Abstract
The R2R3 transcription factor MdMYB88 has previously been reported to function in biotic and abiotic stress responses. Here, we identify BRI1 ETHYLMETHANE SULFONATE SUPRESSOR1 (MdBES1), a vital component of brassinosteroid (BR) signaling in apple (Malus × domestica) that directly binds to the MdMYB88 promoter, regulating the expression of MdMYB88 in a dynamic and multifaceted mode. MdBES1 positively regulated expression of MdMYB88 under cold stress and pathogen attack, but negatively regulated its expression under control and drought conditions. Consistently, MdBES1 was a positive regulator for cold tolerance and disease resistance in apple, but a negative regulator for drought tolerance. In addition, MdMYB88 participated in BR biosynthesis by directly regulating the BR biosynthetic genes DE ETIOLATED 2 (MdDET2), DWARF 4 (MdDWF4), and BRASSINOSTEROID 6 OXIDASE 2 (MdBR6OX2). Applying exogenous BR partially rescued the erect leaf and dwarf phenotypes, as well as defects in stress tolerance in MdMYB88/124 RNAi plants. Moreover, knockdown of MdMYB88 in MdBES1 overexpression (OE) plants decreased resistance to a pathogen and C-REPEAT BINDING FACTOR1 expression, whereas overexpressing MdMYB88 in MdBES1 OE plants increased expression of SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 3 (MdSPL3) and BR biosynthetic genes, suggesting that MdMYB88 contributes to MdBES1 function during BR biosynthesis and the stress response. Taken together, our results reveal multifaceted regulation of MdBES1 on MdMYB88 in BR biosynthesis and stress tolerance.
A vital component of brassinosteroid (BR) signaling in apple directly regulates the expression of transcription factor gene in a dynamic and multifaceted mode, thereby influencing BR biosynthesis and stress tolerance.
Introduction
Many stressors, such as extreme temperature, drought, and plant diseases, threaten plant growth and development, and cause huge economic losses. Brassinosteroids (BRs), a class of plant-specific steroid phytohormones, play multifunctional roles in plant growth and development, including cell elongation, seed germination, vascular differentiation, senescence, and the biotic/abiotic stress response (Nolan et al., 2020). The BR signal transduction pathway has been well established in Arabidopsis (Arabidopsis thaliana; Guo et al., 2013; Nolan et al., 2017). BRs are recognized and bind to the receptor kinase BRASSINOSTERIOD INSENSITIVE1 (BRI1), which is a leucine-rich repeat protein located in the plasma membrane (He et al., 2000). Then, through a series of intracellular phosphorylation cascades, the downstream intermediates are elaborately regulated, including the negative component BRASSINOSTEROID INSENSITIVE2 (BIN2), which is dephosphorylated to activate the transcription factors (TFs) BRI1 ETHYLMETHANE SULFONATE SUPRESSOR1 (BES1) and BRASSINAZOLE-RESISITANT1 (BZR1). Subsequently, activation of BES1/BZR1 further affects the expression of numerous BR response genes (Yin et al., 2002; Guo et al., 2013).
Mutants of BR biosynthesis or perception genes or plants treated with the BR biosynthesis inhibitor brassinazole (BRZ) show stunted growth and shorter hypocotyl elongation (Clouse, 1996; Li and Chory, 1999; Asami et al., 2000). In contrast, the gain-of-function bes1-D promotes stem elongation and resistance to BRZ (a BR biosynthesis inhibitor; Yin et al., 2002). According to previous studies, BR is also involved in the regulation of rice (Oryza sativa) leaf angles, which is associated with abnormal development of the lamina joint (Sun et al., 2015). At the cellular level, BR promotes adaxial side cells to elongate, causing larger leaf angles toward the abaxial side (Hong et al., 2004). Many of the rice BR response components have been characterized, such as REDUCED LEAF ANGLE1 (OsRLA1), OsWRKY53, OsBRI1, OsBZR1, and LEAF AND TILLER ANGLE INCREASED CONTROLLER (OsLIC), which play prominent roles in regulating the development of plant leaf angles (Bai et al., 2007; Wang et al., 2008; Jang et al., 2017; Qiao et al., 2017; Tian et al., 2017; Xiao et al., 2017). Plants with lower endogenous OsBZR1 expression reduce the degree of leaf bend away from the vertical axis, and similar plant architecture appears in oswrky53 and osrla1 mutants (Bai et al., 2007).
