Single-particle analysis of an aquaporin in flg22-induced stomatal movement reveals different protein dynamics and regulation mechanisms between guard and subsidiary cells.
Abstract
Aquaporins such as the plasma membrane intrinsic proteins (PIPs) allow water to move through cell membranes and are vital for stomatal movement in plants. Despite their importance, the dynamic changes in aquaporins during water efflux and influx have not been directly observed in real time in vivo. Here, to determine which factors regulate these changes during the bidirectional translocation of water, we examined aquaporin dynamics during the stomatal immune response to the bacterial flagellin-derived peptide flg22. The Arabidopsis (Arabidopsis thaliana) aquaporin mutant pip2;1 showed defects in the flg22-induced stomatal response. Variable-angle total internal reflection fluorescence microscopy revealed that the movement dynamics and dwell times of AQ6]GFP-AtPIP2;1 in guard cells and subsidiary cells exhibited cell type-specific dependencies on flg22. The cytoskeleton, rather than the cell wall, was the major factor regulating AtPIP2;1 dynamics, although both the cytoskeleton and cell wall might form bounded domains that restrict the diffusion of AtPIP2;1 in guard cells and subsidiary cells. Finally, our analysis revealed the different roles of cortical actin and microtubules in regulating AtPIP2;1 dynamics in guard cells, as well as subsidiary cells, under various conditions. Our observations shed light on the heterogeneous mechanisms that regulate membrane protein dynamics in plants in response to pathogens.
Introduction
Plasma membrane (PM) intrinsic proteins (PIPs) are aquaporins that facilitate the passive diffusion of water and small neutral solutes through the cell membrane in plants (Hachez et al., 2013), which is essential for numerous biological processes (Chevalier and Chaumont, 2015). Aquaporins enable the bidirectional passage of water, and the transport direction is determined by the physical conditions of the medium (Cheng et al., 1997; Meinild et al., 1998). Although the roles of aquaporins in plants have been researched extensively using genetic and physiological technologies (Chaumont and Tyerman, 2014; Grondin et al., 2015), the dynamic changes in aquaporins during water transport have not yet been elucidated.
Stomatal pores on leaves serve as major gateways for gas exchange between plants and their environment (Wang et al., 2007; Hashimoto-Sugimoto et al., 2013; Jin and Pei, 2016). Stomatal movements are regulated by endogenous and environmental stimuli, including light, drought, and pathogen invasion (Melotto et al., 2006; Zhang et al., 2008; Kong et al., 2012). Indeed, most plant pathogens, including fungi and bacteria, efficiently induce stomatal closure in a plant defense mechanism aimed at limiting the entry of the pathogen into leaves (Li et al., 2014). For example, the flagellin-derived peptide flg22 is perceived by the receptor FLAGELLIN SENSITIVE2, resulting in stomatal closure in wild-type (WT) Arabidopsis (Arabidopsis thaliana) plants (Melotto et al., 2008; Xue et al., 2020). In turn, these signals activate several types of cation channels involved in stomatal closure, such as channels that transport Ca2+ and K+ (Zhang et al., 2008; Thor and Peiter, 2014), as well as the PM aquaporin AtPIP2;1 (Rodrigues et al., 2017).
The stomatal complex, comprising two guard cells and the surrounding anisocytic subsidiary cells, constitutes the functional unit for stomatal movement (Serna and Fenoll, 2000). In response to environmental cues, the movement of different ions and water in and out of the guard cells induces a change in guard cell turgor pressure, resulting in stomatal opening and closure (Yao et al., 2013; Higaki et al., 2014). The pip2;1 mutant shows defects in abscisic acid-triggered stomatal movement, suggesting that AtPIP2;1 plays a vital role in stomatal movement (Grondin et al., 2015; Gong et al., 2020). Furthermore, the stomata of the pip2;1 mutant do not close in response to 1 μM flg22, indicating that AtPIP2;1 functions in flg22-induced stomatal closure (Rodrigues et al., 2017).
Subsidiary cells that surround guard cells and support their function have a characteristic shape (Buchsenschutz et al., 2005). In Arabidopsis, three small, simply shaped subsidiary cells around the guard cell participate in rapid stomatal movements. Bulk water transfer also occurs in subsidiary cells during stomatal opening and closure (Higaki et al., 2014). Although subsidiary cells are considered to function as important buffering machinery in the regulation of guard cell turgor and stomatal movement, whether the mechanism regulating hyperpolarization and turgor pressure in these cells during pathogen-triggered stomatal closure is the same as that in guard cells is currently unclear.
During stimulus-induced stomatal movements, the volume of guard cells is adjusted using water, whereas subsidiary cells surrounding the guard cells display no significant changes in shape. In the current study, to gain deeper insight into the molecular mechanisms regulating the dynamics and activity of AtPIP2;1 between the subsidiary and guard cells during water transport, we explored the differences in ion influx in these cells in WT and pip2;1 Arabidopsis plants in response to flg22. Using variable-angle total internal reflection fluorescence microscopy (VA-TIRFM) combined with a single-particle tracking (SPT) assay (Wang et al., 2009, 2013; Cui et al., 2018), we demonstrated that the distribution patterns and dynamic properties of AtPIP2;1 differ between the two cell types both before and after flg22 treatment. Using pharmacological approaches, we demonstrated that the cytoskeleton and cell wall are both involved in constraining the diffusion of AtPIP2;1, with the cytoskeleton having a greater influence on immobilizing AtPIP2;1. Finally, we showed that cortical actin and microtubules differentially regulate AtPIP2;1 between the guard cell and subsidiary cells under various conditions. These findings provide valuable information about the cell type-specific mechanisms regulating water transport within the subsidiary and guard cells during plant defense responses.
Results
Role of AtPIP2;1 in flg22-induced stomatal movements
Stomatal movements in response to environmental stimuli play critical roles in controlling water status in plants (Grondin et al., 2015). To investigate the role of AtPIP2;1 in flg22-induced stomatal movements, we measured stomatal aperture in WT plants (Columbia-0 [Col-0]), the pip2;1 mutant, and plants expressing GFP-AtPIP2;1 before and after flg22 treatment. Stomatal aperture of Col-0 showed a pronounced linear decrease within 30 min of exposure to flg22, with 34.86% narrower apertures than the 0 min control after 40 min of treatment (Figure 1, A and B). A similar response was detected in GFP-AtPIP2;1 plants following flg22 treatment. In contrast, pip2;1 stomata displayed a much smaller decrease in aperture in response to flg22, decreasing by only 16.14% after 40 min of treatment. We also examined water loss in the plants, as shown in Supplemental Figure S1. In the WT and GFP-AtPIP2;1 lines, water loss decreased in response to flg22 treatment compared with the untreated control. In contrast, pip2;1 showed little change in water loss following flg22 treatment, indicating that AtPIP2;1 is involved in flg22-induced stomatal closure.
Figure 1.

AtPIP2;1 functions in flg22-induced immunity responses. A, Representative stomatal apertures of Col-0 (wild-type [WT]), GFP-AtPIP2;1, and pip2;1 plants with or without 1 μM flg22 treatment. Bar= 20 μm. B, Stomatal apertures of WT, GFP-AtPIP2;1, and pip2;1 plants undergoing 1 μM flg22-induced stomatal closure. Data were averaged from three separate experiments; n > 20 stomata from three seedlings per experiment. Error bars represent standard deviations. C, flg22-induced H+ flux in WT, GFP-AtPIP2;1, and pip2;1 guard cells. A continuous 12-min H+ flux recording was conducted for each cell in the test medium. Each point represents the mean value from 8 to 10 individual plants. The positive value indicates H+ efflux, and the negative value indicates H+ influx. The absolute value means the amplitude of H+ flux. Experiments were repeated independently at least twice, with similar results. Error bars represent the standard error of the mean. D, Fluorescence of the voltage-sensitive dye bis-oxonol in representative WT, GFP-AtPIP2;1, and pip2;1 guard cells with or without 1 μM flg22 treatment. Bar= 10 μm. E, Quantified fluorescent signals from the guard cells of each genotype with or without 1 μM flg22 treatment. Error bars represent standard deviations (n > 20). Statistical significance was determined using Student’s t test (***P < 0.001).
