Abstract
Parasitic plants are mostly viewed as pests. This is caused by several species causing serious damage to agriculture and forestry. There is however much more to parasitic plants than presumed weeds. Many parasitic plans exert even positive effects on natural ecosystems and human society, which we review in this paper. Plant parasitism generally reduces the growth and fitness of the hosts. The network created by a parasitic plant attached to multiple host plant individuals may however trigger transferring systemic signals among these. Parasitic plants have repeatedly been documented to play the role of keystone species in the ecosystems. Harmful effects on community dominants, including invasive species, may facilitate species coexistence and thus increase biodiversity. Many parasitic plants enhance nutrient cycling and provide resources to other organisms like herbivores or pollinators, which contributes to facilitation cascades in the ecosystems. There is also a long tradition of human use of parasitic plants for medicinal and cultural purposes worldwide. Few species provide edible fruits. Several parasitic plants are even cultivated by agriculture/forestry for efficient harvesting of their products. Horticultural use of some parasitic plant species has also been considered. While providing multiple benefits, parasitic plants should always be used with care. In particular, parasitic plant species should not be cultivated outside their native geographical range to avoid the risk of their uncontrolled spread and the resulting damage to ecosystems.
Advances
Parasitic plants may act as highways for transferring systemic signals among host plants.
Harmful effects of parasitic plants on individual hosts suppress community dominants including invasive species, reduce competitive pressure, and may increase biodiversity.
Parasitic plants enhance nutrient cycling and provide resources for other organisms thus contributing to facilitation cascades in ecosystems.
Many parasitic plants are recorded to have medicinal values against a broad range of diseases.
There is a long tradition of worldwide human use of parasitic plants, which have been cultivated for their products and aesthetic values.
Introduction
Most plants are photoautotrophic organisms, which need only fundamental abiotic resources for their essential vital processes. An exception to this is parasitic plants, which acquire resources by parasitizing other plants via a specialized organ called the haustorium. Developed as root or stem modifications, haustoria secure unidirectional connections between the vascular systems of host and parasite, enabling resource flow and freeing parasitic plants from many constraints to growth. While parasitism necessarily confers benefits to the parasites, it usually exerts moderate to strong negative effects on host growth and/or reproductive output. Several species of parasitic plants attack agricultural crops or trees used for production forestry. The most important of these are Striga hermonthica attacking cereals like sorghum (Sorghum bicolor), maize (Zea mays), and finger millet (Pennisetum glaucum; Parker, 2013), Phelipanche ramosa attacking a range of crops like tomato (Solanum lycopersicon), tobacco (Nicotiana tabacum), pea (Pisum sativum), lentil (Lens culinaris), carrot (Daucus carota), and sunflower (Helianthus annuus; Parker, 2013), and Cuscuta campestris infesting alfalfa (Medicago sativa), faba bean (Vicia faba), and sugar beet (Beta vulgaris; Parker, 2012). Forestry pests include e.g. Arceuthobium spp. attacking conifers in North America (Hawksworth and Wiens, 1996) and Seymeria cassioides damaging pine plantations (e.g. Pinus clausa, Pinus elliottii, P. strobus) in south-eastern USA (Musselman and Mann, 1978). These weedy species characterize the predominantly negative sentiment toward parasitic plants by the general public and agricultural sector (Pennisi, 2010), exacerbated by the general archetypal attitude toward parasites.
There is, however, much more to parasitic plants than presumed weeds. With ca. 4,500 species in total, parasitic plants represent a specialized yet heterogeneous functional group. Individual species differ in their trophic modes, associated functional traits, and phylogenetic origins (Westwood et al., 2010; Heide-Jørgensen, 2013; Těšitel, 2016). Parasitic plants are a ubiquitous component of terrestrial ecosystems worldwide, though their species richness and frequency varies between biomes and individual habitats (Heide-Jørgensen, 2008; Těšitel et al., 2015; Těšitel, 2016). Their ecological roles are also more complex than just negative effects on the host. Numerous parasitic plant species have been demonstrated to exert multifaceted ecological effects associated with altering competitive relations in the community and release of nutrient-rich litter facilitating nutrient cycling in ecosystems (Press and Phoenix, 2005; March and Watson, 2007; Watson, 2009; Demey et al., 2015; Watson, 2016; Těšitel et al., 2018). A number of species also provide resources for animals including food for herbivores, frugivores or pollinators, and shelter or nesting opportunities. These effects may trigger species composition change (Watson et al., 2011; Hartley et al., 2015) and, eventually, an increase of community diversity (Westbury et al., 2006; Watson and Herring, 2012; Fibich et al., 2017). Parasitic plants have also been used by humans for centuries either whole or as products originating from them. Interestingly, in the first systematic treatment of biology of parasitic plants, Job Kuijt (1969) included a section on “The uses of parasitic plants”, highlighting the many contributions of parasitic plants to human societies.
In this paper, we review the positive effects of parasitic plants and highlight recent advances on this topic. At the organismal level, we consider cases where parasitic plants positively influence host plants or nonhost organisms in terms of their growth, reproductive output or indirectly via modified ecological interactions. We also discuss positive effects on community structure and ecosystem processes. The second half of the paper is dedicated to the benefits to human society provided by parasitic plants. This includes ecosystem effects facilitating crop production and its sustainability, and direct use of parasitic plants as technical crops, food or medicinal resources. Finally, we discuss the use of parasitic plants in horticulture for aesthetic and cultural purposes.
Biology and ecology of positive effects of parasitic plants
Some aspects of parasitism may be positive even for the host
Parasitic plants typically exert negative effects on physiological processes and consequently the fitness of their hosts. However, several cases of positive effects on some host processes have been identified even within the intimate host–parasite interaction. In some root–holoparasitic associations, infected hosts may display higher rates of photosynthesis and nutrient uptake capacity compared to uninfected plants (Hibberd et al., 1998; Irving and Cameron, 2009). Such positive effect on photosynthesis is nevertheless not reflected by host growth although transient increase of host relative growth rate has also been documented (Dale and Press, 1998).
Many parasitic plants parasitize multiple hosts simultaneously; thus, they may serve as a common network connecting multiple individuals in a plant community. This function may be somewhat similar to that of hyphal networks of mycorrhizal fungi, which have been shown to convey systemic signals that facilitate community functions such as adaptation to biotic or abiotic stresses (Barto et al., 2012). Recently, these functions have been demonstrated for a stem-parasitic Cuscuta australis, which was shown to transfer systemic signals between connected hosts, including warning signals against insect herbivores (Hettenhausen et al., 2017) and salt stress (Li et al., 2020). The warning signals formed by one host plant in response to insect attack or salt stress primed the unattacked/unstressed hosts connected by the Cuscuta bridge against subsequent stress induced under greenhouse conditions (Hettenhausen et al., 2017; Li et al., 2020). The transfer of systemic warning signals was shown to be fast (ca. 1 cm·min-1) and far-reaching (> 100 cm), and could occur among conspecific or heterospecific hosts from different families (Hettenhausen et al., 2017). Since plant vasculatures were shown to transmit many types of systemic signals induced by various biotic (Fu and Dong, 2013) and abiotic stresses (Schachtman and Goodger, 2008; Chiou and Lin, 2011), vascular connections in plant clusters via Cuscuta bridging may facilitate inter-plant communication of multiple stress-induced signals. By providing information-based benefits, the Cuscuta vines might alleviate fitness costs of parasitism to their hosts to some extent (Hettenhausen et al., 2017). At present, the nature of the mobile warning signals and the degree of host and Cuscuta control of their delivery remain unknown, as well as the ecological significance of the network in natural environments. Nevertheless, inter-plant communication of warning signals and extensive inter-plant protein transfer with retained biological activities (Liu et al., 2020) in plant clusters connected by Cuscuta suggest important roles of parasitic vines in reshaping multitrophic interactions in the infested community.
Harmful effects on host plants may increase community diversity and suppress plant invasions
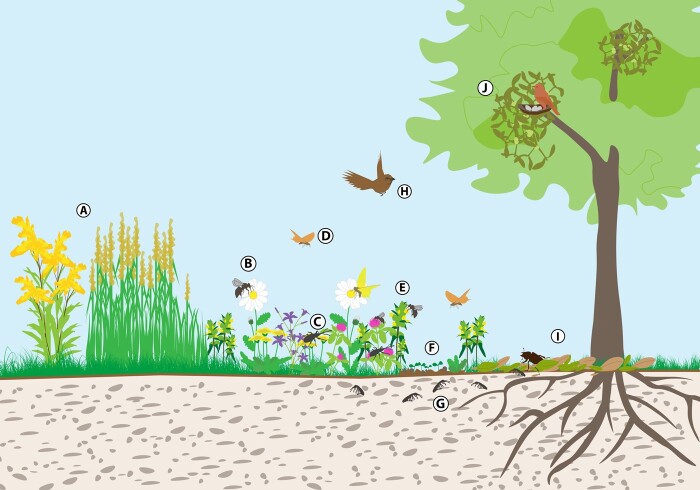
The effects of parasitic plants at the community scale strongly differ between hosts and non-host plants. The presence of parasitic plants in the community generally alters the competitive balance and influences community structure. Typically, host species are reduced and the free space and resources may be exploited by species resistant or tolerant to parasitism (Marvier, 1998; Joshi et al., 2000; Cameron et al., 2005; Mudrák and Lepš, 2010). Many aspects of plant communities, including diversity or reproduction opportunities, are frequently limited by strong dominance of a single or few species of high competitive ability. In herbaceous vegetation, this applies in particular to communities of high primary productivity (Grime, 1973; Fraser et al., 2015). Parasitic plants were repeatedly demonstrated to reduce growth and competitive ability of dominant species, reduce community productivity and facilitate regeneration from seeds by opening gaps for seedling establishment (Davies et al., 1997; Westbury et al., 2006; Demey et al., 2014; Těšitel et al., 2017). Thus, the general negative effect parasitic plants exert on their hosts may be transformed into positive effects on larger plant community scales (Figure 1).