Beyond functions in plant growth and development, BRs and their response genes have been proposed to mediate adaptation to adverse environments (Planas-Riverola et al., 2019). Although the application of exogenous BR improves plant tolerance to a wide spectrum of stressors (Zhou et al., 2014), the mechanism of how BR and its response genes regulate the stress response is complex and not fully understood. It has been demonstrated that BR signaling-defective gene mutants exhibit susceptibility to freezing with or without cold acclimation (Eremina et al., 2016). Overexpressing BIN2 reduces plant freezing tolerance, while the bin2-3 bil1 bil2 triple mutant leads to increased freezing tolerance. In contrast to BIN2, BES1 and BZR1 play positive roles in the cold response (Li et al., 2017). Notably, there are numerous reports about BR action in drought stress, but the relationship between BR and the drought response is still uncertain. Previous studies determined that treatment with exogenous BR strengthens drought tolerance in Arabidopsis, wheat (Triticum aestivum), and tomato (Solanum lycopersicum). However, BRL3, a member of the vascular BR receptor family, contributes to plant drought tolerance without impairing plant growth (Fabregas et al., 2018). These observations indicate that BR plays a positive role in coping with drought (Sairam, 1994; Kagale et al., 2007; Zhou et al., 2014). However, recent studies have reported that some BR signaling components inhibit the drought response. BIN2, a repressor of BR signaling, is activated during abscisic acid treatment or a drought condition. In addition, BIN2 phosphorylates and stabilizes TINY to increase plant drought tolerance (Youn and Kim, 2015; Wang et al., 2018; Xie et al., 2019). Different from its role in cold conditions, BES1 plays a negative role in the drought response and bes1-D plants show significantly lower survival rates than the control under a drought condition (Nolan et al., 2017). Specifically, BES1 interacts with WRKY46/54/70 to reinforce the suppressed expression of drought-inducible genes (Chen et al., 2017). The antagonistic relationship between BES1 and RD26 balances plant growth and the drought response by affecting reciprocal transcriptional activity (Ye et al., 2017).
The MYB family TFs are characterized by a highly conserved MYB domain and are divided into different types according to the number of adjacent MYB repeats (R; Ogata et al., 1996). A tremendous number of MYB proteins participate in various biological processes, including secondary metabolism, growth and development, stress, and the defense response (Abe et al., 2003; Muller et al., 2006; Stracke et al., 2007; Zhang et al., 2019). Ectopic expression of MdMYB30 in Arabidopsis enhances plant resistance against the bacterial strain Pst DC3000 by contributing to surface wax biosynthesis (Zhang et al., 2019). MYB2 and MYB74 are induced under drought conditions (Abe et al., 2003; Denekamp and Smeekens, 2003). AtMYB62 is a key regulator required in the response to phosphate starvation (Devaiah et al., 2009). Moreover, MdMYB88 and MdMYB124 act as atypical R2R3-MYB TFs, which play crucial roles in plant freezing and the drought response. MdMYB88 and MdMYB124 confer cold tolerance by regulating the C-repeat binding factor (CBF)-dependent and CBF-independent pathways, and confer drought tolerance by modulating root development and deposition of the secondary cell wall (Geng et al., 2018; Xie et al., 2018). Several studies have explored how BES1 and MdMYB88 function in response to stress, but the underlying relationship between MdBES1 and MdMYB88 remains unclear.
In this study, MdBES1 acted as an upstream regulator of MdMYB88 and regulated MdMYB88 during growth and the abiotic and biotic stress responses. Overexpression (OE) of MdBES1 improved apple tolerance to cold stress and fungal infection, but reduced tolerance to drought stress, whereas the MdBES1 RNAi plants displayed the opposite phenotypes to those of MdBES1 OE plants. The diverse MdBES1 functions were correlated with its diverse regulation on MdMYB88, which was a positive regulator of BR biosynthesis. Furthermore, a genetic analysis showed that MdBES1 played roles in stress tolerance and BR biosynthesis via MdMYB88.
Results
MdBES1 directly targets the MdMYB88 promoter
Our previous studies have shown that MdMYB88 and its paralog gene, MdMYB124, play vital roles in the response to biotic and abiotic stress (Geng et al., 2018, 2020; Xie et al., 2018). To further explore the mechanism of MdMYB88 during growth and stress, yeast one-hybrid (Y1H) screening assay was used to identify the potential upstream TFs of MdMYB88. Among the several TFs identified in the Y1H screening assay, one was MdBES1, a homolog of BES1 in Arabidopsis, which shares 52.7% identity with BES1 (Supplemental Supplemental Figure S1). As shown in Figure 1A and Supplemental Figure S2, the MdMYB88 promoter contains several E-box (CANNTG) and BRRE (CGTGTG) motifs, which are putative binding motifs of both BES1 and BZR1 (Sun et al., 2010). In a point-to-point Y1H assay, yeast cells containing only the bait vector harboring MdMYB88 P1 (containing five E-boxes), P2 (containing one E-box), and P3 (containing two BRRE boxes) promoter regions grew normally on selective media when co-transformed with MdBES1-AD, but those transformed with empty-AD or the MdMYB88 P2 promoter region did not survive on selective media (Figure 1, B and C). A chromatin immunoprecipitation (ChIP) experiment was carried out to investigate whether MdMYB88 is a direct target of MdBES1 in planta. ChIP was conducted with an anti-BES1 antibody and fragment a, which does not contain BES1 binding elements, was used as a negative control. As MdBES1 was not enriched in MdMYB88 P2, and MdMYB88 P3 contains two BRRE boxes, we further divided MdMYB88 P3 into fragments b and c (Figure 1A). Quantitative real-time PCR (qPCR) with ChIP products showed that MdBES1 was significantly enriched at fragment P1, b, and c (Figure 1D). An electrophoretic mobility shift assay (EMSA) revealed that the MBP–MdBES1 fusion protein directly bound to MdMYB88 P3 fragment (Figure 1E). However, MdBES1 failed to bind the MdMYB124 promoter (Supplemental Figure S3). Taken together, these results indicate that MdBES1 is a direct upstream factor of MdMYB88.