Stomatal closure involves the combined movements of ions and water across the PMs of guard cells and subsidiary cells. We therefore investigated the flux of H+ in the guard cells of WT, pip2;1, and GFP-AtPIP2;1 plants. Compared with the WT, the increase in H+ influx into guard cells was greater in GFP-AtPIP2;1 seedlings but attenuated in the pip2;1 mutant (Figure 1C; Supplemental Figure S2). Cells treated with mock (control) solution showed no significant changes, indicating that H+ influx is indeed induced by flg22 (Figure 1C; Supplemental Figure S2).
To further explore the physical changes in guard cells in response to flg22 treatment, we measured the membrane potential of guard cells using the voltage-sensitive dye bis (1,3-diethylthiobarbiturate acid) trimethine oxonol, as described previously (Guo et al., 2003). The fluorescent signals from Col-0 and GFP-AtPIP2;1 guard cells increased dramatically in response to flg22 treatment, suggesting that their membranes were substantially depolarized; however, no significant increase in fluorescence was observed in the guard cells of the pip2;1 mutant exposed to flg22 treatment (Figure 1, D and E). These results suggest that AtPIP2;1 is involved in maintaining water equilibrium between guard cells and subsidiary cells following the transmembrane diffusion of ions.
The primary response to flg22 is a change in AtPIP2;1 translocation rather than AtPIP2;1 expression
The water transport activity of AtPIP2;1 is regulated at multiple levels, including transcription, translation, and subcellular localization (Li et al., 2011; Hachez et al., 2013). To determine how flg22 influences AtPIP2;1, we measured the expression levels of AtPIP2;1 in plants treated with flg22 for 0, 15, or 30 min via reverse transcription quantitative PCR (RT-qPCR). No significant differences in AtPIP2;1 transcript levels were detected following flg22 treatment compared with untreated WT or GFP-AtPIP2;1 plants (Figure 2A).
Figure 2.
Effects of flg22 on the localization of GFP-AtPIP2;1 in guard cells. A, Relative AtPIP2;1 expression following 1 μM flg22 treatment determined by RT-qPCR, with ACTIN used as the reference gene. AtPIP2;1 expression levels for each genotype were calculated relative to the level before flg22 treatment. Experiments were independently repeated at least twice with similar results. Error bars represent the standard deviation. B, AtPIP2;1 protein levels following 1 μM flg22 treatment, as measured by immunoblot analysis. ACTIN was used as an internal control. C, Relative GFP-AtPIP2;1 contents (per unit of total protein). Error bars represent standard errors. D, Quantification of GFP-AtPIP2;1 fluorescence intensity per unit area in guard cells with or without flg22 treatment (n = 14 cell from three seedlings). E, Confocal images of GFP-AtPIP2;1 guard cells and subsidiary cells with or without flg22 treatment. Bar= 10 μm. F, Quantification of GFP-AtPIP2;1 endocytosis in guard cells or subsidiary cells, as estimated by the ratio of the average signal intensity in the cytosol (C) over the combined signal of the plasma membrane and cytosol (n = 15 cell from three seedlings). Experiments were repeated independently at least three times, with similar results. Error bars represent the standard deviation. Significant differences between the means were determined using Student’s t test (*P < 0.05).
To examine the changes in GFP-AtPIP2;1 abundance in response to flg22, we performed immunoblot analysis of total protein extracts from GFP-AtPIP2;1 plants with an anti-GFP antibody. In addition, to examine the relative abundance of GFP-AtPIP2;1 protein in guard cells, we measured the fluorescence intensity of GFP, which is proportional to the amount of AtPIP2;1 protein. Based on both assays, the level of GFP-AtPIP2;1 did not significantly change in response to 30 min of flg22 treatment (Figure 2, B–D).
Finally, we explored the effect of flg22 on the cellular localization of GFP-tagged AtPIP2;1 in Arabidopsis leaves. In untreated seedlings, the green fluorescent signal from GFP-AtPIP2;1 was mainly localized to the PM, which is consistent with previous findings (Li et al., 2011). However, when the seedlings were treated with flg22, significantly more endosomes were labeled with GFP in cotyledon guard cells compared with the control (Figure 2E). To quantify the internalization of GFP-AtPIP2;1, we calculated the ratio of fluorescence intensity in the cytoplasm related to that of the whole cell (PM + cytoplasm) as previously described, with some modifications (Du et al., 2013). These ratios were 69.3% (guard cells) and 53.9% (subsidiary cells) following flg22 treatment, which were significantly higher than the values under control conditions (54.4% and 44.7%, respectively; Figure 2F). These results suggest that flg22 treatment induced the relocalization of GFP-AtPIP2;1 into the cytoplasm. Together, these results indicate that the subcellular trafficking of AtPIP2;1, rather than transcriptional changes in AtPIP2;1 expression, is largely responsible for regulating the transmembrane diffusion of water during stomatal movements in response to flg22.
Spatiotemporal dynamics of AtPIP2;1 within the PM
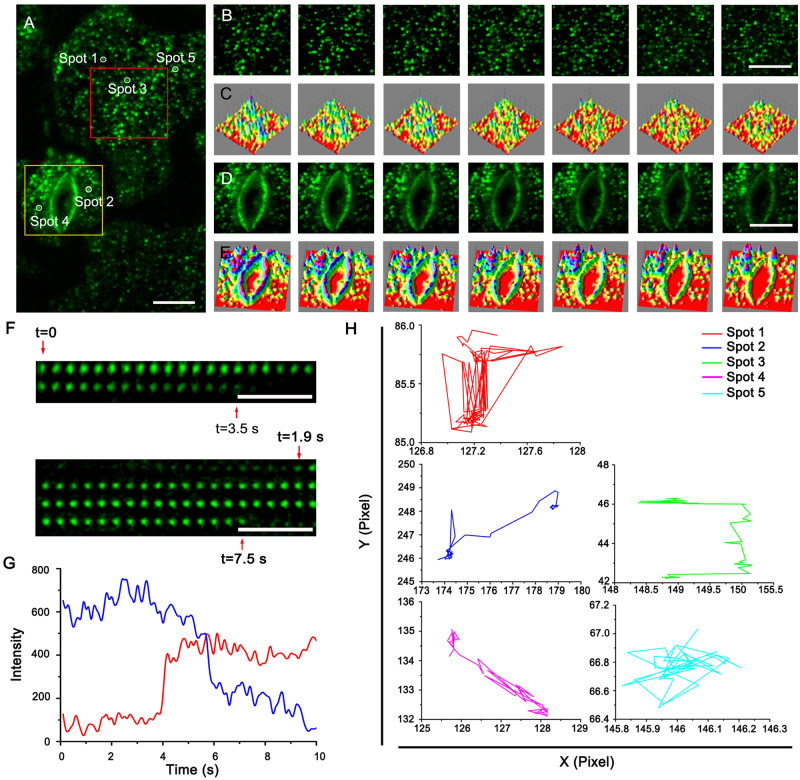
To further explore the role of AtPIP2;1 in water transport in guard and subsidiary cells, we analyzed the dynamics of AtPIP2;1 at single-particle resolution using TIRFM combined with a high-throughput, automated, unbiased analysis pipeline described by Wang et al. (2015). As demonstrated in several recent studies (Cui et al., 2018; Xue et al., 2018), TIRFM allows cellular proteins to be analyzed in a quantitative manner at nanometer spatial resolution and millisecond temporal resolution, making it ideally suited for studying cell surface processes. When the transgenic seedlings were viewed under TIRFM, GFP-AtPIP2;1 fluorophores appeared as small diffraction-limited fluorescent spots, which could be easily detected on the PMs of guard and subsidiary cells of the cotyledon (Figure 3A).
