Improving our knowledge of holoparasitic–host interactions in Mediterranean-type ecosystems will help us to better manage parasitic plants, both in agricultural and natural ecosystems.
Abstract
Although photosynthesis is essential to sustain life on Earth, not all plants use sunlight to synthesize nutrients from carbon dioxide and water. Holoparasitic plants, which are important in agricultural and natural ecosystems, are dependent on other plants for nutrients. Phytohormones are crucial in holoparasitic plant–host interactions, from seed germination to senescence, not only because they act as growth and developmental regulators, but also because of their central role in the regulation of host photosynthesis and source–sink relations between the host and the holoparasitic plant. Here, we compile and discuss current knowledge on the impact and ecophysiology of holoparasitic plants (such as the broomrapes Orobanche sp. and Phelipanche sp.) that infest economically important dicotyledonous crops in Mediterranean agroecosystems (legumes [Fabaceae], sunflowers [Helianthus sp.], or tomato [Solanum lycopersicum] plants). We also highlight the role of holoparasitic plant–host interactions (such as those between Cytinus hypocistis and various shrubs of the genus Cistus) in shaping natural Mediterranean ecosystems. The roles of phytohormones in controlling plant–host interactions, abiotic factors in parasitism, and the biological significance of natural seed banks and how dormancy and germination are regulated, will all be discussed. Holoparasitic plants are unique organisms; improving our understanding of their interaction with hosts as study models will help us to better manage parasitic plants, both in agricultural and natural ecosystems.
Advances
Mediterranean ecosystems represent unique environments to study holoparasitic plant-host interactions
Holoparasitic plants cause severe reductions in productivity, but can also exert positive effects on diversity in natural ecosystems
A bidirectional flux of phytohormones occurs in holoparasitic plant-host interactions
The establishment of seed banks is essential for the success of both Orobanche and Cytinus infection in Mediterranean ecosystems
Introduction
Mediterranean-type climate ecosystems, with generally warm, dry summers, and wet, mild winters, are characterized by a great diversity in vegetation and occupy large natural vegetation areas in different regions of the world (from the well-known chaparral and woodlands ecoregion of California to the sclerophyll forests in south Australia, including the Chilean matorral, the Western Cape of South Africa and the Mediterranean basin). Indeed, a comparison of Mediterranean-type climate ecosystems in different parts of the world not only allows a test for ecological convergence, but also helps in understanding key ecophysiological and population processes (Kalin Arroyo et al., 1995; Rundel, 2019). Furthermore, the declining water balance due to global change has been documented for several Mediterranean natural and agricultural ecosystems in the last decades, to the extreme that in certain North African countries, where rainfed agriculture represents more than 90% of total agricultural land, climate change may result in surface water reductions of more than 35% (Skuras and Psaltopoulos, 2012). Both agricultural and natural Mediterranean ecosystems are subject to similar abiotic factors that shape holoparasitic plant–host interactions in these unique environments and improving our understanding of these interactions can undoubtedly help us manage parasitic plants better both in natural and agricultural ecosystems. About 1% of angiosperms are parasitic plants (Press et al., 1999; Twyford, 2018; Nickrent, 2020). Both agroecosystems and natural ecosystems are shaped by biotic and abiotic factors that modulate the growth and development of organisms that form them. The interaction between holoparasitic plants (see definitions in Glossary Box) and their hosts indirectly but strongly affects the interactions of the host with other elements of these ecosystems. These include other plant species which are important competitors with parasitic plants, pollinators, herbivores, mycorrhizae, nitrogen-fixing microorganisms, and other species that live in contact with the host plant such as insects, as well as other parasitic organisms (Bouwmeester et al., 2007; Bennett and Bever, 2007; Cahill et al., 2008; Zhuang et al., 2018). The study of holoparasites and the interactions with their hosts is therefore essential not only to understand better the delicate balance of Mediterranean ecosystems, a major source of global biodiversity, but also in the search for alternatives to improve crop management and reduce the yield losses due to holoparasitic plants that currently occur in Mediterranean agroecosystems (Runyon et al., 2009).
Glossary Box
Haustorium: a slender projection from the root of a parasitic plant enabling the parasite to penetrate the tissues of its host and absorb nutrients from it.
Hemiparasitic plants: plants that possess chlorophylls and are capable of photosynthesis but that obtain part of nutrients and water from a host plant.
Holoparasitic plants: plants that are not capable of photosynthesis and obtain all nutrients and water from a host plant.
Hormonal crosstalk: interaction between phytohormones in the regulation of a physiological process.
Host range: collection of hosts that a parasite can use.
Phytohormone: signal organic molecules produced at very low amounts by plants that regulate a physiological process.
Seed bank: seeds stored either naturally in the soil (natural seed bank) or by human action (artificial seed bank) that allow population renewal for any given species.
Seed dormancy: the state in which a seed is alive but not actively growing (germination is arrested).
Source–sink relations: relationship between source organs (with export photoassimilates) and sink organs (which receive these assimilates either for growth or storage).
Parasitic plants have evolved independently at least 12 times in angiosperms (Twyford, 2018), and they can be found in almost every ecosystem (Westwood et al., 2010). Since they lack photosynthesis capability, holoparasitic plants obtain all their water, carbon, and nutrients from their hosts, through a basic connective structure: the haustorium. As parasitic plants evolved from autotrophic ancestors, they conserve most of the characteristic features of those plants at the cellular level, such as cell walls and plastids (Clarke et al., 2019); but, in contrast to hemiparasites, holoparasites show extensive reductions in their plastid genome and have lost the capacity to perform photosynthesis (Bungard, 2004; McNeal et al., 2007; Cusimano and Wicke, 2016; Roquet et al., 2016). It has been proposed that the plastid genome in holoparasites could have come from their host through kleptoplasty (Krause, 2015). Despite chloroplasts in holoparasitic plants have experienced genome downsizing and gene loss/pseudogenization, these organisms conserve several of the basic features of autotrophic plants at the genetic, biochemical, and physiological levels, and respond to chemical and tactile signals, volatile organic compounds (VOCs), light, or hormone secretion from the host (Clarke et al., 2019). So holoparasitic plants, which are relatively easy to manipulate (at least compared to several animal parasites), provide a very interesting model through which to study interactions between organisms, which is essential to understand some aspects of basic biology better, as well as allowing us to understanding better their impact on natural and agroecosystems.
Although some aspects of the impact of holoparasitic plants on agriculture and natural ecosystems, including insights into their evolution, have been reviewed earlier ( Bungard, 2004; de Vega et al., 2008, 2009, 2010; Runyon et al., 2009; Roquet et al., 2016), there has been no comparative analysis of parasitic plant–host interactions in Mediterranean ecosystems to date. Here, we aim to go beyond a descriptive analysis of holoparasitic plant–host interactions by discussing these plant–plant interactions at the physiological level (including both ecological and molecular aspects), as central shapers of productivity in both agricultural and natural Mediterranean ecosystems. We will not only discuss recent advances in our understanding of the holoparasitic plant–host interactions, but we will also focus on the role of phytohormones in controlling plant–host interactions and the biological significance of natural seed banks together with how dormancy and germination are regulated in these ecosystems. This knowledge has important applications, not only in agronomy and agri-food biotechnology for improving yields and the quality of produce, but also in environmental management.
Productivity loss in Mediterranean ecosystems
The presence of parasitic plants reduces the aboveground biomass of plant communities, changes the structure of the associated microbial communities, and enhances soil nutrient cycling (resulting, among other effects, in an increased rate of nitrogen mineralization) in both agricultural and natural Mediterranean ecosystems (Quested, 2008; Li et al., 2014). Indeed, the nitrogen content of the host strongly affects the performance of holoparasites in both natural settings and agroecosystems, so that nitrogen fertilization in agroecosystems has generally been shown to reduce the severity of parasitic infestation due to an improvement of host vigor (Jeschke and Hilpert, 1997; Shen et al., 2013). Far more nitrogen is usually available in Mediterranean agroecosystems than in natural ecosystems, due to the crop fertilization treatments (Velthof et al., 2011), and this may have a strong impact on holoparasitic infestations. This human activity that makes more nitrogen available in natural ecosystems can also affect the spread of holoparasites, thus influencing the composition of plant communities within the current framework of global change (Pennings and Simpson, 2008).