Figure 1.
MdBES1 binds to the MdMYB88 promoter in vivo and in vitro. A, Schematic diagram of the MdMYB88 promoter. Solid triangles indicate sites containing the E-box, and the hollow triangles indicate the sites containing BRRE in the MdMYB88 promoter. The MdMYB88 promoter contains different fragments: a (−2,483 to −2,391 bp), b (−1,074 to −907 bp), c (−797 to −632 bp), P1 (−1,634 to −1,401 bp), P2 (−1,398 to −1,227 bp), and P3 (−1,077 to −632 bp). B and C, The Y1H assay shows the binding of MdBES1 to the MdMYB88 promoter. The fragment proMdMYB88 466 (−1,398 to −933 bp) was used for Y1H screening. Yeast cells were grown on SD-Leu plates with 300 ng/mL AbA. D, ChIP-qPCR analysis. Chromatin from GL-3 was immunoprecipitated with or without anti-MdBES1 antibody. Fragment a was used as a negative control. Data are means±sd. Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (***P < 0.001). E, EMSA analysis. Biotin-labeled fragment of the MdMYB88 promoter (P3) that contains BRRE (CGTGTG) motifs was incubated with the MBP or MBP-MdBES1 protein. Competitor fragments were added to examine binding specificity.
To explore the subcellular localization of MdBES1, we transiently expressed MdBES1 fused with GFP in tobacco leaves driven by the cauliflower mosaic virus 35S promoter. Consistent with previous reports, the nuclear localization signal of MdBES1 was detected (Figure 2A;Supplemental Figure S4). Tissue-specific expression pattern showed that MdBES1 was highly expressed in the stem and flowers of apple (Figure 2B). Furthermore, we observed that cold and a pathogen attack induced the expression of MdBES1 but drought repressed (Supplemental Figure S5).
Figure 2.
MdBES1 expression pattern and protein localization. A, MdBES1 is localized in the nucleus of tobacco (N. benthamiana) leaf epidermal cells. GFP, green fluorescent protein. Bars = 50 μm. B, Tissue-specific expression pattern of MdBES1 in GL-3. Data are means ± sd (n = 3). C–E, MdBES1 protein level in the GL-3 under drought (C), cold (D), and disease (E) conditions by Western blot analysis. Total proteins extracted from GL-3 after various treatments were separated on SDS–PAGE gels supplemented with Phos-tag, followed by immunoblotting with anti-MdBES1 antibodies.
BZR1/BES1 can be phosphorylated and deactivated or dephosphorylated and activated to promote expression of target genes depending on the BR levels (He et al., 2019). We then analyzed the dephosphorylated and phosphorylated MdBES1 levels under various stress conditions. We found that drought conditions dramatically decreased both the phosphorylated and dephosphorylated MdBES1. Only a small amount of dephosphorylated MdBES1 was detected after drought, which lead to a remarkably reduced ratio of dephosphorylated to phosphorylated MdBES1 in response to drought stress (Figure 2C). Cold stress slightly increased both the phosphorylated and dephosphorylated MdBES1 levels after 5-h treatment but marginally decreased both forms of MdBES1 after 12- and 24-h treatment. However, under cold stress, the ratio of dephosphorylated to phosphorylated MdBES1 was increased during the whole treatment period (Figure 2D). After pathogen attack for up to 6 h, the phosphorylated MdBES1 slightly decreased, whereas the dephosphorylated MdBES1 increased, resulting in an increased ratio of dephosphorylated to phosphorylated MdBES1 (Figure 2E). These results indicate that MdBES1 may be less active under drought stress than under cold and pathogen attack conditions.