Figure 3.
Distribution and dynamics of GFP-AtPIP2;1. A, A typical single-particle image of GFP-AtPIP2;1 at the PM. Bar = 10 μm. B, C, Dynamic analysis (B) and three-dimensional luminance plots (C) using a time series of the subsidiary cell delimited by the red-boxed area in (A). Bar = 10 μm. D, E, Dynamic analysis (D) and three-dimensional luminance plots (E) using a time series of the guard cells delimited by the yellow boxed area in (A). Bar = 10 μm. F, GFP-AtPIP2;1 spots appeared at different times, stayed for shorter or longer periods of time, and then disappeared. Bar = 2 μm. G, Fluorescence intensity tracks of single GFP-AtPIP2;1 particles, showing their disappearance (the blue line) and appearance (the red line). H, Mean square displacement (MSD) analysis of various GFP-AtPIP2;1 particle trajectories measured from the spots indicated in (A).
To further examine the dynamic parameters of GFP-AtPIP2;1 particles at the PM, we took time-lapse series of GFP-PIP2;1 images with a 100-ms exposure time over a 10-s experimental period and analyzed the sequential images using the SPT algorithm. Most GFP-AtPIP2;1 fluorescent spots in both the guard cells and the subsidiary cells exhibited lateral diffusion within the imaging plane (Figure 3, B–E). Analysis of fluorescent signal intensities suggested that the GFP-AtPIP2;1 particles were present on the PM for a few seconds before rapidly disappearing (Figure 3, F and G).
We then examined the recycling of AtPIP2;1 using Brefeldin A (BFA) as previously described (Cui et al. 2018; Zhu et al. 2020). Following BFA treatment, GFP-AtPIP2;1 fluorescence accumulated in BFA bodies that were co-stained with the endocytic tracer FM4-64 (Supplemental Figure S3), confirming the notion that AtPIP2;1 undergoes constitutive endocytosis. In addition, other GFP-AtPIP2;1 fluorescent spots came into the focal plane during the observation period (Figure 3G). By tracking the diffusion trajectories of the fluorescent spots in the PM, we found that GFP-AtPIP2;1 exhibited multiple types of motion (Figure 3H).
AtPIP2;1 particle dynamics during water translocation
We were interested in determining whether the dynamic behavior of AtPIP2;1 differs between periods of water influx and efflux. Therefore, we monitored the dynamic characters of GFP-AtPIP2;1 in both guard cells and subsidiary cells following flg22 treatment. We measured the velocity and diffusion coefficients of AtPIP2;1 in plants with or without flg22 treatment based on the trajectories of GFP-AtPIP2;1 particles. The data were plotted and fitted to curves using Gaussian functions, in which the Gaussian peaks (represented as Ĝ) were considered to represent the characteristic velocity and diffusion coefficients.
In guard cells, the velocity of GFP-AtPIP2;1 particles significantly increased in response to flg22 treatment (control: 0.44 ± 0.014 μm s−1; flg22-treated: 0.52 ± 0.023 μm s−1; Figure 4, A and B). In addition, the Ĝ value of diffusion coefficients increased to 2.09 × 10−3μm2 s−1 (se: 1.98–2.20 × 10−3μm2 s−1) following flg22 treatment, whereas this value was only 0.87 × 10−3 μm2 s−1 (se: 0.76–0.98 × 10−3μm2 s−1) in the resting state (Figure 4, C and D). In contrast, in subsidiary cells, the velocity of GFP-AtPIP2;1 particles was not significantly affected by flg22 treatment (control: 0.43 ± 0.018; flg22-treated: 0.41 ± 0.015 μm s−1; Figure 4, E and F). Moreover, the diffusion coefficient appeared to slightly decrease in response to flg22 treatment, but this change was not significant (control: 1.15 × 10−3μm2 s−1, se: 0.93–1.37 × 10−3μm2 s−1; flg22: 1.01 × 10−3μm2 s−1, se: 0.89–1.13 × 10−3μm2 s−1; Figure 4, G and H). These data indicate that the changes in the lateral movements of GFP-AtPIP2;1 particles were different in guard cells versus subsidiary cells during stomatal closure in response to flg22. Finally, to exclude the possible side effects of flg22 treatment, we treated cells with flg22Δ2, a flg22-derived peptide lacking the agonist activity of flg22 (Danna et al., 2011). Under this treatment, neither the velocity nor the diffusion coefficients of AtPIP2;1 particles were significantly altered compared with untreated plants (Figure 4, B, D, F, and H).
Figure 4.
Effects of flg22 on GFP-AtPIP2;1 dynamics in guard cells and subsidiary cells. A, Distribution of the velocity of GFP-AtPIP2;1 particles in guard cells under 1 μM flg22 and flg22Δ2 treatment; flg22Δ2 is a flg22-derived peptide lacking agonist activity. B, The average velocity of GFP-AtPIP2;1 particles in guard cells under 1 μM flg22 and flg22Δ2 treatment. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. C, Distribution of the diffusion coefficients of GFP-AtPIP2;1 particles in guard cells under 1 μM flg22 and flg22Δ2 treatment. D, Diffusion coefficients of GFP-AtPIP2;1 particles in guard cells under 1 μM flg22 and flg22Δ2 treatment. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. E, Distribution of the velocity of GFP-AtPIP2;1 particles in subsidiary cells under 1 μM flg22 and flg22Δ2 treatment. F, The average velocity of GFP-AtPIP2;1 particles in subsidiary cells under 1 μM flg22 and flg22Δ2 treatment. No significant differences were identified using one-way ANOVA. Error bars represent the standard deviation. G, Distribution of the diffusion coefficients of GFP-AtPIP2;1 particles in subsidiary cells under 1 μM flg22 and flg22Δ2 treatment. H, Diffusion coefficients of GFP-AtPIP2;1 particles in subsidiary cells under 1 μM flg22 and flg22Δ2 treatment. No significant differences were determined using one-way ANOVA. Error bars represent the standard deviation.
Besides lateral diffusion, some fluorescent spots remained at the cell surface, while others gradually moved out of the focal plane. We therefore analyzed the dwell time of AtPIP2;1 particles on the PMs of guard cells and subsidiary cells. The dwell time is defined as the duration in which a protein remains at the cell surface prior to endocytosis (Flores-Otero et al., 2014). The dwell times of AtPIP2;1 particles on the PM were affected by flg22 treatment in a cell type-specific manner. When the frequency distribution was fitted with an exponential function, the dwell time (represented as τ value) was 2.39 s in guard cells treated with flg22 (Figure 5, B and C), which was significantly higher than the τ value of AtPIP2;1 particles under control conditions (2.17 s; Figure 5, A and C). In contrast, in subsidiary cells, the τ value slightly but significantly decreased in response to flg22 treatment (control: 2.29 s; flg22-treated cells: 2.21 s; Figure 5, D–F). These results indicate that the lifetime of AtPIP2;1 particles on the PM was prolonged in guard cells and shorter in subsidiary cells during stomatal closure in response to flg22.
Figure 5.
Flg22 induces cell type-specific dwell times for GFP-AtPIP2;1 in guard cells and subsidiary cells. A, B, Representative kymographs and traces of normalized fluorescent signals showing individual GFP-AtPIP2;1 dwell times in guard cells in the presence of mock solution (Control; A) and flg22 (B). Bar = 6 s. C, Dwell times in guard cells treated with mock solution and flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. D, E, Representative kymographs and traces of normalized fluorescent signals showing individual GFP-AtPIP2;1 dwell times in subsidiary cells in the presence of mock solution (Control; D) and flg22 (E). Bar = 6 s. F, Dwell times in subsidiary cells treated with mock solution and flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation.
