Abstract
Shoot branching is an important aspect of plant architecture because it substantially affects plant biology and agricultural performance. Sugars play an important role in the induction of shoot branching in several species, including potato (Solanum tuberosum L.). However, the mechanism by which sugars affect shoot branching remains mostly unknown. In the present study, we addressed this question using sugar-mediated induction of bud outgrowth in potato stems under etiolated conditions. Our results indicate that sucrose feeding to detached stems promotes the accumulation of cytokinin (CK), as well as the expression of vacuolar invertase (VInv), an enzyme that contributes to sugar sink strength. These effects of sucrose were suppressed by CK synthesis and perception inhibitors, while CK supplied to detached stems induced bud outgrowth and VInv activity in the absence of sucrose. CK-induced bud outgrowth was suppressed in vinv mutants, which we generated by genome editing. Altogether, our results identify a branching-promoting module, and suggest that sugar-induced lateral bud outgrowth is in part promoted by the induction of CK-mediated VInv activity.
Sugar-induced lateral bud outgrowth is promoted by the induction of cytokinin and vacuolar invertase activity.
Introduction
In plants, the growing shoot apex inhibits the outgrowth of axillary buds further down the stem to control the number of branches. This phenomenon is referred to as apical dominance (Phillips, 1975; Ferguson and Beveridge, 2009; Wingler, 2018; Barbier et al., 2019). In response to decapitation, plants have evolved rapid long-distance signaling—involving sugars—to release axillary buds and replenish the plant with new growing shoot tips (Mason et al., 2014; Fichtner et al., 2017). Since the pattern of shoot branching may reflect the strength of the sugar sink, a clearer understanding of the regulatory mechanisms underlying shoot branching is expected to contribute to an increase in crop yields (Otori et al., 2017; Salam et al., 2017).
Shoot branching is controlled by complex interactions among hormones, nutrients, and environmental cues (Ongaro et al., 2008; Müller and Leyser, 2011; Leduc et al., 2014; Barbier et al., 2015b; Rameau et al., 2015; Roman et al., 2016; Fichtner et al., 2017; Le Moigne et al., 2018). Auxin, strigolactones, and cytokinins (CKs) are three of the main plant hormones involved in the regulation of bud outgrowth, forming a systemic network that orchestrates this process (Ferguson and Beveridge, 2009). The classical view centers on the opinion that a bioactive form of auxin, which is produced in young leaves at the shoot apex (Cline, 1994; Ljung et al., 2001) and is subsequently transported basipetally down the shoot in the polar auxin transport stream (Blakeslee et al., 2005), restricts the development of axillary buds (Sachs and Thimann, 1964; Cline, 1994; Bennett et al., 2016). Strigolactones inhibit shoot branching, as demonstrated by exogenous strigolactone application to the bud and by the strong branching phenotype displayed by strigolactone synthesis and signaling mutants (Rameau et al., 2015). The fact that auxin upregulates strigolactone biosynthesis genes in the stem suggests that strigolactones’ involvement in mediating the branching inhibition by auxin (Saeed et al., 2017). Indeed, auxin requires strigolactones to inhibit bud outgrowth, since exogenous auxin is unable to fully repress decapitation-induced branching in strigolactone-deficient mutants (Beveridge et al., 2000; Arite et al., 2007). In contrast to auxin, a role for CKs in bud outgrowth emerged decades ago when direct CK application onto dormant buds promoted bud outgrowth (Sachs and Thimann, 1967; Hartmann et al., 2011; Dun et al., 2012). Isopentenyltransferase enzymes control a rate-limiting step in CK biosynthesis, and transcript levels of genes encoding these enzymes are modified in response to auxin levels. Repression of CK-biosynthesis genes by auxin is well known (Miyawaki et al., 2004; Nordström et al., 2004; Tanaka et al., 2006).
In parallel, the hormone and genetics era of plant biology research established the nutrient-diversion theory of apical dominance (Wardlaw and Mortimer, 1970), involving the simple idea that bud outgrowth is inhibited by competition for resources (Kebrom, 2017; Barbier et al., 2019). The theory was narrowed down to implicate sugar nutrients, proposing that apical dominance is maintained largely by the sugar demand of the shoot tip, which limits the amount of sugar available to the axillary buds (Mason et al., 2014; Rameau et al., 2015). Sugars are a major source of carbon and energy and are produced by plants in an autotrophic fashion. In vascular plants, three types of sugar accumulate to high levels, namely, the two monosaccharides glucose and fructose, and the disaccharide sucrose (Jung et al., 2015). From the site of their synthesis, sugars are partitioned to sink tissues in a controlled manner via the vascular system.
From a growth perspective, axillary buds are regarded as sink organs that are photosynthetically less active and which need to import sugars to meet their metabolic demand and support their growth (Roitsch and Ehneß, 2000). A bud’s growth capacity is reflected in its sink strength, which represents its ability to acquire and use sugars. Therefore, to sustain its outgrowth, the bud has to compete for sugars, which constitute its main source of carbon and energy. Bud outgrowth occurs concomitantly with (1) starch-reserve mobilization in stem tissues, mostly in perennial plants, (2) high activity of sugar-metabolizing enzymes, and (3) increased sugar absorption in the bud (reviewed by Rameau et al., 2015). The role of sugar as an early signal triggering bud activation has been recently suggested. Mason et al. (2014) showed that sugar initiates rapid outgrowth of the basal bud in pea after shoot decapitation. A strong association between sugar availability and branching has also been observed in studies involving defoliation (Alam et al., 2014; Kebrom and Mullet, 2015), enhanced CO2 supply (Burnett et al., 2016; Otori et al., 2017), and inhibition of sucrose degradation (Salam et al., 2017). Fichtner et al. (2017) demonstrated that changes in the level of bud trehalose 6-phosphate—a signal of sucrose availability in plants—corresponds with the initiation of bud outgrowth following decapitation, suggesting that trehalose 6-phosphate is involved in the release of bud dormancy by sucrose. In addition, the onset of bud outgrowth in various species is tightly associated with the expression of genes involved in sugar transport, metabolism, and signaling (Girault et al., 2010; Rabot et al., 2012; Chao et al., 2016). These findings support the theory that the growing shoot tip inhibits bud outgrowth by being a strong sink for sugars, thereby depriving the axillary buds (reviewed by Barbier et al., 2015b).
A more direct and genetic underpinning of the sucrose connection to bud outgrowth has been achieved through studies of vacuolar invertase (VInv). Salam et al. (2017) showed that silencing VInv in potatoes results in surplus sucrose availability and an increased branching phenotype for the potato tuber. Meristem-specific overexpression of cell-wall or cytosolic invertase in Arabidopsis thaliana changes the shoot branching pattern in a complex manner, differentially affecting the formation of axillary inflorescences, branching of the main inflorescence, and branching of side inflorescences (Heyer et al., 2004; Wingler, 2018). This suggests that the sucrose-to-hexose ratio affects stem branching pattern and might differentially interact with hormones associated with bud growth.
Sugar and hormone networks interact to regulate different developmental processes (LJung et al., 2015). However, the mechanism underlying these interactions is not fully understood. Sucrose has been shown to strongly induce CK synthesis in in vitro grown single nodes in rose (Rosa hybrida), suggesting that this hormone might mediate the sucrose effect (Barbier et al., 2015a). However, replacing sucrose with CK in the growth medium was not enough to trigger bud outgrowth from the rose nodes, suggesting that sucrose also triggers a pathway independent of CKs, or that a minimal amount of sucrose is required for CKs to promote bud outgrowth, or both (Barbier et al., 2015a). In rose, light controls the sugar supply to the axillary buds (Girault et al., 2010). However, in contrast to CKs, sugar supply is unable to restore the decreased branching phenotype triggered by darkness or low light intensity (Rabot et al., 2012; Roman et al., 2016).
Since potato sprouts can grow in the dark, the potato tuber and sprouts serve as an ideal model to study shoot branching under conditions in which most of the sugars are fed exogenously without the involvement of photosynthetic products (Eshel and Teper-Bamnolker, 2012; Teper-Bamnolker et al., 2012; Teper-Bamnolker et al., 2017). Our previous study showed that an exogenous supply of sucrose, glucose, or fructose to detached etiolated sprouts induces their branching in a dose-responsive manner (Salam et al., 2017). Although an increase in sucrose level was observed in tuber parenchyma upon branching induction, sugar analysis of grafted stems showed no distinct differences in sugar levels between branching and nonbranching scions. Furthermore, silencing of the VInv-encoding gene led to increased sucrose levels and branching of the tuber (Salam et al., 2017). The objective of the present study was to decipher the mechanism by which sucrose modulates bud burst and elongation in the etiolated sprout.