Impact of holoparasitic plants on Mediterranean agroecosystems
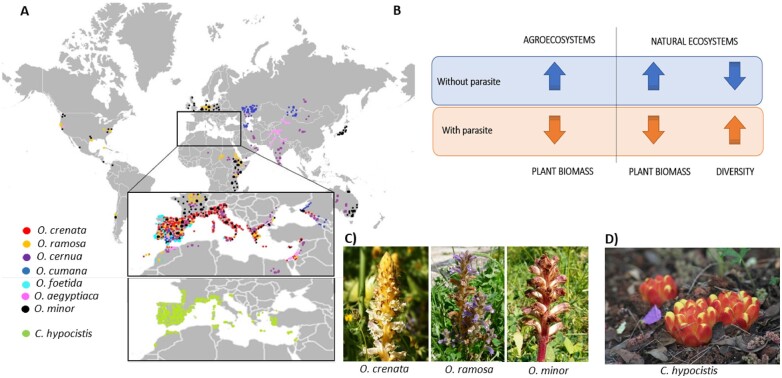
Parasitic plants pose a serious problem for agronomy that affects many parts of the world, due to the limited management tools that are available to control them, and Mediterranean-type ecosystems are not an exception. The most important parasitic plants in agriculture all over the globe are witchweed (Striga spp.), dodder (Cuscuta spp.), and broomrapes (Orobanche spp. and Phelipanche spp.). Striga spp. are root hemiparasites, which cause major problems for cereal production in resource-limited agriculture, as occurs in several areas of Africa (Scholes and Press, 2008). In contrast, Cuscuta spp. are holoparasites that attach themselves to the shoots of host plants, such as sugar beet (Beta vulgaris), onion (Allium cepa), citrus (Rutaceae), or forage legumes, among other crops, causing severe losses, mainly in Asia (Albert et al., 2008; Runyon et al., 2009), although they are widely distributed around the world (Dawson et al., 1994; Holm et al., 1997, Martinčová et al., 2019). Orobanche spp. and Phelipanche spp. are holoparasites that infest the roots of their host, including legumes, tomato, potato (Solanum tuberosum), sunflowers, and other important crops around the world (Parker and Riches, 1993; Parker, 2009), including several agroecosystems with a Mediterranean climate (or Mediterranean-type ecosystems, not only in the Mediterranean basin, but also in California, Chile, and Australia; Figure 1).
Figure 1.
Occurrence of holoparasites in Mediterranean ecosystems. A, Global distribution of Orobanche species and Cytinus hypocistis. B, Graphical representation of the impact of holoparasitic plants on agricultural and natural Mediterranean ecosystems. C, Photographs showing three examples of broomrapes, including O. crenata, O. ramosa and O. minor, which are typical from Mediterranean agroecosystems. D, Photograph of Cytinus hypocistis, which occurs naturally in the Mediterranean basin.
Parasitic plants cause yield losses ranging from 7% to 90% in Mediterranean agroecosystems (Sauerborn, 1991; Ennami et al., 2017, 2020). In Mediterranean agriculture, it has been estimated that some holoparasitic plants such as Orobanche spp. infest 16 million hectares, with an impact on yields of 20%–100% (Parker, 2009). However, the current status of infestation is difficult to assess due to farmers deciding to abandon traditional varieties of crops when they suffer infestations (Westwood et al., 2010). Broomrapes are widely distributed across the Mediterranean region (Figure 1A) and affect important crops in different ways. The most negative influence of bean broomrape (O. crenata) is on faba bean (Vicia faba) cultivars; but pea (Pisum spp.), lentil (Lens esculenta), vetch (Vicia sativa), chickpea (Cicer arietinum), and carrot (Daucus carota), among others, may also be severely affected. It has been estimated that 4 million Ha of legumes could be at risk in the Mediterranean region (reviewed by Parker, 2009). Orobanche foetida also parasitizes faba beans, as well as vetch and chickpea, but with less effect than O. crenata; while O. cumana parasitizing sunflowers has been a serious problem for centuries. O. cernua infests most Solanaceae crops, including tomato, tobacco (Nicotiana tabacum), pepper (Capsicum annuum), and eggplant (Solanum melongena; Mohamed et al., 2006; Parker, 2009). Tomato, potato, and tobacco plants are also affected by O. ramosa (syn. Phelipanche ramosa), with reported losses to tomato production of up to 80% (Díaz et al., 2006). Orobanche aegyptiaca (syn. P. aegyptiaca) shares most hosts with O. ramosa (syn. P. ramosa) in addition of rapeseeds (Brassica napus) and cucurbits (Cucurbitaceae). Finally, O. minor is the Orobanche species that causes the least losses, affecting clovers Trifolium spp.) and alfalfa (Medicago sativa; Parker and Riches, 1993; Parker, 2009).
Key physiological traits of the parasite–host connection make host damage inevitable. Once the haustoria (the vascular system connecting the parasitic plant and the host) is formed, parasitic plants start to receive nourishment from host-derived nutrients. The phloem connection with the host allows broomrapes to take advantage of nutrients, water, carbon and reduced nitrogen, as holoparasites act as a powerful sink for the host (Joel et al., 2007; Pielach et al., 2014). In addition, key biological traits contribute to the success of the parasite. By producing a very large number of seeds, which are small and light, the parasite can achieve great dispersion. Furthermore, hard seeds with water-impermeable coatings (Bouman and Meyer, 1994; Delavault, 2015) helps them retain germination capability for several years, and the need for host root exudates before germination assists the formation of a reserve or “seed bank” in the soil. In addition, rapid fixation to host roots, the synchronization of the parasite life cycle with that of the host, and the notable independence from environmental factors of this interaction at initial stages also contribute to parasite success (Gibot-Leclerc et al., 2012). Eventually, after successful establishment of parasitization, the impact of holoparasitic plants on photosynthetic performance together with the hormonal imbalance produced in the host result in severe growth reductions in the crop (Watling and Press, 2001; Zhuang et al., 2018; Figure 1B). Despite not always being successful, several strategies have been developed to reduce the impact of holoparasitic plants on Mediterranean agroecosystems (Box 1).
Box 1.
Strategies to reduce the impact of holoparasitic plants on Mediterranean agroecosystems
Phytosanitary measures to preventing a lasting seed bank are necessary to deal with broomrapes. Suicidal germination (A) caused by adding synthetic strigolactones (SLs) is a potential control mechanism (Habimana et al., 2014; Zwanenburg et al., 2016).Adding SL deactivators (B) is also a means to deal with broomrapes at later stages. The effectiveness of these treatments is limited by the continuous germination of parasites throughout the season, and their direct host connection; but in some cases, they have been developed and applied with the desired selectivity and efficacy (Zwanenburg et al., 2016).
Rotation with nonhost crops is commonly advised, but not always possible. As an alternative, intercropping (C) with oat has been shown to reduce legume infestation by O. crenata; significant control is achieved in faba bean, pea, lentil, and chickling pea when intercropped with fenugreek; and faba bean and pea benefit from intercropping with berseem clover. Inhibition of host seed germination by allelochemicals released by roots of the second crop may be the mechanism for infection reduction (Fernández-Aparicio et al., 2016).
Soil solarization has shown usefulness in controlling seed banks, since broomrape seeds die at around 48–57ºC when imbibed (Mauromicale et al., 2005). This technique is improved when combined with biocontrol strategies (D).
The fly Phytomyza orobanchia is reportedly host-specific for Orobanche spp., thus it helps to reduce reproductive output and seed dispersal (Abu-Shall and Ragheb, 2014; Bayram and Çikman, 2016).
Alternatively, fungi can be bioherbicides: one Fusarium species used alone or in combination with others can attack the parasite in any season and reduce their seed bank and physiological performance (Shabana et al., 2003; Aybeke, 2017).
Inoculation with compatible Rhizobium can reduce host exudates, preventing parasite attachment and growth in pea (Mabrouk et al., 2006).
Genomic strategies are arousing interest as tools for more sustainable agriculture. While genes resistant against specific varieties of O. cumana have been used in breeding programs (Molinero-Ruiz et al., 2009), the constant evolution of these holoparasitic plants highlights the need for more sustainable resistance (E). Conservation of crop wild relatives offering sources of resistance to support breeding programs will help to achieve this goal (Seiler, 2019).
Mathematical modeling of both seed bank dynamics and competition between parasites and hosts, including interaction studies between variables such as genotype, environment, and management (F), may be effective in developing strategies to control broomrape and assess consequences (Grenz et al., 2005, 2006).

