Brassinosteroids suppress the climacteric fruit ripening by suppressing ethylene biosynthesis.
Abstract
The plant hormone ethylene is important for the ripening of climacteric fruit, such as pear (Pyrus ussuriensis), and the brassinosteroid (BR) class of phytohormones affects ethylene biosynthesis during ripening via an unknown molecular mechanism. Here, we observed that exogenous BR treatment suppressed ethylene production and delayed fruit ripening, whereas treatment with a BR biosynthesis inhibitor promoted ethylene production and accelerated fruit ripening in pear, suggesting BR is a ripening suppressor. The expression of the transcription factor BRASSINAZOLE-RESISTANT 1PuBZR1 was enhanced by BR treatment during pear fruit ripening. PuBZR1 interacted with PuACO1, which converts 1-aminocyclopropane-1-carboxylic acid (ACC) to ethylene, and suppressed its activity. BR-activated PuBZR1 bound to the promoters of PuACO1 and of PuACS1a, which encodes ACC synthase, and directly suppressed their transcription. Moreover, PuBZR1 suppressed the expression of transcription factor PuERF2 by binding its promoter, and PuERF2 bound to the promoters of PuACO1 and PuACS1a. We concluded that PuBZR1 indirectly suppresses the transcription of PuACO1 and PuACS1a through its regulation of PuERF2. Ethylene production and expression profiles of corresponding apple (Malus domestica) homologs showed similar changes following epibrassinolide treatment. Together, these results suggest that BR-activated BZR1 suppresses ACO1 activity and the expression of ACO1 and ACS1, thereby reducing ethylene production and suppressing fruit ripening. This likely represents a conserved mechanism by which BR suppresses ethylene biosynthesis during climacteric fruit ripening.
Introduction
In the life cycle of a fruit, the transformation of a mature green fruit into a ripe fruit is strictly regulated and highly coordinated by various metabolic pathways including hormones (Trainotti et al., 2007). Fruit ripening is a developmental process that can be categorized as climacteric or nonclimacteric (Klee and Giovannoni, 2011). The onset of normal ripening in climacteric fruit requires increased biosynthesis of the gaseous hormone ethylene (Barry and Giovannoni, 2007), and reducing ethylene in climacteric fruit leads to a slow softening rate and longer shelf-life (Osorio et al., 2013). Numerous studies have shown that other hormones are also involved (Zaharah et al., 2011; Chai et al., 2012; Li et al., 2017); however, current understanding of the mechanisms by which these hormones interact with ethylene signaling to regulate fruit ripening is very limited.
The biosynthesis of ethylene includes two critical steps: the formation of 1-aminocyclopropane-1-carboxylic acid (ACC) from S-adenosyl methionine by the enzyme ACC synthase (ACS) and the conversion of ACC to ethylene by ACC oxidase (ACO; Yang and Hoffman, 1984). Previous reports have documented the importance of ACS and ACO genes in ethylene biosynthesis during fruit ripening. For example, silencing of ACS or ACO in transgenic tomato (Solanum lycopersicum) or apple (Malus domestica) fruit results in substantially reduced or undetectable ethylene production (Dandekar et al., 2004; Schaffer et al., 2007; Gupta et al., 2013). The actions of both ACS and ACO are regulated transcriptionally in many species. Examples include a MINICHROMOSOME MAINTENANCE1 from Saccharomyces cerevisiae, AGAMOUS from Arabidopsis thaliana, DEFICIENS from Antirrhinum majus, and SERUM RESPONSE FACTOR from Homo sapiens (MADS-box) transcription factor, RIPENING INHIBITOR, which binds to the promoter of SlACS2 in tomato (Ito et al., 2008), an ethylene response factor, MaERF11, which binds to the promoter of MaACO1 and suppresses its expression in banana (Musa acuminata; Han et al., 2016), and MdERF3, which binds to the promoter of MdACS1 and activates its expression in apple (Li et al., 2016a).
Various phytohormones have been observed to influence ethylene biosynthesis during fruit ripening. Abscisic acid (ABA) concentration increases at the onset of tomato fruit ripening, and application of exogenous ABA promotes the expression of ethylene biosynthetic genes and ethylene production (Zhang et al., 2009). In peach (Prunus persica) fruit, the level of the auxin indole-3-acetic acid increases prior to fruit ripening, and the application of synthetic auxin results in increased PpACS1 expression and ethylene production (Trainotti et al., 2007; Tatsuki et al., 2013). Another well-studied example is jasmonate, which promotes ethylene production in apple fruit via the MdMYC2 transcription factor. This process includes jasmonate-activated MdMYC2 binding to the promoters of both MdACS1 and MdACO1 to induce their expression during fruit ripening (Li et al., 2017). However, in contrast to the above classes of phytohormones, the mechanism by which brassinosteroids (BRs) affect ethylene biosynthesis during ripening is not known.
BRs are involved in regulating a wide range of plant physiological processes and much has been learnt about the BR signaling pathway (Clouse, 2011). Following biosynthesis, BRs are perceived by BRASSINOSTEROID INSENSITIVE 1 (BRI1), leading to association with BRI1-ASSOCIATED KINASE 1 (BAK1). BRI1 and BAK1 transphosphorylate each other, allowing BRI1 to phosphorylate BR SIGNALING KINASE 1 (BSK1). The phosphorylated BSK1 activates BRI SUPPRESSOR 1, which dephosphorylates BIN2 and inactivates it. PROTEIN PHOSPHATASE 2A dephosphorylates BRASSINAZOLE-RESISTANT 1 (BZR1), leading to accumulation of unphosphorylated BZR1 and its homologs in the nucleus. BZR1 and its homologs bind to the promoters of BR-responsive genes and regulate their expression (He et al., 2005; Yin et al., 2005; Li and Jin, 2007; Nolan et al., 2020). The effect of BR on ethylene biosynthesis and fruit ripening has been documented. For example, BR-treated jujube (Zizyphus jujuba) fruit, which is categorized as a climacteric fruit, shows significantly reduced ethylene production during storage (Zhu et al., 2010), while strawberry (Fragaria ananassa), a nonclimacteric fruit, shows delayed fruit ripening after application of epibrassinolide (EBR; Chai et al., 2012). In tomato, another climacteric fruit, treatment with brassinolide promotes the expression of SlACS and SlACO genes, as well as ethylene production (Zhu et al., 2015). More interestingly, overexpression of the BR biosynthetic gene DWARF in tomato results in increased level of endogenous BRs and ethylene production and earlier ripening (Li et al., 2016b), indicating endogenous BR can affect fruit ripening.
These studies indicate that BR is involved in the regulation of ethylene biosynthesis and fruit ripening; however, little is known about how BR signaling genes interact with ethylene biosynthetic genes to regulate ethylene production. In this study, we investigated the effects of BRs on fruit ripening in pear (Pyrus ussuriensis) and apple. The resulting data provide new insights into the molecular basis by which BR suppresses climacteric fruit ripening.
Results
BR suppresses pear fruit ripening
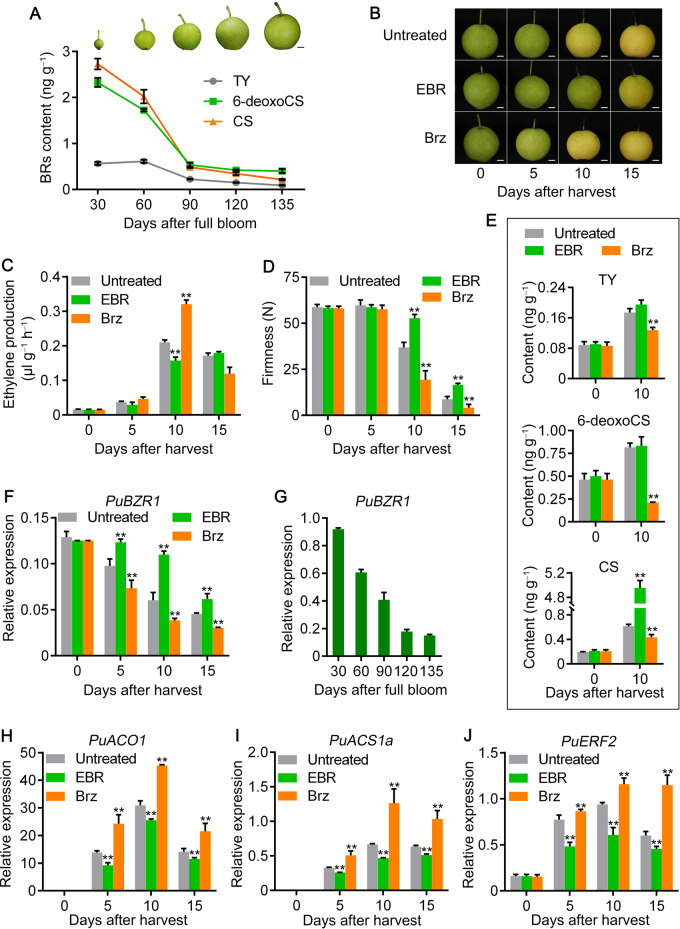
To elucidate the role of BRs in fruit ripening, we first measured the endogenous BR content of pear fruit during development. Fruits were harvested every 30 d from 30 to 135 d after full bloom (DAFB), where 135 DAFB is the day of commercial harvest. Endogenous BRs, such as typhasterol (TY), 6-deoxocastasterone (6-deoxoCS), and castasterone (CS), gradually decreased during fruit development (Figure 1A). The decline of endogenous BRs during fruit development indicated that BR might be a suppressor of fruit ripening.
Figure 1.
BRs suppress pear fruit ripening. Endogenous BR content during pear fruit development was measured in 2020 (A). Fruit collected on the day of commercial harvest (135 DAFB) were treated with EBR or Brz, an inhibitor of BR biosynthesis, and stored at room temperature for 15 d (B). After treatment, ethylene production (C), fruit firmness (D), and endogenous BRs (E) were measured. RT-qPCR was used to examine the PuBZR1 expression level in EBR- and Brz-treated fruit (F) as well as in fruit during development (G). The expression level of PuACO1 (H), PuACS1a (I), and PuERF2 (J) was also investigated. Untreated, fruit not receiving any treatment; EBR, fruit treated with EBR; Brz, fruit treated with Brz. Bars, 1 cm. The x-axes indicate the number of days of storage at room temperature after harvest; 0 indicates the fruit harvested on the day of commercial harvest. Three biological replicates were analyzed as described in the Materials and Methods section. Values represent means ± SE. Statistical significance was determined using a Student’s t test (**P < 0.01).
To investigate the effect of BR on fruit ripening, we designed the following experiments: pear fruits were harvested on the day of commercial harvest, stored at room temperature for 15 d, during which fruit finish the ripening process, and sampled every 5 d; fruits treated with EBR, a BR, were used to compare the ripening behavior with control fruit. In 2015, pear fruits harvested at commercial harvest stage were treated with different concentrations of EBR (0.2, 3, and 10 µM). All treatments significantly inhibited ethylene production and maintained fruit firmness compared with untreated control fruit during the storage period, with 3 µM of EBR treatment having the largest effect (Supplemental Figure S1). In 2016 and 2020, fruits harvested at commercial harvest stage in each year were treated with 3 µM of EBR, and we observed the same effect on ethylene production and fruit firmness as in 2015 (Figure 1, B–D; Supplemental Figure S2). More interestingly, treatment of brassinazole (Brz), an inhibitor of BR biosynthesis, significantly promoted ethylene production and decreased fruit firmness (Figure 1, B–D). EBR treatment enhanced and Brz treatment suppressed endogenous BR biosynthesis (Figure 1E). These results indicated that endogenous BR is a suppressor of ethylene biosynthesis and fruit ripening.