Results
Sucrose induces lateral bud elongation better than hexoses
We recently showed that sucrose and its hydrolytic products induce stem branching in a dose-responsive manner under etiolated conditions (Salam et al., 2017). To distinguish between the effects of sugars on bud burst versus bud elongation, we conducted a detailed time course of the differential effects of sucrose or a mix of glucose and fructose (hexoses) on the number of branches and lateral bud elongation. Tubers were incubated at 14°C until sprouting, and sprouts with three nodes were then manually detached, placed in 300 mM sucrose, hexoses (glucose + fructose, 300 mM each), sorbitol (an osmotic control) or water, and incubated at 14°C for 9 d. Sucrose and hexoses induced branching and lateral bud elongation (Figure 1). Water and the sugar alcohol/osmotic agent sorbitol, which can be imported but is not generally (or is only slowly) metabolized by plant cells (see Klepek et al., 2005), were unable to induce branching and elongation (Figure 1). Sucrose and hexoses yielded similar branching, but lateral bud elongation was significantly higher during the 9 d of sucrose versus hexose feeding. These results suggest that sucrose and its hydrolytic products enhance both stem branching and elongation under etiolated conditions, with a significantly higher effect of sucrose on bud elongation.
Figure 1.
Exogenous sucrose or hexoses induce lateral bud burst and elongation in etiolated stems. Sprouts were detached from the tubers and supplemented with sugars (sucrose, glucose + fructose, sorbitol, each at 300 mM) or water for 9 d in the dark. A, Number of branches. B, Lateral bud length. C, Images showing the lateral node after 7 d of treatment. Bars = 100 μm. Results are means of 10 biological replicates. Error bars represent se. Different letters indicate significant differences between treatments at each time point (one-way ANOVA, P < 0.05).
Sucrose and hexoses translocate to the stem and penetrate the lateral bud
We previously reported that labeled sugars can be transported to the apical bud and lateral node of the etiolated stem following exogenous feeding of tuber parenchyma (Salam et al., 2017). To test whether sucrose and hexoses are translocated into the lateral bud itself or only to its base (node), we fed labeled sugars ([U-14C]sucrose, or [U-14C]glucose and [U-14C]fructose) to the bottom of detached stems (using the same system as above). After 2-h incubation with either sucrose or hexoses, we detected radioactivity at the node and inside the lateral bud (Figure 2). Levels of radiolabel were unchanged in the node and lateral bud between 2 and 4 h of incubation (Figure 2). While these results indicated translocation and entry into the lateral bud, it was not possible to distinguish whether the radioactivity measured in the buds was due to the movement of glucose and fructose, or to their reconversion to sucrose.
Figure 2.
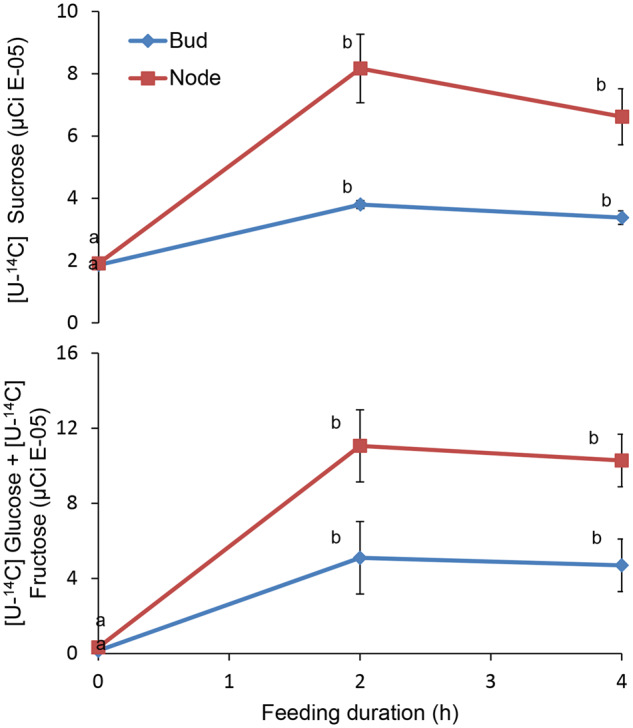
Sucrose and hexoses translocate from the stem into the lateral bud. Detached etiolated stems were fed with a water solution containing A, 1 µCi [U-14C]sucrose or B, 1 µCi [U-14C]glucose + [U-14C]fructose for 0, 2, and 4 h in the dark. Results are means of five biological replicates. Error bars represent se. Different letters indicate significant differences between time points for each treatment (one-way ANOVA, P < 0.05).
Sucrose induces the expression and activity of VInv prior to lateral bud elongation
Since hexoses induced stem branching, we hypothesized that bud burst induced by sucrose is mediated by the activity of invertases, key enzymes involved in sucrose degradation, in the developing bud. To test this hypothesis, etiolated stems were detached and fed with sucrose, hexoses, or sorbitol for 24 h, and VInv and cell-wall invertase (CWInv) transcript level and activity were determined. After 2, 8, and 10 h of sucrose feeding, VInv transcript level in the third lateral stem bud was significantly higher than with the other sugars. No significant difference was found in VInv expression between hexoses and sorbitol over 24 h of feeding (Figure 3A). The expression levels of seven CWInv, found to be expressed in the tuber [StcwINV1-4 (Liu et al., 2011) and PGSC0003DMT400006639, PGSC0003DMT400097344, and PGSC0003DMT400071784 (Potato Genomics database; http://solanaceae.plantbiology.msu.edu/index.shtml)], were not significantly upregulated in response to sucrose feeding, as compared to hexoses or sorbitol feeding (Supplemental Figure S1). In most time points measured, sugars did not increase CWInvs gene expression significantly compared to the osmotic control. Still, activity was enhanced by sucrose and hexoses, suggesting regulation at the post-translation level. VInv and CWInv activity were enhanced in the lateral bud of sucrose-fed stems as early as 2 h into feeding and remained significantly higher until the 24-h measurement (Figure 3, B and C). Altogether, these results show that invertases are associated with sucrose-promoted bud outgrowth. Since VInv was explicitly regulated in both transcript level and enzyme activity, we decide to focus our experiments on that enzyme.
Figure 3.

Sucrose feeding of stems induces higher expression and activity of VInv in the lateral bud. Detached etiolated stems were fed with 300-mM sucrose, hexoses, or sorbitol for 24 h in the dark. A, VInv transcript level at the lateral bud was determined by real-time quantitative polymerase chain reaction (PCR) using gene-specific primers. Gene transcript levels are expressed relative to controls (0 h), which were set to 1 and normalized with the elongation factor 1-α gene (ef1α) transcript level. B, C, VInv and CWInv activity at the stem node, respectively. Results are means of three biological replicates. Error bars represent se. Different letters indicate significant differences between treatments at each time point (one-way ANOVA, P < 0.05). FW, fresh weight.
VInv is involved in branching
To investigate the involvement of VInv in sucrose-induced stem branching and bud elongation, we generated two VInv-CRISPR/Cas9 mutant lines (vinv-7 and vinv-8) characterized by low VInv-activity levels. We compared the effects of sucrose feeding between wild-type (WT) plants and vinv mutants. The number of sucrose-induced branches was substantially reduced, but they were not abolished in vinv-mutant lines compared to the WT, suggesting a role for VInv in the sucrose-induced branching (Figure 4A). There was also a significant reduction in sucrose-induced lateral bud elongation in the vinv mutants (Figure 4B). These results were associated with the low VInv activity, as compared to the WT, in sucrose-fed vinv-7 and vinv-8 (Figure 4C). These results suggest that VInv activity is important for sucrose-induced lateral bud burst and elongation.
Figure 4.

Silencing VInv reduces the effect of sucrose on stem branching. Detached etiolated stems of “Desiree” (WT) and VInv-knockout lines (vinv-7 and vinv-8) were fed with 300-mM sucrose for 20 d at 14°C, 95% relative humidity, in the dark. A, Number of branches. B, Lateral bud length. C, VInv activity at the stem node of the lateral bud. Results are means of eight biological replicates. Error bars represent se. Different letters represent significant differences between treatments at each time point (one-way ANOVA, P < 0.05). FW, fresh weight.