Disrupting the cytoskeleton affects AtPIP2;1 diffusion
We then explored whether the mechanisms regulating AtPIP2;1 dynamics were different in guard cells versus subsidiary cells. The cytoskeleton forms highly organized arrays that affect stomatal morphogenesis (Galatis and Apostolakos, 2004). To determine whether the cytoskeleton influences the mobility of PM proteins, we used oryzalin (Lv et al., 2017) or Lat B (McKenna et al., 2019) treatments to depolymerize the microtubules or microfilaments, respectively.
Compared with GFP-AtPIP2;1 particles in untreated seedlings, the particle density and size significantly increased in response to oryzalin treatment (Figure 6, A–E). Furthermore, the velocity of AtPIP2;1 particles changed from 0.44 ± 0.014 μm s−1 in untreated cells to 0.6 ± 0.026 μm s−1 after oryzalin treatment (Figure 6F; Supplemental Figure S4). Moreover, the diffusion coefficient of AtPIP2;1 particles increased to 2.34 × 10−3μm2 s−1 (se: 2.14–2.54 × 10−3μm2 s−1) compared with 0.87 × 10−3μm2 s−1 in the untreated control (se: 0.76–0.98 × 10−3μm2 s−1; Figure 6G; Supplemental Figure S4). When seedlings were treated with both oryzalin and flg22 (Figure 6, F and G), the velocity of AtPIP2;1 particles increased to 0.89 ± 0.032 μm s−1 and the diffusion coefficient increased to 7.59 × 10−3μm2 s−1 (se: 7.39–7.79 × 10−3μm2 s−1), representing an increase of 71.2% and 263%, respectively, compared with seedlings treated with flg22 alone.
Figure 6.
The dynamics of GFP-AtPIP2;1 particles in guard cells following Lat B and oryzalin treatment. A–C, Single-particle images showing the fluorescence intensity of GFP-AtPIP2;1 on the PM in cells treated with mock solution (Control; A), Lat B (B), and oryzalin (C). Bar = 10 μm. D, GFP-AtPIP2;1 particle density at the PM in leaf epidermal cells treated with mock solution, Lat B, or oryzalin. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. E, Average size of GFP-AtPIP2;1 fluorescent spots under control, Lat B, and oryzalin treatment. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. F, Frequency of the velocity of GFP-AtPIP2;1 particles in guard cells treated with mock solution, Lat B, Lat B + flg22, oryzalin, or oryzalin + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. G, Diffusion coefficients of GFP-AtPIP2;1 particles in guard cells treated with mock solution, Lat B, Lat B + flg22, oryzalin, or oryzalin + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation.
In cells treated with Lat B alone for 30 min, the velocity of GFP-AtPIP2;1 particles was 0.54 ± 0.012 μm s−1 (Figure 6F) and the diffusion coefficient was 1.91 × 10−3μm2 s−1 (Figure 6G); both values were significantly higher than those of untreated guard cells. However, the changes after Lat B treatment were less pronounced than those after oryzalin treatment. We also analyzed GFP-AtPIP2;1 dynamics in seedlings treated with both Lat B and flg22. Unexpectedly, the velocity (0.54 ± 0.019 μm s−1) and diffusion coefficient (2.19 × 10−3μm2 s−1) of AtPIP2;1 particles were not significantly altered compared with the values in cells treated with flg22 alone (Figure 6, F and G). Together, these results indicate that the microtubule cytoskeleton, and not actin microfilaments, plays a crucial role in regulating the dynamics of AtPIP2;1 particles in guard cells, not only in the resting state but also during stomatal closure in response to flg22.
Next, we examined the role of the cytoskeleton in GFP-AtPIP2;1 dynamics in subsidiary cells using kymograph analysis. Under both oryzalin and Lat B treatment, the lines of the kymographs appeared curved (Figure 7, A–C), indicating that the lateral motion of the AtPIP2;1 particles became more active under these treatments. Statistical analysis showed that the changes in the diffusion coefficient and velocity were greater in response to Lat B treatment versus oryzalin treatment. Specifically, the diffusion coefficient increased by 94.78% versus 44.35% and the velocity increased by 11.60% versus 4.65% in response to Lat B versus oryzalin treatment, respectively (Figure 7D; Supplemental Figure S5). These results suggest that the lateral diffusion of AtPIP2;1 in subsidiary cells is mainly confined by actin microfilaments under resting conditions.
Figure 7.
The cytoskeleton affects the dynamics of AtPIP2;1. A–C, Kymographs displaying fluorescence intensity along a predefined path over time. In seedlings treated with mock solution (Control; A), Lat B (B), and oryzalin (C), GFP-AtPIP2;1 particles were detected with an exposure time of 250 ms. Bar = 6 s. D, Frequency of the velocity of GFP-AtPIP2;1 particles in subsidiary cells treated with mock solution, Lat B, Lat B + flg22, oryzalin, or oryzalin + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. E, Diffusion coefficients of GFP-AtPIP2;1 particles in subsidiary cells treated with mock solution, Lat B, Lat B + flg22, oryzalin, or oryzalin + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. F, Time course of water loss from GFP-AtPIP2;1 seedlings treated with oryzalin and Lat B; the loss of fresh weight (FW, %) was used to indicate water loss. Data were averaged from three separate experiments. Error bars represent the standard deviation.
Interestingly, in seedlings treated with both oryzalin and flg22, the distributions of AtPIP2;1 velocity were fitted to a two-subgroup diffusion model, where the higher group represented rather highly mobile molecules (Figure 7D; Supplemental Figure S5). Moreover, the diffusion coefficient in these seedlings increased more than three-fold compared with the value in response to flg22 treatment alone (Figure 7E; Supplemental Figure S5 and S6). A similar effect was observed for seedlings treated with both LatB and flg22. Both the velocity (0.47 μm s−1) and diffusion coefficients (2.09 × 10−3μm2 s−1) significantly increased in these seedlings compared with seedlings treated with flg22 alone. However, the increased amplitude was lower than that of seedlings treated with oryzalin and flg22. Together, these results indicate that microtubules are important regulators of AtPIP2;1 dynamics in subsidiary cells during stomatal closing in response to flg22.
To determine whether the observed changes in AtPIP2;1 dynamics could affect water loss, we measured water loss in various plants. GFP-AtPIP2;1 plants treated with flg22 showed 50% water loss over the course of 3 h, whereas plants treated with cytoskeleton inhibitors together with flg22 showed a greater degree of water loss (67% water loss for Lat B plus flg22 treatment; 75% water loss for oryzalin plus flg22 treatment; Figure 7F). These results suggest that the cytoskeleton and especially microtubules, which are involved in regulating AtPIP2;1 dynamics, play important roles in water loss in response to flg22.
Effect of plant cell walls on AtPIP2;1 movement
The cytoskeleton and cell wall can be thought of as a continuum on the PM (McKenna et al., 2019). To investigate whether the cell wall affects AtPIP2;1 diffusion, we disturbed the organization of the cell wall using epigallocatechin gallate (EGCG) and the herbicide isoxaben, which inhibit native pectin methylesterase and cellulose synthases, respectively (Martinière et al., 2012; McKenna et al., 2019). To estimate the physical limitations associated with the cell wall, we calculated water loss by plotting the reduction in fresh weigh versus time. As shown in Supplemental Figure S7, in seedlings treated with EGCG/isoxaben plus flg22, water loss increased compared with flg22 treatment (63% water loss for EGCG plus flg22 treatment; 60% water loss for isoxaben plus flg22 treatment), suggesting that flg22-induced stomatal closure slowed slightly when the cell wall organization was disturbed.