We also observed that Brz treatment was not able to promote fruit ripening until fruits were developed to a certain stage, because when fruits were harvested before 120 DAFB and treated with Brz, it had no effect on ethylene production and fruit ripening, however, when fruits were harvested at 120 DAFB or later, Brz treatment was able to promote ethylene production and fruit ripening (Supplemental Figure S3).
Given that BZR1 is a key transcription factor in the BR signaling pathway (Kim and Wang, 2010), as an initial step in understanding BR regulated processes associated with ethylene production and fruit ripening, we identified a total of seven PuBZR1 or PuBZR1-like genes from pear (Supplemental Figure S4). Of these, we observed that only the expression of PuBZR1 was significantly enhanced by EBR treatment and repressed by Brz treatment (Figure 1F;Supplemental Figure S2). Moreover, PuBZR1 expression gradually decreased during fruit development (Figure 1G). We, therefore, focused on PuBZR1 and tested the hypothesis that it acts as a BR-induced suppressor of ethylene biosynthesis during pear fruit ripening.
PuBZR1 interacts with PuACO1 and suppresses PuACO1 enzyme activity
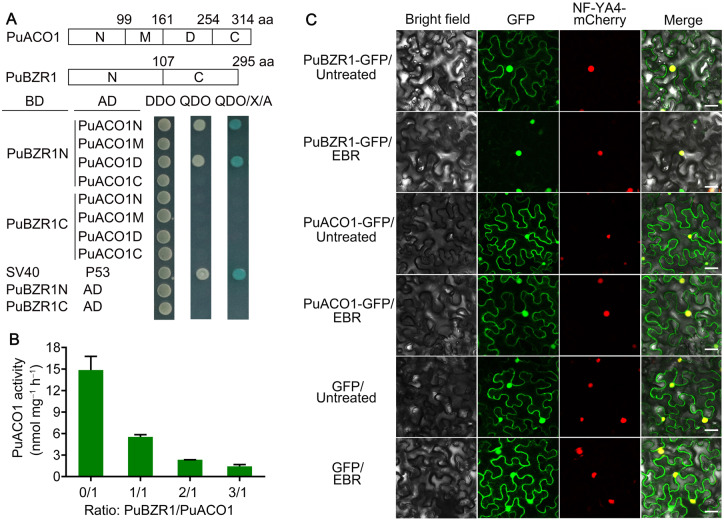
To further characterize the putative role of PuBZR1 in BR-suppressed ethylene biosynthesis, we used PuBZR1 as a bait in yeast two-hybrid (Y2H) screen of a pear fruit cDNA library. A total of 135 positive clones were identified from the screen, corresponding to 23 genes; one of which encoded PuACO1, a key enzyme in ethylene biosynthesis. The potential interaction between PuBZR1 and PuACO1 was then confirmed by co-expressing the two proteins in yeast cells (Supplemental Figure S5A), and this was further validated using a pull-down assay involving PuBZR1-His and PuACO1-GST peptide tagged fusion proteins (Supplemental Figure 5B). We divided the predicted coding region of PuBZR1 into two and the coding region of PuACO1 into four fragments, and used them in a Y2H assay, which showed that the PuBZR1 N terminal region (PuBZR1N) interacts with both the N and D fragments of PuACO1 (PuACO1N and PuACO1D; Figure 2A). Interestingly, the PuACO1D contains Fe2+ binding sites (Supplemental Figure S6) that are essential for ACO enzyme activity (Shaw et al., 1996; Zhang et al., 1997; Rocklin et al., 1999). We then investigated whether the interaction between PuBZR1 and PuACO1 affected PuACO1 enzymatic activity in reactions containing purified PuACO1 mixed with different amounts of purified PuBZR1. PuACO1 activity gradually declined with increasing amounts of PuBZR1 (Figure 2B), suggesting suppression of PuACO1 activity through direct interaction with PuBZR1. These results implied that PuBZR1 might affect PuACO1 activity in vivo.
Figure 2.
BR-activated PuBZR1 interacts with PuACO1 and inhibits PuACO1 enzyme activity. A, The PuBZR1 and PuACO1 protein sequences were divided into two and four fragments, respectively, and their interaction was investigated using a Y2H assay. DDO, SD medium lacking Trp and Leu; QDO, SD medium lacking Trp, Leu, His, and Ade; QDO/X/A, QDO medium containing x-a-gal and aureobasidin A (AbA). SV40 and P53 were used as a positive control, and AD vectors as negative controls. The blue color indicates protein interaction. B, The influence of PuBZR1–PuACO1 interaction on PuACO1 activity was evaluated by adding increased amounts of the PuBZR1 protein. Recombinant His-tagged PuBZR1 and GST-tagged PuACO1 were used. Error bars represent the SE of three independent measurements. C, Subcellular localization of PuBZR1 and PuACO1 with or without EBR treatment. PuBZR1-GFP or PuACO1-GFP was transiently expressed in N. benthamiana leaves, followed by EBR treatment. Transient expression of GFP alone was used as a control. A mCherry-labeled NF-YA4-mCherry vector was used as a nuclear marker. Untreated, N. benthamiana leaves not receiving any treatment; EBR, N. benthamiana leaves treated with EBR. Scale bars, 50 μm.
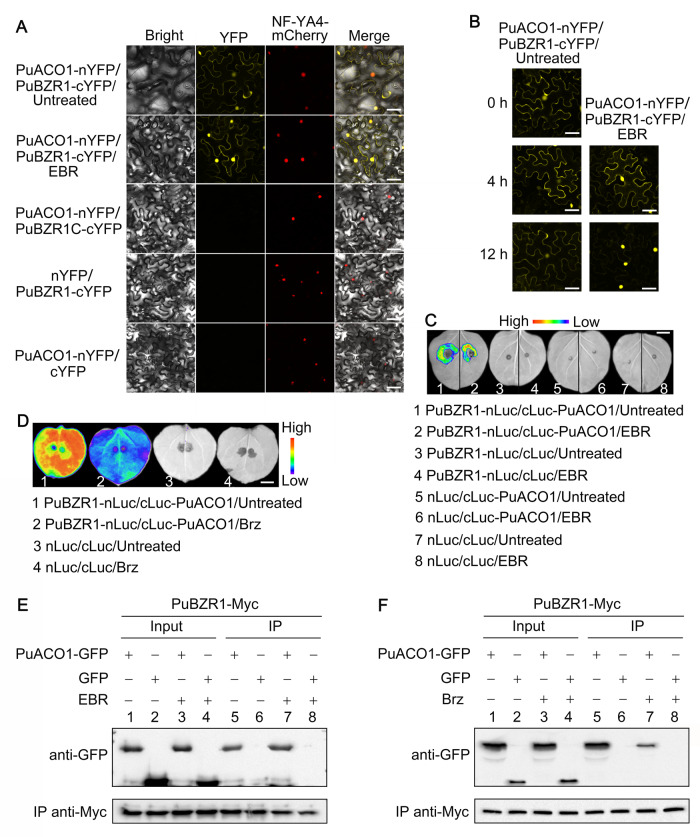
Next, we investigated the intracellular localization of PuBZR1 and PuACO1 to test if they have co-localization. Constructs containing the coding sequence (CDS) of PuBZR1 and PuACO1 fused to a green fluorescent protein (GFP) peptide tag were infiltrated into Nicotiana benthamiana leaves. Both PuBZR1 and PuACO1 localized in the cytoplasm and nucleus (Figure 2C). Under EBR treatment, most PuBZR1 protein moved into the nucleus, but PuACO1 did not (Figure 2C). We then investigated whether the interaction between PuBZR1 and PuACO1 affected their respective intracellular localization under EBR treatment. Constructs containing the CDS of PuBZR1 and PuACO1 (with only one of them harboring a GFP tag) were co-infiltrated into N. benthamiana leaves, and the infiltrated leaves were treated with EBR. The presence of each protein (PBZR1 or PuACO1) did not affect the localization of the other (Supplemental Figure S7). A bimolecular fluorescence complementation (BiFC) assay was performed to see where PuBZR1 and PuACO1 interact in planta. Constructs containing PuBZR1 fused into pSPYCE-35S vector (PuBZR1-cYFP) and PuACO1 fused into pSPYNE-35S vector (PuACO1-nYFP) were co-infiltrated into N. benthamiana leaves, and the infiltrated leaves were treated with EBR. PuBZR1 interacted with PuACO1 in both the cytoplasm and nucleus (Figure 3A). Following EBR treatment, a stronger YFP signal was observed in the nucleus, and a weaker YFP signal was observed in the cytoplasm (Figure 3A). More signals were observed in the nucleus as time passed (Figure 3B). These results suggested that BR enhances PuBZR1 movement into the nucleus, but PuBZR1still interacts with PuACO1 in both cytoplasm and nucleus.
Figure 3.
BR enhances PuBZR1–PuACO1 interaction. A, B, Interaction of PuBZR1 and PuACO1 in a BiFC assay. Nicotiana benthamiana leaves were co-infiltrated with PuBZR1-cYFP and PuACO1-nYFP constructs and kept in the dark for 48 h, and then EBR was injected into the infiltrated leaves. Nicotiana benthamiana leaves were visualized by confocal microscopy 4 h after EBR injection (A). These leaves were also visualized at 0, 4, and 12 h after EBR injection (B). NF-YA4-mCherry was used as a nuclear marker. PuBZR1C-cYFP with PuACO1-nYFP, PuBZR1-cYFP with nYFP, and cYFP with PuACO1-nYFP, were used as negative controls. Scale bars, 50 μM. C, A firefly Luc complementation imaging assay showing that EBR treatment enhanced the interaction between PuBZR1 and PuACO1 in N. benthamiana leaves. Agrobacterium tumefaciens strain EHA105 harboring different constructs was infiltrated into N. benthamiana leaves. Untreated, N. benthamiana leaves not receiving any treatment; EBR, N. benthamiana leaves treated with EBR. Luc activities were recorded in these regions 3 d after infiltration. Bar, 1 cm. The black lines were used to indicate the different regions on each leaf. D, A firefly Luc complementation imaging assay showing that Brz treatment weakened the interaction between PuBZR1 and PuACO1 in N. benthamiana leaves. Bar, 1 cm. E, A co-IP assay showing that EBR treatment enhanced the interaction between PuBZR1 and PuACO1. PuBZR1 fused to a Myc tag (PuBZR1-Myc) and PuACO1 fused to a GFP tag (PuACO1-GFP) were overexpressed in N. benthamiana leaves, and a Myc antibody was used for immunoprecipitation analysis. Myc and GFP antibodies were used in an immunoblot analysis. The band detected by the GFP antibody in the precipitated protein sample indicates the interaction between PuBZR1 and PuACO1 (Lane 5) and EBR treatment enhances the interaction (Lane 7). F, A co-IP assay showing that Brz treatment weakened the interaction between PuBZR1 and PuACO1. The co-IP assay was performed as in (E). The band detected by the GFP antibody in the precipitated protein sample indicates the interaction between PuBZR1 and PuACO1 (Lane 5) and Brz treatment weakens the interaction (Lane 7).
