) Influence of fertilizer microdosing on strigolactone production and Striga hermonthica parasitism in pearl millet. Int J Agric Biol 16: 935–940 [Google Scholar]
- Jamil M, Charnikhova T, Verstappen F, Ali Z, Wainwright H, Bouwmeester HJ (2014b) Effect of phosphate-based seed priming on strigolactone production and Striga hermonthica infection in cereals. Weed Res 54: 307–313 [Google Scholar]
- Jamil M, Kountche BA, Haider I, Guo XJ, Ntui VO, Jia KP, Ali S, Hameed US, Nakamura H, Lyu Y, et al. (2018) Methyl phenlactonoates are efficient strigolactone analogs with simple structure. J Exp Bot 69: 2319–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil M, Kountche BA, Haider I, Wang JY, Aldossary F, Zarban RA, Jia KP, Yonli D, Hameed UFS, Takahashi I, et al. (2019) Methylation at the C-3' in D-Ring of strigolactone analogs reduces biological activity in root parasitic plants and rice. Front Plant Sci 10: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil M, Kountche BA, Wang JY, Haider I, Jia K-P, Takahashi I, Ota T, Asami T, Al-Babili S (2020) A new series of carlactonoic acid based strigolactone analogs for fundamental and applied research. Front Plant Sci 11: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K-P, Kountche BA, Jamil M, Guo X, Ntui VO, Rüfenacht A, Rochange S, Al-Babili S (2016) Nitro-phenlactone, a carlactone analog with pleiotropic strigolactone activities. Mol Plant 9: 1341–1344 [DOI] [PubMed] [Google Scholar]
- Joel DM, Bar H (2013) The seed and the seedling. InJoel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies. Springer; New York, pp 147–165 [Google Scholar]
- Joel DM (2000) The long-term approach to parasitic weeds control: manipulation of specific developmental mechanisms of the parasite. Crop Prot 19: 753–758 [Google Scholar]
- Kamara AY, Menkir A, Chikoye D, Solomon R, Tofa AI, Omoigui LO (2020) Seed dressing maize with imazapyr to control Striga hermonthica in farmers' fields in the savannas of Nigeria. Agriculture Basel 10: 1–9 [Google Scholar]
- Kanampiu F, Makumbi D, Mageto E, Omanya G, Waruingi S, Musyoka P, Ransom J (2018) Assessment of management options on Striga infestation and maize grain yield in Kenya. Weed Sci 66: 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaya H, Njoroge K, Mugo S, Ariga ES, Kanampiu F, Nderitu JH (2012) Determination of levels of Striga germination stimulants for maize gene bank accessions and elite inbred lines. Int J Plant Prod 6: 209–223 [Google Scholar]
- Kawada K, Takahashi I, Arai M, Sasaki Y, Asami T, Yajima S, Ito S (2019) Synthesis and biological evaluation of novel triazole derivatives as strigolactone biosynthesis inhibitors. J Agric Food Chem 67: 6143–6149 [DOI] [PubMed] [Google Scholar]
- Kgosi RL, Zwanenburg B, Mwakaboko AS, Murdoch AJ (2012) Strigolactone analogues induce suicidal seed germination of Striga spp. in soil. Weed Res 52: 197–203 [Google Scholar]
- Khan ZR, Midega CAO, Pittchar JO, Murage AW, Birkett MA, Bruce TJA, Pickett JA (2014) Achieving food security for one million sub-Saharan African poor through push-pull innovation by 2020. Philos Trans R Soc B Biol Sci 369: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kountche BA, Hash CT, Dodo H, Laoualy O, Sanogo MD, Timbeli A, Vigouroux Y, This D, Nijkamp R, Haussmann BIG (2013) Development of a pearl millet Striga-resistant genepool: Response to five cycles of recurrent selection under Striga-infested field conditions in West Africa. Field Crops Res 154: 82–90 [Google Scholar]
- Kountche BA, Jamil M, Yonli D, Nikiema MP, Blanco‐Ania D, Asami T, Zwanenburg B, Al‐Babili S (2019) Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub‐Saharan Africa. Plants People Planet 1: 107–118 [Google Scholar]