Figure 1.
Illustration of functional roles of most significant parasitic plant groups (mistletoes and root hemiparasites) in ecosystems. A, Competitive alien invasive species and native invaders thriving in the absence of parasitic plants. B, Change in the plant community, increasing biodiversity. C, Diversified food supply for herbivores. D, Increased diversity and abundance of pollinators (e.g. Hymenoptera, Lepidoptera, Diptera). E, Root hemiparasite as ecosystem engineer and food opportunity for pollinators and herbivores. F, Sward gap for seedlings establishment. G, Increased soil organic matter and edafauna (Collembola, microorganisms). H, Increased food opportunity for seed herbivores and predators, seed dispersion by herbivores. I, Increased nutrient-rich litter, decompositors, and other animals living in litter. J, Mistletoe – opportunity for nesting, food, shelter.
Empirical evidence of such positive community effects has accumulated mostly for root-hemiparasitic plants over the past ca. 20 years. A series of studies demonstrated the positive effects of root-hemiparasitic Rhinanthus spp. on diversity of European grasslands (Davies et al., 1997; Bardgett et al., 2006; Westbury et al., 2006; Hartley et al., 2015; Fibich et al., 2017; Box 1). Positive effects on diversity based on competitive dominant suppression were also observed in Pedicularis palustris (Decleer et al., 2013) in European wetlands. Facilitation of reproduction of several co-occurring species by improved seedling establishment was demonstrated in a study with hemiparasitic Rhinanthus minor and Pedicularis sylvatica (Demey et al., 2014). In North American prairies, the presence of two hemiparasitic species Comandra umbellata and Pedicularis canadensis was found to be positively correlated with community diversity (Sivicek and Taft, 2011), though subsequent experimental research on the ecology of P. canadensis identified rather complex effects of this species on diversity (Walder et al., 2019; Borowicz et al., 2019).
Box 1. Root-hemiparasitic Rhinanthus species in ecological restoration.
Rhinanthus species are annual root-hemiparasitic herbs native to western Eurasia. They occur in various types of grasslands where they have been recognized as species that reduce productivity in traditional hay-making agriculture. Ecological research has demonstrated that this reduction of productivity is associated with the decrease of dominance of grasses in the community (Davies et al., 1997; Hartley et al., 2015). As a result, competitively inferior dicot forbs are released from competition and increase in abundance. In parallel, Rhinanthus spp. were demonstrated to affect soil properties and processes as well as invertebrate community composition (Bardgett et al., 2006; Hartley et al., 2015). In many cases, the presence of Rhinanthus is associated with increased community diversity (Fibich et al., 2017) or abundance of keystone species such as pollinators or invertebrate predators (Hartley et al., 2015). Blossoming forbs and Rhinanthus itself also improve the aesthetic impression of the grassland which contributed to a great popularity of Rhinanthus spp. as biodiversity-promoting ecosystem engineers (see Figure). Most recently, two Rhinanthus species have been demonstrated as efficient in suppressing the native-invader grass Calamagrostis epigejos in Central Europe. Expansion of this grass supported mostly by land-use change has been one of the most severe nature conservation issues in the region due to its extent and impact on biodiversity and inefficiency of conventional counter measures. Sowing experiments with R. alectorolophus and R. major have demonstrated the ability of these two species to suppress C. epigejos (Těšitel et al., 2017; Těšitel et al., 2018). Sowing of Rhinanthus generally triggered restoration of community composition, and in some cases increased plant diversity or abundance of threatened species. Following this experimental evidence and development of seed-production technology, the use of R. alectorolophus has been implemented as a standard measure in nature conservation in the Czech Republic.
An example of a species-rich meadow patch with R. alectorolophus (white arrows) as a result of grassland restoration on ex-arable land.

On top of the positive community effects, several parasitic plants have recently been demonstrated to act as biotic resistance agents against plant invasions (Figure 1). Invasions of alien plants which colonize various natural habitats and suppress native species (Vilà et al., 2011) represent one of the most important threats to biodiversity (Ichii et al., 2019) and compromise human health and food security (Pejchar and Mooney, 2009). Moreover, expansive native species (native invaders) that uncontrollably spread within their natural geographical range and colonize new habitats may negatively impact ecosystems to a similar extent as alien invaders (Nackley et al., 2017). A common feature of invasive plants (alien or native) with strong negative impact on diversity is high biomass production. Growth of these dominant plants may, however, be suppressed by parasitic plants.
A series of recent studies have identified biotic resistance effects (Levine et al., 2004) in several parasitic plants worldwide (reviewed by Těšitel et al., 2020). In particular, three experimental systems studied in sufficient detail demonstrate the suppressive effect of native parasitic plants to alien or native invasive plants. First, the native invader grass Calamagrostis epigejos that threatens grassland diversity in Europe was successfully suppressed by Rhinanthus species to a degree comparable to or stronger than conventional management (mowing, grazing; see Box 1 for details; Těšitel et al., 2017, 2018). Second, Cassytha pubescens, a stem hemiparasite native to Australia, was demonstrated to attack noxious invaders Ulex europaeus and Cytisus scoparius. Infection by Cassytha pubescens reduced its host’s maximum electron transport rate and photosystem II efficiency, and thus induced chronic photoinhibition in infected plants (Shen et al., 2010; Cirocco et al., 2018). The observed reduction of host biomass by C. pubescens was significantly higher in invasive Ulex europaeus and Cytisus scoparius than in native hosts (Cirocco et al., 2016, 2017). A field study also identified significantly elevated mortality of invasive C. scoparius caused by C. pubescens infection in comparison with the native shrub Leptospermum myrsinoides (Prider et al., 2009). The third system comprises species of the genus Cuscuta, which were tested as possible biological control agents for invasive clonal perennials Ipomoea cairica, Mikania micrantha, Wedelia trilobata, Solidago canadensis, Bidens pilosa, and Humulus scandens in China (Yu et al., 2011; Li et al., 2012; Wu et al., 2019). These alien host species were generally demonstrated to be more vulnerable to parasitic infection than the native ones. In one field experiment, both native species relative abundance and community diversity increased after Cuscuta australis established spontaneously (Yu et al., 2011). This empirical evidence demonstrates the potential of parasitic plants to act as biotic resistance agents to plant invasions, though only the Calamagrostis epigejos–Rhinanthus spp. has hitherto been used in nature conservation/restoration practices. However, such applications of parasitic plants should only consider native species in accordance with biotic resistance theory. Thorough testing in natural community settings should be conducted before any application to identify not only the effects on the target invasive plant but also on the rest of the community. This should minimize possible negative collateral effects occasionally associated with the introduction of a parasitic plant (Walder et al., 2019).
Indirect effects of parasitic plants: enhancing ecosystem processes and providing resources for animal communities
The ecological influence of parasitic plants extends beyond individual hosts and plant communities, having a range of effects that resonate across entire ecosystems and involve higher trophic levels than primary producers (Figure 1; Press and Phoenix, 2005; Watson, 2009). In addition to direct effects mediated by altering competitive dominance of principal hosts discussed in the previous section, indirect effects arise from nutrient reallocation, driven by high rates of enriched litter-fall. Parasitic plants have high fractions of mineral nutrients in their tissues (Quested et al., 2003a; Press and Phoenix, 2005; Gebauer et al., 2012; Fisher et al., 2013; Demey et al., 2014). These nutrients are characteristically not reabsorbed prior to abscission. While most of these nutrients become available to infected hosts (Fisher et al., 2013), they are also accessible to adjacent, uninfected plants. Thus, growth rates of plants growing beneath or beside infected hosts are elevated, a pattern especially prominent in annual and relatively fast-growing plants (Quested et al., 2003b; Watson et al., 2011; Demey et al., 2013). The litter pathway may increase productivity and eventually increase diversity especially in nutrient-poor ecosystems (Spasojevic and Suding, 2011; Griebel et al., 2017), an effect which may extend over several trophic levels of the ecosystem (Watson et al., 2011; Griebel et al., 2017). However, such reallocation may also promote the growth of invasive species (March and Watson, 2010). Some research indicates similar patterns below ground mediated via mycorrhizal networks of fungi that favor infected plants (Mueller and Gehring, 2006). In addition to increased diversity, trees infected with dwarf mistletoes (Arceuthobium spp.) were characterized by greater fungal biomass in their root zone, with consequent effects on decomposition and soil structure (Cullings et al., 2005).
Most parasitic plants rely on animals as vectors, thus providing food resources like nectar for pollinators, or fleshy fruit for seed dispersers (Watson, 2001; Bellot and Renner, 2013). In grasslands, the effects of parasitic plants also tend to increase the abundance ratio between insect- and wind-pollinated plants species (Figure 1; Marvier, 1998; Ameloot et al., 2005; Westbury et al., 2006; Bao et al., 2015; Demey et al., 2015). This further enhances food resources for pollinators, but may have a positive cascade effect on the whole invertebrate community (Hartley et al., 2015). In addition, nutrients are imported by visiting pollinators and seed dispersers. For instance, trees infected with mistletoes are visited by more birds which deposit more excreta beneath them (Mellado and Zamora, 2017). Further, many parasitic plants are highly favored food plants for herbivores, especially insects and mammals which may travel long distances in search of their leaves (Petrović, 2014). Given the aggregated distribution that characterizes many parasitic plants, this leads to increased heterogeneity of nutrient availability, with nutrients becoming concentrated beneath heavily infected hosts. Rather than simply reallocation from the host plants themselves, this is also driven by small- to medium-scale nutrient subsidies (Watson, 2016). Along with nutrients, seeds of other plants are brought in (Mellado and Zamora, 2016), resulting in forests with mistletoes progressively becoming more dominated by other plants with fleshy fruits, and in turn, increasing resource availability and dispersal effectiveness for shared seed vectors (van Ommeren and Whitham 2002; Carlo and Aukema 2005).