MdBES1 plays different roles in biotic and abiotic stress tolerance and regulates MdMYB88 expression
To further investigate the underlying functions of MdBES1 in apple, we generated stable transgenic plants: MdBES1 overexpressing plants (MdBES1 OE) and MdBES1 RNA interfering (RNAi) plants (Supplemental Figure S6). Under short-term drought conditions, MdBES1 RNAi plants showed less ion leakage and higher survival rates than GL-3 (the genetic background for all transgenic apple plants), whereas MdBES1 OE plants displayed the opposite phenotypes, indicating that MdBES1 negatively regulates plant drought tolerance (Figure 3, A–C). In addition, the water loss assay of detached leaves also revealed that MdBES1 OE and RNAi plants displayed stronger and weaker water loss, respectively (Figure 3, D and E).
Figure 3.
MdBES1 decreases drought tolerance in apple. A Whole-plant drought tolerance of MdBES1 RNAi and MdBES1 OE plants. Three-month-old plants were exposed to drought stress until the VWC decreased to 0. During the treatment, the GL-3 and MdBES1 OE plants were supplemented with water to maintain the same VWC with MdBES1 RNAi plants. After 2 d of drought treatment, the status of intact plants was photographed, and plants were recovered under normal conditions for 7 d and survival rates were then calculated. Bars = 10 cm. B, Survival rates of MdBES1 RNAi and MdBES1 OE plants in (A). C, Drought tolerance of MdBES1 RNAi and MdBES1 OE plants determined by leaf electrolyte leakage assays when relative soil water content (RSWC) was 15%. D and E, Water loss assay of MdBES1 RNAi (D) and MdBES1 OE (E) plants. Leaves of 3-month-old MdBES1 transgenic plants were detached and dehydrated for the designated time. Data are means ± sd [n = 6 in (A and B); n = 8 in (C); n = 10 in (D and E)]. Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (*P < 0.05, **P < 0.01, ***P < 000.1).
We also performed a long-term drought stress treatment with the GL-3 and MdBES1 transgenic plants. After drought stress for 3 months, both the MdBES1 OE and RNAi plants were shorter than the nontransgenic GL-3 plants (Figure 4, A and B). However, the MdBES1 RNAi plants maintained higher water content than GL-3, while MdBES1 OE had lower water content than GL-3 (Figure 4C). Root system plays an important role in plant drought defense. Under control conditions, root dry weight of MdBES1 RNAi plants was significantly lower than that of GL-3, whereas it was slightly reduced in MdBES1 OE plants as compared with GL-3. After long-term drought stress, both MdBES1 RNAi and OE plants had lower root dry weight than GL-3 plants. However, the difference between MdBES1 RNAi plants and GL-3 was reduced (Figure 4D;Supplemental Figure S7). As results, after prolonged drought stress, the MdBES1 RNAi plants had higher root hydraulic conductivity and root-to-shoot ratio than the GL-3 plants, whereas the MdBES1 OE plants had lower root hydraulic conductivity and root-to-shoot ratio (Figure 4, E and F). These results indicate that the MdBES1 RNAi plants performed better than the MdBES1 OE plants under long-term drought stress conditions.
Figure 4.
Phenotypes of GL-3 and MdBES1 transgenic plants under long-term drought stress. Three-month-old plants grown in a greenhouse were treated with drought stress for up to 3 months. Pots were weighed every day to maintain a RSWC of 35%–45%. A, Plant shoot morphology. Bars = 10 cm. B, Plant height. Bars = 10 cm. C, Relative water content. D, Root morphology. E, Root hydraulic conductivity; (F) root/shoot ratio. Data in B, C, E, and F are means ± sd (n = 20). Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (*P < 0.05, **P < 0.01, ***P < 000.1).
In contrast, by analyzing ion leakage and the survival rate under freezing conditions, we found that MdBES1 RNAi plants decreased cold tolerance and MdBES1 OE plants significantly increased cold tolerance after exposure to a cold environment, regardless of acclimation (Figure 5). These results indicate that MdBES1 acts as a negative and positive regulator under drought and cold stress in apple, respectively.
Figure 5.
MdBES1 increases cold tolerance in apple. A, Cold tolerance of MdBES1 RNAi and MdBES1 OE plants determined by leaf electrolyte leakage assays before or after cold acclimation. B, Whole-plant freezing tolerance of MdBES1 RNAi and MdBES1 OE before or after cold acclimation. Bars = 10 cm. C, Survival rates of MdBES1 RNAi and MdBES1 OE plants in (B). Data are means ± sd [n = 7 in (A), n = 6 in (B and C)]. Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (*P < 0.05, **P < 0.01, ***P < 000.1).