- Lado A, Hussaini MA, Kamara AY (2018) Effectiveness of imazaquin seed treatment on Striga gesnerioides control and growth traits of seven cowpea genotypes. J Plant Pathol 100: 477–484 [Google Scholar]
- Lendzemo VW, van Ast A, Kuyper TW (2006) Can arbuscular mycorrhizal fungi contribute to Striga management on cereals in Africa? Outlook Agric 35: 307–311 [Google Scholar]
- Lendzemo VW, Kuyper TW, Matusova R, Bouwmeester HJ, Ast Av (2007) Colonization by arbuscular mycorrhizal fungi of sorghum leads to reduced germination and subsequent attachment and emergence of Striga hermonthica. Plant Signal Behav 2: 58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendzemo V, Kuyper T, Vierheilig H (2009) Striga seed-germination activity of root exudates and compounds present in stems of Striga host and nonhost (trap crop) plants is reduced due to root colonization by arbuscular mycorrhizal fungi. Mycorrhiza 19: 287–294 [DOI] [PubMed] [Google Scholar]
- Li J, Timko MP (2009) Gene-for-gene resistance in Striga-cowpea associations. Science 325: 1094–1094 [DOI] [PubMed] [Google Scholar]
- López-Ráez JA, Charnikhova T, Fernández I, Bouwmeester H, Pozo MJ (2011) Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. J Plant Physiol 168: 294–297 [DOI] [PubMed] [Google Scholar]
- Mahuku G, Wosula E, Kanampiu F (2017) Integrated Pest Management in tropical cereal crops. In Rapisarda C, Cocuzza GEM, eds, Integrated Pest Management in Tropical Regions. CAB International, UK, pp 47–74 [Google Scholar]
- Makoi JH, Ndakidemi PA (2012) Allelopathy as protectant, defence and growth stimulants in legume cereal mixed culture systems. N Z J Crop Hortic Sci 40: 161–186 [Google Scholar]
- Makumbi D, Diallo A, Kanampiu F, Mugo S, Karaya H (2015) Agronomic performance and genotype×environment interaction of herbicide‐resistant maize varieties in eastern Africa. Crop Sci 55: 540–555 [Google Scholar]
- Mandumbu R, Mutengwa C, Mabasa S, Mwenje E (2019) Challenges to the exploitation of host plant resistance for Striga management in cereals and legumes by farmers in sub-Saharan Africa: a review. Acta Agric Scand B Soil Plant Sci 69: 82–88 [Google Scholar]
- Mashita O, Koishihara H, Fukui K, Nakamura H, Asami T (2016) Discovery and identification of 2-methoxy-1-naphthaldehyde as a novel strigolactone-signaling inhibitor. J Pestic Sci 41: 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri-Naeini M, Sabbagh SK, Martinez Y, Sejalon-Delmas N, Roux C (2015) Assessment of Ustilago maydis as a fungal model for root infection studies. Fungal Biol 119: 145–153 [DOI] [PubMed] [Google Scholar]
- Mbuvi DA, Masiga CW, Kuria E, Masanga J, Wamalwa M, Mohamed A, Odeny D, Hamza N, Timko MP, Runo S (2017) Novel sources of witchweed (Striga) resistance from wild sorghum accessions. Front Plant Sci 8: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkir A, Chikoye D, Lum F (2010) Incorporating an herbicide resistance gene into tropical maize with inherent polygenic resistance to control Striga hermonthica (Del.) Benth. Plant Breed 129: 385–392 [Google Scholar]
- Menkir A, Meseka S (2019) Genetic improvement in resistance to Striga in tropical maize hybrids. Crop Sci 59: 2484–2497 [Google Scholar]
- Midega CAO, Pickett J, Hooper A, Pittchar J, Khan ZR (2016) Maize landraces are less affected by Striga hermonthica relative to hybrids in Western Kenya. Weed Technol 30: 21–28 [Google Scholar]
- Midega CA, Wasonga CJ, Hooper AM, Pickett JA, Khan ZR (2017) Drought-tolerant Desmodium species effectively suppress parasitic Striga weed and improve cereal grain yields in western Kenya. Crop Prot 98: 94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed KI, Papes M, Williams R, Benz BW, Peterson AT (2006) Global invasive potential of 10 parasitic witchweeds and related Orobanchaceae. AMBIO 35: 281–288 [DOI] [PubMed] [Google Scholar]