In addition to affecting the structure and function of plant communities, the combined effect of these direct and indirect effects (known as a facilitation cascade) can alter overall ecosystem structure (Watson, 2016; Thomsen et al., 2018). Given the extended phenology of many parasitic plants, nectar and fruits are available for longer periods of time, allowing populations of pollinators and seed dispersers to become resident (Fonturbel, 2020, and references therein). This is especially the case in lower productivity systems, such as cool temperate forests and arid ecosystems, where root hemiparasites and mistletoe can frequently be the most reliable sources of nectar and fruit (Meidell, 1944; Simpson et al., 1977; Napier et al., 2013).
Human use of parasitic plants and their products
Parasitic plants have long been exploited for human use, including for food, medicine and cultural purposes in human societies worldwide (Kuijt, 1969; Brand-Miller and Holt, 1998; Büssing, 2004). Australia’s first nation people, for example, have sustained their well-being for some 50–65,000 years on a diet rich in indigenous flora—including a wide range of parasitic plants, primarily from the Santalaceae, Loranthaceae and Lauraceae families (Brand-Miller and Holt 1998; Clarke, 2008). A very different example is ecotourism recently developed around prominent Rafflesia flowers, which contributes substantially to the economies of several South-East Asian regions (Barcelona et al., 2009). The cultural value of parasitic plants is also well known in Europe, where religious use of the mistletoe Viscum album has a long history dating back to ancient Greece and the Celtic period (Büssing, 2004). Viscum album also played an important role in the Roman legend of Aeneas. The origin of the modern mistletoe tradition relates to Celtic pagan rites of the winter solstice (Paine and Harrison, 2018). Christianity later incorporated it into Christmas celebrations as a symbol of love and protection from evil spirits.
Parasitic plants are widely used in both folk and modern medicines
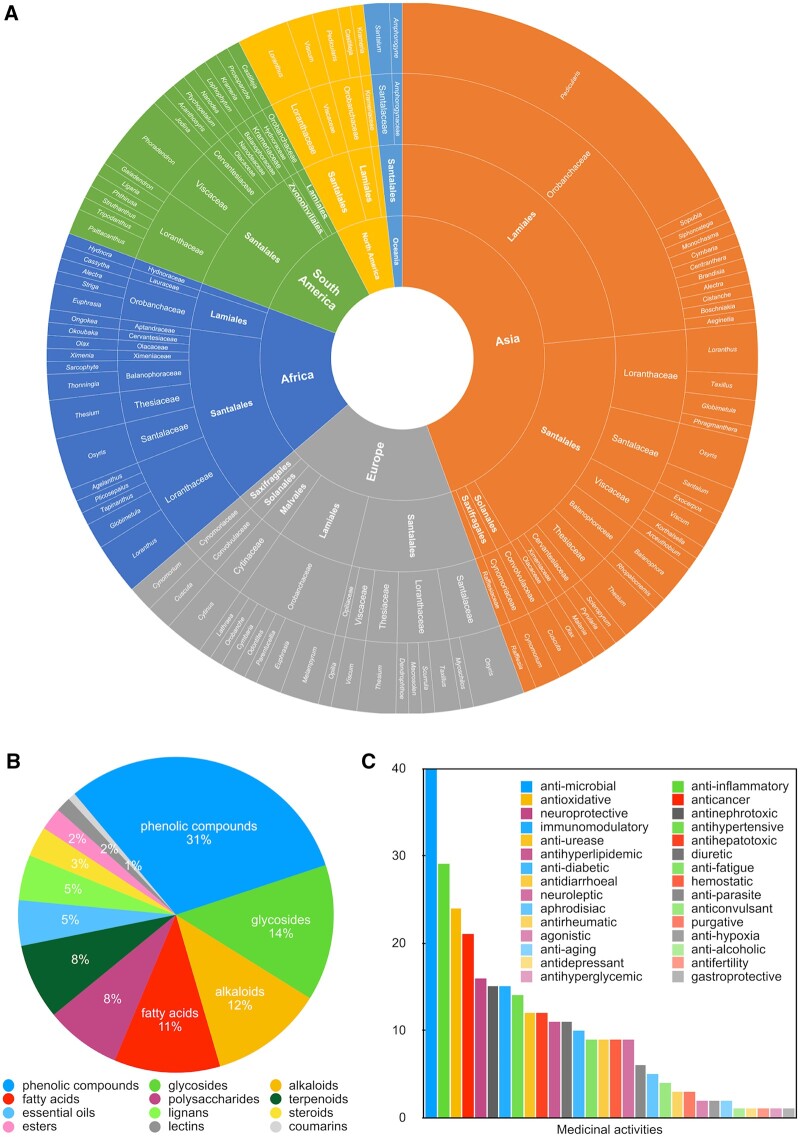
A great proportion of parasitic plant taxa have been used as folk medicines in various regions around the world, particularly Asia, Europe, and Africa (Figure 2). Parasitic plants from the families Orobanchaceae and Loranthaceae are the most reported medicinal plants, with 52 (from 19 genera) and 22 (from 14 genera) experimentally studied species, respectively. Phenolic compounds (particularly flavonoids), glycosides, alkaloids, and fatty acids are the most frequently reported bioactive phytochemical ingredients in parasitic plants (Figure 2). Compounds from parasitic plants have a wide range of medicinal activities, with antimicrobial (Koch et al., 2009; Moghadamtousi et al., 2014; Chabra et al., 2019), anti-inflammatory (Carrillo-Ocampo et al., 2013; Kim et al., 2016; Park et al., 2018), antioxidative (Cui et al., 2013; Fu et al., 2018), anticancer (Kienle et al., 2009; Alonso-Castro et al., 2012), and neuroprotective properties (Moghadamtousi et al., 2014; Li et al., 2016) being the most frequently reported effects (Figure 2).
Figure 2.
Representative taxa, active compounds, and pharmacological activities of medicinal parasitic plants. A, representative parasitic plant taxa used in various continents. B, primary phytochemical ingredients reported in medicinal parasitic plants. C, medicinal activities reported for parasitic plants.
Most medicinal parasitic plants are used in regions where they are indigenous. For example, Cistanche deserticola (Orobanchaceae), a root holoparasitic herb with a high content of phenylethanoid glycosides, has been used as an anti-aging medicinal plant in China for more than 1,800 years to improve the immune system, enhance kidney and skeletal function, and balance hormones for both men and women (Li et al., 2016; Fu et al., 2018). The mistletoe V. album (Viscaceae), a stem hemiparasitic shrub rich in mistletoe lectins and widely distributed in Europe, is a common complementary medicine across Europe for treating breast and gynecological cancers (Kienle et al., 2009). Cuscuta (Convolvulaceae) species are used as anti-aging, anti-inflammatory, pain relieving, immune stimulatory, and aphrodisiac agents in traditional medicines to treat a wide range of diseases in Asia (Noureen et al., 2019). Krameria triandra (Krameriaceae) root extracts have strong anti-inflammatory effects and photoprotective potential. It is used in European and Euro-American traditional medicines to treat intestinal swelling and skin photodamage (Simpson, 1991; Carini et al., 2002). Hydnora abyssinica (Hydnoraceae) rhizomes are used in South African folk medicines against diarrhea, menstrual problems, stomach cramps and intestinal ailments (Williams et al., 2011)
The medicinal properties of many parasitic plants have been validated by phytochemical and pharmacological studies, but knowledge gaps still persist. First, the therapeutic effects of some parasitic plants are still in the exploration stage (Lim et al., 2016). Clinical trials need to be undertaken and mechanisms associated with biological activities of the plants need to be unraveled before their utilization as a source of pharmacological drugs. Second, many medicinal parasitic plants are used as crude extracts with less effective and nonstandard preparation. Improper preparation of the medicinal plants may result in toxic effects (Ojewole and Adewole, 2007), calling for standardization in drug preparation. Third, lack of knowledge about host dependency and host influence on the phytochemistry and toxicological profile of medicinal parasites may lead to suboptimal quality in terms of medicinal effects (Zorofchian Moghadamtousi et al., 2013), or even fatal consequences (Cheung et al., 2018). Further investigations of variation in phytochemistry and toxicological profiles of the medicinal parasites parasitizing different hosts are thus necessary to appraise their medicinal values.
The genus Santalum: parasitic plants exploited and cultivated for their products
Small root-parasitic trees or shrubs of the genus Santalum have provided a range of plant products widely used in medicine, cosmetics, and as food on the global scale. A whole series of Santalum species is exploited by humans, with S. album and S. spicatum in particular harvested by means of industrialized production in the tropical and subtropical regions of South Asia and Australia, respectively. (Kuijt, 1969; Venkatesha Gowda, 2011; Teixeira da Silva et al., 2016). Large quantities of S. album are currently produced in cultivated plantations in South East Asia and northern Australia. Like virtually all of the world’s fragrant sandalwoods, S. album has been heavily exploited across its natural range for many years and is now listed as “vulnerable” on the International Union for Conservation of Nature (IUCN) Red List (Arunkumar et al., 2019; IUCN, 2020).