Sucrose triggers CK accumulation prior to initiation of stem-branching
CKs are able to trigger bud outgrowth, and their accumulation often associate with bud outgrowth in a variety of species, including potato (Bredmose et al., 2005; Shimizu-Sato et al., 2009; Hartmann et al., 2011; Buskila et al., 2016). Moreover, sugars, including sucrose, induce CK synthesis (Barbier et al., 2015b; Kiba et al., 2019). We therefore tested, by quantifying CK accumulation in the stem node following sugar feeding of etiolated stems, whether CK is involved in the sucrose-induced bud outgrowth. Levels of intermediate (zeatin riboside) and active (zeatin) CK forms increased following feeding with sucrose (Figure 5). The response of zeatine level to hexoses or sorbitol feeding was weaker than its response to sucrose (Figure 5), demonstrating that sucrose is most potent in inducing CK accumulation.
Figure 5.

Feeding etiolated stems with sucrose induces higher content of endogenous CK in the node of the lateral bud. Levels of A, zeatin riboside and B, zeatin in untreated sprouts (0 h) or sprouts supplemented with sugars (sucrose, glucose + fructose, sorbitol at 300 mM), at different time intervals at 14°C, 95% relative humidity, in the dark. Results are means of three biological replicates. Error bars represent se. Different letters indicate significant differences between treatments at each time point (one-way ANOVA, P < 0.05). FW, fresh weight.
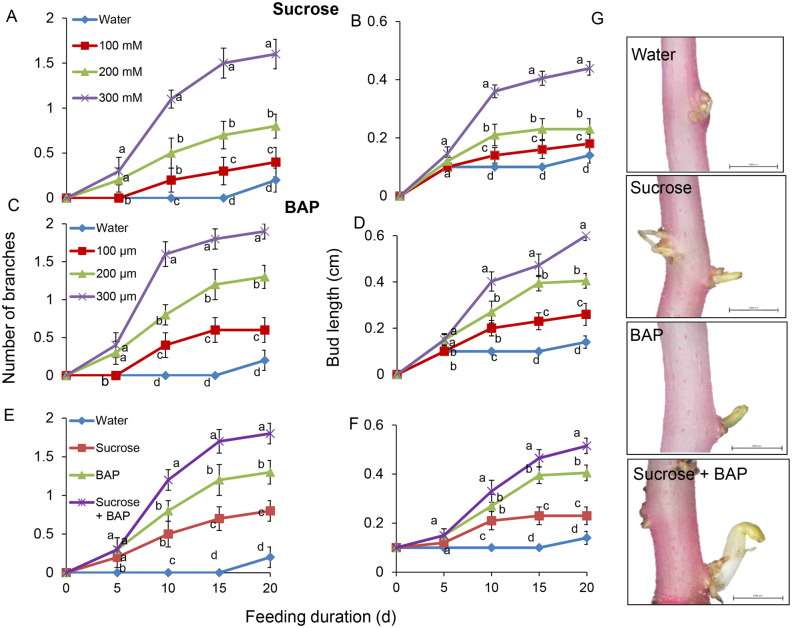
Since sucrose caused CK accumulation (Figure 5), we tested whether exogenous CK application would induce lateral bud outgrowth. Supplying etiolated stems with the synthetic CK 6-benzylaminopurine (BAP) led to a dose-dependent increase in branching and bud elongation, similar to the effect of sucrose feeding (Figure 6). Feeding with a mixture of BAP and sucrose significantly increased branching and lateral bud elongation relative to each treatment alone (Figure 6).
Figure 6.
Sucrose and CK have an additive effect on etiolated stem branching and lateral bud elongation. Sprouts were detached from the tubers, incubated at 14°C, 95% relative humidity, in the dark, and fed for 20 d with A, B, 0-, 100-, 200-, or 300-mM sucrose; C, D, 0-, 100-, 200-, or 300-µM BAP; E, F, 200-mM sucrose with or without 200-µM BAP, BAP alone, or water. The number of branches developed (A, C, E), and lateral bud length (B, D, F) were measured. G, Typical lateral bud after 15 d of feeding. Bars = 1,000 μm. Results are means of 10 biological replicates. Error bars represent se. Different letters indicate significant differences between treatments at each time point (one-way ANOVA, P < 0.05).
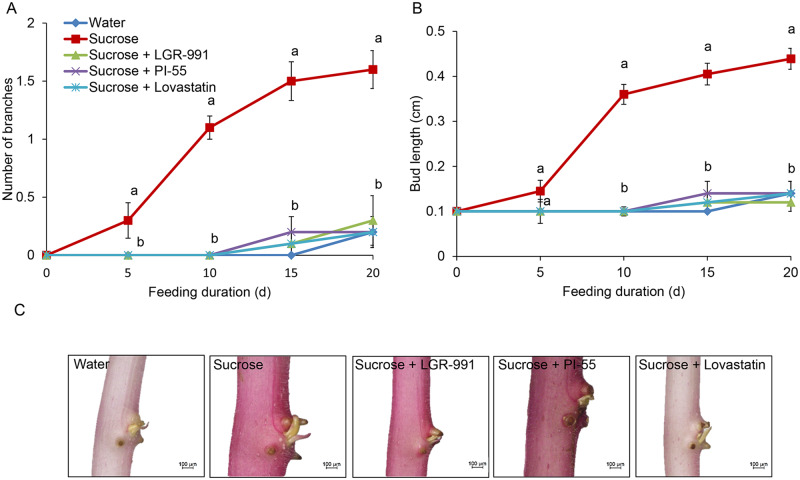
To determine whether CK mediates the effect of sucrose on stem branching, we supplied inhibitors of CK synthesis (lovastatin) or perception (PI-55, LGR-991) to etiolated stems with sucrose. The effects of sucrose on stem branching and lateral bud elongation were completely suppressed by these inhibitors (Figure 7). LGR-991 and PI-55 caused repression of bud outgrowth that could not be overcome by BAP application. In contrast, BAP was able to induce bud outgrowth even after feeding with lovastatin (Supplemental Figure S2). This suggests that sucrose requires CK to induce bud burst and elongation. Supplying etiolated stems with CK degradation inhibitor (INCYDE) increased branching and VInv activity but did not significantly increase sucrose effect (Supplemental Figure S3).
Figure 7.
CK inhibitors eliminate branching induction by sucrose. Etiolated stems were detached from the tubers and fed with 300-mM sucrose, or 300-mM sucrose with CK-synthesis inhibitor (lovastatin, 200 µM) or with CK-perception inhibitors (LGR-991, PI-55, 200 µM), or water for 20 d at 14°C, 95% relative humidity, in the dark. A, Number of branches. B, Lateral bud length. C, Typical lateral bud after 15 d. Bars = 100 μm. Results are means of 10 biological replicates. Error bars represent se. Different letters indicate significant differences between treatments at each time point (one-way ANOVA, P < 0.05).
VInv activity is induced by sucrose and CK
CKs have also been shown to induce VInv expression and nutrient sink strength in various species (Roitsch and Ehneß, 2000; Werner et al., 2008; Liao et al., 2013). We have shown that glucose and fructose, which are the hydrolytic products of sucrose cleavage by VInv, can induce branching (Salam et al., 2017). However, since a mixture of sucrose and CK inhibitors yielded no branching, we hypothesized that VInv activity is also affected by CK, or that VInv-mediated branching requires CK. To test this, we measured the impact of CK inhibitors on CK- and sucrose-induced VInv activity. CK inhibitors reduced the effects of both sucrose and BAP on VInv activity (Figure 8), indicating that sucrose and CK can both trigger VInv activity, and that the impact of sucrose on VInv activity is partially dependent on CK.
Figure 8.

CK inhibitors reduce VInv activity induced by sucrose or BAP. Detached etiolated stems were incubated for 6 h, in the dark and were fed with A, 300-mM sucrose, or 300-mM sucrose with CK-synthesis inhibitor (200-µM lovastatin) or with CK-perception inhibitor (200-µM LGR-991), or water, (B) 200-µM BAP, BAP with CK-synthesis inhibitor (200-µM lovastatin), BAP with CK-perception inhibitor (200-µM LGR-991), or water. Results are means of five biological replicates. Error bars represent se. Different letters indicate significant differences between treatments at each time point (one-way ANOVA, P < 0.05). FW, fresh weight.