Impact of holoparasitic plants on natural Mediterranean ecosystems
Parasitic plants are present in most natural plant communities and play an important role in Mediterranean ecosystems (Musselman and Press, 1995; Press and Phoenix, 2005; Groom and Lamont, 2015). In the Mediterranean basin, the root holoparasitic genus Cytinus, with a wide distribution in the Mediterranean basin (Figure 1A), parasitizes endemic Mediterranean shrubs belonging to the Cistaceae family, such as several species of the genus Cistus, Halimium, Helianthemum, and Fumana. In contrast to what occurs in agroecosystems, in natural systems not only do they have direct negative effects on productivity, reducing host growth, and reproduction output in the short term, but their effects on the ecosystem properties are also indirect and may be positive in the long term (Seel and Press, 1996; Davies and Graves, 2000; Neto et al., 2017). By taking water, carbon, and nutrients from their host, holoparasitic plants can alter the competitive balance between species, altering their composition and eventually increasing the diversity of the ecosystems (Figure 1B). Indeed, holoparasites reduce the growth of competitively dominant species thus allowing a wider pool of species to grow. It has been proposed that the impact of holoparasitic plants on ecosystems depends on the virulence of the parasitic plant, which in turn is influenced by both environmental conditions and the specificity of the holoparasitic plant–host interaction (Gibson and Watkinson, 1991, 1992; Pennings and Callaway 2002).
Holoparasitic plants have countless effects on Mediterranean ecosystems aside from reducing host productivity and reproductive output (de Vega et al., 2010). In the Mediterranean region, Cytinus parasitizing endemic Mediterranean shrubs belonging to the Cistaceae family are abundant. The genetic differentiation of Cytinus has evolved as a result of selective pressure imposed by their host and, despite the classification of Cytinus species being quite controversial due to their morphological similarity, five different genetic groups have been described (de Vega et al., 2008). Following the classification sensude Vega et al. (2008), Cytinus species with ivory-white and pink flowers parasitizing white-leaved rockrose (Cistus albidus) would correspond to C. hypocitis subsp. clusii or C. ruber, and C. hypocitis subsp. hypocistis would correspond to yellow flowered Cytinus, one group parasitizing gum rockrose (C. ladanifer), and the other Montpellier cistus (C. monspeliensis), C. populifolius, and sage-leaved rockrose (C. salvifolius). Yellow flowered Cytinus that parasitizes Halimium sect. Chrysorhodion, would correspond to C. hypocitis subsp. macaranthus, and those that parasitize Halimium, Helianthemum and Fumana would correspond to C. hypocitis subsp. lutescens. In general, holoparasitic plants may cause reductions in plant community biomass in the short term, but they can modulate diversity, heterogeneity, and productivity in both directions in the long term: increasing or reducing them, depending on whether the chosen host is competitively dominant or subordinate. Cytinus hypocistis modulates the ecophysiology of various shrubs of the genus Cistus in natural Mediterranean ecosystems, and these shrubs are competitively dominant and perform an essential role in post-fire succession (Roy and Sonié, 1992; Montès et al., 2004). Therefore, infestation by these holoparasitic plants leads to a decrease in productivity in the short-term during post-fire succession, but also to an increase in diversity in the long term by altering water and nutrient resources in the soil and making other plants more competitive with the dominant Cistus sp. shrubs. Additionally, they can affect vegetation zonation by altering the competitiveness of species: an aspect that has been studied in hemiparasitic plants from the Mediterranean region (Bardgett et al., 2006; Mellado and Zamora, 2017; Griebel et al., 2017), but not in Cytinus. By interacting with other trophic levels, holoparasitic plants can also have an impact on the biotic framework of the ecosystem. For instance, the associations among Cytinus hypocitis, their host plants (including Cistus albidus, C. ladanifer, C. salvifolius, Halimimum halimifolium, and H. ocymoides) and mycorrhizal fungi can be very important in terms of maintaining biodiversity and ecosystem functioning (de Vega et al., 2010; Correia and Ascensao, 2017). Moreover, their interaction with pollinators such as ants, flies, or mammals (depending on their distribution) can contribute to seed dispersal from the host (seeds of Cistus shrubs are usually dispersed by mammals) or other species inhabiting the same ecosystem. Finally, the influence of holoparasitic plants on mycorrhiza can alter the activity of soil microbes and consequently the balance of fungi/bacteria via the input of their nutrient-rich litter to the soil, thus contributing to nutrient cycling: an aspect that deserves further study.
Although the holoparasites Orobanche spp. are well known in agroecosystems, they also inhabit natural Mediterranean ecosystems (Schneeweiss, 2007). While O. cernua L. is most frequently observed parasitizing species of the genus Artemisia in natural ecosystems on the Iberian Peninsula, it has also been found infesting Launaea lanifera in ecological niches that are quite arid and degraded at altitudes between 0 and 1,500 m a.s.l. In contrast, O. cumana, which spread widely across the Iberian Peninsula with the introduction of sunflower cultivars, has not been observed in a natural context; thus, this species only seems to be adapted to cultivated land niches (Pujadas-Salvà and Velasco, 2000). Meanwhile, O. lutea has been reported to be able to grow in polluted soils and even reduces the content of toxic metals in the host, so that the latter increases its photosynthetic rate relative to noninfested plants. This suggests an attenuation role of the parasitic plant when the host is faced with soil metal toxicity (Turnau et al., 2018).
Holoparasitic plant–host interactions
Interactions between holoparasitic plants and their hosts in Mediterranean ecosystems (either natural or agroecosystems) in which the parasites benefit while the hosts are harmed occur at every stage of the relationship. The first interplay occurs in identification of the host, which in most cases leads to parasite germination. It has been demonstrated that holoparasitic plants respond to light and chemical signals when locating their host, and germinate in response to strigolactones (Runyon et al., 2006; Furuhasi et al., 2011). Communication continues through haustorium development, when hautorium-inducing factors (flavonoids, phenolic acids, quinones, cytokinins, and cyclohexene oxides) play a major role in haustorium initiation; while auxin production and cytokinin translocation allow for its correct growth and development (Ishida et al., 2016; Goyet et al., 2017; Clarke et al., 2019). The exchange of microRNAs between holoparasitic plants and their hosts appears to function as interference signals directed at mRNA, such as mRNA related with auxin receptors, development regulators, pathogen defense, and phloem function in the host, as a way to increase parasite fitness. This aspect has been shown to occur in broomrapes (Clarke et al., 2019) but still requires examination in Cytinus. Given that phytohormones regulate several processes in plant development and defense, in-depth understanding of their implication in holoparasitic plant–host interactions in both agricultural and natural Mediterranean ecosystems will help us manage holoparasitic plants better.
Impact of phytohormones on parasitic plant–host interactions
Strigolactones are the best-known phytohormones involved in holoparasitic plant–host interactions in Mediterranean ecosystems. Strigolactones are tricyclic lactones connected with a butyrolactone group by an enol ether bridge, in which their biological activity resides (Zwanenburg et al., 2016). In general, their physiological and ecological relevance stems from their function as promoters of growth and hyphal branching in arbuscular mycorrhizal fungi, as inhibitors of shoot branching, and as stimulators of parasitic plant germination (López-Ráez et al., 2008). Released from the host root into the soil at a very low concentration, strigolactones are thought to form a covalent bond with their receptors, which induces parasitic plant germination (Zwanenburg et al., 2008; Ruyter-Spira et al., 2013). Indeed, a unique combination of strigolactones has been shown to contribute to the host specificity of broomrape germination (Fernández-Aparicio et al., 2011).
Auxin and cytokinins play several roles in holoparasitic plant–host interactions. Not only do auxin and cytokinins play a major role in root architecture, cell division, differentiation, and elongation, as well as in the leaf, flower, vascular, and fruit development of the host (Ljung, 2013), but auxin has also been shown to be involved in broomrapes germination (Slavov et al., 2004). The flow of auxin from the parasitic plant to the host is involved in the formation of a continuous vessel connecting them (Aloni, 2015). Indeed, changes in auxin transport or disturbance of the auxin response has been shown to prevent infection by broomrapes, highlighting the fact that auxin plays a major role in parasite performance (Bar-Nun et al., 2008). Cytokinins not only regulate cell proliferation and differentiation in apical meristems, thereby promoting shoot growth and inhibiting root growth in the host (Werner et al., 2001; Schaller et al., 2015), but they also play a major role in source–sink relationships (Roitsch and Ehneß, 2000), which are essential in holoparasitic plant–host interactions. Cytokinins have been shown to be involved in haustorium induction in holoparasites, in some cases as a result of a signaling pathway initiated by the perception of light and contact signals received during recognition of the host, such as a low red:far red ratio, or mechanical stimulation (Furuhasi et al., 2011). For O. ramosa (syn. P. ramosa), it has been shown that cytokinins present in the common rape host root exudates induce the expression of cytokinin-responsive genes (RESPONSE REGULATOR 5 [PrRR5], CYTOKININ OXIDASE 2 [PrCKX2], CYTOKININ OXIDASE 4 [PrCKX4], and ZINC FINGER PROTEIN 6 [PrZFP6]) in the parasite and play a major role in both induction of haustorium formation and an increase of parasite aggressiveness (Goyet et al., 2017).