The fragrant S. album wood is extensively used to make precious items such as jewelry, jewel cases, boxes, cabinet panels, picture frames, hand fans, pen holders, combs, and letter openers. These items, as well as carvings of gods and mythological figures, command high prices in local and international markets. Nevertheless, the principal S. album product is the valuable aromatic sandalwood oil which is extracted by steam distillation from the heartwood of S. album, the only part of the tree carrying the fragrance (Srinivasan et al., 1992). The oil is a pale yellow, somewhat viscous liquid consisting almost entirely of closely related sesquiterpenoids, particularly alpha-santalol (which comprises approximately 7%–60% of total santalol), and beta-santalol (comprising approximately 7%–33%; Lawrence, 1991). Sandalwood oil is an essential component of many perfumes as it is not only aromatic in itself, but also helps to carry the fragrance of other flowers and herbs (Kumar et al., 2012). The oil is also used in medicine to treat a range of ailments including the common cold, bronchitis, fever, dysentery, piles, scabies, urinary infections, and several other organ complications (Ochi et al., 2005). Research has identified a range of properties of sandalwood oil and its constituents behind these uses, including anti-fungal (Warnke et al., 2009), antiviral against drug-resistant herpes simplex virus (Schnitzler et al., 2007), anti-carcinogenic (Burdock and Carabin, 2008), and anti-influenza HK (H3N2) anti-viral capacities (Paulpandi et al., 2012). S. album oil was also recorded as helping to address pulse rate issues, skin conductance, and cytosolic blood pressure (Heubeger et al., 2006). S. album exploitation and use is part of a long and rich cultural heritage that dates back some 5,000 years and is even mentioned in the ancient Sanskrit manuscripts (Flansda, 2009). Ancient Egyptians are known to have imported the wood for medicinal use, as well as for use in preservation, and for ritual burning to worship their gods (Arctander, 1960). The oil and wood of S. album (and S. spicatum) are used for religious purposes in three of the world’s major religions: Hinduism, Buddhism, and Islam (Kumar et al., 2012). Hindu people believe the goddess Lakshmi resides in the S. album tree (Sensarma, 1989) and use the wood to worship the god Shiva. In Buddhist rituals, the fragrant sandalwood is burnt during worshipping and meditation, and even the sapwood is used––for cremations, and for making offerings at temples.
Several other Santalum species are used in Australia. Santalum acuminatum, known as “Quandong”, is a small hemiparasitic tree that grows throughout southern Australia. It bears large, nutritious, bright-red drupes when ripe, which are considered pre-eminent among Aboriginal peoples’ “bush-foods” (Low, 1988; Pardoe et al., 2019). Referred to as a “super food”, it has been treasured, traded, and transported for millennia (Fuentes-Cross, 2015; Lullfitz, 2017). Rich in energy, protein, fat, minerals, and vitamins (it has twice the level of Vitamin C than oranges), Quandong fruit is considered a staple for Aboriginal people, and is eaten raw or roasted, or rolled into cakes or balls before being dried and stored for later consumption (Meagher, 1974; Brand-Miller and Holt, 1998; Newton, 2016). Partly due to its rich antioxidant and antibacterial characteristics (Zhao and Agboola, 2007), S. acuminatum has also been used for a range of medicinal purposes, with its fruit, kernel, leaves, roots, and bark all applied to treat a variety of health issues from skin disorders and venereal disease, to rheumatism and muscular complaints. A number of other Australian Santalum species have been used as indigenous bushfood or bush-medicine (Karadada, 2011, Fuentes-Cross, 2015). Australian Sandalwood (Santalum spicatum) has predominantly been used for medicinal and spiritual purposes, with its oil-rich seeds (as well as its leaves, branches and trunks) being applied to a variety of medicinal ailments (including the treatment of skin afflictions, cuts, infections, sores, burns, colds, and stiffness), as well as being burned in ceremonies or for individual’s spiritual well-being (Meagher, 1974). Its timber, rich in sandalwood oil, is still being exploited in the wild and currently meets about 40% of the world’s sandalwood timber demand (Tonts and Selwood, 2003). Northern Sandalwood (Santalum lanceolatum) and Bitter Quandong (Santalum murrayanum) have also been used for a variety of purposes (Maiden, 1889; Low, 1988). Other parasitic plants used for bush food and bush medicine by Australia’s Indigenous people include species of the genera Exocarpos, Leptomeria, Amyema, Lysiana, Cassytha, and Nuytsia (Maiden, 1889; Meagher, 1974; Low, 1988). Newton (2016) refers to them as “the oldest foods on Earth”.
Parasitic plants are subject to agronomy and forestry cultivation
Attempts have been made worldwide to cultivate useful local parasitic plants for timber, foods, medicines, or industrial uses. Reported parasitic plants in cultivation include but are not limited to Anacolosa frutescens (as nut trees), Melientha suavis (for vegetables and fruits), Arjona tuberosa (for edible tubers), S. acuminatum (for fruits), S. album (for essential oil), Ximenia americana (for edible fruits and seeds), Orobanche crenata (as vegetables and medicinal plants), V. album (for medicinal uses), and Euphrasia officinalis (for medicinal uses; Pignone and Hammer, 2016). Large plantations have been established for a couple of parasitic plants with high economic values (Figure 3). Apart from providing great economic profits, plantations of parasitic plants play important roles in enhancing ecological revegetation, hence increasing the provision of ecosystem services. Santalum species (particularly S. album and S. spicatum) are the most prominent root hemiparasitic trees being widely planted for precious sandalwood oil in several countries (e.g. Australia, India, China, Indonesia, Malaysia, and Sri Lanka) with modern breeding and propagation technologies greatly enhancing the cultivation efficiency (Box 2). In southern China, plantation of S. album on a large scale began in 2013 and reached 5,000 ha in 2016 with a rapid expanding rate, mainly in mountainous areas (Teixeira da Silva et al., 2016). Recently, great efforts have also been made to grow Malania oleifera, another root hemiparasitic tree species indigenous to karst areas in southwestern China (Li et al., 2019), which is valued not only for high nervonic acid content in seeds (Ma et al., 2004; Tang et al., 2013), but also for its great potential in forest restoration in karst regions (Lü et al., 2016). Cistanche deserticola and C. tubulosa, herbaceous root holoparasites with high medicinal values (Li et al., 2016; Fu et al., 2018), have been planted at large scales in desert regions in Northern China. Since these valuable holoparasites show optimal growth only in sandy soil in arid regions, motivation to establish Cistanche plantations using host plants good for desert revegetation have been increasing. It is estimated that plantation of C. deserticola and C. tubulosa have so far contributed to desert revegetation of more than 40,000 ha by motivating people to grow desert shrubs as their host plants. Due to their great adaptation to disturbed and nutrient-poor habitats, more parasitic plants are becoming incentives for ecological revegetation in regions where revegetation efforts are otherwise scarce.
Figure 3.
Cultivation of parasitic plants with high economic values promotes ecological services. A–B, A sandalwood (S. album) resort area in southern China, photos by Guohua Ma. C, Reforestation using Malania oleifera in karst regions of southwestern China. D, Grassland restoration using Rhinanthus alectorolophus in Czech Republic, photo by Stanislav Hejduk. E, Desert revegetation with root holoparasitic Cistanche tubulosa and its host Tamarix chinensis in northwestern China, photo by Pengfei Tu. F, Desert revegetation with root holoparasitic Cistanche deserticola and its host Haloxylon ammodendron in northeastern China, photo by Pengfei Tu. G, Santalum acuminatum in fruit in Southern Australia, photo by Richard McLellan
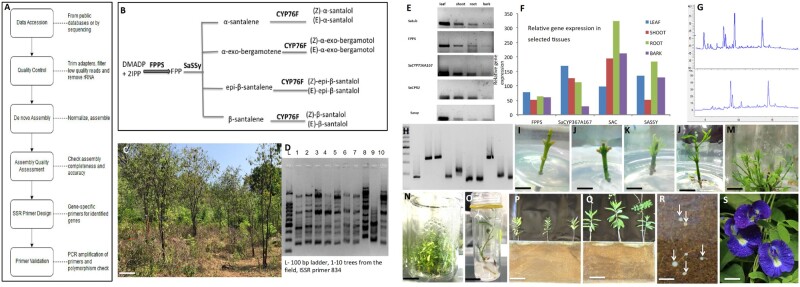
Box 2. Use of transcriptomics for breeding superior sandalwood genotypes.
S. album is among the most valuable trees in the world. Nevertheless, commercial cultivation is limited for several reasons. These include slow growth, variation of oil yield and quality in generatively propagated plants, and a long-time gap between investment into plantation establishment and the revenues from the harvest. A systems biology approach combined with cutting-edge fundamental biology, tissue culture and agronomy was used to solve farmer-level and industrial issues (see Figure). The goal was to identify mother plants providing superior quality oil in large quantity and to develop a micro-propagation protocol for these superior genotypes. Simple sequence repeat (SSR) markers were developed for specific genes in the santalol biosynthesis pathway that are differentially expressed in response to the oil quality and quantity. These genes are differentially expressed in different tissue types of the same plant. The SSR markers were helpful in identification of superior-genotype mother plants in the field. With the development of an efficient tissue culture protocol, a single cutting results in about 1000 plantlets within a year. Additional benefit to the farmer comes from experimental identification of Clitoria ternatea as a high-quality host that promotes the early growth of S. album.
A, Publicly available RNAseq data were used to assemble the S. album transncriptome and a semi-automated system was developed for SSR primer design for specific genes. It can easily be adapted to design SSR primers for breeding programs. B, Several research groups have previously elucidated major steps and genes of the santalol biosynthesis pathway. The major genes of the pathway are dimethylallyl diphosphate (DMADP); isopentenyl diphosphate (IPP); farnesyl diphosphate (FPP); farnesyl diphosphate synthase (FPPS); S. album santalene synthase (SaSSy) and several cytochrome P450 (CYP76F) subfamily members. C, ca. 10-year-old commercial S. album plantation established by seed propagation—the saplings are largely variable despite identical management. D, The genetic variation of 10 seed-propagated plants of the planation (C) assessed at the inter simple sequence region (ISSR). E and F, Differential expression of major genes of the santalol biosynthetic pathway in different tissue types of the same plant (Satub - S. album tubulin was used as the internal control). Expression of major genes in santalol biosynthesis is correlated with the quality and quantity of oil. G, High-performance liquid chromatography chromatograms of two representative plants from plantation (C) showing the variation in chemical composition of the stem extracts. H, Gene-specific polymorphic SSR markers to identify plants with high quality and quantity of oil. I–O, Micropropagation process starting from nodal cuttings to rooted plantlets to multiply superior mother plants avoiding genetic recombination associated with seed propagation. P–Q, Identification of superior hosts for early growth in the rhizotrone system, P: without host, Q: with a legume host – Clitoria ternatea.R, Haustoria (marked with white arrows) formed in the rhizotrone system. S, C. ternatea flowers have economic value and provide additional income for farmers without affecting S. album growth. Size bars: C: 60 cm, I–L: 1 cm, M: 1.5 cm, N: 3 cm, O: 1.5 cm, P–S: 6 cm, R: 1 cm, S: 1.25 cm.