Finally, we performed a firefly luciferase (Luc) complementation imaging assay to investigate how BR affects the PuBZR1–PuACO1 interaction. Constructs containing PuBZR1 fused with the N terminus of Luc (PuBZR1-nLuc) and the C terminus of Luc fused with PuACO1 (cLuc-PuACO1) were co-infiltrated into N. benthamiana leaves, and the infiltrated leaves were treated with EBR or Brz. A luminescence signal was detected in the PuBZR1-nLuc/cLuc-PuACO1 co-expressing region (Figure 3C, Region 1) but not in the negative controls (Figure 3C, Regions 3, 5, and 7), consistent with PuBZR1–PuACO1 protein interaction in planta. Following EBR treatment, a stronger luminescence signal was observed in the PuBZR1-nLuc/cLuc-PuACO1 co-expressing region (Figure 3C, Region 2) but not in the negative controls (Figure 3C, Regions 4, 6, and 8). In contrast, Brz treatment weakened the PuBZR1–PuACO1 protein interaction (Figure 3D, Region 2). A coimmunoprecipitation (co-IP) assay was employed to confirm this result, in which PuBZR1-Myc and PuACO1-GFP were overexpressed in N. benthamiana leaves and treated with EBR or Brz. PuACO1-GFP was detected in the PuBZR1-Myc immunoprecipitated extract, and EBR treatment enhanced the signal, whereas Brz treatment weakened this signal (Figure 3, E and F, Lanes 5 and 7). These results indicated that BR enhances the interaction between PuBZR1 and PuACO1.
We also measured ACO enzyme activity in extracts from pear fruit that had been treated with EBR, or were untreated, and found that EBR treatment significantly inhibited ACO activity (Supplemental Figure S8).
BR-activated PuBZR1 suppresses the expression of PuACO1 and PuACS1a via transcriptional regulation
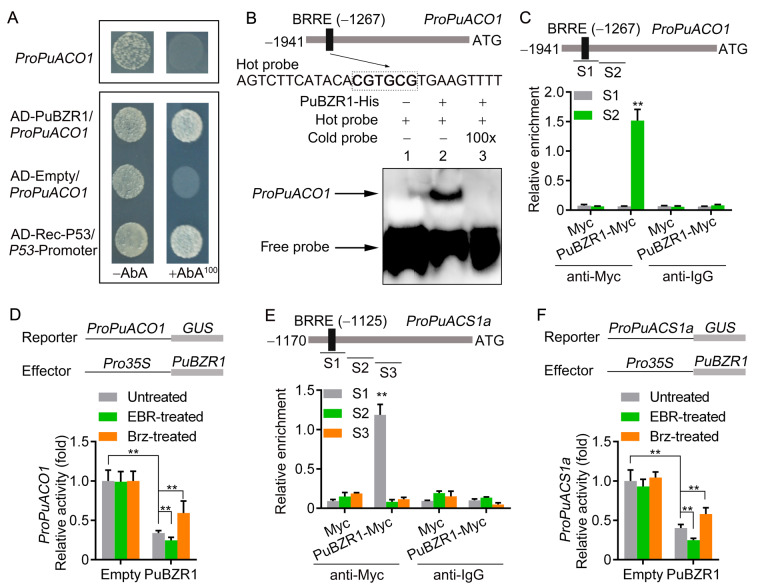
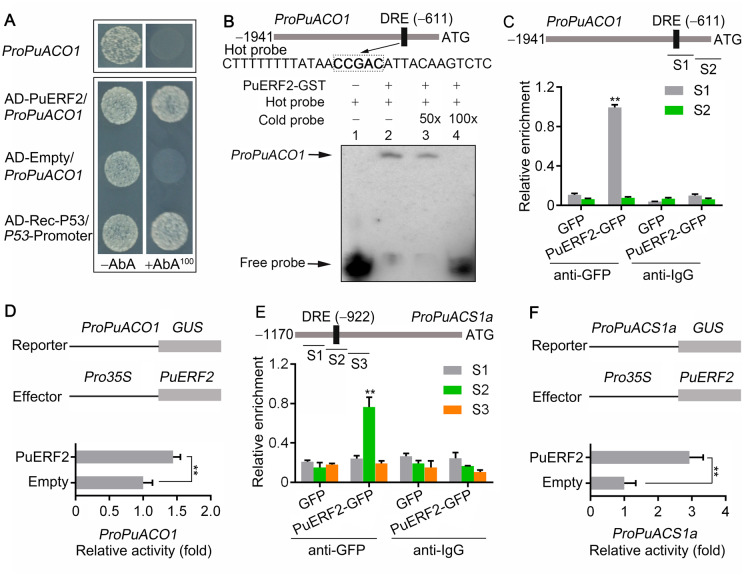
We observed that PuACO1 expression was reduced by EBR treatment and enhanced by Brz treatment (Figure 1H); moreover, EBR treatment enhanced the nuclear translocation of PuBZR1 (Figure 2C), consistent with transcriptional regulation. We identified a BR response element (BRRE) in the promoter of PuACO1 (1,941-bp upstream of the predicted translation start site) that we predicted might be involved in BZR1 binding, and so investigated whether PuBZR1 can bind to the PuACO1 promoter and regulate its expression. This was indeed confirmed using both a yeast one-hybrid (Y1H) assay (Figure 4A) and an electrophoretic mobility shift assay (EMSA; Figure 4B).
Figure 4.
BR-Activated PuBZR1 suppresses the transcription of PuACO1 and PuACS1a. A, Y1H analysis showing that PuBZR1 binds to the promoter of PuACO1 (ProPuACO1). AbA, a yeast cell growth inhibitor, was used as a screening marker. The basal concentration of AbA was 100 ng mL−1. Rec-P53 and the P53-promoter were used as positive controls. The empty vector and the PuACO1 promoter were used as negative controls. B, EMSA showing that PuBZR1 binds to the BRRE motif in the PuACO1 promoter. The hot probe was a biotin-labeled fragment of the PuACO1 promoter containing the BRRE motif, and the cold probe was a nonlabeled competitive probe (100-fold that of the hot probe). His-tagged PuBZR1 was purified. C, ChIP-PCR showing the in vivo binding of PuBZR1 to the PuACO1 promoter. Cross-linked chromatin samples were extracted from PuBZR1-Myc overexpressing pear fruit calli (PuBZR1-Myc) and precipitated with an anti-Myc antibody. Eluted DNA was used to amplify the sequences neighboring the BRRE motif by qPCR. Two regions (S1 and S2) were investigated. Fruit calli overexpressing the Myc sequence (Myc) were used as negative controls. Values are the percentage of DNA fragments that co-immunoprecipitated with the Myc antibody or a nonspecific antibody (pre-immune rabbit IgG) relative to the input DNA. The ChIP assay was repeated 3 times, and the enriched DNA fragments in each ChIP were used as one biological replicate for qPCR. Values represent means ± se. Statistical significance was determined using a Student’s t test (**P < 0.01). D, GUS activity analysis showing that PuBZR1 suppresses the PuACO1 promoter. The PuBZR1 effector vector and the reporter vector containing the PuACO1 promoter were infiltrated into N. benthamiana leaves to analyze the regulation of GUS activity. Untreated, N. benthamiana leaves not receiving any treatment; EBR, N. benthamiana leaves treated with EBR; Brz, N. benthamiana leaves treated with Brz. Three independent transfection experiments were performed. Values represent means ± se. Statistical significance was determined using a Student’s t test (**P < 0.01). E, ChIP-PCR showing the in vivo binding of PuBZR1 to the PuACS1a promoter. F, GUS activity analysis showing that PuBZR1 suppresses the PuACS1a promoter.
In vivo verification was performed by conducting a chromatin immunoprecipitation (ChIP)-PCR (polymerase chain reaction) assay. The CDS of PuBZR1 fused to a sequence encoding a Myc peptide tag was overexpressed in pear fruit calli. The presence of PuBZR1 substantially enhanced the PCR-based detection of the PuACO1 promoter (Figure 4C), indicating that PuBZR1 binds to the PuACO1 promoter in vivo. When the regulation of the PuACO1 promoter by PuBZR1 was examined in N. benthamiana leaves co-transformed with the Pro35S:PuBZR1 and ProPuACO1:GUS constructs using a β-glucuronidase (GUS) activation assay, a significantly reduced GUS signal was observed (Figure 4D), indicating that PuBZR1 suppresses the activity of the PuACO1 promoter. When EBR was applied to the N. benthamiana leaves, the GUS signal was further reduced (Figure 3D). Taken together, these results suggested that PuBZR1 directly suppresses the transcription of PuACO1 and that BR strengthens this suppression.
ACS is also central to ethylene biosynthesis through its role in forming the ethylene precursor, ACC (Yang and Hoffman, 1984; Kende, 1993), and our previous study revealed five ACS genes that were differentially expressed during pear fruit ripening (Huang et al., 2014). When we investigated their expression profiles in EBR-treated fruit in this study, we detected high expression of PuACS1a, which was significantly suppressed by the EBR treatment (Figure 1I;Supplemental Figure S9). Notably, a BRRE motif was identified in the PuACS1a promoter (1,170 bp). We then performed ChIP-PCR and a GUS activation assay in N. benthamiana, which revealed that PuBZR1 bound and reduced the promoter activity of PuACS1a (Figure 4, E and F). When EBR was applied to the N. benthamiana leaves, the GUS signal was reduced further (Figure 4F), suggesting that PuBZR1 directly inhibits the transcription of PuACS1a and that BR strengthens this suppression.
BR-activated PuBZR1 suppresses the expression of PuERF2, and PuERF2 binds to the PuACO1 and PuACS1a promoters
Our previous study showed that four ERF (ethylene response factor) transcription factors were differentially expressed during pear fruit ripening (Huang et al., 2014). Here, we investigated their expression and found that, of the four, only PuERF2 expression was significantly suppressed by the EBR treatment (Figure 1J), while the others showed no significant change compared with the controls (Supplemental Figure S10); moreover, the intracellular localization of PuERF2 was in the nucleus and was not affected by EBR treatment (Supplemental Figure S11). We next confirmed that PuERF2 can bind to the PuACO1 promoter using both Y1H and EMSA analysis (Figure 5, A and B). We further demonstrated binding in vivo by ChIP-PCR, where the CDS of PuERF2 fused to a sequence encoding GFP peptide tag was overexpressed in pear fruit calli. The presence of PuERF2 substantially enhanced the PCR-based detection of the PuACO1 promoter (Figure 5C), indicating that PuERF2 binds to the PuACO1 promoter in vivo. The regulation by PuERF2 of the PuACO1 promoter was examined in N. benthamiana leaves, and PuERF2 activated the PuACO1 promoter (Figure 5D). We tested whether PuACS1a expression was transcriptionally regulated by PuERF2 and found, by ChIP-PCR and a GUS activation assay, that PuERF2 bound to and activated the PuACS1a promoter (Figure 5, E and F).
Figure 5.
PuERF2 regulates PuACO1 and PuACS1a transcription. A, Y1H analysis showing that PuERF2 binds to the promoter of PuACO1 (ProPuACO1). See Figure 4A for additional details. B, EMSA showing that PuERF2 binds to the DRE motif of the PuACO1 promoter. The hot probe was a biotin-labeled fragment of the PuACO1 promoter containing the DRE motif, and the cold probe was a non-labeled competitive probe (50- and 100-fold that of the hot probe). GST-tagged PuERF2 was purified. C, ChIP-PCR showing the in vivo binding of PuERF2 to the PuACO1 promoter. Cross-linked chromatin samples were extracted from PuERF2-GFP overexpressing fruit calli (PuERF2-GFP) and precipitated with an anti-GFP antibody. Eluted DNA was used to amplify the sequences neighboring the DRE motif by qPCR. Two regions (S1 and S2) were investigated. Fruit calli overexpressing the GFP sequence (GFP) were used as negative controls. Values are the percentage of DNA fragments that co-immunoprecipitated with the GFP antibody or a nonspecific antibody (pre-immune rabbit IgG) relative to the input DNA. The ChIP assay was repeated 3 times and the enriched DNA fragments in each ChIP were used as one biological replicate for qPCR. Values represent means ± se. Statistical significance was determined using a Student’s t test (**P < 0.01). D, GUS activity analysis showing that PuERF2 promotes the activity of the PuACO1 promoter. The PuERF2 effector vector and the reporter vector containing the PuACO1 promoter were infiltrated into N. benthamiana leaves to analyze the regulation of GUS activity. Three independent transfection experiments were performed. Values represent means ± se. Statistical significance was determined using a Student’s t test (**P < 0.01). E, ChIP-PCR showing the in vivo binding of PuERF2 to the PuACS1a promoter. F, GUS activity analysis showing that PuERF2 promotes the PuACS1a promoter.