Because MdBES1 directly binds to the MdMYB88 promoter, we examined the MdMYB88 expression level in MdBES1 transgenic plants. Under normal conditions, MdBES1 negatively regulated MdMYB88 expression; however, the situation changed when plants encountered different stressors (Figure 6A). Under drought conditions, MdMYB88 expression was still repressed by MdBES1, whereas its expression increased by MdBES1 under cold conditions (Figure 6, A and C). Previous reports showed that SPL3 positively regulated drought tolerance, and MdSPL3 level was also found reduced in the RNA-seq data of MdMYB88/124 RNAi plants under drought (Wang et al., 2015). Here, we found expression of MdSPL3 was elevated in MdBES1 RNAi but repressed in MdBES1 OE plants under the drought treatment (Figure 6B). We previously found that MdMYB88 positively regulates the expression of CIRCADIAN CLOCK ASSOCIATED 1 (MdCCA1), COLD SHOCK DOMAIN PROTEIN 3 (MdCSP3), and MdCBF1 under cold conditions (Xie et al., 2018). Here, we determined that the expression of these three genes was upregulated in MdBES1 OE plants but downregulated in MdBES1 RNAi plants under 4°C treatment for 8 h (Figure 6, D–F). Taken together, we deduced that the regulatory mode of MdBES1 on MdMYB88 varies, based on the changing environments.
Figure 6.
Transcription levels of stress-responsive genes in MdBES1 transgenic apple plants under drought and cold conditions. A and B, The expression of MdMYB88 (A) and MdSPL3 (B) in MdBES1 transgenic plants under drought conditions. C–F, The expression of MdMYB88 (C) and MdCBF1 (D), MdCCA1 (E), and MdCSP3 (F) in MdBES1 transgenic plants under cold conditions. Data are means ± sd (n = 3). Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (*P < 0.05, **P < 0.01, ***P < 000.1).
As reported in our previous study, MdMYB88 enhances plant resistance to Alternaria alternata f.sp mali and Valsa mali by mediating the contents of flavonoids and phenylpropanoids (Geng et al., 2020). Hence, we examined the response of MdBES1 to pathogen attacks by inoculating transgenic plant leaves with V. mali for 7 d. As results, MdBES1 RNAi plants showed greater lesion area than GL-3 after the pathogen infection, while MdBES1 OE plants showed milder disease symptoms (Figure 7, A and B). We also inoculated plant stems with V. mali for 9 d and observed similar results (Figure 7, C and D). The RT-qPCR results revealed that MdMYB88 expression was repressed in MdBES1 RNAi plants, compared with MdBES1 OE and GL-3 (Figure 7E). In contrast, HORISMATE MUTASE 2 (MdCM2), one of the HORISMATE MUTASE (CM) that catalyze phenylalanine biosynthesis, was induced in MdBES1 OE plants but repressed in MdBES1 RNAi plants at 5 d post-infection (dpi), compared with GL-3 (Figure 7F). As expected, more phenylalanine accumulated in MdBES1 OE plants but less in MdBES1 RNAi plants at 5 dpi (Figure 7G). These results suggest that MdBES1 positively regulates plant resistance to V. mali.
Figure 7.
MdBES1 increases the disease resistance of apple to Valsa mali. A, Disease resistance of GL-3, MdBES1 RNAi, and MdBES1 OE plants. Leaves were incubated with V. mali for 7 d. Bar = 2 cm. B, Quantification of data shown in (A). C, Disease resistance of GL-3, MdBES1 RNAi, and MdBES1 OE plants. Stems were incubated with V. mali for 9 d. Bar = 2 cm. D, Quantification of data shown in (C). E and F, The expression levels of MdMYB88 (E) and HORISMATE MUTASE 2 (MdCM2) (F) in MdBES1 transgenic plants at 5 dpi. G, The phenylalanine content in MdBES1 transgenic plants at 5 dpi. Data are means ± sd [n = 6 in (A – D), n = 3 in (E, F, and G)]. Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (*P < 0.05, **P < 0.01, ***P < 000.1).