Aesthetics of parasitic plants in horticulture
Many parasitic plants show great aesthetic value with striking colors, unusual corolla shapes, showy fruits, or other unique morphologies, giving them great potential for application in landscape architecture and planting design (Figure 4). Still, horticultural uses of parasitic plants are scarce. The general impression that parasitic plants are harmful may have discouraged people to grow parasitic plants in their gardens. Another likely reason is the complexity in cultivation of parasitic plants, optimal performance of which depends on not only host identity but also on various environmental factors (Gawler et al., 1987; Mellado and Zamora, 2014; Liu et al., 2017). Nevertheless, with increasing demand in diverse horticultural industries, the most underutilized parasitic plants may serve as valued resources for novel horticultural plants, thanks to their unique characteristics and intriguing biological traits.
Figure 4.
High aesthetic values of parasitic plants, which are used or are suitable for use in horticulture. A–C, Himalayan Pedicularis species (P. olivcriana, P. oxycarpa, and P. tricolor from left to right). D, Nuytsia floribunda, photo by Owen Roberts. E, Melampyrum arvense. Photo by Jakub Těšitel. F, Castilleja latifolia, photo by Huiting Zhang. G, Taxillus delavayi, photo by Yang Niu
Current trends in horticulture are focused on naturalistic planting design, self-regulation, diversity and ecological functionality (Dunnett, 2019; Oudolf and Kingsbury, 2013). With wide distribution, high diversity, and great potential as ecosystem engineers, parasitic plants are definitely included in new horticulture ideas. Landscape architecture can use parasitic plants as a cheap and ecological autoregulation mechanism to improve aesthetic value, diversity and to reduce maintenance of present lawns. Root hemiparasitic Rhinanthus minor was used this way in the United Kingdom (Pywell et al., 2004; Westbury et al., 2006). A promising use of hemiparasitic plants in designed meadows was performed by Hitchmough (2017) and his colleagues in Great Britain who demonstrated successful cultivation of the appealing American root hemiparasites Castilleja coccinea and C. integra with common garden plants like Penstemon species or Phlox species as hosts. Sowing mixtures for semi-natural meadows including root-hemiparasitic Melampyrum arvense were tested in Austria (Brocks et al., 2016). Efforts have also been taken in China for horticultural cultivation of Pedicularis species (Li et al., 1997; Wang and Tang, 2005; Li and Guan, 2007), a large group (ca. 800 species worldwide) of root hemiparasitic plants with interesting flowers of high morphological diversity.
Some parasitic plants may also be used in floral design, though with some limits such as limited shelf life due to fast water loss because of high transpiration rates by nature (Stewart and Press, 1990). The most popular and commonly used parasitic plants for decoration are stem hemiparasitic mistletoes (Viscum, Phoradendron; Paine and Harrison, 2018). Apart from Christmas season decorations, modern use of mistletoe as a wedding decoration or a bouquet element can be interesting and promising. Wood roses also represent mistletoe-related products used for ornamental purposes. They are not formed by mistletoes themselves but consist of proliferated host tissue typical of haustorial attachments of some African and American Loranthaceae (Mathiasen et al., 2008; Dzerefos et al., 2009).
Conclusions and risk assessment
Naturally growing parasitic plants should primarily be regarded as an important functional component of terrestrial ecosystems. Many parasitic plant species may have positive effects on biodiversity across multiple groups of organisms. Available empirical evidence points particularly to the effects of root hemiparasites and mistletoes. This evidence is, however, still limited both taxonomically and geographically. Broadening this scope by including other parasitic plants and their host associations in hitherto underinvestigated areas (e.g. mistletoes in tropics, and root hemiparasites in alpine systems and tropical savannas) may thus reveal additional fascinating ecological stories.
Human use of parasitic plants has a long history, but novel applications have emerged in recent years thanks to intense research. In particular, the use of parasitic plants in ecological restoration, invasive plant suppression and horticulture seem promising. Additional research is, however, still required to optimize the methodical approaches and application protocols (see Outstanding questions). This also applies to more traditional medicinal use of parasitic plants.
In this paper, we highlighted the bright side of parasitic plants, but it is important not to forget about their dark side. Therefore, all applications of parasitic plants should undergo a detailed risk assessment, which is crucial in large-scale or uncontrolled applications like agricultural production of commodities, ecological restoration and horticultural use. Invasions of weedy parasitic plants into new areas are well known (Parker, 2013). Similarly, non-weedy parasitic plants beneficial for biodiversity in their native range may spread as alien invaders and cause harm to ecosystems in other parts of the world (van Hulst et al., 1987; Kennedy, 2011). Therefore, we strongly advocate against introductions of parasitic plants outside their native range. This should apply also on horticultural use because many serious plant invasions have started from gardens (Reichard and White, 2001). For instance, we strongly advocate against the suggestion to use the noxious weed Striga hermonthica as a possible ornamental plant for Europe (Gladis et al., 2000) but also express concern on the use of American Castilleja species in European ornamental horticulture (Hitchmough, 2017). Instead, we believe that research should aim at revealing the potential of indigenous parasitic plants for such applications. Nevertheless, even using native parasitic species is not risk-free. Dangerous pests, like Striga hermonthica, Arceuthobium spp., Seymeria cassioides, or recently emerged Rhamphicarpa fistulosa are harmful in agroecosystems or production forests and also within in their native range. Therefore, the risk assessment should be based on detailed knowledge of biology and ecology of specific parasitic plant species.
Acknowledgments
We thank all photograph contributors who kindly provided their work to this paper and two anonymous reviewers who provided valuable comments on a previous version of the manuscript.
Funding
J.T. was supported by Czech Science Foundation project no. 19-28491X. A.-R.L. was supported by National Natural Science Foundation of China project no. 31971536, Yunnan Ten Thousand Talents Plan Young and Elite Talents Project, and Youth Innovation Promotion Association of Chinese Academy of Sciences. P.C.G.B. was supported by Sri Lanka Council for Agricultural Research Policy project no. NARP/16/UP/AG/01. DW and RM were supported by the Hermon Slade Foundation (HSF 18/3).
Conflict of interest statement. None declared.
Outstanding questions
May ecological agriculture and landscape design benefit from the community and ecosystem effects of parasitic plants?
What are the long-term effects of parasitic plants on soil permeability and carbon sequestration?
May improved use of genetic resources and plant breeding techniques contribute to more efficient use of parasitic plants as ecosystem engineers?
How to standardize utilization of parasitic plants as medicinal resources?
J.T., A-R.L., K.K., R.M., P.C.G.B., and D.M.W. wrote the manuscript. The work was coordinated by J.T.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Jakub Těšitel (tesitel@sci.muni.cz).
References
- Alonso-Castro AJ, Juarez-Vazquez Mdel C, Dominguez F, Gonzalez-Sanche z I, Estrada Castillon E, Lopez-Toledo G, Chavez M, Cerbon MA, Garcia-Carranca A (2012) The antitumoral effect of the American mistletoe Phoradendron serotinum (Raf.) M.C. Johnst. (Viscaceae) is associated with the release of immunity-related cytokines. J Ethnopharmacol 142:857–864 [DOI] [PubMed] [Google Scholar]
- Ameloot E, Verheyen K, Hermy M (2005) Meta-analysis of standing crop reduction by Rhinanthus spp. and its effect on vegetation structure. Folia Geobot 40:289–310 [Google Scholar]
- Arctander S (1960) Sandalwood oil East India. In S Arctander, ed, Perfume and Flavor Materials of Natural Origin. S Arctander, Elizabeth, NJ, pp 574–576 [Google Scholar]
- Arunkumar AN, Dhyani A, Joshi G (2019) Santalum album. The IUCN Red List of Threatened Species 2019: e.T31852A2807668. http://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T31852A2807668.en (May 20, 2020)
- Bao G, Suetsugu K, Wang H, Yao X, Liu L, Ou J, Li C (2015) Effects of the hemiparasitic plant Pedicularis kansuensis on plant community structure in a degraded grassland. Ecol Res 30:507–515 [Google Scholar]
- Bardgett RD, Smith RS, Shiel RS, Peacock S, Simkin JM, Quirk H, Hobbs PJ (2006) Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature 439:969–972 [DOI] [PubMed] [Google Scholar]
- Barcelona JF, Pelser PB, Balete DS, Co LL (2009) Taxonomy, ecology, and conservation status of Philippine Rafflesia (Rafflesiaceae). Blumea 54:77–93 [Google Scholar]
- Barto EK, Weidenhamer JD, Cipollini D, Rillig MC (2012) Fungal superhighways: do common mycorrhizal networks enhance below ground communication? Trends Plant Sci 17:633–637 [DOI] [PubMed] [Google Scholar]
- Bellot S, Renner SS (2013) Pollination and mating systems of Apodanthaceae and the distribution of reproductive traits in parasitic angiosperms. Am J Bot 100:1083–1094 [DOI] [PubMed] [Google Scholar]
- Borowicz VA, Walder MR, Armstrong JE (2019) Coming undone: hemiparasite presence and effects in a prairie grassland diminish over time. Oecologia 190:679–688 [DOI] [PubMed] [Google Scholar]
- Brand-Miller JC, Holt SH (1998) Australian Aboriginal plant foods: a consideration of their nutritional composition and health implications. Nutr Res Rev 11:5–23 [DOI] [PubMed] [Google Scholar]
- Brocks J, Plenk S, Schwingesbauer S (2016) Nachhaltige Pflanzenmischungen für den pflegeleichten Einsatz im niederösterreichischen Gemeindegrün – “Natürlich bunt!”. Wien.