Given the presence of a BRRE motif in the PuERF2 promoter (1,979 bp), we investigated whether PuBZR1 can bind the PuERF2 promoter and regulate its expression. Binding was indeed confirmed using both Y1H assay (Figure 6A) and EMSA analysis (Figure 6B), while a ChIP-PCR assay demonstrated that PuBZR1 can bind to the PuERF2 promoter in vivo (Figure 6C). The regulation of the PuERF2 promoter by PuBZR1 was examined in N. benthamiana leaves, and we determined that PuBZR1 suppresses the activity of the PuERF2 promoter, while BR strengthens this suppression (Figure 6D). These results suggest that BR-activated PuBZR1 indirectly suppresses the expression of PuACO1 and PuACS1a through transcriptional regulation of PuERF2.
Figure 6.
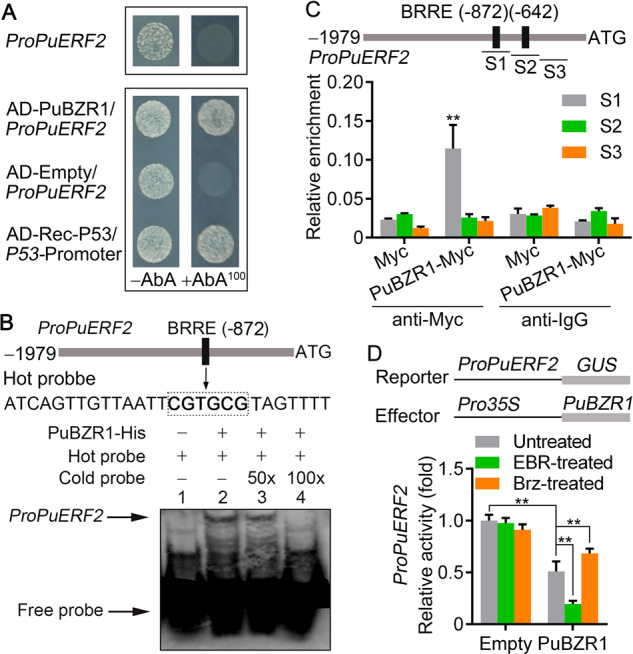
BR-activated PuBZR1 suppresses PuERF2 transcription. A, Y1H analysis showing that PuBZR1 binds to the promoter of PuERF2 (ProPuERF2). See Figure 4A for additional details. B, EMSA showing that PuBZR1 binds to the BRRE motif of the PuERF2 promoter. The hot probe was a biotin-labeled fragment of the PuERF2 promoter containing the BRRE motif, and the cold probe was a nonlabeled competitive probe (50- and 100-fold that of the hot probe). His-tagged PuBZR1 was purified. C, ChIP-PCR showing the in vivo binding of PuBZR1 to the PuERF2 promoter. Cross-linked chromatin samples were extracted from PuBZR1-Myc-overexpressing pear fruit calli (PuBZR1-Myc) and precipitated with an anti-Myc antibody. Eluted DNA was used to amplify the sequences neighboring the BRRE motif by quantitative (q)-PCR. Three regions (S1–S3) were investigated. Fruit calli overexpressing the Myc sequence (Myc) were used as negative controls. Values are the percentage of DNA fragments that co-immunoprecipitated with the Myc antibody or a nonspecific antibody (pre-immune rabbit IgG) relative to the input DNA. The ChIP assay was repeated 3 times and the enriched DNA fragments in each ChIP were used as one biological replicate for qPCR. Values represent means ± se. Statistical significance was determined using a Student’s t test (**P < 0.01). D, GUS activity analysis showing that PuBZR1 suppresses the PuERF2 promoter. The PuBZR1 effector vector and the reporter vector containing the PuERF2 promoter were infiltrated into N. benthamiana leaves to analyze the regulation of GUS activity. Untreated, N. benthamiana leaves not receiving any treatment; EBR, N. benthamiana leaves treated with EBR; Brz, N. benthamiana leaves treated with Brz. Three independent transfection experiments were performed. Values represent means ± se. Statistical significance was determined using a Student’s t test (**P < 0.01).
Next, we investigated whether PuERF2 can bind to the promoter of PuBZR1 in vivo, since it contains a DRE motif, but we found evidence through ChIP-PCR that it does not (Supplemental Figure S12A). We also showed in N. benthamiana leaves that PuERF2 regulation of the PuACO1 and PuACS1a promoters was not EBR dependent (Supplemental Figure S12, B and C), suggesting that PuERF2 does not respond to EBR treatment without the presence of PuBZR1. We also investigated the potential interaction between PuBZR1 and PuERF2 using Y2H and a co-IP assay and determined that they do not interact with each other (Supplemental Figure S13). We concluded that PuERF2 likely does not regulate the transcription of PuBZR1, and that PuBZR1 works upstream of PuERF2 in response to BR.
Given the interaction between PuBZR1 and PuACO1 in the nucleus (Figure 3A), we investigated the influence of this interaction on the binding of PuBZR1 to its target promoters. EMSA analyses showed that the increase of PuACO1 protein did not obviously influence the capacity of PuBZR1 binding to the promoters of PuACO1, PuACS1a, and PuERF2 (Supplemental Figure S14).
Since both PuBZR1 and PuERF2 can bind to the promoters of PuACO1 and PuACS1a, we then investigated if PuBZR1 and PuERF2 interfered with each other in binding their target promoters. Because the molecular weight of PuBZR1 is very similar to that of PuERF2, we divided the PuBZR1 into two (PuBZR1N and PuBZR1C) and PuERF2 into three fragments (PuERF2N, PuERF2D, and PuERF2C), and performed Y1H assay to determine which fragment binds to the target promoter. The results showed that PuBZR1N and PuERF2D were the regions that bound their target promoters (Supplemental Figure S15). Then PuBZR1N and the full length of PuERF2 were used to test the interference between PuBZR1 and PuERF2 in binding promoters. The PuBZR1 binding site (BBRE) is 656 bp away from the PuERF2 binding site (DRE) in the PuACO1 promoter, and 203 bp away in the PuACS1a promoter (Supplemental Figure S16), thus it is hard to enclose both binding sites in a single EMSA probe. Thus, we selected 25-bp sequence flanking the BBRE or DRE motif from the PuACO1 or PuACS1a promoter and synthesized a 50-bp probe for EMSA analysis. The binding of PuBZR1N or PuERF2 to the promoters (PuACO1 or PuACS1) was not obviously affected when the amount of the other protein increased (Supplemental Figure S16). This finding indicated that PuBZR1 and PuERF2 do not interfere with each other in binding PuACO1 or PuACS1 promoters.
PuBZR1 plays a significant role in BR-suppressed ethylene biosynthesis
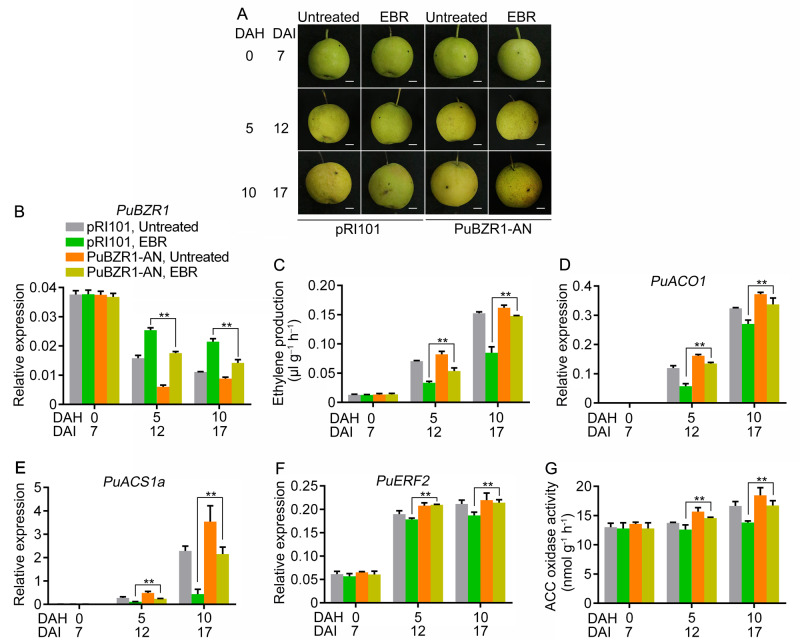
To further confirm the role of PuBZR1 in BR-suppressed ethylene biosynthesis, we transiently silenced PuBZR1 expression in pear fruit. The full PuBZR1 CDS was ligated into the pRI101 vector in the reverse direction, and the resulting construct was introduced into Agrobacterium tumefaciens, cultures of which were infiltrated into fruit still attached to trees. Fruits infiltrated with the empty pRI101 vector were used as a control. The infiltrated fruits were harvested at 7 d after infiltration (DAI), treated with EBR, and stored at room temperature for 10 d (Figure 7A). In the PuBZR1-suppressed pear fruit (PuBZR1-AN), PuBZR1 transcript levels were significantly reduced compared with the control fruits (Figure 7B), and after EBR treatment, PuBZR1-AN fruits showed significantly higher ethylene production compared with the control fruit (Figure 7C). In addition, the expression levels of PuACO1, PuACS1a, and PuERF2 (Figure 7, D–F), and enzyme activity of ACO (Figure 7G) were higher in PuBZR1-AN fruit than in control fruit. These findings indicate that PuBZR1 action is important for BR-suppressed ethylene production in pear fruit.
Figure 7.
PuBZR1 is involved in BR-suppressed ethylene biosynthesis in pear fruit. PuBZR1 was silenced in pear fruit (PuBZR1-AN) by A. tumefaciens-mediated transient transformation. Fruit infiltrated with an empty pRI101 vector were used as controls (pRI101). PuBZR1-AN and control fruits were harvested 7 DAI, treated with EBR immediately, and then stored at room temperature for 10 d (A). PuBZR1 expression was examined by RT-qPCR (B). Ethylene production (C), the expression levels of PuACO1 (D), PuACS1a (E), and PuERF2 (F), and the ACO enzyme activity (G) were investigated. Bars in (A), 1 cm. Untreated, fruit not receiving any treatment; EBR, fruit treated with EBR; DAH, days after harvest; For RT-qPCR, three biological replicates were analyzed as described in the Materials and Methods section. Values represent means ± se. Statistical significance was determined using a Student’s t test (**P < 0.01).