To test whether VInv mediates the effect of CK on branching, we investigated the impact of BAP on stem branching in vinv mutants. vinv-7 and -8 mutants were treated with 100- or 200-µM BAP for 15 d in the dark. Both VInv knockout lines showed reduced branching (Figure 9, A and B) and elongation (Figure 9, C and D) response to BAP, with differences becoming significant after 15 d. These results support the notion that CK induces branching in part through induction of VInv activity.
Figure 9.
Silencing VInv activity reduces CK effect on stem branching. Etiolated stems were detached from tubers of WT (Désirée) and vinv mutants (vinv-7 and vinv-8). Stems were supplied with 100- or 200-μM BAP or water for 15 d at 14°C in 95% relative humidity, in the dark. A, B, Number of branches. C, D, Lateral bud length. Results are means of eight biological replicates. Error bars represent se. * Indicates significant differences between WT and each vinv mutant at each time point (Student’s t test, P < 0.05).
To summarize, we showed that sucrose promoted branching better than hexoses or sorbitol (Figures 1–2). We showed that sucrose induced a higher level of VInv activity and CK (zeatin) in the lateral bud (Figures 3, 5). Exogenous application of CK (BAP) induced branching in a dose–response manner, and the CK inhibitors we used reduced this effect (Figure 6; Supplemental Figure S2). CK inhibitors dramatically reduced the effect of sucrose as well as VInv activity (Figures 7–8), whereas a CK degradation inhibitor increased branching and VInv activity (Supplemental Figure S3). Finally, CK had a lower effect on vinv mutants (Figure 9). Altogether, these results suggest the proposed sucrose–CK-VInv connection.
Discussion
Sucrose moves into the lateral bud to induce its burst and elongation
Sugars play a major role in plant growth and development; they provide energy and a source of carbon for protein and cell-wall synthesis (Patrick et al., 2013). Independent of their nutritional role, sugars also play a signaling role and can therefore interact with other regulatory networks to control plant development (Lastdrager et al., 2014; Yadav et al., 2014; Li and Sheen, 2016; Sakr et al., 2018). Recent studies have suggested that sugars are important signaling regulators of bud outgrowth. Using an elegant set of experiments, Mason et al. (2014) demonstrated the systemic movement of sucrose as a branching signal that moves from the leaf to the lateral bud after decapitation. Barbier et al. (2015a) demonstrated that sugar availability, together with auxin (Bertheloot et al., 2020), control the entrance of buds into the sustained growth stage. Recently, we showed that sucrose and its hydrolytic products can induce etiolated stem branching in a dose-responsive manner (Salam et al., 2017). Taken together, these results suggest that stem branching and lateral bud elongation are closely linked to the mobilization of sugars toward the buds. Here, we show a differential effect of sucrose and hexoses feeding on invertase activities, the number of branches and lateral bud elongation (Figures 1, 3). Treatment with water or the osmotic control sorbitol did not induce significant activation of invertases or lateral bud growth (Figures 1, 3). The lower effect of sucrose-degradation products suggests that sucrose not only acts as an energy source, but also through other pathways to initiate branching.
Sucrose cleavage to hexoses is required for stem branching
Sucrose feeding of etiolated stems induced increased activities of all tested invertases. Sucrose also induced a higher level of VInv expression after 2, 8, and 10 h, as compared to hexoses or sorbitol (Figure 3). The expression levels of CWInvs were not differentially upregulated by sucrose feeding. These CWInv genes show homology to Arabidopsis AtcwINV1 (At3g13790), AtcwINV4 (At2g36190), and AtcwINV3 (At1g55120), respectively; these are CWInv genes that have been previously reported to be expressed during shoot development (Tymowska-Lalanne and Kreis, 1998; Sherson et al., 2003). These results suggest that several invertases are involved in mediating the effect of sucrose on branching. Interestingly while sucrose affected the activities of all tested invertases, it affected the expression of only VInv, suggesting that sucrose may affect most invertases at the post-transcriptional level, and in addition, affects VInv at the transcription level (Figure 3;Supplemental Figure S1). In cellular interfaces lacking the plasmodesmata connection, sucrose can only be transported apoplastically across the cell-wall matrix and plasma membranes (Patrick, 1997). In that case, sucrose is often unloaded from the sieve element companion cell to the surrounding cell-wall matrix, and is then taken up by recipient sink cells via sucrose transporters and hydrolyzed by CWInv at the cellular space (Braun et al., 2014; Li et al., 2017).
VInv has been shown to be a major component of organ sink strength (Nägele et al., 2010; Albacete et al., 2015) and cell elongation (Morris and Arthur, 1984; Morey et al., 2018). The imported sucrose can contribute to cellular growth processes by contributing to the carbon skeleton and energy, and by providing osmotically active molecules for cell expansion. In addition, the sucrose imported into the bud, or its cleavage products (hexoses) derived from the action of VInv, may serve as signal molecules to regulate development (Gibson, 2005; Li and Sheen, 2016; Wang et al., 2018; Barbier et al., 2019).
Silencing of VInv results in inhibition of sucrose cleavage (Bhaskar et al., 2010; Zhu et al., 2016). Salam et al. (2017) showed an association between higher sucrose level and higher branching of transgenic potato tubers. Here, vinv mutants suppressed the effect of sucrose on branching (Figure 4), supporting a role for hexoses, produced by VInv in the lateral bud, in sucrose-induced stem-branching. Heyer et al. (2004) overexpressed cell wall or cytosolic invertase in Arabidopsis, and this led to changes in the shoot-branching pattern in a composite manner, differentially affecting the formation of axillary inflorescences, branching of the main inflorescence, and branching of side inflorescences. The essential role of acid invertases in regulating sink strength was analyzed in transgenic carrot plants by antisense suppression of VInv under control of the 35S-CaMV promoter that is predominantly active in carrot tap roots. The resulting lowered carbohydrate content in the roots and severe impairment of both growth and development demonstrated the important function of VInv in sucrose partitioning (Tang et al., 1999). In addition to modulating sink strength, antisense suppression of VInv in tomato led to differential growth and changes in fruit size (Klann et al., 1996). Goetz et al. (2001) reported that antisense repression of the CWInv Nin88 results in assimilation blockage and developmental arrest during the early stages of pollen development, leading to a distorted and invaginated morphology. The transgenic lines revealed an association between reduced enzymatic activity and decreased germination efficiency. Exogenous supply of glucose or sucrose partly rescued developmental arrest (Goetz and Roitsch, 2006), suggesting that the function of invertase is not only to provide carbohydrates to sustain growth, but also to create a delicate, fine-tuned balance between the sucrose and hexose sugars required as metabolic signals that regulate growth and development. It may also suggest a role for VInv as a sugar sensor. Indeed, enzymes catalyzing sugars, such as hexokinase 1 or fructose-1,6-bisphosphatase, have been shown to play a sensor role in sugar signaling (Moore et al., 2003; Cho and Yoo, 2011). In addition, CWInv has been recently suggested to play a role in ovule development, independent of its catalytic activity (Liao et al., 2020). It would be interesting to test whether VInv acts as a sensor for sucrose during the regulation of bud outgrowth.
Sucrose promotes sugar sink strength in the bud through CK-induced VInv
CKs are known to promote bud release from dormancy in intact plants (Sachs and Thimann, 1964, 1967; Dun et al., 2012). However, how their accumulation is induced during bud outgrowth remains unclear. Our results demonstrate that sucrose upregulates CK accumulation in stem nodes (Figure 5). Compared to hexoses and water, sucrose induced the accumulation of intermediate and active forms of CKs in the bud node prior to lateral bud burst. Feeding with a mixture of BAP and sucrose increased the effect over that of each component alone with respect to both branching level and lateral bud elongation (Figure 6). The effect of sugars on CK production has been reported for lily flowers (Arrom and Munné-Bosch, 2012) and Arabidopsis seedlings (Kushwah and Laxmi, 2014; Kiba et al., 2019). Sucrose has been reported to strongly induce CK synthesis in in vitro-grown single nodes of rose in the absence of auxin, suggesting that CKs might mediate the effect of sucrose, although the authors concluded that CKs alone are not sufficient to stimulate bud outgrowth in rose single nodes (Barbier et al., 2015a). In the presence of auxin in the growth medium, sucrose could not promote CK accumulation, and the CK content did not corresponds with the onset of bud outgrowth (Bertheloot et al., 2020). Here, we report that CKs play an important role in mediating sucrose-promoted bud outgrowth in etiolated stem. Indeed, sucrose-induced bud outgrowth was significantly suppressed by inhibitors of CK synthesis or perception (Figure 7).