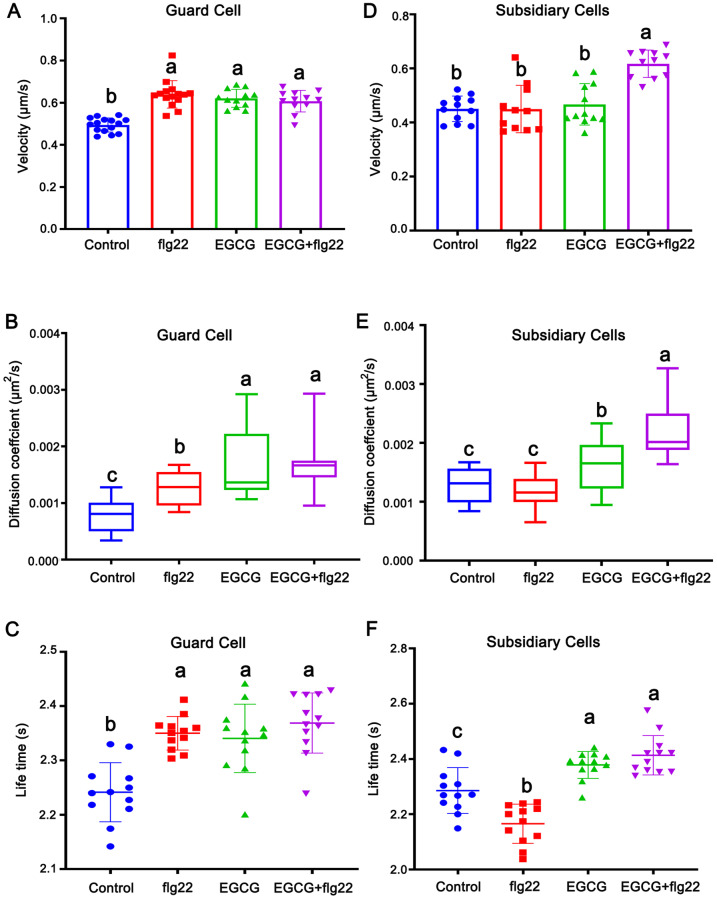
We then directly addressed the role of the cell wall in AtPIP2;1 dynamics in guard cells and subsidiary cells. Compared with untreated guard cells, as shown in Figure 8, A and B, a greater than two-fold increase in the mean value of diffusion coefficient was detected in cells treated with EGCG. The velocity also significantly increased (by 25.5%) after EGCG treatment. We then analyzed AtPIP2;1 dynamics in guard cells treated with EGCG and flg22. Intriguingly, the diffusion coefficient slightly but significantly increased in response to this treatment compared with flg22 treatment, whereas the velocity did not significantly change, suggesting that disturbing the cell wall changed the diffusion area of AtPIP2;1 particles (Figure 8, A and B). Similar effects were observed for isoxaben treatment (Supplemental Figure S8, A and B). These results confirm the notion that walls of guard cells primarily participate in confining the motion area of AtPIP2;1 particles and have limited effects on velocity, especially during stomatal movement in response to flg22.
Figure 8.
Dynamics of GFP-AtPIP2;1 particles at the PM under EGCG treatment. A, Frequency of the velocity of GFP-AtPIP2;1 particles in guard cells treated with mock solution (Control), EGCG, or EGCG + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. B, Diffusion coefficients of GFP-AtPIP2;1 particles in guard cells treated with mock solution, EGCG, or EGCG + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. C, Dwell times of AtPIP2;1 in guard cells treated with mock solution, EGCG, or EGCG + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. D, Frequency of the velocity of GFP-AtPIP2;1 particles in subsidiary cells treated with mock solution, EGCG, or EGCG + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. E, Diffusion coefficients of GFP-AtPIP2;1 particles in subsidiary cells treated with mock solution, EGCG, or EGCG + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation. F, Dwell times of AtPIP2;1 in subsidiary cells treated with mock solution, EGCG, or EGCG + flg22. Different letters indicate significant differences (P < 0.05) by one-way ANOVA. Error bars represent the standard deviation.
We then investigated the role of the cell wall in AtPIP2;1 dynamics in subsidiary cells. In seedlings treated with EGCG, the diffusion coefficient increased by 25.6% compared with the control, although the velocity was not significantly altered by this treatment. However, in seedlings treated with EGCG plus flg22, the diffusion coefficient of AtPIP2;1 particles increased by 88.7% and the velocity increased by 37.3% compared with the seedlings treated with flg22 alone (Figure 8, D and E). We further validated this phenomenon using isoxaben, finding that the changes were similar to those after EGCG treatment, although the values were not exactly the same (Supplemental Figure S8, D and E).
Finally, we analyzed the dwell time of AtPIP2;1 particles on the PM. EGCG as well as isoxaben treatment increased the dwell time in both guard cells and subsidiary cells in the resting state (Figure 8, C and F; Supplemental Figure S8, C and F). In addition, when combined with flg22 treatment, the dwell time of AtPIP2;1 particles in guard cells was not significantly altered. However, the dwell time was slightly but significantly prolonged in subsidiary cells in response to this treatment. Together, these results indicate that the movements of AtPIP2;1 particles in subsidiary cells are related to the cell wall, especially during stomatal closure in response to flg22, which is different from the situation for AtPIP2;1 particles in guard cells.
Discussion
Plant growth and development are dependent on the regulated movement of water (Chaumont and Tyerman, 2014). The diffusion of water across the cell membrane is tightly controlled by the number and activity of water channels known as aquaporins. In the current study, we demonstrated that flg22 treatment induces stomatal closure; however, this did not occur in the pip2;1 mutant, suggesting that aquaporins play a central role in stomatal movements, which is consistent with previous findings (Rodrigues et al., 2017; Ding and Chaumont, 2020). Stomatal opening and closure are driven by ion fluxes in guard cells, which are in turn mediated by electrogenic proton pumps in the PM (Shimazaki et al., 2007). Here, we examined transmembrane ion flux in guard cells using a noninvasive microtest. In WT (Col-0) plants, flg22 significantly affected the efflux of H+ out of the guard cell cytoplasm; however, this process was significantly repressed in pip2;1 guard cells. Using the voltage-sensitive dye bis-oxonol, we showed that flg22 induces the depolarization of guard cell membranes in WT but not pip2;1 plants. This observation is consistent with the finding that in maize (Zea mays), guard cells are depolarized during stomatal closure (Roelfsema et al., 2001), whereas transient hyperpolarization occurs in subsidiary cells (Mumm et al., 2011).
Chevalier and Chaumont (2015) reported that PIPs are trafficked through the secretory pathway under normal conditions, whereas abiotic stress conditions modify this subcellular PIP transport pattern. Here, we analyzed AtPIP2;1 trafficking under flg22 treatment, which represents a biotic stress, finding that this stimulus induced the relocalization of GFP-AtPIP2;1. We compared the fluorescence intensity of GFP-AtPIP2;1 in the guard cell cytoplasm relative to whole cell (PM + cytoplasm). This ratio was significantly higher in flg22-treated plants than the untreated controls, suggesting that flg22 induces the internalization of AtPIP2;1. Neither AtPIP2;1 expression levels nor AtPIP2;1 protein levels were significantly affected by 30 min of flg22 treatment. Based on these results, we conclude that the relocalization of AtPIP2;1, rather than changes in AtPIP2;1 gene or protein expression, is responsible for regulating water transport during flg22-induced stomatal closure.
Aquaporins participate in stomatal movement via mediating bulk water efflux in the guard cells as well as mediating water influx in the subsidiary cells. Therefore, the stomate is an excellent system for studying AtPIP2;1 dynamics during bidirectional transport. Protein dynamics are closely involved in many fundamental biological processes (Shinozaki et al., 2009; Zhang et al., 2009). The use of VA-TIRFM techniques to study membrane protein dynamics in Arabidopsis guard cells and subsidiary cells could provide insight to help decipher these complex systems in other tissues and organisms. This technique has a high signal-to-noise ratio, which allowed us to examine the dynamics of membrane proteins at the single-particle level (Konopka et al., 2008; Hao et al., 2014; Fan et al., 2015; Yu et al., 2017). Sequential VA-TIRFM observations revealed numerous GFP-AtPIP2;1 particles in guard cells and subsidiary cells, which were visualized as well-dispersed diffraction-limited fluorescent particles (Figure 3, A–E). In addition, some GFP-AtPIP2;1 particles appeared to “drop off” from the PM, while others appeared to “rise up” from the cytosol, revealing the exocytosis or endocytosis of PM components. In addition, most GFP-AtPIP2;1 particles diffused laterally across the PM for several seconds in both guard cells and subsidiary cells. These results suggest that GFP-AtPIP2;1 was mobile and distributed in guard cells and subsidiary cells in a highly heterogeneous manner.