Other phytohormones involved in holoparasitic plant–host interactions are the typical stress-related hormones jasmonic acid, salicylic acid, and abscisic acid (ABA). The involvement of jasmonic acid and salicylic acid in host defense response against broomrapes at initial stages of the holoparasitic plant–host interaction has been reported in several studies (reviewed by Gutjahr and Paszkowski, 2009; see also Torres-Vera et al., 2016). In addition, given the observed enhanced expression of ABA biosynthetic (LeNCED1) and responsive genes (Le4) in the tomato host roots, it has been proposed that this phytohormone is involved in host defense response at early stages of the infection between O. ramosa and tomato plants (Torres-Vera et al., 2016).
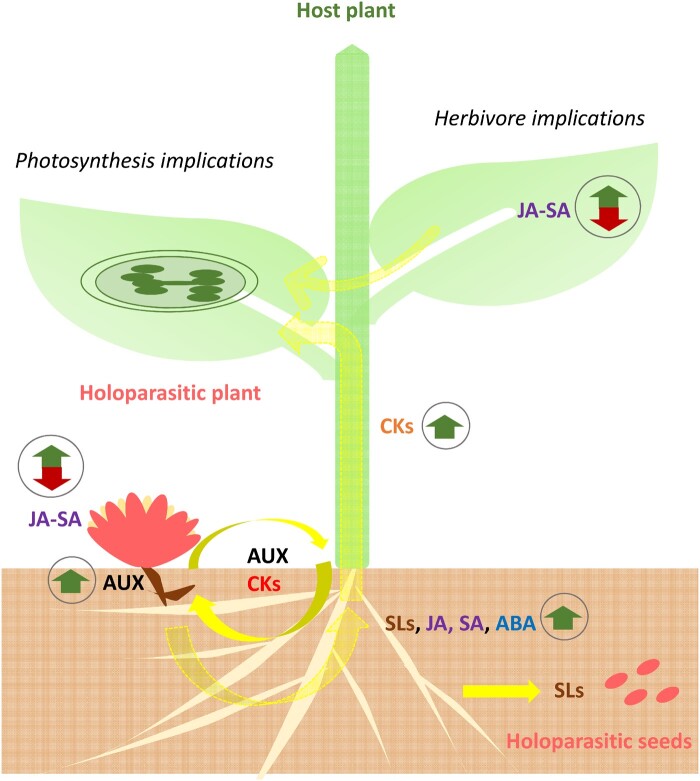
Since complex crosstalk occurs between phytohormones, and each phytohormone can mediate different responses depending on the plant species and the prevailing environmental conditions, it is necessary to study hormonal responses in the holoparasitic plant–host system through integrating what occurs in the whole ecosystem to understand better the role of phytohormones in both agricultural and natural Mediterranean ecosystems (Figure 2). This is not only important to understand better how these interactions occur at the physiological level in natural ecosystems, but also to arrive at better management of holoparasitic plants in Mediterranean agroecosystems. Specifically, important points of control in Mediterranean agroecosystems include strigolactones, to produce suicidal germination and thereby control seed banks (Habimana et al., 2014; Zwanenburg et al., 2016, see also Box 1). Other hormonal approaches with potential application also cover ABA and auxins, for preventing germination (Slavov et al., 2004); cytokinins, which prevent parasite and host connection once the parasitic plant has germinated (Furuhasi et al., 2014; Goyet et al., 2017); and salicylic acid, and to a lesser extent jasmonic acid, involved in resistance against parasites (Buschmann et al., 2005; Yoder and Scholes, 2010).
Figure 2.
Schematic representation of holoparasitic plant–host interactions, placing special interest on phytohormone flux and its implications. Solid yellow arrows represent demonstrated fluxes of hormones, while translucent yellow arrows represent possible fluxes. Small arrows inside circles represent reported hormone increases/decreases. ABA: abscisic acid, AUX: auxin, CKs: cytokinins, JA: jasmonic acid, SA: salicylic acid, SLs: strigolactones
Impact of phytohormones on photosynthesis in the host
Holoparasites represent an extra sink for photosynthates in the host. Although in most cases the holoparasites enhance the host photosynthesis rate, this compensation effect is limited and a constraint on the number and size of parasites infecting a host can emerge. Furthermore, in most cases, this source–sink relationship benefits the parasite rather than the host (Watling and Press, 2001). It has been reported that infection by O. ramosa causes upregulation of ABA biosynthesis and signaling in tomato roots (Torres-Vera et al., 2016). ABA is known to regulate guard cells, promoting stomatal closure (Acharya and Assmann, 2009), so we might expect that photosynthesis could be reduced as a stress response to the holoparasite, as it has been recently shown in red clover–O. minor host–parasite interaction (Jokinen and Irving, 2019). However, it has been reported that holoparasitic plant–host systems can partly compensate for this, or even maintain productivity, by greater leaf area and by delaying leaf senescence in response to the extra demand for carbon by the holoparasite (Hibberd et al., 1998). So ABA increases in the host root in response to the O. ramosa do not entail negative effects of the parasite, but positive effects, most likely related to an improved root: shoot ratio in the host, which may improve vegetative growth and delay senescence in the long term.
It has been shown that auxin flows from parasitic plants to their hosts (Aloni, 2015). The link between auxins and photosynthesis has been reported to involve leaf venation, since altered auxin homeostasis affects leaf hydraulic conductance and leaf gas exchange (McAdam et al., 2017). Furthermore, it has also been shown that an auxin-responsive factor in tomato (AUXIN RESPONSE FACTOR 6A [SlARF6A]) regulates chlorophyll content and chloroplast development, which result in improved photosynthesis (Yuan et al., 2019). The significance of the auxin flow to the host has also been related to haustorial development and the formation of continuous vessels connecting parasite and host (Bar-Nun et al., 2008; Aloni, 2015; Ishida et al., 2016). It may also play a role in the improvement of host photosynthesis, given the benefit that this represents for the holoparasite. In addition, increased cytokinin contents have been reported in both holoparasitic plant and host during infection (Furuhasi et al., 2014); and translocation of cytokinins from the parasite to the host has been shown to cause changes in host architecture (Watling and Press, 2001). Moreover, cytokinins are involved in source–sink relationships, and so alterations in host cytokinin levels may have an impact on photosynthesis at the whole-plant level (Acharya and Assmann, 2009).
Altered salicylic acid and jasmonic acid contents have been reported in tomato during the initial stages of O. ramosa infection (Torres-Vera et al., 2016). Also, changes in superoxide dismutase activity have been reported in the host during enhanced reactive oxygen species (ROS) production due to broomrapes infection. ROS detoxification seemed to be related to host resistance to holoparasites (Demirbaş and Acar, 2008). Since both salicylic acid and jasmonic acid have been reported to improve antioxidant mechanisms under various plant stress conditions (Sirhindi et al., 2015; Bali et al., 2018), both phytohormones could also be involved, together with ABA, in alleviation of photoinhibition and improvement of photosynthesis in the holoparasite–host system. This aspect requires further study in Mediterranean ecosystems, particularly in the Cytinus-Cistus system, which is still poorly understood in this respect. Another aspect that requires more research is the critical lack of information on the selection pressures that may be acting on the holoparasite. For example, is the use of each hormone (such as salicylic acid) selected for in the parasite, or are these passively being used? Further investigations are needed to shed light on this question, and more specifically to identify putative genes that might be under selection pressure.
Natural seed banks: dormancy versus germination
The establishment of persistent seed banks and an efficient control of seed dormancy and germination are essential for the success of holoparasitic plants in Mediterranean natural and agroecosystems. Indeed, understanding the dynamics of the seed bank has been shown to be crucial to solving the problem of broomrapes infestations in these agroecosystems. Most Orobanche seeds present physiological dormancy (Fernández-Aparicio et al., 2016); studies of O. ramosa seeds have revealed seasonal physiological dormancy. While the short-term seed mortality was about 4%–7% per year, seed dormancy was observed to be synchronized with the host cycle, since low seed dormancy was recorded in the period when their host seeds were set in the ground, but high dormancy in the period between the appearance of host seeds in the soil. This means that O. ramosa is able to infect its host early in its development (Pointurier et al., 2019; Figure 3A). However, intraspecific variability has been demonstrated. In another population of O. ramosa, opportunistic behavior has been suggested. The population showed a shorter dormancy cycle, with quicker and massive spontaneous germination, probably making them able to parasitize both winter and summer crops (Pointurier et al., 2019).