BR also suppresses apple fruit ripening
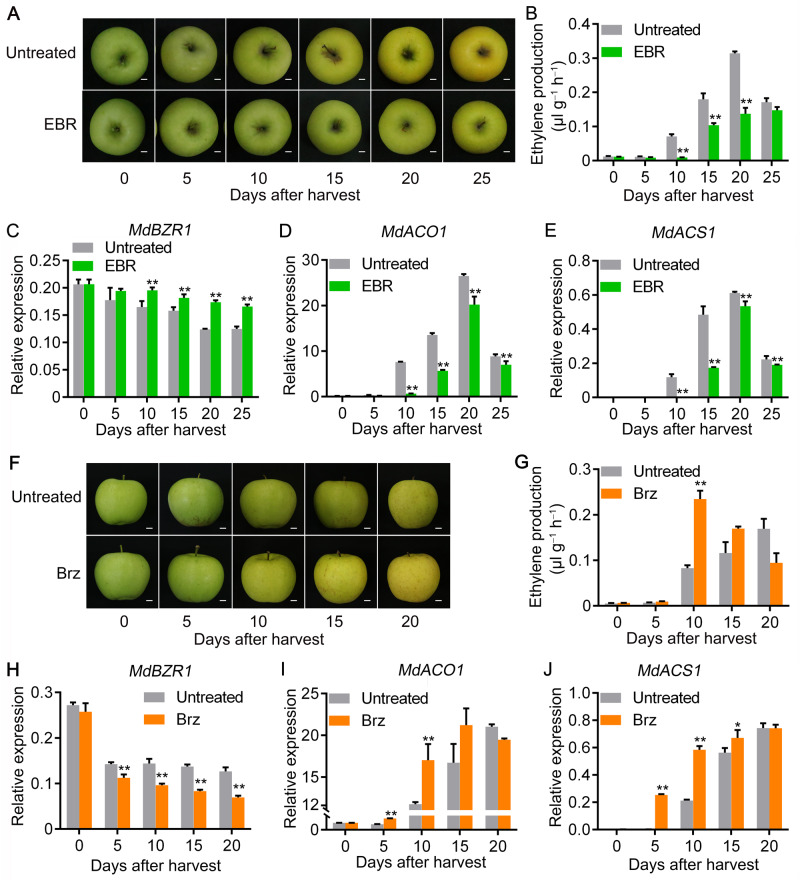
To complement the studies in pear, we investigated the influence of BR on ripening of apple fruit, which is also climacteric. We sampled apple fruits at the commercial harvest stage and treated them with EBR. Fruits were then stored at room temperature for 25 d (Figure 8A). Ethylene production in apple fruit treated with EBR was significantly inhibited compared with that of the control fruit (Figure 8B). We compared the transcriptome of apple fruit stored at room temperature for 10 d and treated with or without EBR using RNA sequencing (RNA-seq). The transcript level of MdBZR1 increased, and that of the ethylene biosynthetic genes MdACO1 and MdACS1 decreased as a consequence of EBR treatment (Supplemental Figure S17; Supplemental Dataset S1). Reverse transcription-quantitative PCR (RT-qPCR) detected expression of these genes confirmed the results of RNA-seq (Figure 8, C–E). Moreover, we observed from the RNA-seq data that the expression of four ERF transcription factors was suppressed by the EBR treatment (Supplemental Dataset S2), indicating their involvement in BR-suppressed ethylene biosynthesis. Finally, we sampled apple fruits at the commercial harvest stage and treated them with Brz. Fruits were then stored at room temperature for 20 d (Figure 8F). We observed that ethylene production was significantly promoted and a climacteric ethylene peak appeared earlier in apple fruit treated with Brz compared with that of the control fruit (Figure 8G). The transcript level of MdBZR1 decreased, and that of MdACO1 and MdACS1 increased as a consequence of Brz treatment (Figure 8, H–J). Taken together, these results suggest that the mechanism for BR-suppressed ethylene biosynthesis in apple and pear fruit may be conserved.
Figure 8.
BR suppresses apple fruit ripening. A–E, Apple fruit were collected on the day of commercial harvest (145 DAFB), treated with EBR, and then stored at room temperature for 25 d (A). Ethylene production was measured (B), and the expression levels of MdBZR1 (C), MdACO1 (D), and MdACS1 (E) were investigated by RT-qPCR. Bars 1 cm. F–J, Apple fruit were collected on the day of commercial harvest, treated with Brz, and then stored at room temperature for 20 d (F). Ethylene production was measured (G), and the expression levels of MdBZR1 (H), MdACO1 (I), and MdACS1 (J) were investigated by RT-qPCR. Bar 1 cm. Untreated, fruit not receiving any treatment; EBR, fruit treated with EBR. Brz, fruit treated with Brz. Numbers under the x-axis indicate the number of days of storage at room temperature after harvest; 0 indicates the fruit harvested on the day of commercial harvest. Three biological replicates were analyzed as described in the Methods section. Values represent means ± se. Statistical significance was determined using a Student’s t test (**P < 0.01).
Discussion
BR has been reported to participate in various aspects of plant development (Kim and Wang, 2010); however, although many studies have described the involvement of BR in fruit ripening in various species, including tomato (Zhu et al., 2015; Li et al., 2016b), persimmon (Diospyros kaki; He et al., 2018), mango (Mangifera indica; Zaharah et al., 2011), jujube (Zhu et al., 2010), strawberry (Chai et al., 2012), and grape (Vitis vinifera) berry (Symons et al., 2006), these studies only investigated changes in ethylene production after exogenous BR treatment and the expression profile of genes involved in ethylene biosynthesis and signal transduction. How endogenous BRs affect fruit ripening was unclear.
In our study, we dissected the regulatory network involving PuBZR1 association with ethylene signaling genes in BR-suppressed ethylene production. We observed that application of BR biosynthesis inhibitor Brz decreased endogenous BR content and accelerated fruit ripening (Figure 1), suggesting that BR is a suppressor of fruit ripening. By exogenously applying EBR to fruit, we observed that BR-activated PuBZR1 interacts with PuACO1 and suppresses its enzyme activity (Figure 2) and that PuBZR1 binds to the PuACO1 and PuACS1a promoters, directly downregulating their expression (Figure 4). In addition, we showed that PuBZR1 binds to the PuERF2 promoter and also downregulates its expression (Figure 6), while PuERF2 in turn binds to the promoters of PuACO1 and PuACS1a (Figure 5). Thus, PuBZR1 indirectly suppresses the expression of PuACO1 and PuACS1a through PuERF2.
We observed that PuBZR1 suppressed PuACO1 activity by directly interacting with the PuACO1 protein (Figure 2). ACO is a member of the Fe2+-dependent family of oxidases or oxygenases (Zhang et al., 1997) and it requires Fe2+ as a cofactor to catalyze the formation of ethylene (Dong et al., 1992). The ACO amino acid (aa) sequences are highly conserved between many species, and H177, D179, and H234 in ACOs of tomato, apple, and avocado (Persea americanna) are Fe2+ binding sites that are essential for enzyme activity (Shaw et al., 1996; Zhang et al., 1997; Rocklin et al., 1999). In these studies, substitutions of H177, D179, and H234 by site-directed mutagenesis result in complete loss of ACO activity. We observed that PuBZR1 interacted with the D fragment of PuACO1, which contains the three Fe2+ binding sites (Figure 2; Supplemental Figure S6). Therefore, we propose that BR-enhanced PuBZR1/PuACO1 interaction might hinder the binding of Fe2+ to PuACO1, thereby suppressing PuACO1 activity to suppress ethylene biosynthesis.
Previous studies have demonstrated that the presence of BR results in dephosphorylation of BZR1, and the dephosphorylated BZR1 then enters into the nucleus to regulate expression of its target genes (Bishop and Koncz, 2002; Ryu et al., 2007; Nolan et al., 2020). In our data, PuBZR1 interacted with PuACO1 at the protein level in the cytoplasm, and this interaction did not influence the nuclear translocation of PuBZR1 (Supplemental Figure S7A). Moreover, although PuBZR1–PuACO1 interaction also occurred in the nucleus, this interaction did not affect PuBZR1 binding to its target promoter (Supplemental Figure S14). These findings suggested that PuACO1 does not affect PuBZR1 action. Although EBR treatment enhanced the nuclear translocation of PuBZR1, PuBZR1 still interacted with PuACO1 and suppressed its enzyme activity (Figure 2) in the cytoplasm where ACO converts ACC to ethylene (Guy and Kende, 1984), suggesting the cytoplasm-retained PuBZR1 still can suppress ethylene biosynthesis by interacting with PuACO1. In combination with the above findings that PuBZR1 suppressed the transcription of PuACO1 and PuACS1a, the translocation of PuBZR1 suggested that the nucleus-localized PuBZR1 suppresses the transcription of ethylene biosynthetic genes (PuACO1 and PuACS1a), and the cytoplasm-retained PuBZR1 suppresses PuACO1 enzyme activity. In both ways, BR-activated PuBZR1 suppresses ethylene biosynthesis and fruit ripening in pear.
We noticed that endogenous BR content gradually declined along with fruit development, and the level of PuBZR1 transcription paralleled with the changes of BRs content (Figure 1); moreover, Brz treatment decreased endogenous BR levels (Figure 1) and weakened the PuBZR1–PuACO1 interaction (Figure 3, D and F). Based on these findings, we propose that, along with the decline of endogenous BR levels during fruit development, the suppression of PuBZR1 on PuACO1 enzyme activity and on the transcription level of PuACO1 and PuACS1a is gradually impaired, which leads to a large amount of ethylene production and initiation of fruit ripening; during fruit ripening, although PuBZR1 still interacts with PuACO1 in the cytoplasm, the limited amount of PuBR1 protein might not be able to hinder the PuACO1 activity due to the increasing transcription level of PuACO1. Our findings explained why fruits are not able to ripen until they reach a certain development stage. We noticed that endogenous BR content increased during fruit ripening (Day 0 compared with Day 10 in Figure 1E), but PuBZR1 expression declined in this process (Figure 1F). This result implied that ethylene might have a feedback regulation on BR biosynthesis during fruit ripening, since ethylene production reached a peak at Day 10 (Figure 1C). On the other hand, ethylene inhibited PuBZR1 expression during fruit ripening (Supplemental Figure S18). These findings indicated that ethylene could inhibit PuBZR1 expression to further weaken its suppression on the action of PuACO1 and PuACS1a, once fruit initiate the ripening process and produce a large amount of ethylene. In addition, Arabidopsis (Arabidopsis thaliana) AtBZR1 activates BR response in its unphosphorylated form (Ryu et al., 2007). Thus the phosphorylated or unphosphorylated form of PuBZR1 might influence the interaction between PuBZR1 and PuACO1. To test this possibility, we identified two phosphorylation residues (S102 and S106) from PuBZR1N, the fragment that interacted with PuACO1, according to the study on AtBZR1. These two residues were mutated into unphosphorylated forms (S102A and S106A), and the mutant PuBZR1S102/106A was used in a pull-down assay. We observed that the mutant form of PuBZR1 did not change its interaction with PuACO1 (Supplemental Figure S19). Therefore, BR-enhanced PuBZR1–PuACO1 interaction is not dependent on the unphosphorylated form of PuBZR1.
Some studies have revealed that exogenous BR can promote ethylene production and accelerate fruit ripening in tomato (Vardhini and Rao, 2002; Zhu et al., 2015), persimmon (He et al., 2018), and mango (Zaharah et al., 2011). Although these fruits are also climacteric, the results of our studies of pear and apple fruits were opposite to these previous reports. This might be due to differences in species or the dose of BR applied: Zhu et al. (2010) reported that 5 μM of brassinolide suppressed ethylene production and fruit ripening in jujube, also a climacteric fruit, while application of 10 μM of brassinolide had the opposite result. In strawberry and grape berry, both categorized as nonclimacteric fruit, application of BR accelerated fruit ripening (Symons et al., 2006; Chai et al., 2012). In Arabidopsis seedlings, low concentration (10 or 100 nM) of exogenous BR suppresses ethylene biosynthesis, while high concentration (>500 nM) promotes ethylene biosynthesis (Lv et al., 2018). These findings suggest that the influence of BR on ethylene biosynthesis and fruit ripening is different between species and might vary in a dose-dependent manner.