The lateral diffusion of GFP-AtPIP2;1 particles along the guard cell PM markedly increased in response to flg22 treatment. In contrast, in subsidiary cells, the velocity and diffusion coefficients of GFP-AtPIP2;1 particles were not affected by flg22 treatment. In addition, flg22 induced contrasting changes in the dwell time of AtPIP2;1 in guard cells versus subsidiary cells. On the basis of these observations, we suggest that the association between the movements of AtPIP2;1 and the bidirectional transport of water is regulated in a cell type-specific manner.
Over the past few years, many studies have uncovered a role for the cytoskeleton in stomatal movement. During stomatal opening under white light, the actin filaments below the periclinal walls are radially arranged around the stomatal pore, resembling the arrangement of microtubules (Galatis and Apostolakos, 2004). In contrast, when stomata close in the dark or in response to other stimuli, the radial actin filaments disintegrate (Eun and Lee, 2000, Hwang and Lee, 2001). The cytoskeleton also influences the lateral mobility of PM proteins in plant cells (Bücherl et al., 2017). In the present study, we demonstrated that disturbing the organization of the cytoskeleton using specific inhibitors increased the velocity and diffusion coefficient of AtPIP2;1 in both guard cells and subsidiary cells. Intriguingly, in guard cells, the effects of microtubules on regulating AtPIP2;1 dynamics were more pronounced than those of actin. However, in subsidiary cells, actin filaments had stronger effects than microtubules on regulating the diffusion of AtPIP2;1 under normal growth conditions.
We then monitored the effects of disrupting microfilament or microtubule organization using Lat B or oryzalin, respectively, combined with flg22 treatment. The changes of velocity and diffusion coefficients in guard cells indicate that the dynamics of AtPIP2;1 are primarily regulated by microtubules rather than microfilaments during stomatal closure in response to flg22, which is consistent with their regulation under normal conditions. Furthermore, in subsidiary cells, the changes in the velocity and diffusion coefficients of AtPIP2;1 were more pronounced in plants treated with oryzalin plus flg22. These findings demonstrate that microtubules play a primary role in regulating AtPIP2;1 dynamics during stomatal closure, in contrast to their regulation under normal conditions. Analysis of stomatal closure (represented by water loss) further confirmed the roles of microtubules and microfilaments in regulating AtPIP2;1 dynamics. More importantly, the dynamic regulation of AtPIP2;1 dynamics by microtubules and actin microfilaments in guard cells is distinct from that in subsidiary cells.
In addition to the cytoskeleton (microtubules and microfilaments), the cell wall is also responsible for restricting the lateral mobility of PM proteins in plant cells (Martinière et al., 2012; McKenna et al., 2019), which is also detected in AtPIP2;1 dynamics under resting condition. We also determined that the diffusion area of AtPIP2;1, but not its velocity, increased when the cell wall was disturbed in plants under flg22 treatment. These findings suggest that the cell wall is mainly involved in constraining the diffusion of this protein along the guard cell PM. In contrast, the dynamics of AtPIP2;1 in the subsidiary cells significantly changed when the cell wall was disordered both with and without flg22 treatment. In addition, the changes in the dwell time of AtPIP2;1 in guard cells and subsidiary cells under various conditions were consistent with the changes in diffusion characters. Our analysis of water loss from seedlings under different treatments further demonstrated that the cell wall is also involved in regulating stomatal movement, although this effect is less significant compared with the cytoskeleton.
Collectively, our single-molecule analysis provided new insight into the regulation of AtPIP2;1 dynamics at the PM at unprecedented spatial and temporal resolution. Our results suggest that the subcellular localization of AtPIP2;1 is regulated by flg22-induced regulatory signals. Using SPT analysis, we demonstrated that the partitioning and dynamics of AtPIP2;1 are cell type-specific. In addition, in guard cells, microtubules play primary roles in regulating AtPIP2;1 dynamics at the PM in both resting and closing stomata. However, in subsidiary cells, the primary regulators of AtPIP2;1 dynamics are microfilaments under normal conditions and microtubules during stomatal closure. Although the cell wall structure constrains the diffusion of AtPIP2;1 protein in both guard cells and subsidiary cells, the effect of the cell wall is less pronounced compared with the cytoskeleton. Based on these results, we conclude that AtPIP2;1-related stomatal movement plays a positive role in pathogen-associated molecular pattern-triggered immunity and that AtPIP2;1 dynamics are regulated in a cell type-specific manner.
Materials and methods
Plant materials
All experiments were performed using the Arabidopsis (A. thaliana) ecotype Columbia-0 (Col-0), the aquaporin mutant pip2;1 (CS813476), and the GFP-AtPIP2;1 line (Li et al., 2011). Before being sown on agar plates, the seeds were surface-sterilized for 30 s in 85% EtOH/H2O2. The seeds were grown vertically on half-strength Murashige and Skoog (1/2MS) medium solidified with 1% (w/v) agar (pH 5.8) and stratified at 4°C in the dark for 2 d. The plants were grown at 22°C under a 16-h/8-h light/dark cycle on 1/2 MS plates.
Drug treatments
The flagellin peptide flg22 or flg22Δ2 was synthesized by GL Biochem (Shanghai) Ltd. and used at a concentration of 1 μM (stock solution was 1 mM in ddH2O). The inhibitor BFA (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO) to make a 50 mM stock solution. FM4-64 was acquired from Molecular Probes (5 mM water stock). Lat B (Sigma-Aldrich) was dissolved in DMSO to make a 5-mM stock solution. Oryzalin (Sigma-Aldrich) was dissolved in 100% ethanol to make a 10-mM stock solution. EGCG (Sigma-Aldrich) and isoxaben (Sigma-Aldrich) were dissolved in ddH2O to prepare a 5-mM stock solution and a 2 mM stock solution, respectively. The inhibitors were diluted in liquid 1/2 MS medium, and the final DMSO concentration was 0.1% (v/v) in all experiments. The mock treatments used only ½MS medium. Vertically grown 4-d-old seedlings were incubated in ½MS medium containing 1 μM flg22 or flg22Δ2 for 15 min for TIRF detection. For treatment with BFA, the seedlings were incubated in ½MS medium containing 50 μM BFA for 45 min. For treatment with Lat B or oryzalin, the seedlings were incubated in ½MS medium containing 5 μM Lat B or 10 μM oryzalin for 30 min. For treatment with EGCG or isoxaben, the seedlings were incubated in ½MS medium containing 50 μM EGCG or 20 μM isoxaben for 60 min.
Stomatal aperture measurement
Measurements of the stomatal aperture were performed as described previously (Suhita et al., 2004). Briefly, plants were kept under light for 2 h to ensure that most stomata were opened before treatment. The stomata of WT, GFP-AtPIP2;1, and pip2;1 were imaged at 10, 20, 30, 40, 50, and 60 min after treatment with 1 μM flg22 using a microscope. Then, the stomatal aperture was measured using ImageJ software. At least 20 stomata were measured from each seedling. The ratio of transverse length to longitudinal length was calculated and used as an index of stomatal opening.
Measurements of membrane potential
Arabidopsis leaves were incubated in loading buffer (5 mM Mes-KOH, pH 5.7, 0.25 mM KCl, and 1 mM CaCl2) containing 1 μM dye (bis-[1,3-dibutylbarbituric acid] trimethine oxonol [B-438; Molecular Probes, Eugene, OR]) for 10 min. B-438 fluorescent signals were detected using a confocal microscope. The dye was excited using a fluorescein isothiocyanate filter set (488 nm), and images were collected using an emission filter (530 ± 15 nm; Guo et al., 2003). The fluorescence intensity was quantified with ImageJ software.