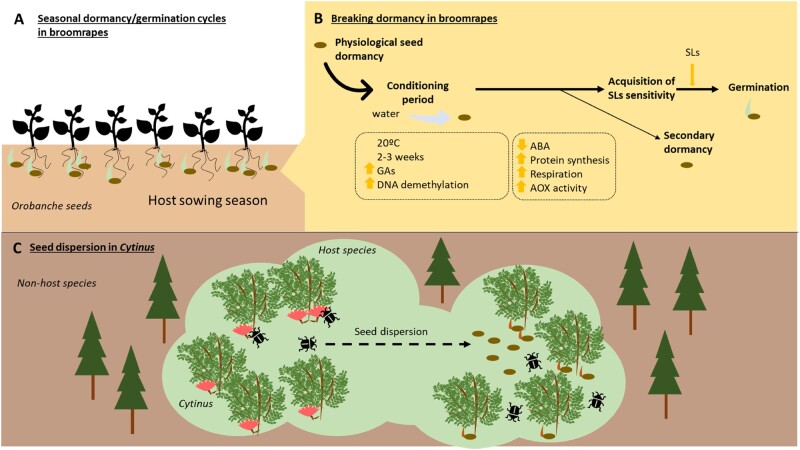
Figure 3.
Seed dormancy and germination in holoparasitic plants. Schematic representation of (A) seasonal physiological seed dormancy: Orobanche germination during the season when host seeds were in the ground, B, process of breaking seed dormancy and germination demonstrated in Orobanche, C, seed dispersion in Cytinus by beetles. ABA: abscisic acid, AOX: alternative oxidase, GAs: gibberellins, SLs: strigolactones.
Germination of broomrapes is a two-step process involving a conditioning period that allows a proper response to germination stimulants, followed by biochemical stimulation of germination (Lechat et al., 2015; Figure 3B). The wake from seed dormancy in O. ramosa requires a minimum of 4 d conditioning at 21°C for water to enter and imbibition to occur (Mauromicale et al., 2005; Lechat et al., 2012). On the first day, ABA seed content decreases sharply (Lechat et al., 2015), protein synthesis occurs, and alternative oxidase activity increases as does respiration (Bar-Nun et al., 2003). The ABA decrease has been shown to be mediated by the activation of CYP707A1 (an ABA catabolic gene) by a strigolactone analog (GR 24), but it can only occur after the conditioning period, with a few exceptions (Plakhine et al., 2009; Lechat et al., 2012). However, the acquisition of strigolactone sensitivity during the conditioning period is not only mediated by the ABA content, but also by the DNA methylation status (Lechat et al., 2015; Figure 3B). Under typical Mediterranean conditions, such as a temperature of 20°C, responsiveness to strigolactones increases with the duration of the conditioning period, becoming optimal after 2–3 weeks. However, when conditioned at sub-optimal temperatures, a secondary dormancy may occur. Similar requirements for the conditioning period and for entrance to secondary dormancy have been shown for several species of the genus, including O. crenata, O. cumana, O. aegyptiaca, and O. minor; and it has been shown that the gibberellin effect of promoting seed germination is species specific (Kebreab and Murdoch 1999; Matusova et al., 2004; Moral et al., 2015; Ye et al., 2017; Figure 3B). Oxygen tension and water potential also influence the seed germination of broomrapes, so their distribution across the globe is limited by factors governing soil conditions. These requirements make germination of this species possible in all Mediterranean climate areas (Gibot-Leclerc et al., 2004; Grenz et al., 2006). Taken together, the physiological mechanisms involved in the control of seed dormancy and germination in broomrapes have important agronomic implications in Mediterranean agroecosystems (Grenz et al., 2005, 2006). Good examples include the use of catch crops, species grown between the cultivation of main crops when seed dormancy of broomrapes is low (Pointurier et al., 2019); trap crops, which induce holoparasite germination without being parasitized (Rubiales et al., 2009); or gibberellins, which help retain high sensitivity in seeds for efficient suicidal germination strategies (Ye et al., 2017). Soil solarization (Mauromicale et al., 2005), biocontrol strategies (Mabrouk et al., 2006; Abu-Shall and Ragheb, 2014; Bayram and Çikman, 2016), and bioherbicides (Shabana et al., 2003; Aybeke, 2017) have also shown usefulness in controlling holoparasite seed banks. Finally, genomic strategies (Molinero-Ruiz et al., 2009; Seiler 2019) and mathematical modeling (Grenz et al., 2005, 2006) may also be very useful tools to achieve a more sustainable agriculture in seed bank management (Box 1).
Knowledge of the mechanisms of seed dispersal and germination adopted by species occurring in natural Mediterranean ecosystems, such as Cytinus, is also useful for better management of holoparasitic plants in both agricultural and natural ecosystems. Cytinus produce fruit that give rise to a large amount of dust-like seeds with a rigid thick coat. These are consumed by beetle species, as well as rodents, ants and lagomorphs that defecate intact and viable seeds far away from the parent plant, although seed dispersal by Cytinus hypocistis in Mediterranean ecosystems has mainly been associated with endozoochory by beetles (de Vega et al., 2011). Beetles can be considered an efficient seed dispersal agent given that, contrary to other fruit-consuming species, they are likely to leave the seeds, once eaten, near to host roots, underground (Figure 3C). This mutualistic relationship between the holoparasitic plant and the beetle has been shown to be limited by the distribution and population size of beetles, which in turn is influenced by environmental and ecological factors such as soil type, or by the presence of other consumers of Cytinus fruits such as wood mice (de Vega et al., 2011). However, the seed dispersal area seems to be fairly small due to the reduced mobility of this insect, which on the one hand may be contributing to the small size populations of C. hypocistis, but on the other hand may ensure the placement of the seeds in areas where their host is present (de Vega et al., 2008, 2011; Figure 3C). Although the germination requirements of C. hypocistis seeds remain unknown, the knowledge acquired to date of the germination of other holoparasitic species may lead to the discovery of similar physiological mechanisms that allow the control of Cytinus seed banks. Taken together with the information available on seed dispersal, this could allow us to better understand the physiological and ecological mechanisms underlying the spread of Cytinus in Mediterranean ecosystems.
Conclusions and future prospects
Major advances have taken place in our understanding of holoparasitic plant–host interactions, the role of phytohormones in these interactions, and how these influence host photosynthesis and control source–sink relations. Furthermore, the physiological mechanisms involved in seed dormancy and germination of holoparasitic seed banks have been reported in detail at the physiological and molecular levels. There are still, however, many gaps in our knowledge of the physiological processes and mechanisms underlying holoparasitic plant–host interactions in Mediterranean ecosystems, most particularly in Cytinus and to a lesser extent in broomrapes. These include aspects related to hormonal crosstalk in holoparasitic plant–host interactions, to what extent diversity increases in natural ecosystems due to holoparasitic plants both in the short and long term, and the role of VOCs in holoparasitic plant–pollinator interactions. Although several advances have recently been made in the latter question (see Box 2), some questions remain unsolved, including how the host influence VOCs release by holoparasites in plant–pollinator interactions in Mediterranean ecosystems. Furthermore, it will also be challenging to investigate in the near future how the mode and tempo of evolution of holoparasitic plant–host interactions differ in agricultural systems relative to natural environments; and finally, in order to gain a holistic understanding of ecosystem processes, how ecophysiology is linked to its underpinning genetic basis in holoparastic plant–host interactions (see Outstanding questions). Better understanding of how the whole holoparasitic plant–host system is regulated, including as well its relation to the environment, pollinators, and microbial communities in a holistic view, is not only essential to expand our knowledge of basic biology, but it will also have important economic implications in the agricultural, horticultural and agri-food biotechnology sectors, as well as in environmental management.
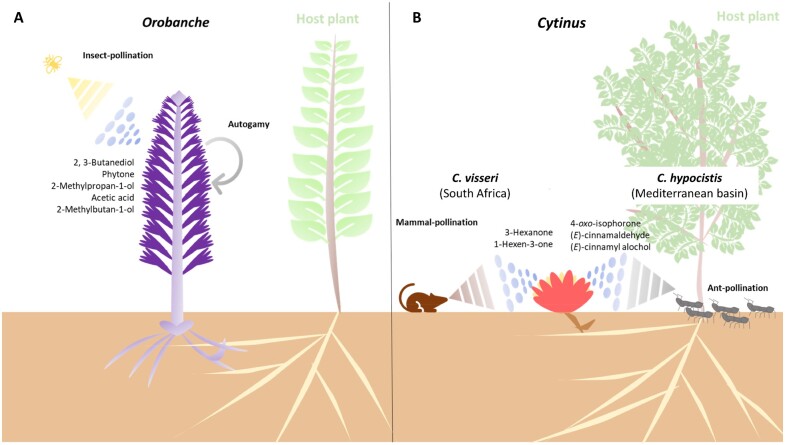
Box 2.
Schematic representation of holoparasitic plant–pollinators interaction through the release of VOCs in Mediterranean ecosystems. VOCs play a key role both in insect pollination of Orobanche (A) and mammal- and ant-pollination of Cytinus (B)
Funding
Part of this work was supported by the Spanish Government (through the grant BFU2015-64001-P/MINECO/FEDER) and the Generalitat de Catalunya (ICREA Academia award to S.M.-B.).