- Mohamed AH, Housley TL, Ejeta G (2010a) Inheritance of hyper sensitive response to Striga parasitism in sorghum Sorghum bicolor (L.) Moench. Afr J Agric Res 5: 2720–2729 [Google Scholar]
- Mohamed AH, Housley TL, Ejeta G (2010b) An in vitro technique for studying specific Striga resistance mechanisms in sorghum. Afr J Agric Res 5: 1868–1875 [Google Scholar]
- Mohammadi G (2019) Can soil microorganisms reduce Broomrape (Orobanche spp.) infestation in cropping systems?Microbiome in Plant Health and Disease. Springer, pp 385–402 [Google Scholar]
- Mohemed N, Charnikhova T, Fradin EF, Rienstra J, Babiker AGT, Bouwmeester HJ (2018) Genetic variation in Sorghum bicolor strigolactones and their role in resistance against Striga hermonthica. J Exp Bot 69: 2415–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moumouni K, Kountche B, Jean M, Hash C, Vigouroux Y, Haussmann B, Belzile F (2015) Construction of a genetic map for pearl millet, Pennisetum glaucum (L.) R. Br., using a genotyping-by-sequencing (GBS) approach. Mol Breed 35: 1–5 [Google Scholar]
- Mounde LG, Boh MY, Cotter M, Rasche F (2015) Potential of rhizobacteria for promoting sorghum growth and suppressing Striga hermonthica development. J Plant Dis Prot 122: 100–106 [Google Scholar]
- Mrema E, Shimelis H, Laing M, Mwadzingeni L (2018) Genetic analysis of the maximum germination distance of Striga under Fusarium oxysporum f. sp strigae biocontrol in sorghum. J Integr Agric 17: 1585–1593 [Google Scholar]
- Mrema E, Shimelis H, Laing M (2020) Combining ability of yield and yield components among Fusarium oxysporum f. sp. Strigae-compatible and Striga-resistant sorghum genotypes. Acta Agric Scand B Soil Plant Sci 70: 95–108 [Google Scholar]
- Müller‐Stöver D, Nybroe O, Baraibar B, Loddo D, Eizenberg H, French K, Sønderskov M, Neve P, Peltzer D, Maczey N (2016) Contribution of the seed microbiome to weed management. Weed Res 56: 335–339 [Google Scholar]
- Murage AW, Obare G, Chianu J, Amudavi DM, Pickett J, Khan ZR (2011) Duration analysis of technology adoption effects of dissemination pathways: a case of ‘push-pull’ technology for control of Striga weeds and stemborers in Western Kenya. Crop Prot 30: 531–538 [Google Scholar]
- Muranaka S, Fatokun C, Boukar O (2011) Stability of Striga gesnerioides resistance mechanism in cowpea under high-infestation level, low soil fertility and drought stresses. J Food Agric Environ 9: 313–318 [Google Scholar]
- Mutinda SM, Masanga J,, Mutuku JM, Runo S, Alakonya A (2018) KSTP 94, an open-pollinated maize variety has postattachment resistance to purple witchweed (Striga hermonthica). Weed Sci 66: 525–529 [Google Scholar]
- Mutuku JM, Yoshida S, Shimizu T, Ichihashi Y, Wakatake T, Takahashi A, Seo M, Shirasu K (2015) The WRKY45-dependent signaling pathway is required for resistance against Striga hermonthica parasitism. Plant Physiol 168: 1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku JM, Cui S, Hori C, Takeda Y, Tobimatsu Y, Nakabayashi R, Mori T, Saito K, Demura T, Umezawa T (2019) The structural integrity of lignin is crucial for resistance against Striga hermonthica parasitism in rice. Plant Physiol 179: 1796–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwakaboko AS, Zwanenburg B (2011) Single step synthesis of strigolactone analogues from cyclic keto enols, germination stimulants for seeds of parasitic weeds. Bioorg Med Chem 19: 5006–5011 [DOI] [PubMed] [Google Scholar]
- Nakamura H, Hirabayashi K, Miyakawa T, Kikuzato K, Hu W, Xu Y, Jiang K, Takahashi I, Niiyama R, Dohmae N (2019) Triazole ureas covalently bind to strigolactone receptor and antagonize strigolactone responses. Mol Plant 12: 44–58 [DOI] [PubMed] [Google Scholar]