CKs have been reported to enhance sugar sink strength in the tissues in which they accumulate, notably through upregulation of invertases (Figure 8) and cell-cycle promotion (Roitsch and Ehneß, 2000; Peleg et al., 2011; Wang et al., 2016). This might explain the additive effect of CK and sucrose in enhancing stem branching and bud elongation (Figure 6). Here, reduction in branching and elongation in VInv CRISPR/Cas9 mutants emphasized the notion that part of the branching induced by CK is through enhancement of VInv activity (Figure 9). Similar to our findings, Wang et al. (2016) reported that when a wheat (Triticum aestivum L.) stay-green mutant, tasg1, are treated with the CK inhibitor lovastatin, the activity of invertase is inhibited; this was associated with a premature senescence phenotype. The activity of invertase was partially recovered in tasg1 treated with BAP, suggesting that CKs might regulate the invertase activity involved in sucrose remobilization. Our findings are consistent with previous studies in which CKs were shown to adjust the sugar partitioning and sink strength of some organs through the regulation of sugar transporters and invertases (Thomas, 1986; Roitsch and Ehneß, 2000; Guivarc’h et al., 2002; Werner et al., 2008; Proels and Roitsch, 2009; Liao et al., 2013). In addition, our results are in agreement with recent results obtained by Roman et al. (2016) in rose buds showing that exogenous feeding of CK induces SUC2 and VInv, although an environmental cue—light—was integral to their system. Taken together, our data strongly suggest a crucial role for CKs in sucrose-induced axillary bud outgrowth in etiolated stems, and indicate that this occurs via increased VInv activity in the bud, which possibly leads to an increase in sink strength.
In summary, our study demonstrates that sucrose induces bud growth and elongation better than its moieties. Sucrose, but not hexoses, activates CK accumulation. Elevated sucrose (directly and indirectly) and CK were associated with higher invertase activities, which contribute to bud outgrowth. The CK/VInv pathway plays a role in increasing the capacity of sugars to promote bud outgrowth. This property may be important in the competition between axillary buds. Further studies are needed to determine how this mechanism affects the establishment of shoot architecture in plants.
Materials and methods
Plant material and storage conditions
Freshly harvested tubers of potato (Solanum tuberosum ‘Désirée’) were obtained from a potato-growing field in the northern Negev, Israel, stored at 14°C for 2 weeks for curing, and transferred to 4°C until use. WT and CRISPR mutants were grown in a greenhouse with a controlled atmosphere (10-h d length at a temperature of 18°C –22°C; extra light was supplied by lamps with intensity ranging from 600 to 1,000 μmol m−2 s−1). Water and mineral nutrients were provided by sub-irrigation for 5 min d−1. For sprouting induction, tubers were transferred from 4°C to 14°C, both under dark conditions. In all experiments, sprouts with three nodes were selected unless otherwise stated. Tubers and sprouts in all treatments were maintained at 95% relative humidity.
Exogenous application of sugars, CK, and CK Inhibitors
Sprouts were detached manually from tubers stored at 14°C, and surface cleaned by washing with sterile water for 5 min. Sprouts were dried for 3 min on a filter paper, and placed in a sterile Eppendorf rack containing 300-mM sucrose, sorbitol or a mixture of glucose and fructose (300 mM each). These were then incubated at 14°C in the dark for up to 16 d, unless otherwise stated.
To evaluate the effects of CK on bud outgrowth, the synthetic CK BAP (Duchefa, Netherlands), as well as the CK-synthesis inhibitor lovastatin (Sigma, Israel) and CK-perception inhibitors LGR-991 and PI-55 CK-degradation inhibitor INCYDE (Spíchal et al., 2009; Nisler et al., 2010; Gemrotová et al., 2013) were exogenously supplied to sprouts at 200 µM, unless otherwise stated. Bud length was measured with a millimeter-scale held perpendicular to the stem. Branches were defined as lateral buds longer than 0.2 cm.
RNA extraction and cDNA synthesis
The lateral bud located at the third node from the apical bud was sampled from five sprouts per replicate and immediately frozen at −80°C. The buds were ground, and RNA was extracted according to Chen et al. (2015) with slight modifications. The powdered tissue was added to 800-μL pre-warmed (65°C) extraction buffer (100-mM Tris–HCl, pH 8.0, 25.0-mM EDTA, 2.0-M NaCl, 3% w/v cetyl-trimethylammonium bromide, 4% w/v polyvinylpyrrolidone 40, 3% w/v β-mercaptoethanol) and incubated for 45 min at 65°C. Chloroform:isoamylalcohol (24:1, v/v) was added when the mixture had cooled to room temperature. The mixture, in centrifuge tubes, was allowed to stand for 10 min and then centrifuged at 12,400g for 20 min at 4°C. The above steps were repeated. RNA was precipitated by the addition of 2-mL LiCl at a final concentration of 3.0 M and incubation for 2 h at −20°C. Following another centrifugation at 12,400g, 4°C for 20 min, the pellet was washed twice with 2 mL of 70% ethanol, centrifuged for 10 min, and air-dried at room temperature. Finally, the pellet was suspended in 1% DEPC-treated H2O. The quality and quantity of the extracted RNA were, respectively, assessed by spectrometer (Thermo NanoDrop 2000, USA). DNA was removed by incubating the RNA with DNase (Invitrogen, USA) for 10 min at 37°C (1-μL DNase for 10-μg RNA). The reaction was stopped by adding DNase-deactivation buffer (Invitrogen) and incubating for 5 min at 70°C. cDNA was obtained by reverse transcription performed on 400 ng of RNA using reverse transcriptase (PCR Biosystems, USA).
Gene-expression analyses
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) was performed with SYBR Green mix (Thermo Fisher Scientific, USA) using cDNA as a template, with the following program: 2 min at 50°C, 10 min at 95°C, then 40 cycles of 15 s at 95°C, and 60 s at 60°C. The primers used for the RT-qPCR are given in Supplemental Table S1A. Specific sets of primers were selected according to their melting curves. Fluorescence detection was performed using a Step One Plus Real-Time PCR system (Applied Biosytems, USA). Quantification of relative gene expression was normalized using Ef1α expression as an internal control (Nicot et al., 2005).
Enzyme extraction and activity
VInv activity was measured as described previously (Miron and Schaffer, 1991), with minor modifications. Nodal stems carrying buds (250-mg fresh weight) were ground in liquid nitrogen and subsequently dissolved in 1-mL extraction buffer containing 25-mM HEPES–NaOH, 7-mM MgCl2, 0.5 mM EDTA, 3-mM dithiothreitol, and 2-mM diethyldithiocarbamic acid, pH 7.5. After centrifugation at 18,000g for 30 min, the supernatant was dialyzed overnight against 25-mM HEPES–NaOH and 0.25-mM EDTA, pH 7.5, and used as a crude extract. The pellet was used for CWInv activity assay, as described previously. After centrifugation at 18,000g for 30 min, the collected pellet was resuspended in extraction buffer, including 1% (vol/vol) Triton X-100. The suspension was shaken vigorously for 10 min at 4°C. After another centrifugation, the pellet was washed in extraction buffer without Triton X-100 and finally resuspended in assay buffer. Both VInv and CWInv activities were measured by incubating 0.3 mL of 0.1-M citrate/phosphate buffer (pH 5.0), 0.1-mL extract and 0.1 mL of 0.1-M sucrose. After 30 min incubation at 37°C, glucose liberated from the hydrolysis of sucrose was quantified by adding 500-μL Sumner’s reagent (3,5-dinitrosalicylic acid) and immediately transferring the sample to heating at 100°C for 10 min to terminate the reaction, then chilling at 4°C (Sumner and Graham, 1921). The reduction of dinitrosalicylic acid to 3-amino-5-nitrosalicylic acid by glucose was measured by absorbance at 550 nm in a spectrophotometer (Amersham Biosciences, UK). Quantification of glucose in each sample was based on glucose standards. VInv and CWInv activities were expressed as nanomoles glucose formed per gram fresh weight per minute.