Measurement of net H+ fluxes
The net H+ fluxes were measured as described by Yan et al. (2015). The ion-selective electrodes were constructed as follows: prepulled and silanized glass micropipettes (2–4-μm aperture) were filled with a backfilling solution (H+: 40 mM KH2PO4 and 15 mM NaCl, pH 7.0) to about 1 cm from the tip. Then the micropipettes were front-filled with selective liquid ion exchange cocktails to a column length of approximately 25 μm. The procedure of measuring the ion flux was divided into three periods. The basic ion flux measurement took place before the addition of flg22, defined here as the pre-exposure period. The period between the addition of flg22 and the point at which the ion flux returned to the baseline was defined as the peak response period. The subsequent period, during which the recovered ion-flux phase remained stable, was defined as the postexposure period.
Confocal laser scanning microscopy and image analysis
Confocal microscopy was performed on an Olympus FV10-ASW 3.0 microscope fitted with a 60× water-immersion objective. Confocal imaging of Arabidopsis cotyledons of 4-d-old seedlings expressing GFP was performed via excitation with 488-nm light and detection of fluorescence emissions at 505–545 nm. The image analysis was performed using the Olympus FV10-ASW 3.0 software package and ImageJ software (NIH).
Reverse transcription quantitative PCR
Total RNA was extracted from 10-d-old whole seedlings, which had been incubated in half-strength liquid MS medium containing 1 μM flg22 for 0, 15, and 30min, using a Qiagen RNeasy plant mini kit with an on-column DNase treatment (RNase-free DNAse; Qiagen). Reverse transcription was performed using a First Strand cDNA Synthesis kit (TaKaRa) and an oligo (dT) primer. RT-qPCR was performed using SYBR Green Sensimix (Quantace) on a Roche Light Cycler 480 apparatus. The 384-well optical reaction plates were heated to 95°C for 1 min, followed by 40 cycles of denaturation at 95°C for 5 s, annealing at 62°C for 8 s, and an extension at 72°C for 30 s. The AtPIP2;1 primer sequences were 5′-GTCTACTGCACCGCCGGTAT-3′ and 5′-GTTGGCTCCACCTCCGTAAC-3′.
Immunoblot analysis
Ten-day-old seedlings expressing GFP-AtPIP2;1 were incubated in 1/2 MS containing 1 μM flg22 for 15 and 30 min. The total proteins from whole seedlings were extracted in 2× extraction buffer. The proteins were separated on an 8% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane, which was then blotted with an anti-GFP antibody. The intensity of each band was detected with ImageJ and normalized with respect to the control.
VA-TIRFM and single-particle fluorescence image analysis
The GFP-AtPIP2;1 signal from Arabidopsis cotyledons of 4-d-old seedlings was recorded using the VA-TIRFM technique with an inverted microscope (IX-71; Olympus), a total internal reflective fluorescence illuminator, and a 100× oil-immersion objective (Olympus; numerical aperture = 1.45). The position of the GFP-AtPIP2;1 particles was determined by calculating the weighted-centroid with subpixel accuracy following determination of the local maxima with a mask of 3 × 3 pixels. Any spot with a peak pixel very close to another spot (<3 pixels) was not used in the calculation. The SPT method described by Cui et al. (2018) was employed to analyze the kinetic parameters of GFP-AtPIP2;1. The dwell time, velocity, and diffusion coefficients were determined according to the methods previously described by Cui et al. (2018). The single GFP-AtPIP2;1 particles were detected in a time-lapse series of up to 100 images per sequence, which were acquired using a 100-ms exposure time.
Water loss assays
For water loss measurements of WT GFP-AtPIP2;1 and pip2;1 mutant plants, the 2-week-old plants were exposed to cool white light (125 μmol m−2·s−1) at 25°C and 50% relative humidity. The seedlings were treated with inhibitor after 30 min, and weighed at 20 min time intervals. The loss of fresh weight (%) was used to indicate water loss.
Accession numbers
Sequence data from this article can be found in the Arabidopsis Information Resource (TAIR) database under the following accession numbers: AtPIP2;1 (AT3G53420).
Supplemental data
Supplemental Figure S1. Time courses of water loss from Col-0, GFP-AtPIP2;1, and pip2;1 plants before and after flg22 treatment.
Supplemental Figure S2. Net H+ fluxes in the guard cells of Col-0, GFP-AtPIP2;1, and pip2;1 plants.
Supplemental Figure S3. The internalization of AtPIP2;1 under BFA treatment.
Supplemental Figure S4. Effects of cytoskeleton inhibitors on the dynamics of GFP-AtPIP2;1 particles in guard cells.
Supplemental Figure S5. Effects of cytoskeleton inhibitors on the dynamics of GFP-AtPIP2;1 particles in subsidiary cells.
Supplemental Figure S6. Disrupting the microtubule affects the trajectories of GFP-AtPIP2;1 in Arabidopsis.
Supplemental Figure S7. Time courses of water loss from GFP-AtPIP2;1 after cell wall inhibitor treatments.
Supplemental Figure S8. Dynamics of GFP-AtPIP2;1 at the PM under isoxaben treatment.
Supplementary Material
Acknowledgments
We thank Yan-Li Zhang (Imaging Core Facility of Protein Research Center for Technology Development, Tsinghua University) for FV1200 LSCM technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (91954202, 31871349, 31622005, 32000483), Beijing Forestry University Outstanding Young Talent Cultivation Project (2019JQ03003).
Conflict of interest statement. None of the authors have any conflict of interest.
X.J.L. and J.X.L. designed the project; Y.N.C., Y.X.Z., and Y.Q.L. performed the experiments; Y.Y.C. and Y.B.S. helped with some experiments; Y.N.C., Y.X.Z., and X.S. analyzed the data; Y.N.C. and X.J.L. wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Author (https://academic.oup.com/plphys/pages/general-instructions) is: Xiaojuan Li (lixj@bjfu.edu.cn).