- Ndambi B, Cadisch G, Elzein A, Heller A (2011) Colonization and control of Striga hermonthica by Fusarium oxysporum f. sp strigae, a mycoherbicide component: an anatomical study. Biol Control 58: 149–159 [Google Scholar]
- Ndambi B, Cadisch G, Elzein A, Heller A (2012) Tissue specific reactions of sorghum roots to the mycoherbicide Fusarium oxysporum f. sp strigae versus the pathogenic F. proliferatum. Biocontrol Sci Technol 22: 135–150 [Google Scholar]
- Neondo JO, Alakonya AE, Kasili RW (2017) Screening for potential Striga hermonthica fungal and bacterial biocontrol agents from suppressive soils in Western Kenya. BioControl 62: 705–717 [Google Scholar]
- Nickrent DL, Musselman LJ (2004) Introduction to parasitic flowering plants. Plant Health Instr 13: 300–315 [Google Scholar]
- Nyakurwa CS, Gasura E, Setimela PS, Mabasa S, Rugare JT, Mutsvanga S (2018) Reaction of new quality protein maize genotypes to Striga asiatica. Crop Sci 58: 1201–1218 [Google Scholar]
- Nzioki HS, Oyosi F, Morris CE, Kaya E, Pilgeram AL, Baker CS, Sands DC (2016) Striga biocontrol on a Toothpick: a readily deployable and inexpensive method for smallholder farmers. Front Plant Sci 7: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea F, Georgescu E, Matusova R, Georgescu F, Nicolescu A, Raut I, Jecu M-L, Vladulescu M-C, Vladulescu L, Deleanu C (2017) New strigolactone mimics as exogenous signals for rhizosphere organisms. Molecules 22: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson EW, Timko MP (2020) Race structure of cowpea witchweed (Striga gesnerioides) in West Africa and its implications for Striga resistance breeding of cowpea. Weed Sci 68: 125–133 [Google Scholar]
- Omoigui LO, Kamara AY, Ajeigbe HA, Akinwale RO, Timko MP, Oyekunle M, Bello LL (2017) Performance of cowpea varieties under Striga gesnerioides (Willd.) Vatke infestation using biplot analysis. Euphytica 213: 1–16 [Google Scholar]
- Parker C (2009) Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag Sci 65: 453–459 [DOI] [PubMed] [Google Scholar]
- Parker C (2012) Parasitic weeds: a world challenge. Weed Sci 60: 269–276 [Google Scholar]
- Pennisi E (2010) Armed and dangerous. Science 327: 804–805 [DOI] [PubMed] [Google Scholar]
- Pickett JA, Hamilton ML, Hooper AM, Khan ZR, Midega CA (2010) Companion cropping to manage parasitic plants. Annu Rev Phytopathol 48: 161–177 [DOI] [PubMed] [Google Scholar]
- Pickett JA, Woodcock CM, Midega CA, Khan ZR (2014) Push–pull farming systems. Curr Opin Biotechnol 26: 125–132 [DOI] [PubMed] [Google Scholar]
- Prandi C, McErlean CSP (2019) The chemistry of strigolactones. In Koltai H, Prandi C, eds, Strigolactones—Biology and Applications, Vol 1. Springer International Publishing, Springer Nature Switzerland AG, pp 163–198 [Google Scholar]
- Randrianjafizanaka MT, Autfray P, Andrianaivo AP, Ramonta IR, Rodenburg J (2018) Combined effects of cover crops, mulch, zero-tillage and resistant varieties on Striga asiatica (L.) Kuntze in rice-maize rotation systems. Agric Ecosyst Environ 256: 23–33 [Google Scholar]
- Ransom J, Kanampiu F, Gressel J, De Groote H, Burnet M, Odhiambo G (2012) Herbicide applied to imidazolinone resistant-maize seed as a Striga control option for small-scale African farmers. Weed Sci 60: 283–289 [Google Scholar]
- Rebeka G, Shimelis H, Laing MD, Tongoona P, Mandefro N (2013) Evaluation of sorghum genotypes compatibility with Fusarium oxysporum under Striga infestation. Crop Sci 53: 385–393 [Google Scholar]
- Rodenburg J, Riches CR, Kayeke JM (2010) Addressing current and future problems of parasitic weeds in rice. Crop Prot 29: 210–221 [Google Scholar]