- Burdock G, Carabin IG (2008) Safety assessment of sandalwood oil (Santalum album L.). Food Chem Toxicol 46:421–432 [DOI] [PubMed] [Google Scholar]
- Büssing A (2004) Introduction: history of mistletoe uses. InBüssing A, ed, Mistletoe, The Genus Viscum. CRC Press, Boca Raton, FL, pp 1–6 [Google Scholar]
- Cameron DD, Hwangbo J, Keith AM, Kraushaar D, Rowntree J, Seel WE (2005) Interactions between the hemiparasitic Angiosperm Rhinanthus minor and its hosts: from cell to the ecosystem. Folia Geobot 40:217–229 [Google Scholar]
- Carlo TA, Aukema JE (2005) Female-directed dispersal and facilitation between a tropical mistletoe and a dioecious host. Ecology 86:3245–3251 [Google Scholar]
- Carrillo-Ocampo D, Bazaldua-Gomez S, Bonilla-Barbosa JR, Aburto-Amar R, Rodriguez-Lopez V (2013) Anti-inflammatory activity of iridoids and verbascoside isolated from Castilleja tenuiflora. Molecules 18:12109–12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini M, Aldini G, Orioli M, Facino RM (2002) Antioxidant and photoprotective activity of a lipophilic extract containing neolignans from Krameria triandra roots. Planta Med 68:193–197 [DOI] [PubMed] [Google Scholar]
- Chabra A, Monadi T, Azadbakht M, Haerizadeh SI (2019) Ethnopharmacology of Cuscuta epithymum: a comprehensive review on ethnobotany, phytochemistry, pharmacology and toxicity. J Ethnopharmacol 231:555–569 [DOI] [PubMed] [Google Scholar]
- Cheung WL, Law CY, Lee HCH, Tang CO, Lam YH, Ng SW, Chan SS, Chow TC, Pang KS, Mak TWL (2018) Gelsemium poisoning mediated by the non-toxic plant Cassytha filiformis parasitizing Gelsemium elegans. Toxicon 154:42–49 [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206 [DOI] [PubMed] [Google Scholar]
- Cirocco RM, Facelli JM, Watling JR (2016) Does light influence the relationship between a native stem hemiparasite and a native or introduced host? Ann Bot 117:521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirocco RM, Facelli JM, Watling JR (2017) Does nitrogen affect the interaction between a native hemiparasite and its native or introduced leguminous hosts? New Phytol 213:812–821 [DOI] [PubMed] [Google Scholar]
- Cirocco RM, Facelli JM, Watling JR (2018) A native parasitic plant affects the performance of an introduced host regardless of environmental variation across field sites. Funct Plant Biol 45:1128–1137 [DOI] [PubMed] [Google Scholar]
- Clarke PA (2008) Aboriginal healing practices and Australian bush medicine. J Anthropol Soc South Aust 33:3–38 [Google Scholar]
- Cui Z, Guo Z, Miao J, Wang Z, Li Q, Chai X, Li M (2013) The genus Cynomoriumin in China: an ethnopharmacological and phytochemical review. J Ethnopharmacol 147:1–15 [DOI] [PubMed] [Google Scholar]
- Cullings K, Raleigh C, Vogler DR (2005) Effects of severe dwarf mistletoe infection on the ectomycorrhizal community of a Pinus contorta stand in Yellowstone Park. Can J Bot 83:1174–1180 [Google Scholar]
- Dale H, Press MC (1998) Elevated atmospheric CO2 influences the interaction between the parasitic angiosperm Orobanche minor and its host Trifolium repens. New Phytol 140:65–73 [Google Scholar]
- Davies D, Graves J, Elias C, Williams P (1997) The impact of Rhinanthus spp. on sward productivity and composition: implications for the restoration of species-rich grasslands. Biol Conserv 82:87–93 [Google Scholar]
- Decleer K, Bonte D, Van Diggelen R (2013) The hemiparasite Pedicularis palustris: “Ecosystem engineer” for fen-meadow restoration. J Nat Conserv 21:65–71 [Google Scholar]
- Demey A, De Frenne P, Baeten L, Verstraeten G, Hermy M, Boeckx P, Verheyen K (2015) The effects of hemiparasitic plant removal on community structure and seedling establishment in semi-natural grasslands. J Veg Sci 26:409–420 [Google Scholar]
- Demey A, Rütting T, Huygens D, Staelens J, Hermy M, Verheyen K, Boeckx P (2014) Hemiparasitic litter additions alter gross nitrogen turnover in temperate semi-natural grassland soils. Soil Biol Biochem 68:419–428 [Google Scholar]
- Demey A, Staelens J, Baeten L, Boeckx P, Hermy M, Kattge J, Verheyen K (2013) Nutrient input from hemiparasitic litter favors plant species with a fast-growth strategy. Plant Soil 371:53–66 [Google Scholar]
- Dunnett NP (2019) Naturalistic Planting Design: The Essential Guide. Filbert Press, London [Google Scholar]
- Dzerefos ACM, Shackleton CM, Witkowski ETF (2009) Sustainable utilization of woodrose-producing mistletoes (Loranthaceae) in South Africa. Econ Bot 53:439–447 [Google Scholar]
- Fibich P, Lepš J, Chytrý M, Těšitel J (2017) Root hemiparasitic plants are associated with high diversity in temperate grasslands. J Veg Sci 28:184–191 [Google Scholar]
- Fisher JP, Phoenix GK, Childs DZ, Press MC, Smith SW, Pilkington MG, Cameron DD (2013) Parasitic plant litter input: a novel indirect mechanism influencing plant community structure. New Phytol 198:222–231 [DOI] [PubMed] [Google Scholar]
- Flansda R (2009) The outlook for non wood forest products in Asia and the Pacific, Working Paper No. APFSOS II/WP/2009/18, Food and Agriculture Organisations Regional Office, Bangkok: 89 [Google Scholar]
- Fonturbel FE (2020) Mistletoes in a changing world: a premonition of a non-analog future? Botany 98
- Fraser LH, Pither J, Jentsch A, Sternberg M, Zobel M, Askarizadeh D, Bartha S, Beierkuhnlein C, Bennett JA. , Bittel A (2015) Worldwide evidence of a unimodal relationship between productivity and plant species richness. Science 349:302–306 [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64:839–863 [DOI] [PubMed] [Google Scholar]
- Fu Z, Fan X, Wang X, Gao X (2018) Cistanches herba: an overview of its chemistry, pharmacology, and pharmacokinetics property. J Ethnopharmacol 219:233–247 [DOI] [PubMed] [Google Scholar]
- Fuentes-Cross P (2015) New approaches to explore the past and present diversity of Australian sandalwood species—from palaeobotany to next generation sequencing. PhD thesis, University of Adelaide, Adelaide, South Australia
- Gawler SC, Waller DM, Menges ES (1987) Environmental factors affecting establishment and growth of Pedicularis furbishiae, a rare endemic of the St. John River Valley, Maine. Bull - Torrey Bot Club 114:280–292 [Google Scholar]
- Gebauer R, Volařík D, Urban J (2012) Quercus pubescens and its hemiparasite Loranthus europaeus: nutrient dynamics of leaves and twigs. Acta Physiol Plant 34:1801–1809 [Google Scholar]
- Gladis T, Hammer K, Roose K, Knüpffer H, Azurdia C, Leiva JM (2000) Hemerophyta—a special case of invasive organisms. Nutzung genetischer Ressourcen - okologischer Wert der Biodiversitat. Symp. AG Genet. Ressourcen GPZ 23-24. Witzenhausen, pp 23–29
- Griebel A, Watson D, Pendall E (2017) Mistletoe, friend and foe: synthesizing ecosystem implications of mistletoe infection. Environ Res Lett. 12:115012. doi: 10.1088/1748-9326/aa8fff [Google Scholar]
- Grime J (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344–347 [Google Scholar]
- Hartley S, Green J, Massey F (2015) Hemiparasitic plant impacts animal and plant communities across four trophic levels. Ecology 96:2408–2416 [DOI] [PubMed] [Google Scholar]
- Hawksworth F, Wiens D (1996) Dwarf Mistletoes: Biology, Pathology, and Systematics. United States Department of Agriculture, Washington
- Heide-Jørgensen HS (2008) Parasitic Flowering Plants. Brill, Leiden, The Netherlands [Google Scholar]
- Heide-Jørgensen HS (2013) Introduction: the parasitic syndrome in higher plants. In Joel D, Musselman LJ, Gressel J, eds, Parasitic Orobanchaceae. Springer, Berlin, Heidelberg, pp 1–20 [Google Scholar]
- Hettenhausen C, Li J, Zhuang H, Sun H, Xu Y, Qi J, Zhang J, Lei Y, Qin Y, Sun G. , et al. (2017) Stem parasitic plant Cuscuta australis(dodder) transfers herbivory-induced signals among plants. Proc Natl Acad Sci USA 114:E6703–E6709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heubeger E, Hongratanaworakit T, Buchbauer G (2006) East Indian Sandalwood and a-santalol odor increase physiological and self-rated arousal in humans. Planta Med 72:792–800 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Quick WP, Press MC, Scholes JD (1998) Can source–sink relations explain responses of tobacco to infection by the root holoparasitic angiosperm Orobanche cernua? Plant Cell Environ 21:333–340 [Google Scholar]
- Hitchmough J (2017) Sowing Beauty: Designing Flowering Meadows from Seed. Timber Press, Portland [Google Scholar]
- Ichii K, Molnár Z, Obura D, Purvis A, Willis K (2019) Status and Trends - Nature. IPBES Glob. Assess. Biodivers. Ecosyst. Serv. p 170
- Irving LJ, Cameron DD (2009) You are what you eat: interactions between root parasitic plants and their hosts. Adv Bot Res 50:87–138 [Google Scholar]
- International Union for Conservation of Nature (IUCN) (2020) The IUCN Red List of Threatened Species. Version 2019-3. IUCN website. https://www.iucnredlist.org/species/31852/2807668 (accessed 6 April 2020)