Although we did not dissect the details of MdBZR1 regulation of MdACO1 or MdACS1 activity in apple, we observed that the EBR treatment also resulted in reduced ethylene production, reduced expression of MdACO1 and MdACS1, and increased expression of MdBZR1 in apple fruit (Figure 8). Moreover, four ERF transcription factors down-regulated by an EBR treatment were identified from RNA-seq data (Supplemental Dataset S2). Thus the mechanism by which BR suppresses ethylene biosynthesis in apple fruit is likely similar to that in pear fruit.
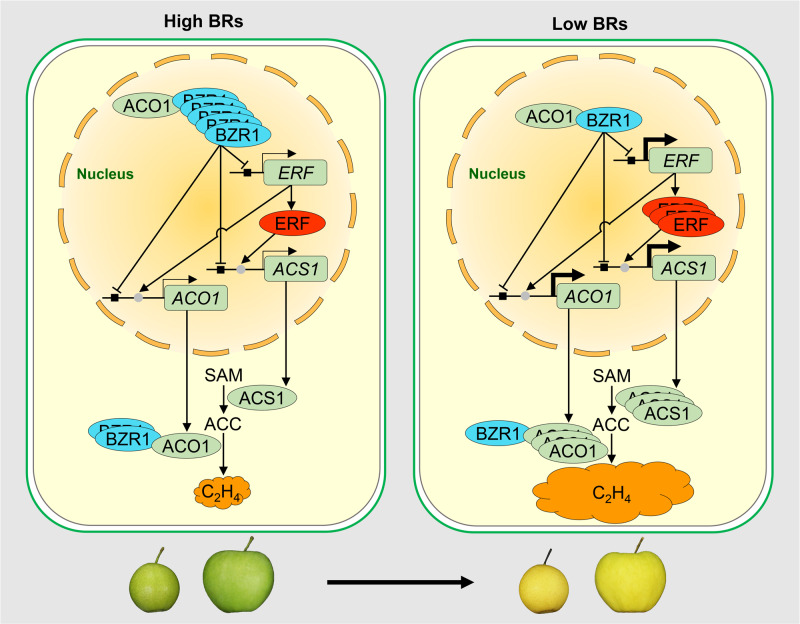
We finally generated a model for how BRs regulate climacteric fruit ripening (Figure 9). When endogenous BR levels are high, BZR1 expression is enhanced, and most of the BZR1 protein moves into the nucleus to suppress the transcription of ethylene biosynthetic genes (ACO1 and ACS1) by direct promoter binding or through ERF(s). The cytoplasm-retained BZR1 suppresses ACO1 enzyme activity by direct protein interaction, thus ethylene biosynthesis is inhibited and fruit cannot ripen; when endogenous BR levels decline, BZR1 expression declines, which weakens the suppression of BZR1 on ACO1 enzyme activity and on the transcription of ACO1 and ACS1, leading to a burst of ethylene production and fruit ripening.
Figure 9.
A model showing the regulation of BRs on fruit ripening. When endogenous BR levels are high, BZR1 expression is enhanced, and most of the BZR1 protein moves into the nucleus to suppress the transcription of ethylene biosynthetic genes (ACO1 and ACS1) by direct promoter binding or through ERF(s). The cytoplasm-retained BZR1 suppresses ACO1 enzyme activity by direct protein interaction, leading to inhibition of ethylene biosynthesis, and fruit cannot ripen; when endogenous BR levels decrease, BZR1 expression also decreases, which weakens the suppression of BZR1 on ACO1 enzyme activity and transcription of ACO1 and ACS1, leading to a burst of ethylene production and fruit ripening. arrow symbol, promotion; up tack symbol, suppression; filled square, BZR1-binding site; filled circle, ERF-binding site; C2H4, ethylene.
Materials and methods
Plant materials and treatments
Pear (P. ussuriensis cv Nanguo) fruits were obtained from the experimental farm of Shenyang Agricultural University. Fruits were collected on the day of commercial harvest (135 DAFB) when the content of total soluble solids reached 12% and immediately brought to the laboratory. For BR treatment, fruits were immersed in 0.2, 3, or 10 μM EBR (Cat. no. 78821-43-9, Yuanye Biotechnology, Shanghai, China) for 2 h. For the BR biosynthesis inhibitor treatment, fruits were immersed in 10 μM (Brz, Cat. no. 224047-41-0, Yuanye Biotechnology) for 12 h. Ethephon (an ethylene releaser), and 1-methylcyclopropene (1-MCP, an ethylene inhibitor) treatments were performed as previously reported (Tan et al., 2013). Fruits that did not receive any treatment were used as a control. After the treatments, the fruits were stored at room temperature for 15 d and sampled every 5 d. At each sampling time, nine fruits were randomly collected and divided into three groups (three fruit per group), resulting in three biological replicates. Ethylene production and fruit firmness were measured as previously described (Li et al., 2014), and a total of three individual replicates were assayed. Statistical significance was determined using a Student’s t test. Following ethylene measurement, flesh of the three fruits from each group was sliced, pooled, frozen in liquid nitrogen, and stored at −80°C for RNA extraction and gene expression analysis. The pooled flesh in each group was used as one biological replicate for gene expression analysis, and a total of three replicates were used.
Apple (M. domestica cv Golden Delicious) fruits were obtained from the experimental farm of Liaoning Pomology Institute (Xiongyue, China). Fruits were collected on the day of commercial harvest (145 DAFB), treated with 3 μM EBR or 10 μM Brz as above. Fruits that did not receive any treatment were used as a control. Control and treated fruits were stored at room temperature for 20 or 25 d and sampled every 5 d. The sampling regime was similar to that of the pear fruit. At each sampling time, nine fruits were divided into three groups (as three biological replicates) for ethylene production measurement. Statistical significance was determined using a Student’s t test. Following ethylene measurement, flesh from each group of fruits was pooled for RNA extraction and gene expression analysis, and a total of three replicates was used.
Pear fruit calli were prepared as previously described (Alayón-Luaces et al., 2008). Briefly, pear fruits harvested at Day 75 after full bloom were used to generate primary calli, which were cultivated on basal Murashige and Skoog (MS) medium supplemented with 2 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D, Sangon Biotech, Shanghai, China; http://www.life-biotech.com) and 1.5 mg L−1 6-benzyladenine (6-BA, Sangon Biotech). Calli were subcultured on a proliferation medium consisting of basal MS medium supplemented with 2.5 mg L−1 2,4-D and 1 mg L−1 6-BA.
Quantification of endogenous BRs content
The quantification of endogenous BRs was performed according to the previously reported method with some modification in sample pretreatment (Xin et al., 2013). The fruit flesh was ground to a fine powder, and 200 mg of the powder was extracted with of 90% (v/v) aqueous methanol (MeOH) in an ultrasonic bath for 1 h. Simultaneously, D3-CS, D3-6-deoxo-CS, and D3-TY were added to the extract as internal standards for BR content measurement. After the MCX cartridge was activated and equilibrated with MeOH, water, and 40% MeOH in sequence, the crude extracts reconstructed in 40% MeOH were loaded onto the cartridge. The MCX cartridge was washed with 40% MeOH, and then BRs were eluted with MeOH. After drying with N2 stream, the eluent was redissolved with ACN to be derivatized with 2-methoxypyridine-5-boronic acid prior to ultra-performance liquid chromatography mass spectrometry (UPLC–MS/MS) analysis. BR analysis was performed on a quadrupole linear ion trap hybrid MS (QTRAP 6500; AB SCIEX, Framingham, MA, USA) equipped with an electrospray ionization source coupled with a UPLC (Waters, Milford, MA, USA; Xin et al., 2020). As for CS, D3-CS, 6-deoxo-CS, D3-6-deoxo-CS, TY, and D3-TY, the MRM transition 582.4 > 178.1, 585.4 > 178.1, 568.4 > 178.1, 571.4 > 178.1, 566.4 > 548.3, and 569.4 > 548.3 was used for quantification.
Gene expression analysis
Total RNA was extracted according to the method of Li et al. (2014), and cDNA synthesis and RT-qPCR was performed as previously described (Li et al., 2017). RT-qPCR was conducted using an Analytik Jena qTOWER3 G PCR System. RNA extracted from each group of flesh (as described above) was used as one biological replicate, and a total of three biological replicates were conducted. Statistical significance was determined using a Student’s t test. Specific primers (Supplemental Dataset S3) for each gene were designed using Primer3 (http://frodo.wi.mit.edu). The pear and apple Actin genes (PuActin and MdActin, respectively) were used as internal controls. Standard PCR was performed according to the method of (Li et al., 2017), and the PCR product was separated on a 1% agarose gel and photographed with a GelDoc XR System (Bio-Rad, Hercules, CA, USA).
Y2H assay
A cDNA library was constructed with mRNA from pear fruit harvested at commercial maturity in 2015, using a Make Your Own Mate & Plate Library System (Cat. no. 630489; Clontech, Mountain View, CA, USA). The PuBZR1 CDS was introduced into the pGBKT7 vector enclosed in this kit using EcoRI and BamHI sites. The recombinant plasmid was used as bait to screen the cDNA library using the Matchmaker™ Gold Y2H Library Screening System kit (Cat. no. 630489; Clontech).
The PuACO1 (314 aa), PuACO1N (aa 1–99), PuACO1M (aa 100–161), PuACO1D (aa 162–254), and PuACO1C (aa 255–314) sequences were introduced into the activation domain (AD) vector (pGADT7) using the NdeI and EcoRI restriction sites. The PuBZR1 (295 aa), PuBZR1N (aa 1–107), and PuBZR1C (aa 108–295) sequences were ligated to the binding domain (BD) in the pGBKT7 vector using the NdeI and EcoRI restriction sites. The BD and AD vectors were co-transformed into the Y2HGold yeast (Saccharomyces cerevisiae) strain. The detection of protein interactions between two proteins was conducted using the Matchmaker™ Gold Y2H Library Screening System kit.
Protein expression and purification
The PuBZR1 CDS was inserted into the pEASY-E1 vector (Transgen Biotech, Beijing, China; http://www.transgen.com.cn) resulting in its downstream fusion to a His-tag sequence. The PuACO1 or PuERF2 CDSs were inserted into the pGEX4T-1 vector (GE Healthcare, Chicago, IL, USA;http://www3.gehealthcare.com) downstream from GST. The resulting plasmids were transformed into Escherichia coli BL21 (DE3) competent cells. Recombinant fusion proteins were purified as described in Li et al. (2016a).
Pull-down assay
To confirm the interaction between PuBZR1 and PuACO1, 5 μg of purified His fusion protein (PuBZR1-His) was bound to Ni-NTA His binding resin (Novagen, Madison, WI, USA). GST fusion proteins containing PuACO1 (PuACO1-GST) were added and incubated for 1 h at 4°C with the subsequent immunoblot analysis performed as previously described (Li et al., 2017). GST protein was used as the negative control.