- Rodenburg J, Cissoko M, Kayeke J,, Dieng I, Khan ZR, Midega CAO, Onyuka EA, Scholes JD (2015) Do NERICA rice cultivars express resistance to Striga hermonthica (Del.) Benth and Striga asiatica (L.) Kuntze under field conditions? Field Crops Res 170: 83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg J, Demont M, Zwart SJ, Bastiaans L (2016) Parasitic weed incidence and related economic losses in rice in Africa. Agric Ecosyst Environ 235: 306–317 [Google Scholar]
- Rodenburg J, Cissoko M, Kayongo N, Dieng I, Bisikwa J, Irakiza R, Masoka I, Midega CAO, Scholes JD (2017) Genetic variation and host-parasite specificity of Striga resistance and tolerance in rice: The need for predictive breeding. New Phytol 214: 1267–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg J, Randrianjafizanaka MT, Büchi L, Dieng I, Andrianaivo AP, Ravaomanarivo LHR, Autfray P (2020) Mixed outcomes from conservation practices on soils and Striga-affected yields of a low-input, rice–maize system in Madagascar. Agron Sustain Dev 40: 1–11 [Google Scholar]
- Rubiales D, Fernández-Aparicio M, Vurro M, Eizenberg H (2018) Advances in parasitic weed research. Front Plant Sci 9: 236–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima H, Babiker AG, Mustafa A, Sugimoto Y (2016a) Identification of Striga hermonthica-resistant upland rice varieties in Sudan and their resistance phenotypes. Front Plant Sci 7: 634–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima H, Babiker AG, Takikawa H, Sasaki M, Sugimoto Y (2016b) Practicality of the suicidal germination approach for controlling Striga hermonthica. Pest Manag Sci 72: 2035–2042 [DOI] [PubMed] [Google Scholar]
- Samejima H, Sugimoto Y (2018) Recent research progress in combatting root parasitic weeds. Biotechnol Biotechnol Equip 32: 221–240 [Google Scholar]
- Satish K, Gutema Z, Grenier C, Rich PJ, Ejeta G (2012) Molecular tagging and validation of microsatellite markers linked to the low germination stimulant gene (lgs) for Striga resistance in sorghum Sorghum bicolor (L.) Moench. Theor Appl Genet 124: 989–1003 [DOI] [PubMed] [Google Scholar]
- Sattler FT, Sanogo MD, Kassari IA, Angarawai II, Gwadi KW, Dodo H, Haussmann BIG (2018) Characterization of West and Central African accessions from a pearl millet reference collection for agro-morphological traits and Striga resistance. Plant Genet Resour 16: 260–272 [Google Scholar]
- Schaub B, Marley P, Elzein A, Kroschel J (2006) Field evaluation of an integrated Striga hermonthica management in Sub-Saharan Africa: synergy between Striga-mycoherbicides (biocontrol) and sorghum and maize resistant varieties. J Plant Dis Prot 20: 691–699 [Google Scholar]
- Scholes JD, Press MC (2008) Striga infestation of cereal crops—an unsolved problem in resource limited agriculture. Curr Opin Plant Biol 11: 180–186 [DOI] [PubMed] [Google Scholar]
- Screpanti C, Fonné-Pfister R, Lumbroso A, Rendine S, Lachia M, De Mesmaeker A (2016) Strigolactone derivatives for potential crop enhancement applications. Bioorg Med Chem Lett 26: 2392–2400 [DOI] [PubMed] [Google Scholar]
- Shayanowako AI, Shimelis H, Laing MD, Mwadzingeni L (2020) Striga resistance and compatibility of maize genotypes to a biocontrol agent, Fusarium oxysporum f. sp. strigae. J Crop Improv 1: 1–18 [Google Scholar]
- Shen H, Ye W, Hong L, Huang H, Wang Z, Deng X, Yang Q, Xu Z (2006) Progress in parasitic plant biology: host selection and nutrient transfer. Plant Biol 8: 175–185 [DOI] [PubMed] [Google Scholar]
- Sibhatu B (2016) Review on Striga weed management. Int J Life Sci Scienti Res 2: 110–120 [Google Scholar]
- Su C, Liu H, Wafula EK, Honaas L, de Pamphilis CW, Timko MP (2020) SHR4z, a novel decoy effector from the haustorium of the parasitic weed Striga gesnerioides, suppresses host plant immunity. New Phytol 226: 891–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbrick P, Huang K, Liu G, Slate J, Press M, Scholes J (2008) Global patterns of gene expression in rice cultivars undergoing a susceptible or resistant interaction with the parasitic plant Striga hermonthica. New Phytol 179: 515–529 [DOI] [PubMed] [Google Scholar]