- Joshi J, Matthies D, Schmid B (2000) Root hemiparasites and plant diversity in experimental grassland communities. J Ecol 88:634–644 [Google Scholar]
- Karadada J (2011) Uunguu plants and animals: aboriginal biological knowledge from Wunambal Gaambera Country in the north-west Kimberley, Australia. Wunambal Gaambera Aboriginal Corporation, Department of Natural Resources, Environment, the Arts and Sport, Darwin, Northern Territory
- Kennedy BN (2011) The new invasive Odontites serotina: impacts, responses and predictive model. Master thesis. University of Manitoba, Winnipeg, Canada
- Kienle GS, Glockmann A, Schink M, Kiene H (2009) Viscum album L. extracts in breast and gynaecological cancers: a systematic review of clinical and preclinical research. J Exp Clin Cancer Res 28:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MK, Yun KJ, Lim DH, Kim J, Jang YP (2016) Anti-inflammatory properties of flavone di-C-glycosides as active principles of Camellia mistletoe, Korthalsella japonica. Biomol Ther (Seoul) 24:630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Bugni TS, Pond CD, Sondossi M, Dindi M, Piskaut P, Ireland CM, Barrows LR (2009) Antimycobacterial activity of Exocarpos latifolius is due to exocarpic acid. Planta Med 75:1326–1330 [DOI] [PubMed] [Google Scholar]
- Kuijt J (1969) The biology of parasitic flowering plants. University of California Press, Berkeley, CA and Los Angeles, CA [Google Scholar]
- Kumar AA, Joshi G, Ram HM (2012) Sandalwood: history, uses, present status and the future. Curr Sci 103:1408–1416 [Google Scholar]
- Lawrence BM (1991) Recent progress in essential oils. Perfumer & Flavorist 16:49–58 [Google Scholar]
- Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989 [Google Scholar]
- Li S, Zhang J, Liu H, Liu N, Shen G, Zhuang H, Wu J (2020) Dodder-transmitted mobile signals prime host plants for enhanced salt tolerance. J Exp Bot 71:1171–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AR, Guan KY (2007) On cultivation of Pedicularis L. Acta Hortic Sin 34:1050–1054 [Google Scholar]
- Li AR, Mao P, Li YJ (2019) Root hemiparasitism in Malania oleifera (Olacaceae), a neglected aspect in research of the highly valued tree species. Plant Divers 41:347–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Jin Z, Song W (2012) Do native parasitic plants cause more damage to exotic invasive hosts than native non-invasive hosts? An implication for biocontrol. PLoS One 7:e34577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Guan KY, Li YC, Kuang J, Kong FC (1997) Study on introduction and cultivation of Pedicularis tricolor. Guihaia 17:255–258 [Google Scholar]
- Li Z, Lin H, Gu L, Gao J, Tzeng CM (2016) Herba Cistanche (Rou Cong-Rong): one of the best pharmaceutical gifts of traditional Chinese medicine. Front Pharmacol 7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YC, Rajabalaya R, Lee SH, Tennakoon KU, Le QV, Idris A, Zulkipli IN, Keasberry N, David SR (2016) Parasitic mistletoes of the genera Scurrula and Viscum: from bench to bedside. Molecules 21:E1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Shen G, Xu Y, Liu H, Zhang J, Li S, Li J, Zhang C, Qi J, Wang L. , et al. (2020) Extensive inter-plant protein transfer between Cuscuta parasites and their host plants. Mol Plant 13:573–585 [DOI] [PubMed] [Google Scholar]
- Liu Y, Taxipulati T, Gong Y, Sui X, Wang X, Parent SE, Hu Y, Guan K, Li A (2017) N-P Fertilization inhibits growth of root hemiparasite Pedicularis kansuensis in natural grassland. Front Plant Sci 8:2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low T (1988) Wild Food Plants of Australia. Angus and Robertson, North Ryde, NSW [Google Scholar]
- Lv SH, Wei CQ, Huang FZ, He YL, Zeng DJ, Li XK. , Deng ZH (2016) Fruit and seed traits and adaptability to rocky desertification mountain of rare tree species Malania oleifera. Chin J Ecol 35:57–62 [Google Scholar]
- Lullfitz A, Dortch J, Hopper SD, Pettersen C, Guilfoyle D (2017) Human niche construction: Noongar evidence in pre-colonial southwestern Australia. Conserv Soc 15:201–216 [Google Scholar]
- Ma BL, Liang SF, Zhao DY, Xu AX, Zhang KJ (2004) Study on plants containing nervonic acid. Acta Bot Boreali-Occidentalia Sin 24:2362–2365 [Google Scholar]
- Maiden JH (1889) The Useful Native Plants of Australia. Trubner and Co., London; Turner and Henderson, Sydney [Google Scholar]
- March WA, Watson DM (2007) Parasites boost productivity: effects of mistletoe on litterfall dynamics in a temperate Australian forest. Oecologia 154:339–347 [DOI] [PubMed] [Google Scholar]
- March WA, Watson DM (2010) The contribution of mistletoes to nutrient returns in a temperate eucalypt forest: evidence for a critical role in nutrient cycling. Austral Ecol 35:713–721 [Google Scholar]
- Marvier MA (1998) Parasite impacts on host communities: plant parasitism in a California coastal prairie. Ecology 79:2616–2623 [Google Scholar]
- Mathiasen R, Shaw D, Nickrent DL, Watson DM (2008) Mistletoes: pathology, systematics, ecology, and management. Plant Dis 92:988–1006 [DOI] [PubMed] [Google Scholar]
- Meagher SJ (1974) The food resources of the Aborigines of the south-west of Western Australia. WA Museum. Rec West Aust Mus 3:14–65 [Google Scholar]
- Meidell O (1944) Notes on the pollination of Melampyrum pratense and the “honeystealing” of humble-bees and bees. Bergen Museum Årb 58: 5–12 [Google Scholar]
- Mellado A, Zamora R (2014) Linking safe sites for recruitment with host-canopy heterogeneity: the case of a parasitic plant, Viscum album subsp. austriacum (Viscaceae). Am J Bot 101:957–964 [DOI] [PubMed] [Google Scholar]
- Mellado A, Zamora R (. 2016) Spatial heterogeneity of a parasitic plant drives the seed‐dispersal pattern of a zoochorous plant community in a generalist dispersal system. Funct Ecol 30:459 [Google Scholar]
- Mellado A, Zamora R (2017) Parasites structuring ecological communities: the mistletoe footprint in Mediterranean pine forests. Funct Ecol 31: 2167–2176 [Google Scholar]
- Moghadamtousi SZ, Kamarudin MN, Chan CK, Goh BH, Kadir HA (2014) Phytochemistry and biology of Loranthus parasiticus Merr, a commonly used herbal medicine. Am J Chin Med 42:23–35 [DOI] [PubMed] [Google Scholar]
- Mudrák O, Lepš J (2010) Interactions of the hemiparasitic species Rhinanthus minor with its host plant community at two nutrient levels. Folia Geobot 45:407–424 [Google Scholar]
- Mueller RC, Gehring CA (2006) Interactions between an above-ground plant parasite and below-ground ectomycorrhizal fungal communities on pinyon pine. J Ecol 94:276–284 [Google Scholar]
- Musselman L, Mann WF (1978) Root parasites of southern forests. U.S. Department of Agriculture, Forest Service
- Nackley LL, West AG, Skowno AL, Bond WJ (2017) The nebulous ecology of native invasions. Trends Ecol Evol 32:814–824 [DOI] [PubMed] [Google Scholar]
- Napier KR, Mather SH, McWhorter TJ, Fleming PA (2013) Do bird species richness and community structure vary with mistletoe flowering and fruiting in Western Australia? Emu 114:13–22 [Google Scholar]
- Newton J (2016) The Oldest Foods on Earth: A History of Australian Native Foods with Recipes. New South Publishing, Sydney, NSW [Google Scholar]
- Noureen S, Noreen S, Ghumman SA, Batool F, Bukhari SNA (2019) The genus Cuscuta (Convolvolaceac): an updated review on indigenous uses, phytochemistry, and pharmacology. Iran J Basic Med Sci 22:1225–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi T, Shibata T, Higuti T, Kodama K, Kusumi T, Takaishi Y (2005) Anti Helicobacter pylori compounds from Santalum album. J Nat Prod 68:819–824 [DOI] [PubMed] [Google Scholar]
- Ojewole JA, Adewole SO (2007) Hypoglycaemic and hypotensive effects of Globimetula cupulata (DC) Van Tieghem (Loranthaceae) aqueous leaf extract in rats. Cardiovasc J S Afr 18:9–15 [PubMed] [Google Scholar]
- Oudolf P, Kingsbury N (2013) Planting: A New Perspective. Timber Press, Portland, OR [Google Scholar]
- Paine LK, Harrison HC (2018) Mistletoe: Its role in horticulture and human life. Hort Technol 2:324–330 [Google Scholar]
- Pardoe C, Fullagar R, Hayes E (2019) Quandong stones: A specialised Australian nut-cracking tool. PLoS One 14:e0222680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SB, Park GH, Kim HN, Son HJ, Song HM, Kim HS, Jeong HJ, Jeong JB (2018) Anti-inflammatory effect of the extracts from the branch of Taxillus yadoriki being parasitic in Neolitsea sericea in LPS-stimulated RAW264.7 cells. Biomed Pharmacother 104:1–7 [DOI] [PubMed] [Google Scholar]
- Parker C (2012) Parasitic weeds: a world challenge. Weed Sci 60:269–276 [Google Scholar]
- Parker C (2013) The parasitic weeds of the Orobanchaceae. In Joel D, Gressel LJ, Musselmann J, eds, Parasitic Orobanchaceae. Springer, Heidelberg, pp 313–344 [Google Scholar]
- Paulpandi M, Kannan S, Thangam R, Kaveri K, Guna Sekaran P, Rejeeth C (2012) In vitro anti-viral effect of/7-santalol against influenza viral replication. Phytomedicine 19:231–235 [DOI] [PubMed] [Google Scholar]