Translocation and accumulation of labeled sugars
To determine sugar translocation and accumulation, sprouts were detached and cut at the base to expose the vascular tissues, and then incubated in 1 µCi of [U-14C]sucrose or a mixture of [U-14C]glucose + [U-14C]fructose, to a depth of 1 cm, supplemented with a mixture of 100-mM glucose and fructose, or sucrose. Sprouts were fed for 2 or 4 h. A total of 100-mg tissue was subsequently collected from the node, five buds per replicate. Radioactive counts of sucrose and the glucose–fructose mixture were determined by liquid-scintillation counting after crushing the tissue and diluting in Ultima Gold liquid scintillation cocktail (PerkinElmer, Israel) using a Packard Tri-Carb 2100TR counter analyzer (Packard BioScience, USA).
Analysis of CK content
For each sample, 200 mg of freeze-dried pulverized tissue was extracted with 1 mL of isopropanol: methanol: glacial acetic acid (79:20:1, v:v:v), and two stably labeled isotopes were used as internal standards and added as follows: 1 ng of [15N]trans-zeatin, 1 ng of [2H5]trans-zeatin riboside. The extract was vigorously shaken for 60 min at 4°C in a Thermomixer (Eppendorf), and then centrifuged (14,000g, 4°C, 15 min). The supernatants were collected, and the pellets were re-extracted twice with 0.5 mL of the same extraction solution, then vigorously shaken (1 min). After centrifugation, the three supernatants were pooled and dried (final volume 1.5 mL). Each dry extract was dissolved in 2,000-μL methanol: water (50:50, v/v), filtered, and analyzed by UPLC-Triple Quadrupole-MS (Waters Xevo TQ MS, USA). Separation was performed in a Waters Acquity UPLC BEH C18 1.7 µm 2.1 × 100 mm2 column with a VanGuard precolumn (BEH C18 1.7 µm 2.1 × 5 mm2). Chromatographic and MS parameters for the CK analysis were as follows: the mobile phase consisted of water (phase A) and acetonitrile (phase B), both containing 0.1% formic acid in gradient-elution mode. The solvent gradient was applied as follows [t (min), A (%)]: (0.5, 95), (14, 50), (15, 5), (18, 5), (19, 95), (22, 95); [t (min), B (%)]: (0.5, 95), (14, 50), (15, 5), (18, 5), (19, 95), (22, 95); flow rate was 0.3 mL min−1, and column temperature was kept at 35°C. CK analyses were performed using the ESI source in positive ion mode with the following settings: capillary voltage 3.1 kV, cone voltage 30 V, desolvation temperature 400°C, desolvation gas flow 565 l h−1, source temperature 140°C. The parameters used for multiple reaction monitoring quantification of the different hormones are shown in Supplemental Table S1B.
VInv sgRNA design and cloning
The sgRNA9 target region in exon 2 of VInv (PGSC0003DMG400013856) was identified using the publicly available CRISPR-design web-based tool (http://crispr.hzau.edu.cn/CRISPR2/). The target sequence, known as the protospacer adjacent motif (VInv-sgRNA9: GGACACATCATATAACGGCC), was located upstream to the NGG trinucleotide (Supplemental Figure S4A). The VInv target sequence, together with the gRNA scaffold, was amplified using two primers (see sequences below) and pRCS‐35S: Cas9‐AtU6: sgRNA as a template (Chandrasekaran et al., 2016). The forward primer contained SalI (underline) site as part of the U6 Arabidopsis promoter and VInv-sgRNA9 target site (bold and underline); StVInv9-F 5'AGAGTCGAC ATAGCGATTGGACACAT CATATA ACGGCC GTTTTAGAGC TAGAAATAGCA. The reverse primer of the PolIII‐terminator sequence contained a HindIII (underline) site; StVInv9-R 3' GCTAAGCTTC GATCTAAAAAA AGCAC. The amplified DNA (138 bp) was cloned into SalI and HindIII sites of the pRCS‐35S: Cas9‐AtU6: sgRNA binary plasmid (Supplemental Figure S4, B and C). The clone obtained was confirmed by sequencing.
Agrobacterium-mediated transformation
Potato leaves (cv Désirée) were used for Agrobacterium-mediated leaf disc infection, as described previously (Rocha‐Sosa et al., 1989). Transgenic plants were selected on 50-mg L−1 kanamycin (Duchefa, Haarlem, The Netherlands). Well-rooted plants were transferred to soil and grown at 25°C in a greenhouse. After 100 d, tubers were harvested and stored at 4°C in 95% humidity for further analysis.
Mutant screening and genotyping
Genomic DNA was isolated from 50 T0 potato plants by the method of Dellaporta et al. (1983). Screening for VInv mutated lines was done using primers flanking VInv- sgRNA9 target region VI_F 2052 5′-ACCATCCTACCCGATGGTCA′3 and VI_R 2705 5′ CAGGSTCAGCAGATTCACTAT '3. PCR products were digested with the restriction enzyme BsuRI. The digested products were separated on 1.5% agarose gel, searching for an uncut band indicating changes in the DNA. Two positive transgenic lines (vinv-7 and vinv-8), out of 50 regenerated plants, were detected (Supplemental Figure S5). The undigested PCR products were purified, cloned into pGEM-T (Promega, Biological Industries, Kibbutz Beit-Haemek, Israel), and digested again with BsuRI enzyme for genotype validation (Supplemental Figure S5A). For lines vinv-7 and vinv-8, 20 and 14 colonies were sequenced, respectively, and aligned to the intact VInv using the ClustalW BioEdit software program (Copyright©1997–2013, Tom Hall Ibis Biosciences, Carlsbad, CA, USA; Supplemental Figure S5B). The Désirée cultivar is tetraploid; according to the ratio obtained between the sequenced colonies, the four allele mutation composition was determined. Genotyping revealed that vinv-7 contains four mutant alleles, each encoding a stop codon (Supplemental Figure S5C). vinv-8 contains three mutant alleles encoding a stop codon, and fourth allele containing a 15-bp deletion (Supplemental Figure S5C).
Ten plants from each of the two CRISPR mutants, vinv-7 and vinv-8, were grown in greenhouse conditions and their phenotypes were characterized. This characterization showed that the two mutants exhibit typical development, morphology, and tuber yield, with slightly more compact structure of the stems in comparison with wild type plants (Supplemental Figure S6).
Data analysis
Data were analyzed using Microsoft Excel 2010. ANOVA and Tukey–Kramer test were performed using JMP software (version 3 for windows; SAS Institute).
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL/Potato Genomics Resource data libraries under accession numbers provided in the Supplementary Table S1A.
Funding
This research was supported by US–Israel Binational Agricultural Research and Development fund (BARD) project IS-5038-17C and by the European Regional Developmental Fund (ERDF) project “Plants as a tool for sustainable global development” (No. CZ.02.1.01/0.0/0.0/16_019/0000827).
Conflict of interest statement. None declared.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Sucrose feeding of stems does not induce higher expression of CWInv in the lateral bud.
Supplemental Figure S2. Effects of CK, CK inhibitors, or a mixture of CK and its inhibitors on bud outgrowth and elongation.
Supplemental Figure S3. Effects of inhibitor of cytokinin degradation (INCYDE) on stem branching.
Supplemental Figure S4. Cloning of VInv-sgRNA9 to pRCS‐35S:Cas9‐AtU6:sgRNA binary vector.
Supplemental Figure S5. Genotyping of vinv-7 and vinv-8 mutant lines generated in cv Désirée.
Supplemental Figure S6.Aerial tissue and tubers phenotype of potato (cv Desirre) VInv knockout lines (vinv-7 and vinv-8) grown in greenhouse conditions.
Supplemental Table S1. RT-qPCR primers and parameters for hormones measurements.
Supplementary Material
B.B.S., D.E., N.O., and F.B. conceived the original screening and research plans; D.E., J.J. N.O., A.G.-O., and C.B. supervised the experiments; B.B.S., R.D., C.Z., L.S., K.A. Y.S., D.L., F.S., and M.C-W. performed the experiments; P.T-B. provided assistance to B.B.S.; B.B.S. designed the experiments and analyzed the data; B.B.S. and D.E. conceived the project and wrote the article with contributions of all the authors; D.E., N.O., C.B., and F.B. supervised and completed the writing.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Dani Eshel (dani@agri.gov.il).