MdMYB88 transgenic plants display BR-related phenotypes
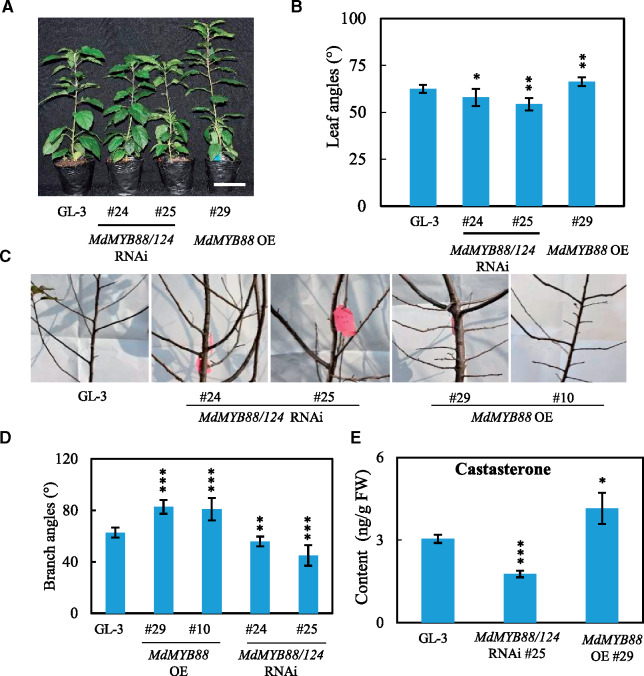
Numerous studies have established that BR plays an essential role in shaping plant architecture, such as stem elongation and leaf angles (Bai et al., 2007; Tian et al., 2017). The phenotype of MdBES1 OE plants displayed greater plant height and larger leaf angles than GL-3, which resembled many of the typical BR-response mutants, such as osbzr1-D and OsBU1-OE (Tanaka et al., 2009; Qiao et al., 2017), while MdBES1 RNAi plants displayed the opposite growth phenotype (Supplemental Figure S8, A–D). These typical BR-response phenotypes prompted us to determine endogenous levels of BR in MdBES1 transgenic plants. Brassinolide (BL) content was below the detectable level in leaves, but the level of castasterone (CS), a BL precursor was detectable. MdBES1 RNAi and MdBES1 OE plants, respectively, accumulated greater and less CS than GL-3 (Supplemental Figure S8E). We also observed that MdMYB88/124 RNAi transgenic plants displayed smaller leaf angles, whereas the MdMYB88 OE plants showed larger angles (Figure 8, A and B). Additionally, the 3-year-old MdMYB88 OE plants displayed larger branch angles than GL-3, but MdMYB88/124 RNAi plants had smaller branch angles (Figure 8, C and D). The measurement of endogenous level of BR in MdMYB88 transgenic plants showed that MdMYB88 OE and MdMYB88/124 RNAi plants accumulated more and less CS, respectively, compared with GL-3 (Figure 8E). In addition, MdMYB88/124 RNAi plants contained less BR under cold and drought conditions (Supplemental Figure S9). Hence, we supposed that MdMYB88 may function as a potential component of BR biosynthesis or in the signaling pathway, thereby influencing plant architecture.
Figure 8.
MdMYB88 enlarges leaf and branch angles. A, Morphology of GL-3, MdMYB88 RNAi, and MdMYB88 OE plants. Bars = 5 cm. B, Quantification of data shown in (A). C, The branch phenotype of 3-year-old MdMYB88 transgenic plants. D, Quantification of data shown in (C). E, The endogenous content of leaf fresh weight (FW) CS in GL-3, MdMYB88 RNAi, and MdMYB88 OE plants. Data are means ± sd [n = 8 in (B), n = 9 in (D), n = 3 in (E)]. Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (*P < 0.05, **P < 0.01, ***P < 000.1).
MdMYB88 positively regulates BR biosynthesis in apple
To verify the hypothesis and further elucidate the mechanism of an altered BR level in MdMYB88 transgenic plants, we examined the transcription levels of several BR biosynthetic genes, including MdDWF4, MdBR6OX2, and MdDET2, which are homologs of DWF4, BR6OX2, and DET2 in Arabidopsis. As shown in Figure 9, A–C, the expression levels of MdDWF4, MdBR6OX2, and MdDET2 were upregulated in MdMYB88 OE plants compared with GL-3 but downregulated in MdMYB88/124 RNAi.
Figure 9.
MdMYB88 positively and directly regulates BR biosynthetic genes. A–C, The expression of BR biosynthetic genes [MdDET2 (A), MdDWF4 (B), and MdBR6OX2 (C)] in MdMYB88 transgenic plants. D–F, Schematic diagram of the MdDET2 (D), MdDWF4 (E), and MdBR6OX2 (F) promoters. Fragment b in MdDET2 promoter (D) contains AACCG cis-element, fragment b in MdDWF4 promoter (E) contains AGCCG (red arrowhead), and fragment d in MdBR6OX2 promoter (J) contains TTCCG/C. G–I, ChIP-qPCR analysis with MdDET2 (G), MdDWF4 (H), and MdBR6OX2 (I) promoters. Chromatin from GL-3 was immunoprecipitated with or without the anti-MdMYB88 antibody. Fragments a, c, and d were used as negative controls in (G), fragments c and d were used as negative controls in (H). Fragments a, b, and c were used as negative controls in (I), (J–L) EMSA analysis with MdDET2 (J), MdDWF4 (K), and MdBR6OX2 (L) probes. Biotin-labeled fragments [fragments b in (J) and (K), fragment d in (L)] were incubated with or without the MdMYB88-His protein. Competitor fragments and mutant (Mut) probes were added to examine binding specificity. Mut1 and mut2 in (L) indicate the mutations were separately made in two individual probes in the MdBR6OX2 promoter, while mut indicates the mutation was simultaneously made in the two probes. Data are means ± sd (n = 3). Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (*P < 0.05, **P < 0.01, ***P < 000.1).