- Swarbrick PJ, Scholes JD, Press MC, Slate J (2009) A major QTL for resistance of rice to the parasitic plant Striga hermonthica is not dependent on genetic background. Pest Manag Sci 65: 528–532 [DOI] [PubMed] [Google Scholar]
- Tadesse F (2018) Effect of Striga trap crops and nitrogen fertilizer application on yield and yield related traits of Sorghum [Sorghum bicolor (L.) Moench] at Fedis District, Eastern Ethiopia. Open Access Lib J 5: 1–17 [Google Scholar]
- Takahashi I, Fukui K, Asami T (2016) Chemical modification of a phenoxyfuranone-type strigolactone mimic for selective effects on rice tillering or Striga hermonthica seed germination. Pest Manag Sci 72: 2048–2053 [DOI] [PubMed] [Google Scholar]
- Tank DC, Beardsley PM, Kelchner SA, Olmstead RG. ( 2006) Review of the systematics of Scrophulariaceae s.l. and their current disposition. Aust Syst Bot 19: 289–307 [Google Scholar]
- Tignegre JBS, Ouedraogo JT, Melis R, Tongoona P, Sibiya J, Makanda I, Drabo I (2013) Identification of new sources of resistance to Striga gesnerioides in cowpea germplasm. Plant Breed 132: 330–336 [Google Scholar]
- Timko MP, Singh B (2008) Cowpea, a multifunctional legume. Genomics of Tropical Crop Plants. Springer, New York, USA, pp 227–258 [Google Scholar]
- Timko MP, Huang K, Lis KE (2012) Host resistance and parasite virulence in Striga–host plant interactions: a shifting balance of power. Weed Sci 60: 307–315 [Google Scholar]
- Toh S, Holbrook-Smith D, Stokes ME, Tsuchiya Y, McCourt P (2014) Detection of parasitic plant suicide germination compounds using a high-throughput Arabidopsis HTL/KAI2 strigolactone perception system. Chem Biol 21: 988–998 [DOI] [PubMed] [Google Scholar]
- Toh S, Holbrook-Smith D, Stogios PJ, Onopriyenko O, Lumba S, Tsuchiya Y, Savchenko A, McCourt P (2015) Structure-function analysis identifies highly sensitive strigolactone receptors in Striga. Science 350: 203–207 [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Yoshimura M, Hagihara S (2018) The dynamics of strigolactone perception in Striga hermonthica: a working hypothesis. J Exp Bot 69: 2281–2290 [DOI] [PubMed] [Google Scholar]
- Tyc O, Song C, Dickschat JS, Vos M, Garbeva P (2017) The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol 25: 280–292 [DOI] [PubMed] [Google Scholar]
- Uraguchi D, Kuwata K, Hijikata Y, Yamaguchi R, Imaizumi H, Sathiyanarayanan AM, Rakers C, Mori N, Akiyama K, Irle S, et al. (2018) A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica. Science 362: 1301–1305 [DOI] [PubMed] [Google Scholar]
- Van Mourik TA, Stomph TJ, Murdoch AJ (2011) Purple witchweed (Striga hermonthica) germination and seedbank depletion under different crops, fallow, and bare soil. Weed Biol Manag 11: 100–110 [Google Scholar]
- Vurro M, Boari A, Evidente A, Andolfi A, Zermane N (2009) Natural metabolites for parasitic weed management. Pest Manag Sci 65: 566–571 [DOI] [PubMed] [Google Scholar]
- Vurro M, Prandi C, Baroccio F (2016) Strigolactones: how far is their commercial use for agricultural purposes? Pest Manag Sci 72: 2026–2034 [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Hamana M, Mori A, Akiyama R, Ueno K, Osakabe K, Osakabe Y, Suzuki H, Takikawa H, Mizutani M (2019) Direct conversion of carlactonoic acid to orobanchol by cytochrome P450 CYP722C in strigolactone biosynthesis. Sci Adv 5: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T, Shida K, Kitano Y, Takikawa H, Mizutani M, Sugimoto Y (2020) CYP722C from Gossypium arboreum catalyzes the conversion of carlactonoic acid to 5-deoxystrigol. Planta 251: 1–6 [DOI] [PubMed] [Google Scholar]