References
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 [DOI] [PubMed] [Google Scholar]
- Arrom L, Munné-Bosch S (2012) Sucrose accelerates flower opening and delays senescence through a hormonal effect in cut lily flowers. Plant Sci 188: 41–47 [DOI] [PubMed] [Google Scholar]
- Barbier F, Péron T, Lecerf M, Perez-Garcia M-D, Barrière Q, Rolčík J, Boutet-Mercey S, Citerne S, Lemoine R, Porcheron B (2015a) Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66: 2569–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA (2019) An update on the signals controlling shoot branching. Trends Plant Sci 24: 220–236 [DOI] [PubMed] [Google Scholar]
- Barbier FF, Lunn JE, Beveridge CA (2015b) Ready, steady, go! A sugar hit starts the race to shoot branching. Curr Opin Plant Biol 25: 39–45 [DOI] [PubMed] [Google Scholar]
- Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljung K, Leyser O (2016) Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biol 14: e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertheloot J, Barbier F, Boudon F, Perez‐Garcia MD, Péron T, Citerne S, Dun E, Beveridge C, Godin C, Sakr S (2020) Sugar availability suppresses the auxin‐induced strigolactone pathway to promote bud outgrowth. New Phytol 225: 866–879 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Turnbull CGN (2000) Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 andRms2. Plant Physiol 123: 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar PB, Wu L, Busse JS, Whitty BR, Hamernik AJ, Jansky SH, Buell CR, Bethke PC, Jiang J (2010) Suppression of the vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiol 154: 939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee JJ, Peer WA, Murphy AS (2005) Auxin transport. Curr Opin Plant Biol 8: 494–500 [DOI] [PubMed] [Google Scholar]
- Bredmose N, Kristiansen K, Nørbæk R, Christensen LP, Hansen-Møller J (2005) Changes in concentrations of cytokinins (CKs) in root and axillary bud tissue of miniature rose suggest that local CK biosynthesis and zeatin-type CKs play important roles in axillary bud growth. J Plant Growth Regul 24: 238 [Google Scholar]
- Braun DM, Wang L, Ruan Y-L (2014) Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J Exp Bot 65: 1713–1735 [DOI] [PubMed] [Google Scholar]
- Burnett AC, Rogers A, Rees M, Osborne CP (2016) Carbon source–sink limitations differ between two species with contrasting growth strategies. Plant Cell Environ 39: 2460–2472 [DOI] [PubMed] [Google Scholar]
- Buskila Y, Sela N, Teper-Bamnolker P, Tal I, Shani E, Weinstain R, Gaba V, Tam Y, Lers A, Eshel D (2016) Stronger sink demand for metabolites supports dominance of the apical bud in etiolated growth. J Exp Bot 67: 5495–5508 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, Pearlsman M, Sherman A, Arazi T, Gal‐On A (2016) Development of broad virus resistance in non‐transgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol 17: 1140–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao WS, Doğramaci M, Horvath DP, Anderson J V, Foley ME (2016) Phytohormone balance and stress-related cellular responses are involved in the transition from bud to shoot growth in leafy spurge. BMC Plant Biol 16: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Guo Y, Bai G, Sun J, Li Y (2015) Effect of 5-aminolevulinic acid and genistein on accumulation of polyphenol and anthocyanin in ‘Qinyang’ apples. J Anim Plant Sci 25: 68–79 [Google Scholar]
- Cho Y-H, Yoo S-D (2011) Signaling role of fructose mediated by FINS1/FBP in Arabidopsis thaliana. PLoS Genet 7: e1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MG (1994) The role of hormones in apical dominance. New approaches to an old problem in plant development. Physiol Plant 90: 230–237 [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Report 1: 19–21 [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158: 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel D, Teper-Bamnolker P (2012) Canloss of apical dominance potato tuber serve as marker of physiologicalage? 7: 1158–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Beveridge CA (2009) Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol 149: 1929–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner F, Barbier FF,, Feil R, Watanabe M, Annunziata MG, Chabikwa TG, Höfgen R, Stitt M, Beveridge CA, Lunn JE (2017) Trehalose 6‐phosphate is involved in triggering axillary bud outgrowth in garden pea (Pisum sativum L.). Plant J 92: 611–623 [DOI] [PubMed] [Google Scholar]
- Gemrotová M, Kulkarni MG, Stirk WA, Strnad M, Van Staden J, Spíchal L (2013) Seedlings of medicinal plants treated with either a cytokinin antagonist (PI-55) or an inhibitor of cytokinin degradation (INCYDE) are protected against the negative effects of cadmium. Plant Growth Regul 71: 137–145 [Google Scholar]
- Gibson SI (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8: 93–102 [DOI] [PubMed] [Google Scholar]
- Girault T, Abidi F, Sigogne M, Pelleschi-Travier S, Boumaza R, Sakr S, Leduc N (2010) Sugars are under light control during bud burst in Rosa sp. Plant Cell Environ 33: 1339–1350 [DOI] [PubMed] [Google Scholar]
- Goetz M, Godt DE, Guivarc’h A, Kahmann U, Chriqui D, Roitsch T (2001) Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc Natl Acad Sci USA 98: 6522–6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner S, Rausch T, Sonnewald U, Herbers K (1999) Ectopic expression of a tobacco invertase inhibitor homolog prevents cold-induced sweetening of potato tubers. Nat Biotechnol 17: 708–711 [DOI] [PubMed] [Google Scholar]
- Guivarc’h A, Rembur J, Goetz M, Roitsch T, Noin M, Schmülling T, Chriqui D (2002) Local expression of the ipt gene in transgenic tobacco (Nicotiana tabacum L. cv SR1) axillary buds establishes a role for cytokinins in tuberization and sink formation. J Exp Bot 53: 621–629 [DOI] [PubMed] [Google Scholar]
- Hartmann A, Senning M, Hedden P, Sonnewald U, Sonnewald S (2011) Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol 155: 776–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer AG, Raap M, Schroeer B, Marty B, Willmitzer L (2004) Cell wall invertase expression at the apical meristem alters floral, architectural, and reproductive traits in Arabidopsis thaliana. Plant J 39: 161–169 [DOI] [PubMed] [Google Scholar]
- Jung B, Ludewig F, Schulz A, Meißner G, Wöstefeld N, Flügge U-I, Pommerrenig B, Wirsching P, Sauer N, Koch W (2015) Identification of the transporter responsible for sucrose accumulation in sugar beet taproots. Nat Plants 1: 14001. [DOI] [PubMed] [Google Scholar]
- Kalousek P, Buchtová D, Balla J, Reinöhl V, Procházka S (2014) Cytokinins and polar transport of auxin in axillary pea buds. Acta Univ Agric Silvic Mendelianae Brun 58: 79–88 [Google Scholar]
- Kiba T, Takebayashi Y, Kojima M, Sakakibara H (2019) Sugar-induced de novo cytokinin biosynthesis contributes to Arabidopsis growth under elevated CO 2. Sci Rep 9: 7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann EM, Hall B, Bennett AB (1996) Antisense acid invertase (TIV1) gene alters soluble sugar composition and size in transgenic tomato fruit. Plant Physiol 112: 1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepek Y-S, Geiger D, Stadler R, Klebl F, Landouar-Arsivaud L, Lemoine R, Hedrich R, Sauer N (2005) Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose. Plant Cell 17: 204–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwah S, Laxmi A (2014) The interaction between glucose and cytokinin signal transduction pathway in A rabidopsis thaliana. Plant Cell Environ 37: 235–253 [DOI] [PubMed] [Google Scholar]
- Lastdrager J, Hanson J, Smeekens S (2014) Sugar signals and the control of plant growth and development. J Exp Bot 65: 799–807 [DOI] [PubMed] [Google Scholar]
- Leduc N, Roman H, Barbier F, Péron T, Huché-Thélier L, Lothier J, Demotes-Mainard S, Sakr S (2014) Light signaling in bud outgrowth and branching in plants. Plants 3: 223–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sheen J (2016) Dynamic and diverse sugar signaling. Curr Opin Plant Biol 33: 116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wu L, Foster R, Ruan Y (2017) Molecular regulation of sucrose catabolism and sugar transport for development, defence and phloem function. J Integr Plant Biol 59: 322–335 [DOI] [PubMed] [Google Scholar]
- Liao S-C, Lin C-S, Wang A-Y, Sung H-Y (2013) Differential expression of genes encoding acid invertases in multiple shoots of bamboo in response to various phytohormones and environmental factors. J Agric Food Chem 61: 4396–4405 [DOI] [PubMed] [Google Scholar]