The expression changes of MdDWF4, MdBR6OX2, and MdDET2 by MdMYB88 prompted us to analyze their promoter sequences. As expected, the promoter region of these three genes contained MdMYB88 binding sites (Figure 9, D, G, and J; Supplemental Figures S10–12). ChIP-qPCR analysis showed that MdMYB88 was enriched in the promoter region of these three genes (Figure 9, E, H, and K). Subsequently, the direct binding of MdMYB88 to MdDET2, MdDWF4, and MdBR6OX2 was observed by the EMSA assays (Figure 9, F, I, and L). These results indicate that MdMYB88 affects the endogenous level of BR by directly regulating BR biosynthetic genes.
Due to the negative feedback regulation of the expression of BR biosynthesis genes by BR signaling (He et al., 2005), the bioactive BR 24-epiBL treatment repressed gene expression of MdMYB88 (Supplemental Figure S13), suggesting negative feedback regulation of MdMYB88 by BL.
MdMYB88-mediated plant development and stress tolerance partially depends on BR
BR is a multidimensional phytohormone associated with plant growth, development, and stress response. To determine whether BR is responsible for differences in the developmental phenotype of MdMYB88 transgenic plants, we exogenously applied 250 nmol/L BL on the MdMYB88 transgenic plants for two months. The BL treatment completely eliminated the difference in leaf angles between MdMYB88 OE plants and GL-3, and mostly reduced the leaf angles difference between GL-3 and MdMYB88/124 RNAi plants (Figure 10, A and B). Applying BL partially rescued the dwarf phenotype of MdMYB88/124 RNAi plants (Figure 10C). These results suggest that disrupting MdMYB88 does not produce enough endogenous BR for plant growth or to maintain leaf angles.
Figure 10.
Application of BL partially rescues morphologic phenotypes and stress tolerance of MdMYB88/124 RNAi plants. A, Morphology of MdMYB88 transgenic plants under the control (upper) and BL (lower) treatment. Bars = 5 cm. B and C, Quantification of leaf angles (B) and plant height (C) of plants shown in (A). D and E, Drought (D) and cold (E) tolerance of GL-3 and MdMYB88 transgenic plants with or without BL treatment as determined by leaf electrolyte leakage assays. F, Disease symptoms of GL-3 and MdMYB88 RNAi plants with (lower panel) or without (upper panel) BL treatment under V. mali infection. Bars = 2 cm. G, Quantification of data shown in (F). Data are means ± sd [n = 12 in (A–C), n = 10 in (D and E), n = 8 in (F and G)]. Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (*P < 0.05, **P < 0.01, ***P < 000.1).
To investigate the effects of BR on the MdMYB88 stress response, we examined stress tolerance of MdMYB88 transgenic plants with or without BL pretreatment. As shown in Figure 10, D and E, BL significantly decreased ion leakage in all plants under drought and cold conditions. BL also partially rescued drought stress tolerance and completely recovered cold stress tolerance of MdMYB88/124 RNAi plants. Both MdMYB88/124 RNAi plants and GL-3 strikingly alleviated infectious symptoms after the BL pretreatment. Importantly, the significant differences in the lesion area between MdMYB88/124 RNAi plants and GL-3 were reduced (Figure 10, F and G).
Genetic analysis of MdBES1 and MdMYB88
To further understand the genetic regulation of MdMYB88 and MdBES1 in BR biosynthesis and various environmental stresses, we transiently silenced or overexpressed MdMYB88 gene in MdBES1 OE plants (Supplemental Figure S14). After treating MdBES1 OE MdMYB88/124 RNAi plants with cold (4°C) stress, we found disruption of MdMYB88 significantly decreased the expression of MdCBF1 in MdBES1 OE plants (Figure 11A). We also examined disease resistance of MdBES1 OE MdMYB88/124 RNAi plants by infecting leaves with V. mali for 3 d, and the results showed that MdBES1 OE MdMYB88/124 RNAi plants were more sensitive to V. mali than MdBES1 OE plants (Figure 11, B and C). In addition, overexpressing MdMYB88 in MdBES1 OE plants partially rescued the expression of MdSPL3 and the BR biosynthesis genes (MdDWF4 and MdDET2) in MdBES1 OE plants (Figure 11, D and E). These results further suggest that MdMYB88 acts as a downstream target of MdBES1.
Figure 11.