Co-IP Assay
For the co-IP assay, the PuBZR1 CDS was ligated into the pCAMBIA1307 vector (BioVector, http://www.biovector.net) to allow expression of the PuBZR1 protein with a Myc tag driven by the CaMV 35S promoter, using the XbaI and BamHI sites. The CDS of PuACO1 was cloned into the KpnI and EcoRI sites downstream of the GFP sequence and the CaMV 35S promoter in the pRI101 vector (TaKaRa, Kyoto, Japan). The recombinant Pro35S:Myc-PuBZR1 and Pro35S:GFP-PuACO1 constructs were infiltrated into N. benthamiana leaves using A. tumefaciens-mediated infiltration as previously described (Li et al., 2017). EBR (10 μM) was injected into infiltrated N. benthamiana leaves 3 h before sampling. For Brz treatment, 10 μM of Brz was sprayed on N. benthamiana leaves 2 d before infiltration. Protein extracted from the infiltrated N. benthamiana leaves was used for co-IP analysis. A Pierce co-IP kit (catalog no. 26149; Thermo Scientific, Waltham, MA, USA) was used to immunoprecipitate Myc-PuBZR1 using 10 μL of anti-Myc antibody (1 mg mL−1; Transgen Biotech). The precipitate was analyzed by immunoblot analysis with the anti-GFP antibody (1 mg mL−1; Transgen Biotech) diluted 1:3,000.
Subcellular localization
The PuACO1 or PuBZR1 coding region was cloned into the KpnI and EcoRI sites downstream of GFP in the pRI101 vector to form the Pro35S:GFP-PuACO1 or Pro35S:GFP-PuBZR1 construct. The construct was co-infiltrated with a mCherry-labeled nuclear marker NF-YA4-mCherry (Zhang et al., 2019) into N. benthamiana leaves using A. tumefaciens-mediated infiltration. The N. benthamiana plants were kept in the dark for 48 h after infiltration. Then EBR (10 μM) was injected into the infiltrated N. benthamiana leaves and imaging was performed 12 h after EBR treatment. GFP fluorescence was observed under a confocal microscope (TCS SP8; Leica Wetzlar, Germany). For green fluorescence observation, the excitation wavelength was 488 nm and the emission wavelengths were 520–540 nm; for red fluorescence observation, the excitation wavelength was 561 nm and the emission wavelengths were 610–630 nm. Pro35S:GFP was used as a control. All transient expression assays were repeated at least 3 times, and the representative results were shown.
BiFC assay
The PuACO1 CDS was ligated into the pSPYNE-35S vector (Walter et al., 2004) using the BamHI and KpnI sites. The PuBZR1 CDS was ligated into the pSPYCE-35S vector using the BamHI and KpnI sites. The resulting plasmids were introduced into A. tumefaciens strain EHA105, and then infiltration of N. benthamiana leaves was performed. All N. benthamiana plants were kept in the dark for 48 h, then EBR (10 μM) was injected into the infiltrated N. benthamiana leaves. Nicotiana benthamiana leaves were visualized at 0, 4, and 12 h after EBR treatment. YFP fluorescence was observed under a confocal laser scanning microscope (TCS SP8; Leica). For yellow fluorescence observation, the excitation wavelength was 488 nm and the emission wavelengths were 520–540 nm; for red fluorescence observation, the excitation wavelength was 561 nm and the emission wavelengths were 610–630 nm. Fragments of PuBZR1C and PuACO1 were used as a negative control. All transient expression assays were repeated at least 3 times, and the representative results were shown.
Measurements of ACO activity
PuACO1 enzyme activity was measured as previously described (Zhang et al., 1997). Purified PuACO1-GST protein (0.2 μg) was added to 2 mL of incubation buffer (pH 7.2) containing 10% (v/v) glycerol (Solarbio, http://www.solarbio.com), 5 mM Na-ascorbate (Sangon Biotech), 0.1 mM ACC (Sigma-Aldrich, St Louis, MO, USA), 80 μM FeSO4 (Sangon Biotech), 15 mM NaHCO3 (Sangon Biotech), 500 μg catalase (Worthington, http://www.worthington-biochem.com), and 2 mM dithiothreitol (DTT; Solarbio, Beijing, China), and the mixture was incubated at 30°C for 2 h in a 15-mL gas-tight glass tube with a septum, shaking at 120 rpm, and then 1 mL of gas was extracted from the headspace of the tube with a 1-mL syringe for measurement of ethylene production as previously described (Li et al., 2014). To investigate the effect of PuBZR1 on PuACO1 activity, different amounts of purified PuBZR1-His (0.2, 0.4, and 0.6 μg) were mixed with 0.2 μg of PuACO1-GST and incubated on ice for 1 h, shaking at 100 rpm. The mixture was then added to incubation buffer and incubated at 30°C for 2 h to measure ethylene production, which was defined as the amount of ethylene produced at 30°C in 1 h.
The ACO activity of extracts from pear fruit was measured as described in Ververidis and John (1991) with a few modifications. Briefly, 1 g of fruit flesh was ground into a fine powder in liquid nitrogen and suspended in 2 mL of extraction buffer containing 0.1 M Tris–HCl (pH 7.5, Sangon Biotech), 10% (v/v) glycerol, 5% polyvinylpolypyrrolidone (PVP, Solarbio), 5 mM DTT, 30 mM Na-ascorbate, and 0.1 mM FeSO4. The suspension was centrifuged at 4°C and 11,000 rpm for 10 min, and the supernatant was collected as a crude extract. To determine ACO activity, 400 μL of crude extract was incubated with 3,600 μL of a solution containing 0.1 M Tris–HCl (pH 7.5), 10% (v/v) glycerol, 1 mM ACC, 30 mM NaHCO3, 30 mM Na-ascorbate, and 0.1 mM FeSO4 in a 30°C water bath for 1 h in a 15-mL gas-tight glass tube with a septum. Ethylene production was measured to calculate the ACO activity as described above. Each experiment was repeated independently at least 3 times, and a Student’s t test was employed to determine the statistical significance.
Y1H assay
The sequences of PuBZR1 (295 aa), PuBZR1N (aa 1–107), PuBZR1C (aa 108–295), PuERF2 (342 aa), PuERF2N (aa 1–159), PuERF2D (aa 160–257), and PuERF2C (aa 258–342) were ligated into the pGADT7 vector using the NdeI and EcoRI restriction sites. The PuERF2 (1,979-bp upstream of the predicted translation start site), PuACO1 (1,941-bp upstream of the predicted translation start site), or PuACS1a (1,170-bp upstream of the predicted translation start site) promoter fragments were cloned into the pAbAi vector using the KpnI and XhoI restriction sites. The binding of transcription factor to the promoters was assayed using the Matchmaker™ Gold Y1H Library Screening System kit (Cat. no. 630491; Clontech).
Electrophoretic mobility shift assay
For the EMSA, recombinant His-tagged PuBZR1, His-tagged PuBZR1N, GST-tagged PuERF2, GST-tagged PuACO1, or MBP-tagged PuERF2 were expressed in E. coli BL21 (DE3) cells and purified as described above. The biotin-labeled PuACO1, PuACS1a, or PuERF2 promoter regions contained a BRRE or DRE motif as shown in Figures 4 and 5;Supplemental Figure S16. Corresponding unlabeled regions were used as competitors. The EMSA analysis was completed as previously described (Li et al., 2016a).
ChIP-PCR analysis
The PuBZR1 CDS was ligated into the pCAMBIA1307 vector as in the co-IP assay. The PuERF2 CDS was cloned into the pRI101 vector (TakaRa) to allow expression of PuERF2 as a fusion with GFP driven by the CaMV 35S promoter, using the KpnI and EcoRI sites. The resulting Pro35S:Myc-PuBZR1 or Pro35S:GFP-PuERF2 constructs were transformed into pear calli, and ChIP assays were performed as previously described (Li et al., 2017) with anti-Myc or an anti-GFP antibodies. The amount of immunoprecipitated chromatin was determined by qPCR as described in Li et al. (2017). Each ChIP assay was repeated 3 times and the enriched DNA in each time was used as one biological replicate for qPCR. At least three biological replicates were performed, and a Student’s t test was employed to determine the statistical significance. Primers used are listed in Supplemental Dataset S3.
GUS analysis
The PuBZR1 or PuERF2 CDS regions were cloned into the pRI101 vector (Xiao et al., 2013) using restriction enzyme sites (NdeI and EcoRI for PuBZR1, NdeI, and BamHI for PuERF2) to generate the effector constructs. The reporter constructs were generated using the PuACO1 (1,941 bp), PuACS1a (1,170 bp), and PuERF2 (1,979 bp) promoter sequences cloned upstream of the GUS reporter gene in the pBI101 vector. The reporter and effector vectors were transformed into A. tumefaciens strain EHA105, and N. benthamiana leaves were used for co-infiltration. The co-infiltration and examination of GUS activity were performed according to Li et al. (2017). The infiltration in each assay was repeated 3 times as three biological replicates, and a Student’s t test was employed to determine the statistical significance.
Firefly Luc complementation imaging assay
The PuBZR1 or PuACO1 CDS regions were inserted into the pCAMBIA1300-nLuc/-cLuc vectors (Chen et al., 2008) using the KpnI and SalI or KpnI and PstI restriction enzyme sites, respectively. Agrobacterium tumefaciens strain EHA105 carrying the indicated constructs was cultured to OD600 0.5 (optical density measured at a wavelength of 600 nm equals 0.5) and incubated at room temperature for 3 h before being infiltrated into N. benthamiana leaves. The EBR (10 μM) was injected into infiltrated N. benthamiana leaves 3 h before imaging. For Brz treatment, 10 μM of Brz was sprayed on N. benthamiana leaves 2 d before infiltration. Luc activity was detected as previously described (Li et al., 2017). The infiltration in each assay was repeated 3 times as three biological replicates.
Agrobacterium tumefaciens infiltration
To silence PuBZR1 expression in pear fruit, the full PuBZR1 CDS was ligated into the pRI101 vector in the reverse direction to generate the antisense pRI101-PuBZR1-AN construct. The recombinant construct was transformed into A. tumefaciens strain EHA105. The infection suspension was prepared as in Li et al. (2016a). Infiltration assay was performed on pear fruit still attached to trees ∼7 d before commercial harvest. For silencing of PuBZR1 expression, 100 μL of the suspension was taken using a 1-mL sterile syringe and injected into fruit at a depth of 0.5 cm, and 4–5 injections were performed on each fruit. Infiltrated fruits were harvested 7 DAI, treated with 3 μM of EBR as above, stored at room temperature for 10 d and sampled every 5 d. One fruit was used as a biological replicate, and at least three fruits were used for measurement of ethylene production or gene expression at each sampling point. A Student’s t test was employed to determine the statistical significance.
RNA-seq of apple fruit
Control and EBR-treated apple fruits sampled at Day 10 (stored at room temperature for 10 d after harvest) were used for RNA-seq. RNA extracted from control or EBR-treated fruits (three biological replicates for each) was used for library construction, and a total of six libraries were constructed. cDNA synthesis and library construction were performed as previously described (Huang et al., 2014). RNA-seq was performed using an Illumina HiSeq2500 by BIOMARKER (http://www.biomarker.com.cn/). The FPKM (fragments per kb per million reads) method was used to calculate the rate of differential expressed genes. The false-discovery rate (FDR) was used to determine the P-value thresholds via multiple testing. All genes with a Log2FC (Fold Change) >1.5 or less than −1.5 with a P <0.05 were selected. The Unique gene identifier (Gene ID), log2FC, FDR, and annotation are indicated in Supplemental Dataset S1. The heat map for differentially expressed genes between untreated and EBR treated apple fruits was constructed using Cluster version 3.0 software. All the raw data were deposited into NCBI Sequence Read Archive under accession number PRJNA557322.