- Liao S, Wang L, Li J, Ruan Y-L (2020) Cell wall invertase is essential for ovule development through sugar signaling rather than provision of carbon nutrients. Plant Physiol 183: 1126–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang C, Ou Y, Lin Y, Song B, Xie C, Liu J, Li XQ (2011) Systematic analysis of potato acid invertase genes reveals that a cold-responsive member, StvacINV1, regulates cold-induced sweetening of tubers. Mol Genet Genomics 286: 109–118 [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28: 465–474 [DOI] [PubMed] [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci 111: 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron D, Schaffer AA (1991) Sucrose phosphate synthase, sucrose synthase, and invertase activities in developing fruit of Lycopersicon esculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol 95: 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Matsumoto‐Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37: 128–138 [DOI] [PubMed] [Google Scholar]
- Morey SR, Hirose T, Hashida Y, Miyao A, Hirochika H, Ohsugi R, Yamagishi J, Aoki N (2018) Genetic evidence for the role of a rice vacuolar invertase as a molecular sink strength determinant. Rice 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moigne M-A, Guérin V, Furet P-M, Billard V, Lebrec A, Spíchal L, Roman H, Citerne S, Morvan-Bertrand A, Limami A (2018) Asparagine and sugars are both required to sustain secondary axis elongation after bud outgrowth in Rosa hybrida. J Plant Physiol 222: 17–27 [DOI] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng W-H, Liu Y-X, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Morris DA, Arthur ED (1984) Invertase activity in sinks undergoing cell expansion. Plant Growth Regul 2: 327–337 [Google Scholar]
- Müller D, Leyser O (2011) Auxin, cytokinin and the control of shoot branching. Ann Bot 107: 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägele T, Henkel S, Hörmiller I, Sauter T, Sawodny O, Ederer M, Heyer AG (2010) Mathematical modeling of the central carbohydrate metabolism in Arabidopsis reveals a substantial regulatory influence of vacuolar invertase on whole plant carbon metabolism. Plant Physiol 153: 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot N, Hausman J-F, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56: 2907–2914 [DOI] [PubMed] [Google Scholar]
- Nisler J, Zatloukal M, Popa I, Doležal K, Strnad M, Spíchal L (2010) Cytokinin receptor antagonists derived from 6-benzylaminopurine. Phytochemistry 71: 823–830 [DOI] [PubMed] [Google Scholar]
- Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. Proc Natl Acad Sci USA 101: 8039–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongaro V, Bainbridge K, Williamson L, Leyser O (2008) Interactions between axillary branches of Arabidopsis. Mol Plant 1: 388–400 [DOI] [PubMed] [Google Scholar]
- Otori K, Tamoi M, Tanabe N, Shigeoka S (2017) Enhancements in sucrose biosynthesis capacity affect shoot branching in Arabidopsis. Biosci Biotechnol Biochem 81: 1470–1477 [DOI] [PubMed] [Google Scholar]
- Patrick JW (1997) Phloem unloading: sieve element unloading and post-sieve element transport. Annu Rev Plant Biol 48: 191–222 [DOI] [PubMed] [Google Scholar]
- Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011) Cytokinin‐mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water‐stress. Plant Biotechnol J 9: 747–758 [DOI] [PubMed] [Google Scholar]
- Phillips Idj (1975) Apical dominance. Annu Rev Plant Physiol 26: 341–367 [Google Scholar]
- Proels RK, Roitsch T (2009) Extracellular invertase LIN6 of tomato: a pivotal enzyme for integration of metabolic, hormonal, and stress signals is regulated by a diurnal rhythm. J Exp Bot 60: 1555–1567 [DOI] [PubMed] [Google Scholar]
- Rabot A, Henry C, Ben Baaziz K, Mortreau E, Azri W, Lothier J, Hamama L, Boummaza R, Leduc N, Pelleschi-Travier S (2012) Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol 53: 1068–1082 [DOI] [PubMed] [Google Scholar]
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, Sakr S (2015) Multiple pathways regulate shoot branching. Front Plant Sci 5: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha‐Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L (1989) Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J 8: 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, Ehneß R (2000) Regulation of source/sink relations by cytokinins. Plant Growth Regul 32: 359–367 [Google Scholar]
- Roman H, Girault T, Barbier F, Péron T, Brouard N, Pěnčík A, Novák O, Vian A, Sakr S, Lothier J (2016) Cytokinins are initial targets of light in the control of bud outgrowth. Plant Physiol 172: 489–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T, Thimann K V (1964) Release of lateral buds from apical dominance. Nature 201: 939 [Google Scholar]
- Sachs T, Thimann K V (1967) The role of auxins and cytokinins in the release of buds from dominance. Am J Bot 54: 136–144 [Google Scholar]
- Saeed W, Naseem S, Ali Z (2017) Strigolactones biosynthesis and their role in abiotic stress resilience in plants: A critical review. Front Plant Sci 8: 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S, Wang M, Dédaldéchamp F, Perez-Garcia M-D, Ogé L, Hamama L, Atanassova R (2018) The sugar-signaling hub: overview of regulators and interaction with the hormonal and metabolic network. Int J Mol Sci 19: 2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam BB, Malka SK, Zhu X, Gong H, Ziv C, Teper-Bamnolker P, Ori N, Jiang J, Eshel D (2017) Etiolated stem branching is a result of systemic signaling associated with sucrose level. Plant Physiol 175: 734–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54: 525–531 [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin–cytokinin interactions in the control of shoot branching. Plant Mol Biol 69: 429. [DOI] [PubMed] [Google Scholar]
- Spíchal L, Werner T, Popa I, Riefler M, Schmülling T, Strnad M (2009) The purine derivative PI‐55 blocks cytokinin action via receptor inhibition. FEBS J 276: 244–253 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45: 1028–1036 [DOI] [PubMed] [Google Scholar]
- Tang G-Q, Lüscher M, Sturm A (1999) Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell 11: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper-Bamnolker P, Buskila Y, Lopesco Y, Ben-Dor S, Saad I, Holdengreber V, Belausov ED, Zemach H, Ori N, Lers A, Eshel D (2012) Release of apical dominance in potato tuber is accompanied byprogrammed cell death in the apical bud meristem. Plant Physiology 158: 2053–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper-Bamnolker P, Buskila YBelausov E, Wolf D, Doron-Faigenboim A, Ben-Dor S, Van der Hoorn RA, Lers A, Eshel D (2017) Vacuolar processing enzyme (VPE) activates programmed cell death in the apical meristem inducing loss of apical dominance. Plant, Cell & Environment 40: 2381–2392 [DOI] [PubMed] [Google Scholar]
- Thomas TH (1986) Hormonal control of assimilate movement and compartmentation. In MBopp, ed, Plant Growth Substances. Springer, Berlin–Heidelberg–New York–Tokyo, pp 350–359 [Google Scholar]
- Tymowska-Lalanne Z, Kreis M (1998) Expression of the Arabidopsis thaliana invertase gene family. Planta 207: 259–265 [DOI] [PubMed] [Google Scholar]
- Patrick JW, Botha FC, Birch RG (2013) Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol J 11: 142–156 [DOI] [PubMed] [Google Scholar]
- Wang B, Smith SM, Li J (2018) Genetic regulation of shoot architecture. Annu Rev Plant Biol 69: 437–468 [DOI] [PubMed] [Google Scholar]
- Wang W, Hao Q, Tian F, Li Q, Wang W (2016) Cytokinin-regulated sucrose metabolism in stay-green wheat phenotype. PLoS One 11: e0161351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw IF, Mortimer DC (1970) Carbohydrate movement in pea plants in relation to axillary bud growth and vascular development. Can J Bot 48: 229–237 [Google Scholar]
- Werner T, Holst K, Pörs Y, Guivarc’h A, Mustroph A, Chriqui D, Grimm B, Schmülling T (2008) Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J Exp Bot 59: 2659–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A (2018) Transitioning to the next phase: the role of sugar signaling throughout the plant life cycle. Plant Physiol 176: 1075–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten H-M, Stitt M (2014) The sucrose–trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 65: 1051–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Gong H, He Q, Zeng Z, Busse JS, Jin W, Bethke PC, Jiang J (2016) Silencing of vacuolar invertase and asparagine synthetase genes and its impact on acrylamide formation of fried potato products. Plant Biotechnol J 14: 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.