References
- Buchsenschutz K, Marten I, Becker D, Philippar K, Ache P, Hedrich R (2005) Differential expression of K+ channels between guard cells and subsidiary cells within the maize stomatal complex. Planta 222: 968–976 [DOI] [PubMed] [Google Scholar]
- Bücherl CA, Jarsch IK, Schudoma C, Segonzac C, Mbengue M, Robatzek S, MacLean D, Ott T, Zipfel C (2017) Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. Elife 6: e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Tyerman SD (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164: 1600–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, van Hoek AN, Yeager M, Verkman AS, Mitra AK (1997) Three-dimensional organization of a human water channel. Nature 387: 627–630 [DOI] [PubMed] [Google Scholar]
- Chevalier AS, Chaumont F (2015) Trafficking of plant plasma membrane aquaporins: multiple regulation levels and complex sorting signals. Plant Cell Physiol 56: 819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Li X, Yu M, Li R, Fan L, Zhu Y, Lin J (2018) Sterols regulate endocytic pathways during flg22-induced defense responses in Arabidopsis. Development 145: dev165688. [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang X, Yu M, Zhu Y, Xing J, Lin J (2019) Techniques for detecting protein-protein interactions in living cells: principles, limitations, and recent progress. Sci China Life Sci 62: 619–632 [DOI] [PubMed] [Google Scholar]
- Danna C, Millet Y, Koller T, Han S, Bent A, Ronald P, Ausubel F (2011) The Arabidopsis flagellin receptor FLS2 mediates the perception of Xanthomonas Ax21 secreted peptides. Proc Natl Acad Sci USA 108: 9286–9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Chaumont F (2020) Are aquaporins expressed in stomatal complexes promising targets to enhance stomatal dynamics?. Front Plant Sci 11: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Tejos R, Beck M, Himschoot E, Li H, Robatzek S, Vanneste S, Friml J (2013) Salicylic acid interferes with clathrin-mediated endocytic protein trafficking. Proc Natl Acad Sci USA 110: 7946–7951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun SO, Lee Y (2000) Stomatal opening by fusicoccin is accompanied by depolymerization of actin filaments in guard cells. Planta 210: 1014–1017 [DOI] [PubMed] [Google Scholar]
- Fan L, Li R, Pan J, Ding Z, Lin J (2015) Endocytosis and its regulation in plants. Trends Plant Sci 20: 388–397 [DOI] [PubMed] [Google Scholar]
- Flores-Otero J, Ahn KH, Delgado-Peraza F, Mackie K, Kendall DA, Yudowski GA (2014) Ligand-specific endocytic dwell times control functional selectivity of the cannabinoid receptor 1. Nat Commun 5: 4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatis B, Apostolakos P (2004) The role of the cytoskeleton in the morphogenesis and function of stomatal complexes. New Phytol 161: 613–639 [DOI] [PubMed] [Google Scholar]
- Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella L, Xu G, Chao D, Li J, Wang P, Qin F, et al. (2020) Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci 63: 635–674 [DOI] [PubMed] [Google Scholar]
- Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C (2015) Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 27: 1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Young J, Crawford N (2003) The Nitrate Transporter AtNRT1.1 (CHL1) Functions in Stomatal Opening and Contributes to Drought Susceptibility in Arabidopsis. Plant Cell 15: 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Besserer A, Chevalier AS, Chaumont F (2013) Insights into plant plasma membrane aquaporin trafficking. Trends Plant Sci 18: 344–352 [DOI] [PubMed] [Google Scholar]
- Hao H, Fan L, Chen T, Li R, Li X, He Q, Botella M, Lin J (2014) Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 26: 1729–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Sugimoto M, Higaki T, Yaeno T, Nagami A, Irie M, Fujimi M, Miyamoto M, Akita K, Negi J, Shirasu K, et al. (2013) A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat Commun 4: 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki T, Hashimoto-Sugimoto M, Akita K, Iba K, Hasezawa S (2014) Dynamics and environmental responses of PATROL1 in Arabidopsis subsidiary cells. Plant Cell Physiol 55: 773–780 [DOI] [PubMed] [Google Scholar]
- Hwang JU, Lee Y (2001) Abscisic acid-induced actin reorganization in guard cells of dayflower is mediated by cytosolic calcium levels and by protein kinase and protein phosphatase activities. Plant Physiol 125: 2120–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Pei Y (2016) Hydrogen sulfide: the shutter button of stomata in plants. Sci China Life Sci 11: 1187–1188 [DOI] [PubMed] [Google Scholar]
- Kong X, Pan J, Cai G, Li D (2012) Recent insights into Brassinosteroid signaling in plants: its dual control of plant immunity and stomatal development. Mol Plant 5: 1179–1181 [DOI] [PubMed] [Google Scholar]
- Konopka C, Backues S, Bednarek S (2008) Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell 20: 1363–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al. (2014) The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338 [DOI] [PubMed] [Google Scholar]
- Li X, Wang X, Yang Y, Li R, He Q, Fang X, Luu D, Maurel C, Lin J (2011) Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 23: 3780–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Jing Y, Xiao J, Zhang Y, Zhu Y, Julian R, Lin J (2017) Membrane microdomains and the cytoskeleton constrain AtHIR1 dynamics and facilitate the formation of an AtHIR1-associated immune complex. Plant J 90: 3–16 [DOI] [PubMed] [Google Scholar]
- Martinière A, Lavagi I, Nageswaran G, Rolfe DJ, Maneta-Peyret L, Luu DT, Botchway SW, Webb SED, Mongrand S, Maurel C, et al. (2012) Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc Natl Acad Sci USA 109: 12805–12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna J, Rolfe D, Webb S, Tolmie A, Botchway S, Martin-Fernandez M, Hawes C, Runions J (2019) The cell wall regulates dynamics and size of plasma-membrane nanodomains in Arabidopsis. Proc Natl Acad Sci USA 116: 12857–12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinild A, Klaerke D, Zeuthen T (1998) Bidirectional water fluxes and specificity for small hydrophilic molecules in aquaporins 0-5. J Biol Chem 273: 32446–32451 [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He S (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, He SY (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46: 101–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm P, Wolf T, Fromm J, Roelfsema MRG, Marten I (2011) Cell type-specific regulation of ion channels within the maize stomatal complex. Plant Cell Physiol 52: 1365–1375 [DOI] [PubMed] [Google Scholar]
- Rodrigues O, Reshetnyak G, Grondin A, Saijo Y, Leonhardt N, Maurel C, Verdoucq L (2017) Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc Natl Acad Sci USA 114: 9200–9205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema M, Steinmeyer R, Staal M, Hedrich R (2001) Single guard cell recordings in intact plants: light-induced hyperpolarization of the plasma membrane. Plant J 26: 1–13 [DOI] [PubMed] [Google Scholar]
- Serna L, Fenoll C (2000) Stomatal development and patterning in Arabidopsis leaves. Physiol Plant 109: 351–358 [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Shinozaki Y, Sumitomo K, Tsuda M, Koizumi S, Inoue K, Torimitsu K (2009) Direct observation of ATP-induced conformational changes in single P2X(4) receptors. Plos Biol 7: e1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, , Raghavendra AS, , Kwak JM, , Vavasseur A ( 2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K, Peiter E (2014) Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol 204: 873–881 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao Y, Luo W, Li R, He Q, Fang X, De Michele R, Ast C, von Wiren N, Lin J (2013) Single-particle analysis reveals shutoff control of the Arabidopsis ammonium transporter AMT1;3 by clustering and internalization. Proc Natl Acad Sci USA 110: 13204–13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li X, Deng X, Luu D, Maurel C, Lin J (2015) Single-molecule fluorescence imaging to quantify membrane protein dynamics and oligomerization in living plant cells. Nat Protoc 10: 2054–2063 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen X, Xiang CB (2007) Stomatal density and bio-water saving. J Integr Plant Biol 49: 1435–1444 [Google Scholar]
- Wang Y, Zhang JZ, Chen Y, Jiang L, Wei J, Xu T (2009) Characterization of GLUT4-containing vesicles in 3T3-L1 adipocytes by total internal reflection fluorescence microscopy. Sci China Ser 52: 665–671 [DOI] [PubMed] [Google Scholar]
- Xue J, Gong B, Yao X, Huang X, Li J (. 2020) BAK1-mediated phosphorylation of canonical G protein alpha during flagellin signaling in Arabidopsis. J Integr Plant Biol 62: 690–701 [DOI] [PubMed] [Google Scholar]
- Xue Y, Xing J, Wan Y, Lv X, Fan L, Zhang Y, Song K, Wang L, Wang X, Deng X, et al. (2018) Arabidopsisblue light receptor phototropin 1 undergoes blue light-induced activation in membrane microdomains. Mol Plant 11: 846–859 [DOI] [PubMed] [Google Scholar]
- Yan S, Mclamore ES, Dong S, Gao H, Taguchi M, Wang N, Zhang T, Su X, Shen Y (2015) The role of plasma membrane H(+)-ATPase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. Plant J 83: 638–649 [DOI] [PubMed] [Google Scholar]
- Yao Y, Liu X, Li Z, Ma X, Rennenberg H, Wang X, Li H (2013) Drought-induced H2O2 accumulation in subsidiary cells is involved in regulatory signaling of stomatal closure in maize leaves. Planta 238: 217–227 [DOI] [PubMed] [Google Scholar]
- Yu M, Liu HJ, Dong Z, Xiao J, Su B, Fan L, Komis G, Samaj J, Lin J, Li R (2017) The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. J Plant Physiol 215: 73–84 [DOI] [PubMed] [Google Scholar]
- Zhu D, Zhang M, Gao C, Shen J (2020) Protein trafficking in plant cells: tools and markers. Sci China Life Sci 63: 343–363 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien R (2009) The Dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science 323: 1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He S, Assmann S (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56: 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.