Conflict of interest statement. Authors declare no conflicts of interest.
Outstanding Questions
How phytohormone crosstalk during the holoparastic plant-host interaction determine host tolerance?
What physiological mechanisms in the host may influence VOCs release by holoparasitic plants in holoparasitic plant-pollinator interactions?
Do the mode and tempo of evolution of holoparasitic plant-host interactions differ in agricultural systems relative to natural environments?
How ecophysiology is linked to its underpinning genetic basis in holoparastic plant-host interactions?
S.M.-B. conceived the idea and prepared the outline. A.C. wrote the manuscript with the help of S.M.-B. A.C. prepared figures and boxes. S.M.-B. revised and edited the final manuscript.
The author responsible for the distribution of materials integral to the findings presented in this article, in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Sergi Munné-Bosch (smunne@ub.edu).
References
- Abu-Shall AMH, Ragheb EIM (2014) Management of Orobanche crenata using trap crops and Phytomyza orobanchia Kalt. in broad bean (Vicia faba) field in Egypt. Egyptian J Biol Pest Control 24: 217–223 [Google Scholar]
- Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol 69: 451–462 [DOI] [PubMed] [Google Scholar]
- Albert M, Belastegui-Macadam X, Bleischwitz M, Kaldenhoff R (2008) Cuscuta spp: parasitic plants in the spotlight of plant physiology, economy and ecology. Prog Bot 69: 267–275 [Google Scholar]
- Aloni R (2015) Eophysiological implications of vascular differentiation and plant evolution. Trees 29: 1–16 [Google Scholar]
- Aybeke M (2017) Fusarium infection causes phenolic accumulations and hormonal disorders in Orobanche spp. Ind J Microbiol 57: 416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali S, Kaur P, Sharma A, Ohri P, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2018) Jasmonic acid-induced tolerance to root-knot nematodes in tomato plants through altered photosynthetic and antioxidative defense mechanisms. Protoplasma 255: 471–484 [DOI] [PubMed] [Google Scholar]
- Bardgett RD, Smith RS, Shiel RS, Peacock S, Simkin JM, Quirk H, Hobbs PJ (2006) Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature 439: 969–972 [DOI] [PubMed] [Google Scholar]
- Bar-Nun N, Plakhine D, Joel DM, Mayer AM (2003) Changes in the activity of the alternative oxidase in Orobanche seeds during conditioning and their possible physiological function. Phytochemistry 64: 235–241 [DOI] [PubMed] [Google Scholar]
- Bar-Nun N, Sachs T, Mayer AM (2008) A role for IAA in the infection of Arabidopsis thaliana by Orobanche aegyptiaca. Ann Bot 101: 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram Y, Çikman E (2016) Efficiency of Phytomyza orobanchia Kaltenbach (Diptera: Agromyzidae) on Orobanche crenata Forsk. (Orobanchaceae) in lentil fields at Diyarbakır and Mardin Provinces, Turkey. Egypt J Biol Pest Control 26: 365–371 [Google Scholar]
- Bennett AE, Bever JD (2007) Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 88: 210–218 [DOI] [PubMed] [Google Scholar]
- Bouman F, Meyer W (1994) Comparative structure of ovules and seeds in Rafflesiaceae. Plant Syst Evol 193: 187–212 [Google Scholar]
- Bouwmeester HJ, Roux C, López-Ráez JA, Bécard G (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12: 224–230 [DOI] [PubMed] [Google Scholar]
- Bungard RA (2004) Photosynthetic evolution in parasitic plants: insight from the chloroplast genome. BioEssays 26: 235–247 [DOI] [PubMed] [Google Scholar]
- Buschmann H, Fan SW, Sauerborn J (2005) Effect of resistance-inducing agents on sunflower (Helianthus annuus L.) and its infestation with the parasitic weed Orobanche cumana Wallr. J Plant Dis Prot 112: 386–397 [Google Scholar]
- Cahill JF, Elle E, Smith GR, Shore BH (2008) Disruption of a belowground mutualism alters interactions between plants and their floral visitors. Ecology 89: 1791–1801 [DOI] [PubMed] [Google Scholar]
- Clarke CR, Timko MP, Yoder JI, Axtell MJ, Westwood JH (2019) Molecular dialog between parasitic plants and their hosts. Annu Rev Phytopathol 57: 279–299 [DOI] [PubMed] [Google Scholar]
- Correia O, Ascensão L (2017) Summer semi-deciduous species of the Mediterranean landscape: a winning strategy of Cistus species to face the predicted changes of the Mediterranean climate. In Ansari AA, Gill SS, Abbas ZK, Naeem M, ed, Plant Biodiversity: Monitoring, Assessment and Conservation. CABI Publishing. [Google Scholar]
- Cusimano N, Wicke S (2016) Massive intracellular gene transfer during plastid genome reduction in nongreen Orobanchaceae. New Phytol 210: 680–693 [DOI] [PubMed] [Google Scholar]
- Davies DM, Graves JD (2000) The impact of phosphorus on interactions of the hemiparastitic angiosperm Rhianthus minor and its host Lolium perenne. Oecologia 124: 100–106 [DOI] [PubMed] [Google Scholar]
- Dawson JH, Musselman LI, Wolswinkel P, Dorr I (1994) Biology and control of Cuscuta. Rev Weed Sci 6: 265–317 [Google Scholar]
- Delavault P (2015) Knowing the parasite: biology and genetics of Orobanche. Helia 38: 15–29 [Google Scholar]
- Demirbaş S, Acar O (2008) Superoxide dismutase and peroxidase activities from antioxidative enzymes in Helianthus annuus L. roots during Orobanche cumana Wallr. Penetratin Fres Environ Bul 17: 8a [Google Scholar]
- Díaz JS, Norambuena HM, López-Granados F (2006) Characterization of the holoparasitism of Orobanche ramosa on tomatoes under field conditions. Agric Tec 66: 223–234 [Google Scholar]
- Ennami M, Briache FZ, Gaboun F, Abdelwahd R, Ghaouti L, Belqadi L, Westwood J, Mentag R (2017) Host differentiation and variability of Orobanche crenata populations from legume species in Morocco as revealed by cross-infestation and molecular analysis. Pest Manag Sci 73: 1753–1763 [DOI] [PubMed] [Google Scholar]
- Ennami M, Mbasani-mansi J, Briache FZ, Oussible N, Gaboun F, Ghaouti L, Belqadi L, Ghanem ME, Aberkani K, Westwood J, et al. (2020) Growth-defense tradeoffs and source-sink relationship during both faba bean and lentil interactions with Orobanche crenata Forsk. Crop Prot 127: 104924 [Google Scholar]
- Fernández-Aparicio M, Yoneyama K, Rubiales D (2011) The role of strigolactones in host specificity of Orobanche and Phelipanche seed germination. Seed Sci Res 21: 55–61 [Google Scholar]
- Fernández-Aparicio M, Flores F, Rubiales D (2016) The effect of Orobanche crenata infection severity in faba bean, field pea, and grass pea productivity. Front Plant Sci 7: 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhasi T, Kojima M, Sakakibara H, Fukushima A, Hirai MY, Furuhashi K (2014) Morphological and plant hormonal changes during parasitization by Cuscuta japonica on Momordica charantia. J Plant Inter 9: 220–232 [Google Scholar]
- Gibot-Leclerc S, Corbineau F, Sallé G, Côme D (2004) Responsiveness of Orobanche ramosa L. seeds to GR 24 as related to temperature, oxygen availability and water potential during preconditioning and subsequent germination. Plant Growth Regul 43: 63–71 [Google Scholar]
- Gibot-Leclerc S, Sallé G, Reboud X, Moreau D (2012) What are the traits of Phelipanche ramosa (L.) Pomel that contribute to the success of its biological cycle on its host Brassica napus L.? Flora 207: 512–521 [Google Scholar]
- Gibson CC, Watkinson AR (1991) Host selectivity and the mediation of competition by the root hemiparasite Rhianthus minor. Oecologia 86: 81–87 [DOI] [PubMed] [Google Scholar]