- Wang JY, Haider I, Jamil M, Fiorilli V, Saito Y, Mi J, Baz L, Kountche BA, Jia K-P, Guo X (2019) The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nat Commun 10: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Jamil M, Lin P-Y, Ota T, Fiorilli V, Novero M, Zarban RA, Kountche BA, Takahashi I, Martínez C (2020) Efficient mimics for elucidating zaxinone biology and promoting agricultural applications. Mol Plant 13: 1654–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Scaffidi A, Sun YK, Flematti GR, Smith SM (2014) The karrikin response system of Arabidopsis. Plant J 79: 623–631 [DOI] [PubMed] [Google Scholar]
- Watson AK (2013) Biocontrol. In Joel Daniel M., Gressel Jonathan, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies, Vol 1. Springer, New York, pp 469–497 [Google Scholar]
- Wilson J, Hess D, Hanna W (2000) Resistance to Striga hermonthica in wild accessions of the primary gene pool of Pennisetum glaucum. Phytopathology 90: 1169–1172 [DOI] [PubMed] [Google Scholar]
- Wilson J, Hess D, Hanna W, Kumar K, Gupta S (2004) Pennisetum glaucum subsp. monodii accessions with Striga resistance in West Africa. Crop Prot 23: 865–870 [Google Scholar]
- Xiang HB, Yao RF, Quan TF, Wang F, Chen L, Du XX, Zhang WH, Deng HT, Xie DX, Luo TP (2017) Simple beta-lactones are potent irreversible antagonists for strigolactone receptors. Cell Res 27: 1525–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XN, Yoneyama K, Yoneyama K (2010) The Strigolactone story. Annu Rev Phytopathol 48: 93–117 [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y (2010) Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol 51: 1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Arakawa R, Ishimoto K, Kim HI, Kisugi T, Xie X, Nomura T, Kanampiu F, Yokota T, Ezawa T (2015) Difference in Striga‐susceptibility is reflected in strigolactone secretion profile, but not in compatibility and host preference in arbuscular mycorrhizal symbiosis in two maize cultivars. New Phytol 206: 983–989 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shirasu K (2012) Plants that attack plants: molecular elucidation of plant parasitism. Curr Opin Plant Biol 15: 708–713 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Cui S, Ichihashi Y, Shirasu K (2016) The haustorium, a specialized invasive organ in parasitic plants. Annu Rev Plant Biol 67: 643–667 [DOI] [PubMed] [Google Scholar]
- Zarafi A, Elzein A, Abdulkadir D, Beed F, Akinola O (2015) Host range studies of Fusarium oxysporum f. sp. strigae meant for the biological control of Striga hermonthica on maize and sorghum. Arch Phytopathol Plant Protect 48: 1–9 [Google Scholar]
- Zheng X, Giuliano G, Al-Babili S (2020) Carotenoid biofortification in crop plants: citius, altius, fortius. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biol Lipids 1865: 1–17 [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Musyoki MK, Cadisch G, Rasche F (2016) Biocontrol agent Fusarium oxysporum f.sp Strigae has no adverse effect on indigenous total fungal communities and specific AMF taxa in contrasting maize rhizospheres. Fungal Ecol 23: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanenburg B, Mwakaboko AS (2011) Strigolactone analogues and mimics derived from phthalimide, saccharine, p-tolylmalondialdehyde, benzoic and salicylic acid as scaffolds. Bioorg Med Chem 19: 7394–7400 [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Nayak SK, Charnikhova TV, Bouwmeester HJ (2013) New strigolactone mimics: structure-activity relationship and mode of action as germinating stimulants for parasitic weeds. Bioorg Med Chem Lett 23: 5182–5186 [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Mwakaboko AS, Kannan C (2016) Suicidal germination for parasitic weed control. Pest Manag Sci 72: 2016–2025 [DOI] [PubMed] [Google Scholar]