Accession numbers
Sequence data from this article can be found in the Genome Database for Rosaceae (https://www.rosaceae.org) or GenBank libraries under accession numbers PuBZR1 (MH188908), PuERF2 (MH188911), PuERF3 (MH188907), PuERF106 (MH188910), PuERF113 (MH188909), PuACO1 (MH188913), PuACS1a (EF566865), PuACS1b (KC632527), PuACS1-like (XM018643584), PuACS10 (XM009375065), PuACS12 (XM009376269), PuActin (AB190176), MdBZR1 (MDP0000306427), MdACS1 (U89156), MdACO1 (AF030859), MdActin (EB136338), SlACO1 (EF501822), and PaACO1 (M32692).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 Ethylene production and fruit firmness in pear fruit treated with EBR.
Supplemental Figure S2 Ethylene production and gene expression in pear fruit treated with EBR.
Supplemental Figure S3 Brz promoted pear fruit ripening.
Supplemental Figure S4 Expression of PuBZR1 and its homologs in pear fruit treated with EBR.
Supplemental Figure S5 The interaction between PuBZR1 and PuACO1 proteins.
Supplemental Figure S6 Sequence alignment of PuACO1 with its homologs from tomato (S. lycopersicum), apple (M. domestica), and avocado (P. americanna).
Supplemental Figure S7 Subcellular localization of PuBZR1 and PuACO1.
Supplemental Figure S8 ACO activity in pear fruit treated with EBR.
Supplemental Figure S9 Expression of PuACSs in pear fruit treated with EBR.
Supplemental Figure S10 Expression of PuERFs in pear fruit treated with EBR.
Supplemental Figure S11 Subcellular localization of PuERF2.
Supplemental Figure S12 PuBZR1 works upstream of PuERF2.
Supplemental Figure S13 PuBZR1 does not interact with PuERF2 in yeast cells.
Supplemental Figure S14 The interaction between PuBZR1 and PuACO1 does not affect the binding of PuBZR1 to its target promoters.
Supplemental Figure S15 The binding of PuBZR1 and PuERF2 on PuACO1 or PuACS1a promoters.
Supplemental Figure S16 PuBZR1 and PuERF2 do not interfere with each other on the binding to their target promoters.
Supplemental Figure S17 Heat map of differentially expressed genes between untreated and EBR-treated apple fruits from RNA-seq data.
Supplemental Figure S18 The expression of PuBZR1 was suppressed by ethylene during pear fruit ripening.
Supplemental Figure S19 Interaction between unphosphorylated PuBZR1 and PuACO1.
Supplemental Table S1 Differentially expressed genes identified from RNA-seq data of apple fruit treated with or without EBR.
Supplemental Table S2 BR-suppressed ERF transcription factors identified from RNA-seq data of apple fruit treated with or without EBR.
Supplemental Table S3 Primers used in this study.
Supplementary Material
Acknowledgments
We thank Professor Nan Ma from China Agricultural University for kindly providing the NF-YA4-mCherry and BiFC vectors, and Professor Zhi Liu from Liaoning Pomology Institute for kindly providing the apple fruit samples. We also thank PlantScribe (http://www.plantscribe.com) for editing this manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFD1000105), the National Natural Science Foundation of China (31722047), and the Strategic Priority Research Program of Chinese Academy of Sciences ( XDA24040202).
Conflict of interest statement: The authors declare no conflict of interest.
A.W. conceived and Y.J. designed this research. Y.J., Y.Q., and M.X. performed most of the experiments. Z.J. generated the Y2H library and performed the library screening. J.Y. and J.C. measured endogenous BR content. H.Y. and X.S. performed protein purification. A.W., Y.J., and Y.Q. wrote the article. All authors analyzed the data and discussed the article.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instruction for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Aide Wang (awang@syau.edu.cn).
References
- Alayón-Luaces P, Pagano EA, Mroginski LA, Sozzi GO (2008) Four glycoside hydrolases are differentially modulated by auxins, cytokinins, abscisic acid and gibberellic acid in apple fruit callus cultures. Plant Cell Tiss Org 95:257–263 [Google Scholar]
- Barry CS, Giovannoni JJ (2007) Ethylene and fruit ripening. J Plant Growth Regul 26:143–159 [Google Scholar]
- Bishop GJ, Koncz C (2002) Brassinosteroids and plant steroid hormone signaling. Plant Cell 14 (Suppl): S97–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai YM, Zhang Q, Tian L, Li CL, Xing Y, Qin L, Shen YY (2012) Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul 69:63–69 [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM (2008) Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol 146:368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD (2011) Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 23:1219–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar AM, Teo G, Defilippi BG, Uratsu SL, Passey AJ, Kader AA, Stow JR, Colgan RJ, James DJ (2004) Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res 13:373–384 [DOI] [PubMed] [Google Scholar]
- Dong JG, Fernandez-Maculet JC, Yang SF (1992) Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci USA 89:9789–9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Pal RK, Rajam MV (2013) Delayed ripening and improved fruit processing quality in tomato by RNAi-mediated silencing of three homologs of 1-aminopropane-1-carboxylate synthase gene. J Plant Physiol 170:987–995 [DOI] [PubMed] [Google Scholar]
- Guy M, Kende H (1984) Conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene by isolated vacuoles of Pisum sativum L. Planta 160:281–287 [DOI] [PubMed] [Google Scholar]
- Han YC, Kuang JF, Chen JY, Liu XC, Xiao YY, Fu CC, Wang JN, Wu KQ, Lu WJ (2016) Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol 171:1070–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307:1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Li J, Ban Q, Han S, Rao J (2018) Role of brassinosteroids in persimmon (Diospyros kaki L.) fruit ripening. J Agric Food Chem 66:2637–2644 [DOI] [PubMed] [Google Scholar]
- Huang G, Li T, Li X, Tan D, Jiang Z, Wei Y, Li J, Wang A (2014) Comparative transcriptome analysis of climacteric fruit of Chinese pear (Pyrus ussuriensis) reveals new insights into fruit ripening. PLoS One 9:e107562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Kitagawa M, Ihashi N, Yabe K, Kimbara J, Yasuda J, Ito H, Inakuma T, Hiroi S, Kasumi T (2008) DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. Plant J 55:212–223 [DOI] [PubMed] [Google Scholar]
- Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Biol 44:283–307 [Google Scholar]
- Kim TW, Wang ZY (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61:681–704 [DOI] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59 [DOI] [PubMed] [Google Scholar]
- Li J, Jin H (2007) Regulation of brassinosteroid signaling. Trends Plant Sci 12:37–41 [DOI] [PubMed] [Google Scholar]
- Li T, Jiang Z, Zhang L, Tan D, Wei Y, Yuan H, Li T, Wang A (2016a) Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J 88:735–748 [DOI] [PubMed] [Google Scholar]
- Li T, Li X, Tan D, Jiang Z, Wei Y, Li J, Du G, Wang A (2014) Distinct expression profiles of ripening related genes in the ‘Nanguo’ pear (Pyrus ussuriensis) fruits. Sci Hortic 171:78–82 [Google Scholar]
- Li T, Xu Y, Zhang L, Ji Y, Tan D, Yuan H, Wang A (2017) The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 29:1316–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Chen XJ, Guo X, Yin LL, Ahammed GJ, Xu CJ, Chen KS, Liu CC, Xia XJ, Shi K, et al. (2016b) DWARF overexpression induces alteration in phytohormone homeostasis, development, architecture and carotenoid accumulation in tomato. Plant Biotechnol J 14:1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv B, Tian H, Zhang F, Liu J, Lu S, Bai M, Li C, Ding Z (2018) Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet 14:e1007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TM, Vukasinovic N, Liu D, Russinova E, Yin Y (2020) Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32:295–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S, Scossa F, Fernie AR (2013) Molecular regulation of fruit ripening. Front Plant Sci 4:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin AM, Tierney DL, Kofman V, Brunhuber NMW, Hoffman BM, Christoffersen RE, Reich NO, Lipscomb JD, Que L (1999) Role of the nonheme Fe(II) center in the biosynthesis of the plant hormone ethylene. Proc Natl Acad Sci USA 96:7905–7909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Kim K, Cho H, Park J, Choe S, Hwang I (2007) Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell 19:2749–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer RJ, Friel EN, Souleyre EJ, Bolitho K, Thodey K, Ledger S, Bowen JH, Ma JH, Nain B, Cohen D,. et al.. (2007) A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol 144:1899–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JF, Chou YS, Chang RC, Yang SF (1996) Characterization of the ferrous ion binding sites of apple 1-aminocyclopropane-1-carboxylate oxidase by site-directed mutagenesis. Biochem Bioph Res Co 225:697–700 [DOI] [PubMed] [Google Scholar]
- Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR (2006) Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol 140:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D, Li T, Wang A (2013) Apple 1-aminocyclopropane-1-carboxylic acid synthase genes, MdACS1 and MdACS3a, are expressed in different systems of ethylene biosynthesis. Plant Mol Biol Rep 31:204–209 [Google Scholar]
- Tatsuki M, Nakajima N, Fujii H, Shimada T, Nakano M, Hayashi K, Hayama H, Yoshioka H, Nakamura Y (2013) Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch). J Exp Bot 64:1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainotti L, Tadiello A, Casadoro G (2007) The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot 58:3299–3308 [DOI] [PubMed] [Google Scholar]
- Vardhini BV, Rao SSR (2002) Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 61:843–847 [DOI] [PubMed] [Google Scholar]
- Ververidis P, John P (1991) Complete recovery in vitro of ethylene-forming enzyme activity. Phytochemistry 30:725–727 [Google Scholar]
- Walter M, Chaban C, Schutze K, Batistic O, Weckermann K, Nake C, Blazevic D, Grefen C, Schumacher K, Oecking C,. et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40:428–438 [DOI] [PubMed] [Google Scholar]
- Xiao YY, Chen JY, Kuang JF, Shan W, Xie H, Jiang YM, Lu WJ (2013) Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J Exp Bot 64:2499–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin P, Guo Q, Li B, Cheng S, Yan J, Chu J (2020) A tailored high-efficiency sample pretreatment method for simultaneous quantification of 10 classes of known endogenous phytohormones. Plant Commun 1:100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin P, Yan J, Fan J, Chu J, Yan C (2013) An improved simplified high-sensitivity quantification method for determining brassinosteroids in different tissues of rice and arabidopsis. Plant Physiol 162:2056–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189 [Google Scholar]
- Yin Y, Vafeados D, Tao Y, Yoshida S, Asami T, Chory J (2005) A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120:249–259 [DOI] [PubMed] [Google Scholar]
- Zaharah SS,, Singh Z, Symons GM, Reid JB (2011) Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. J Plant Growth Regul 31:363–372 [Google Scholar]
- Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60:1579–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Feng M, Chen W, Zhou X, Lu J, Wang Y, Li Y, Jiang CZ, Gan SS, Ma N. et al. (2019) In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat Plants 5:290–299 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Barlow JN, Baldwin JE, Schofield CJ (1997) Metal-catalyzed oxidation and mutagenesis studies on the iron(II) binding site of 1-aminocyclopropane-1-carboxylate oxidase. Biochemistry 36:15999–16007 [DOI] [PubMed] [Google Scholar]
- Zhu T, Tan WR, Deng XG, Zheng T, Zhang DW, Lin HH (2015) Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biol Technol 100:196–204 [Google Scholar]
- Zhu Z, Zhang Z, Qin G, Tian S (2010) Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol Technol 56: 50–55 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.