- Gibson CC, Watkinson AR (1992) The role of the hemiparasitic annual Rhianthus minor in determining grassland community structure. Oecologia 89: 62–68 [DOI] [PubMed] [Google Scholar]
- Goyet V, Billard E, Pouvreau JB, Lechat MM, Pelletier S (2017) Hautorium initiation in the obligate parasitic plant Phelipanche ramosa involves a host-exudated cytokinin signal. J Exp Bot 68: 5539–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz JH, Manschadi AM, De Voli P, Meinke H, Sauerborn J (2005) Assessing strategies for Orobanche sp. control using a combined seedbank and competition model. Agron J 97: 1551–1559 [Google Scholar]
- Grenz JH, Manschadi AM, De Voli P, Meinke H, Sauerborn J (2006) Simulating crop-parasitic weed interactions using APSIM: model evaluation and application. Eur J Agron 24: 257–267 [Google Scholar]
- Griebel A, Watson D, Pendall E (2017) Mistletoe, friend and foe: synthesizing ecosystem implications of mistletoe infection. Environ Res Lett 12: 115012 [Google Scholar]
- Groom PK, Lamont B (2015) Plant Life of Southwestern Australia: Adaptations for Survival. De Gruyter, Berlin, Germany [Google Scholar]
- Gutjahr C, Paszkowski U (2009) Weights in the balance: jamonic acid and salicylic acid signaling in root-biotroph interactions. Mol Plant Mol Interact 22: 763–772 [DOI] [PubMed] [Google Scholar]
- Habimana S, Nduwumuremyi A, Chinama R (2014) Management of orobanche in field crops - a review. J Soil Sci Plant Nutr 14: 43–62 [Google Scholar]
- Hibberd JM, Quick WP, Press MC, Scholes JD (1998) Can source–sink relations explain responses of tobacco to infection by the root holoparasitic angiosperm Orobanche cernua? Plant Cell Environ 21: 333–340 [Google Scholar]
- Holm LG, Phucknett DL, Pancho JV, Herberger JP (1997) The World’s Worst Weeds: Distribution and Biology. University Press of Hawaii, Honolulu, HI [Google Scholar]
- Ishida HK, Wakatake T, Yoshida S, Takebayashi Y, Kasahara H, Wafula E, dePamphilis CW, Namba S, Shirasu K (2016) Local auxin biosynthesis mediated bya YUCCA flavin monooxyenase regulates haustorium development in the parasitic plant Phtheirospermum japonicum. Plant Cell 28: 1795–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke WD, Hilpert A (1997) Sink-stimulated photosynthesis and sink-dependent increase in nitrate uptake: nitrogen and carbon relations of the parasitic association Cuscuta reflexa-Ricinus communis. Plant Cell Environ 20: 47–56 [Google Scholar]
- Joel DM, Hershenhorn J, Eizenberg H, Aly R (2007) Biology and management of weedy root parasites. Hort Rev 33: 267–347 [Google Scholar]
- Jokinen JI, Irving LJ (2019) Effects of light level and nitrogen supply on the red clover–Orobanche minor host–parasite interaction. Plants 8: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin Arroyo MT, Zedler PH, Fox MD (1995) Ecology and Biogeography of Mediterranean Ecosystems in Chile, California, and Australia. Springer, Berlin, Germany [Google Scholar]
- Kebreab E, Murdoch AJ (1999) A quantitative model for los of primary dormancy and induction of secondary dormancy in imbibed seeds of Orobanche spp. J Exp Bot 50: 211–219 [Google Scholar]
- Krause K (2015) Grand-scale theft: Kleptoplasty in parasitic plants? Trends Plant Sci 20: 4. [DOI] [PubMed] [Google Scholar]
- Lechat MM, Brun G, Montiel G, Véronési C, Simier P, Thoiron S, Pouvreau J-B, Delavault P (2015) Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in the obligate root parastitic plant, Phelipanche ramosa L. Pomel. J Exp Bot 66: 3129–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat MM, Pouvreau JB, Péron T, Gauthier M,, Montiel G, Véronési C, Todoroki Y, Le Bizec B, Monteau F, Macherel F, et al. (2012) PrCYP707A1, and ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J Exp Bot 63: 5311–5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-M, Jin Z-X, Hagedorn F, Li M-H (2014) Short-term parasite-infection alters already the biomass, activity and functional diversity of soil microbial communities. Sci Rep 4: 6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Matusova R, Cardoso C, Jamil M, Charnikhova T, Kohlen W, Ruyter-Spira C, Verstappen F, Bouwmeester H (2008) Strigolactones: ecological significance and use as a target for parasitic plant control. Pest Manag Sci 64: 471–477 [DOI] [PubMed] [Google Scholar]
- Ljung K (2013) Auxin metabolism and homeostasis during plant development. Development 140: 943–950 [DOI] [PubMed] [Google Scholar]
- Mabrouk Y, Zourgui L, Sifi B, Delavault P, Simier P, Belhadj O (2006) Some compatible Rhizobium leguminosarum strains in peas decrease infections when parasitized by Orobanche crenata. Weed Res 47: 44–53 [Google Scholar]
- Martinčová M, Kaštier P, Krasylenko YA, Gajadoš P, Čertík M, Matusikova I, Blehová A (2019) Species-specific differences in architecture and chemical composition of dodder seeds. Flora 256: 61–68 [Google Scholar]
- Matusova R, van Mourik T, Bouwmeester HJ (2004) Changes in the sensitivity of parasitic weed seeds to germination stimulants. Seed Sci Res 14: 335–344 [Google Scholar]
- Mauromicale G, Lo Monaco A, Longo AMG, Restuccia A (2005) Soil solarization, a non-chemical method to control branched broomrape (Orobanche ramosa) and improve the yield of greenhouse. Weed Sci 53: 877–883 [Google Scholar]
- McAdam SAM, Eléouët MP, Best M, Brodribb TJ, Murphy MC, Cook SD, Dalmais M, Dimitriou T, Gélinas-Marion A, Gill WM, et al. (2017) Linking auxin with photosynthetic rate via leaf venation. Plant Physiol 175: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal JR, Kuehl JV, Boore JL, de Pamphilis CW (2007) Complete plastid genome sequences suggest strong selection for retention of photosynthetic genes in the parasitc plant genus Cuscuta. BMC Plant Biol 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado A, Zamora R (2017) Parasites structuring ecological communities: the mistletoe footprint in Mediterranean pine forests. Funct Ecol 31: 2167–2176 [Google Scholar]
- Mohamed KI, Papes M, Williams R, Benz BW, Peterson AT (2006) Global invasive potential of 10 parasitic witchweeds and related Orobanchaceae. Amibio 35: 281–288 [DOI] [PubMed] [Google Scholar]
- Molinero-Ruiz L, García-Ruiz R, Melero-Vara JM, Domínguez J (2009) Orobanche cumana race F: performance of resistant sunflower hybrids and aggressiveness of populations of the parasitic weed. Weed Res 49: 469–478 [Google Scholar]
- Montès N, Ballini C, Bonin G, Faures J (2004) A comparative study of aboveground biomass of three Mediterranean species in a post-fire succession. Acta Oecol 25: 1–6 [Google Scholar]
- Moral J, Lozano-Baena MD, Rubiales D (2015) Temperature and water stress during conditioning and incubation phase affecting Orobanche crenata seed germination and radicle growth. Front Plant Sci 6: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LJ, Press MC (1995) Introduction to parasitic plants. InPress MC, Graves JD, eds, Parasitic Plants. Chapman and Hall, London, pp 1–13 [Google Scholar]
- Neto OC, Leal IR, Santos JC, Lopes AV (2017) A holoparasitic plant severely reduces the vegetative and reproductive performance of its host plant in the Caatinga, a Brazilian seasonally dry forest. Acta Bot Brasilica 31: 147–152 [Google Scholar]
- Nickrent DL (2020) Parasitic plant genera and species. Parasitic Plant Connection. http://www.parasiticplants.siu.edu/ (accessed April 27, 2020).