- Pejchar L, Mooney HA (2009) Invasive species, ecosystem services and human well-being. Trends Ecol Evol 24:497–504 [DOI] [PubMed] [Google Scholar]
- Pennisi E (2010) Armed and dangerous. Science 327:804–805 [DOI] [PubMed] [Google Scholar]
- Petrović K (2014) Herbivory of the common brushtail possum (Trichosurus vulpecula, Marsupialia: Phalangeridae) at different scales of resource heterogeneity. PhD thesis, Charles Sturt University, Sydney, Australia
- Pignone D, Hammer K (2016) Parasitic angiosperms as cultivated plants? Genet Resour Crop Evol 63:1273–1284 [Google Scholar]
- Press MC, Phoenix GK (2005) Impacts of parasitic plants on natural communities. New Phytol 166:737–751 [DOI] [PubMed] [Google Scholar]
- Prider J, Watling JR, Facelli JM (2009) Impacts of a native parasitic plant on an introduced and a native host species: implications for the control of an invasive weed. Ann Bot 103:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pywell RF, Bullock JM, Walker KJ, Coulson SJ, Gregory SJ, Stevenson MJ (2004) Facilitating grassland diversification using the hemiparasitic plant Rhinanthus minor. J Appl Ecol 41:880–887 [Google Scholar]
- Quested H, Cornelissen J, Press MC, Callaghan T, Aerts R, Trosien F, Riemann P, Gwyn-Jones D, Kondratchuk A, Jonasson S (2003a) Decomposition of sub-arctic plants with differing nitrogen economies: a functional role for hemiparasites. Ecology 84:3209–3221 [Google Scholar]
- Quested HM, Press MC, Callaghan TV (2003b) Litter of the hemiparasite Bartsia alpina enhances plant growth: evidence for a functional role in nutrient cycling. Oecologia 135:606–614 [DOI] [PubMed] [Google Scholar]
- Reichard SH, White P (2001) Horticulture as a pathway of invasive plant introductions in the United States. BioScience 51:103–113 [Google Scholar]
- Schachtman DP, Goodger JQ (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287 [DOI] [PubMed] [Google Scholar]
- Schnitzler P, Koch C, Reichling J (2007) Susceptibility of drug resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop and sandalwood. Antimicrob Agents Chemother 51:1859–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensarma P (1989) Plants in the Indian Puranas: An Ethnobotanical Investigation. Naya Prokash, Kolkata, India [Google Scholar]
- Shen H, Prider J, Facelli J, Watling JR (2010) The influence of the hemiparasitic angiosperm Cassytha pubescens on photosynthesis of its host Cytisus scoparius. Funct Plant Biol 37:14–21 [Google Scholar]
- Simpson BB (1991). The past and present uses of rhatany (Krameria, Krameriaceae). Econ Bot 45:397–409 [Google Scholar]
- Simpson BB, Neff JL, Seigler D (1977) Krameria, free fatty acids and oil-collecting bees. Nature 267:150–151 [DOI] [PubMed] [Google Scholar]
- Sivicek VA, Taft JB (2011) Functional group density as an index for assessing habitat quality in tallgrass prairie. Ecol Indic 11:1251–1258 [Google Scholar]
- Spasojevic M, Suding K (2011) Contrasting effects of hemiparasites on ecosystem processes: can positive litter effects offset the negative effects of parasitism? Oecologia 165:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan VV, Sivaramakrishnan VR, Rangaswamy CR, Ananthapadmanabha HS, Shankaranarayana KH (1992) Sandal (Santalum album L.). ICFRE, Dehradun, India.
- Stewart GR, Press MC (1990) The physiology and biochemistry of parasitic angiosperms. Annu Rev Plant Physiol Plant Mol Biol 41:127–151 [Google Scholar]
- Tang TF, Liu XM, Ling M, Lai F, Zhang L, Zhou YH, Sun RR (2013) Constituents of the essential oil and fatty acid from Malania oleifera. Ind Crops Prod 43:1–5 [Google Scholar]
- Teixeira da Silva JA, Kher MM, Soner D, Page T, Zhang X, Nataraj M,, Ma G (2016) Sandalwood: basic biology, tissue culture, and genetic transformation. Planta 243:847–887 [DOI] [PubMed] [Google Scholar]
- Těšitel J (2016) Functional biology of parasitic plants: a review. Plant Ecol Evol 149:5–20 [Google Scholar]
- Těšitel J, Cirocco RM, Facelli JM, Watling JR (2020) Native parasitic plants: biological control for plant invasions? Appl Veg Sci 23: 464–469. 10.1111/avsc.12498 [DOI] [Google Scholar]
- Těšitel J, Fibich P, de Bello F, Chytrý M, Lepš J (2015) Habitats and ecological niches of root-hemiparasitic plants: an assessment based on a large database of vegetation plots. Preslia 87:87–108 [Google Scholar]
- Těšitel J, Mládek J, Fajmon K, Blažek P, Mudrák O (2018) Reversing expansion of Calamagrostis epigejos in a grassland biodiversity hotspot: Hemiparasitic Rhinanthus major does a better job than increased mowing intensity. Appl Veg Sci 21:104–112 [Google Scholar]
- Těšitel J, Mládek J, Horník J, Těšitelová T, Adamec V, Tichý L (2017) Suppressing competitive dominants and community restoration with native parasitic plants using the hemiparasitic Rhinanthus alectorolophus and the dominant grass Calamagrostis epigejos. J Appl Ecol 54:1487–1495 [Google Scholar]
- Thomsen MS, Altieri AH, Angelini C, Bishop MJ, Gribben PE, Lear G, He Q, Schiel BR, South PM, Watson DM. , et al. (2018) Secondary foundation species enhance biodiversity. Nat Ecol Evol 2:634–639 [DOI] [PubMed] [Google Scholar]
- Tonts M, Selwood J (2003) Niche markets, regional diversification and the reinvention of Western Australia’s sandalwood industry. Tijdsch Econ Soc Geogr 94:564–575 [Google Scholar]
- van Hulst R, Shipley B, Thériault A (1987) Why is Rhinanthus minor (Scrophulariaceae) such a good invader? Can J Bot 65:2373–2379 [Google Scholar]
- van Ommeren RJ, Whitham TG (2002) Changes in interactions between juniper and mistletoe mediated by shared avian frugivores: parasitism to potentialmutualism. Oecologia 130:281–288 [DOI] [PubMed] [Google Scholar]
- Venkatesha Gowda VS (2011) Global emerging trends on sustainable production of natural sandalwood. In Proceedings of the Art and Joy of Wood Conference, 19–22 October 2011, Bangalore, India, pp 3–38
- Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708 [DOI] [PubMed] [Google Scholar]
- Walder M, Armstrong JE, Borowicz VA (2019) Limiting similarity, biotic resistance, nutrient supply, or enemies? What accounts for the invasion success of an exotic legume? Biol Invasions 21:435–449 [Google Scholar]
- Wang J, Tang Y (2005) Feasibility study of the introduction of the wild Pedicularis. Forest By-Product and Speciality in China 1:5–7 [Google Scholar]
- Warnke PH, Becker ST, Podschun R, Sivananthan S, Springer IN, Russo PAJ, Wiltfang J, Fickenscher H, Sherry E (2009) The battle against multi-resistant strains: renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J Cranio-Maxillofacial Surg 37:392–397 [DOI] [PubMed] [Google Scholar]
- Watson DM (2001) Mistletoe-a keystone resource in forests and woodlands worldwide. Annu Rev Ecol Syst 32:219–249 [Google Scholar]
- Watson DM (2009) Parasitic plants as facilitators: more Dryad than Dracula? J Ecol 97:1151–1159 [Google Scholar]
- Watson DM (2016) Fleshing out facilitation – reframing interaction networks beyond top-down versus bottom-up. New Phytol 211:803–808 [DOI] [PubMed] [Google Scholar]
- Watson DM, Herring M (2012) Mistletoe as a keystone resource: an experimental test. Proc R Soc B Biol Sci 279:3853–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DM, McGregor HW, Spooner PG (2011) Hemiparasitic shrubs increase resource availability and multi-trophic diversity of eucalypt forest birds. Funct Ecol 25:889–899 [Google Scholar]
- Westbury DB, Davies A, Woodcock BA, Dunnett NP (2006) Seeds of change: the value of using Rhinanthus minor in grassland restoration. J Veg Sci 17:435–446 [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, DePamphilis CW (2010) The evolution of parasitism in plants. Trends Plant Sci 15:227–235 [DOI] [PubMed] [Google Scholar]
- Williams VL, Falcão MP, Wojtasik EM (2011) Hydnora abyssinicia: ethnobotanical evidence for its occurrence in southern Mozambique. S Afr J Bot 77:474–478 [Google Scholar]
- Wu AP, Zhong W, Yuan JR, Qi LY, Chen FL, Liang YS, He FF, Wang YH (2019) The factors affecting a native obligate parasite, Cuscuta australis, in selecting an exotic weed, Humulus scandens, as its host. Sci Rep 9:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Liu J, He WM, Miao SL, Dong M (2011) Cuscuta australis restrains three exotic invasive plants and benefits native species. Biol Invasions 13:747–756 [Google Scholar]
- Zhao J, Agboola SO (2007) Functional Properties of Australian Bushfoods: A Report for the Rural Industries Research and Development Corporation. Rural Industries Research and Development Corporation, Canberra, ACT [Google Scholar]
- Zorofchian Moghadamtousi S, Hajrezaei M, Abdul Kadir H, Zandi K ( 2013) Loranthus micranthus Linn.: biological activities and phytochemistry. Evid Based Complement Alternat Med 2013:273712. [DOI] [PMC free article] [PubMed] [Google Scholar]