- Parker C (2009) Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag Sci 65: 453–459 [DOI] [PubMed] [Google Scholar]
- Parker C, Riches CR (1993) Parasitic Weeds of the World: Biology and Control. CAB International, Walingrod
- Pennings SC, Callaway RM (2002) Parasitic plants: parallels and contrasts with herbivores. Oecologia 131: 479–489 [DOI] [PubMed] [Google Scholar]
- Pennings SC, Simpson JC (2008) Like herbivores, parasitic plants are limited by host nitrogen content. Plant Ecol 196: 245–250 [Google Scholar]
- Pielach A, Leroux O, Domozych DS, Knox P, Popper ZA (2014) Arabinogalactan protein-rich cell walls, paramural deposits and ergastic globules define the hyaline bodies of rhinanthoid Orobanchaceae haustoria. Ann Bot 114: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakhine D, Ziadna H, Joel DM (2009) Is seed conditioning essential for Orobanche germination? Pest Manag Sci 65: 492–496 [DOI] [PubMed] [Google Scholar]
- Pointurier O, Gibot-Leclerc S, Le Corre V, Reibel C, Strbik F, Colbach N (2019) Intraspecific seasonal variation of dormancy and mortality of Phelipanche armosa seeds. Weed Res 59: 407–418 [Google Scholar]
- Press MC, Phoenix GK (2005) Impacts of parasitic plants on natural communities. New Phytol 166: 737–751 [DOI] [PubMed] [Google Scholar]
- Press MC, Scholes JD, Qatling JR (1999) Parasitic plants: physiological and ecological interactions with their hosts. InPress MC, Scholes JD, Barker MG, eds. Physiological Plant Ecology. Blackwell Scientific Ltd, Oxford, pp 175–197 [Google Scholar]
- Pujadas-Salvà A, Velasco L (2000) Comparative studies on Orobanche cernua L. and O. cumana Wallr. (Orobanchaceae) in the Iberian Peninsula. Bot J Linean Soc 134: 513–527 [Google Scholar]
- Quested H (2008). Parasitic plants - impacts on nutrient cycling. Plant Soil 311: 269–272 [Google Scholar]
- Roitsch T, Ehneß R (2000) Regulation of source/sink relations by cytokinins. Plant Growth Regul 32: 359–367 [Google Scholar]
- Roquet C, Coissac E, Cruaud C, Boleda M, Boyer F, Alberti A, Gielly L, Taberlet P, Thuiller W, Van Es J, et al. (2016) Understanding the evolution of holoparasitic plants: the complete genome of the holoparasites Cytinus hypocistis (Cytinaceae). Ann Bot 118: 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J, Sonié L (1992) Germination and population dynamics of Cistus species in relation to fire. J Appl Ecol 29: 647–655 [Google Scholar]
- Rubiales D, Fernández-Aparicio M, Wegmann K, Joel DM (2009) Revisiting strategies for reducing the seedbank of Orobanche and Phelipanche spp. Weed Res 49: 23–33 [Google Scholar]
- Rundel PW (2019) A neogene heritage: conifer distributions and endemism in Mediterranean-climate ecosystems. Front Ecol Evol 7: 364 [Google Scholar]
- Runyon JB, Mescher MC, De Moraes CM (2006) Volatile chemical cues guide host location and host selection by parasitic plants. Science 313: 1964–1967 [DOI] [PubMed] [Google Scholar]
- Runyon JB, Tooker JF, Mescher MC, De Moraes CM (2009) Parasitic plants in agriculture: chemical ecology of germination and host-plant location as targets for sustainable control: a review. InLichtfouse E, ed, Organic Farming, Pest Control and Remediation of Soil Pollutants, Sustainable Agriculture Reviews. Springer Science+Business Media B.V., Berlin, Germany, pp 123–136 [Google Scholar]
- Ruyter-Spira C, Al-Babili A, van der Krol A, Bouwmeester H (2013) The biology of strigolactones. Trends Plant Sci 18: 24. [DOI] [PubMed] [Google Scholar]
- Sauerborn J (1991) Parasitic Flowering Plants: Ecology and Management. Verlag Josef Margraf, Weikershiem, Germany [Google Scholar]
- Schaller GE, Bishopp A, Kieber JJ (2015) The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27: 44–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes JD, Press MC (2008) Striga infestation of cereal crops – an unsolved problem in resource limited agriculture. Curr Opin Plant Biol 11: 180–186 [DOI] [PubMed] [Google Scholar]
- Schneeweiss GM (2007) Correlated evolution of life history and host range in the nonphotosynthetic parasitic flowering plants Orobanche and Phelipanche (Orobanchaceae). J Evol Biol 20: 471–478 [DOI] [PubMed] [Google Scholar]
- Seel WE, Press MC (1996) Effects of repeated parasitism by Rhianthus minor on the growth and photosynthesis of the perennial grass, Poa alpine. New Phytol 134: 495–502 [Google Scholar]
- Seiler GJ (2019) Genetic resources of the sunflower crop wild relatives for resistance to sunflower broomrape. Helia 42: 127–143 [Google Scholar]
- Shabana YM, Müller-Stöver D, Sauerborn J (2003) Granular Pesta formulation of Fusarium oxysporum f.sp. orthoceras for biological control of sunflower broomrape: efficacy and shelf-life. Biol Control 26: 189–201 [Google Scholar]
- Shen H, Xu SJ, Hong L, Wang ZM, Ye WH (2013) Growth but not photosynthesis response of a host plant to infection by a holoparasitic plant depends on nitrogen supply. PLoS One 8: e75555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirhindi G, Mira MA, Sharma P, Gill SS, Kaur H, Mushtaq R (2015) Modulatory role of jasmonic acid on photosynthetic pigments, antioxidants and stress markers of Glycine max L. under nickel stress. Physiol Mol Biol Plants 21: 559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuras D, Psaltopoulos D (2012) A broad overview of the main problems derived from climate change that will affect agricultural production in the Mediterranean area. InBuilding Resilience for Adaptation to Climate Change in the Agriculture Sector. Food and Agriculture Organization of the United Nations, Rome, Italy [Google Scholar]
- Slavov S, van Onckelen H, Batchvarova R, Atanassov A, Prinsen E (2004) IAA production during germination of Orobanche spp. seeds. J Plant Physiol 161: 847–853 [DOI] [PubMed] [Google Scholar]
- Torres-Vera R, García JM, Pozo MJ, López-Ráez JA (2016) Expression of molecular markers associated to defense signaling pathways and strigolactone biosynthesis during the early interaction tomato-Phelipanche ramosa. Physiol Mol Plant Phatol 94: 100–107 [Google Scholar]
- Turnau K, Jędrzejczyk R, Domka A, ANielska T, Piwowarczyk R (2018) Expansion of a holoparasitic plant, Orobanche lutea (Orobanchaceae), in post-industrial areas – a possible Zn effect. Sci Total Environ 639: 714–724 [DOI] [PubMed] [Google Scholar]
- Twyford AD (2018) Parasitic plants. Curr Biol 28: PR857–R859 [DOI] [PubMed] [Google Scholar]
- de Vega C, Arista M, Ortiz PL, Herrera CM, Talavera S (2009) The ant-pollination system of Cytinus hypocistis (Cytinaceae), a Mediterranean root holoparasites. Ann Bot 103: 1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega C, Arista M, Ortiz PL, Talavera S (2010) Anatomical relations among endophytic holoparasitic angiosperms, autothrophic host plants and mycorrhizal fungi: a novel tripartite interaction. Am J Bot 97: 730–737 [DOI] [PubMed] [Google Scholar]
- de Vega C, Arista M, Ortiz PL, Herrera CM, Talavera S (2011) Endozoochory by beetles: a novel seed dispersal mechanism. Ann Bot 107: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega C, Berjano E, Arista M, Ortiz PL, Talavera S, Stuessy TF (2008) Genetic races associated with the genera and sections of host species in the holoparasitic plant Cytinus (Cytinaceae) in the Western Mediterranean basin. New Phytol 178: 875–887 [DOI] [PubMed] [Google Scholar]
- Velthof G, Barot S, Bloem J, Butterbach-Bahi K, de Vries W, Kros H, Lavelle P, Olesen JE, Oenema O (2011) Nitrogen as a threat to European soil quality. InSutton MA, Howard CM, Willem J, Billen G, Bleeker A, Grennfelt P, van Grinsven H, Grizzetti B, eds, The European Nitrogen Assessment. Cambridge University Press, Cambridge [Google Scholar]
- Watling JR, Press MC (2001) Impacts of infection by parasitic angiosperms on host photosynthesis. Plant Biol 3: 244–250 [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinins. Proc Natl Acad Sci 98: 10487–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW (2010) The evolution of parasitism in plants. Trends Plant Sci 15: 4. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhang M, Dong S, Ma Y (2017) Conditioning duration and agents involved in broomrape seeds responding to germination stimulants. Plant Growth Regul 81: 221–230 [Google Scholar]
- Yoder JI, Scholes JD (2010) Host plant resistance to parasitic weeds; recent progress and bottlenecks. Curr Opin Plant Biol 13: 478–484 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Xu X, Gong Z, Tang Y, Wu M, Yan F, Zhang X, Zhang Q, Yang F, Hu X, et al. (2019) Auxin response factor 6A regulates photosynthesis, sugar accumulation, and fruit development in tomato. Hort Res 6: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H, Li J, Song J, Hettenhausen C, Schuman MC, Sun G, Zhang C, Li J, Song D, Wu J (2018) Aphid (Myzus persicae) feeding on the parasitic plant dodder (Cuscuta austalis) activates defense responses in both the parasite and soybean host. New Phytol 218: 1586–1596 [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Mwakaboko AS, Reizelman A, Anilkumar G, Sethumadhavan D (2008) Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Plant Manag Sci 65: 478–491 [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Pospíšil T, Zeljković SC (2016) Strigolactones: new plant hormones in action. Planta 243: 1311–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]