Genetic analysis of MdBES1 and MdMYB88. A, The expression of MdCBF1 in GL-3, MdBES1 OE, MdMYB88/124 RNAi, and MdBES1 OE MdMYB88/124 RNAi under cold stress. MdMYB88/124 was transiently knocked down in MdBES1 OE plants. B, Disease symptoms of plants under V. mali infection. Bars = 1 cm. C, Quantification of data shown in (B). D, The expression of MdSPL3 in GL-3, MdBES1 OE, MdMYB88 OE, and MdBES1 OE MdMYB88 OE under drought stress. MdMYB88 was transiently overexpressed in MdBES1 OE plants and treated with drought stress. E and F, The expression of BR biosynthetic genes [MdDET2 (E) and MdDWF4 (F)] in plants shown in (D). Data are means ± sd (n = 3). Asterisks indicate significant differences as assessed by one-way ANOVA (Tukey’s test) (***P < 0.001, **P < 0.01, *P < 0.05).
Discussion
Plants have evolved sophisticated mechanisms to cope with various adverse conditions, such as extreme temperature, water deprivation, and disease, to minimize damage. In this study, we uncovered a regulatory module of MdBES1–MdMYB88 in apple growth and stress tolerance.
Several lines of evidence indicate that MdMYB88 is a direct target of MdBES1. First, the Y1H, ChIP-qPCR, and EMSA results suggested that MdBES1 directly bound to the MdMYB88 promoter (Figure 1). Second, we carried out a genetic analysis of MdBES1 and MdMYB88, and confirmed that MdMYB88 contributed to stress tolerance and BR biosynthesis of MdBES1. Transiently knocking down MdMYB88 in MdBES1 OE plants increased lesion areas after V. mali infection compared with MdBES1 OE plants, suggesting weaker stress tolerance (Figure 11, B and C). In addition, increased expression of MdMYB88 in MdBES1 OE plants rescued expression of MdSPL3 and the BR biosynthetic genes (Figure 11, D and E).
The mechanisms of BR signaling in regulating stress are complex. Exogenous application of BR enhances tolerance to cold, drought, and pathogen attack (Zhou et al., 2014; Marcos et al., 2015), and we also obtained similar results in apple (Figures 3, 4, 5, and 7). The relationship between the BR signaling network and various stresses is more complex. OE of the BR receptor gene BRL3 increases plant drought tolerance in Arabidopsis without impairing growth (Fabregas et al., 2018). OE of the BR receptor gene SIBRI1 in tomato decreases drought tolerance in a manner opposite to exogenous BR treatment (Nie et al., 2019), In addition, BIN2, a repressor of BR signaling positively regulates drought tolerance, whereas it negatively regulates cold tolerance (Li et al., 2017; Xie et al., 2019). Similarly, BZR1/BES1, an essential component in the BR signaling pathway, plays diverse roles in different stress responses. Arabidopsis BZR1 positively regulates freezing tolerance by regulating the expression of CBFs and genes uncoupled with CBF genes (Li et al., 2017). Under drought stress, Arabidopsis bes1-D, a gain-of-function mutant, displays reduced drought tolerance (Ye et al., 2017). However, BR signaling is not always a negative regulator of the drought response. TaBZR1, a BES1 and BZR1 homolog in wheat, is a positive regulator of the drought response by mediating O2– scavenging and antioxidant enzyme gene expression (Cui et al., 2019). In addition, maize ZmBES1/BZR1-5 positively regulates salt and drought stress tolerance (Sun et al., 2020). In this study, MdBES1 played a positive role in cold tolerance and disease resistance but a negative role in drought tolerance, further suggesting the complexity of MdBES1 in the stress responses of different plant species.
MdMYB88 has been reported to promote cold, drought, and disease responses via diverse mechanisms (Geng et al., 2018, 2020; Xie et al., 2018; Geng et al., 2020). In this study, MdBES1 directly regulated MdMYB88 by binding to its promoter. Interestingly, the regulation of MdBES1 on MdMYB88 was dynamic when plants suffered from different stressors. A genome-wide analysis of BZR1/BES1 target genes showed that thousands of genes are activated or repressed by BZR1/BES1, and the binding sites in the promoters determined their activation versus repressive activity (Sun et al., 2010; Yu et al., 2011). BRRE (CGTGT/CG) is more dominant in promoters of BZR1/BES1-repressed genes, whereas BZR1/BES1 induces gene expression by binding to the E-box (CANNTG; Sun et al., 2010; Yu et al., 2011). Here, we found that both BRRE and the E-box existed in the MdMYB88 promoter; therefore, we hypothesized that MdBES1 regulates MdMYB88 under different conditions by dynamically adjusting the enrichment of MdBES1 in the BRRE (CGTGT/CG) or E-box (CANNTG) of MdMYB88. By performing ChIP-qPCR experiment with plants pretreated with drought or cold stresses, we observed that MdBES1 had priority to bind BRRE motifs (fragments b and c) of the MdMYB88 promoter under control and drought conditions (Figure 1D;Supplemental Figure S15A). On the other hand, MdBES1 preferred to enrich in the E-box (Fragment P1) of MdMYB88 promoter under cold conditions (Supplemental Figure S15B). Hence, it is possible that the dynamic regulation of MdBES1 on MdMYB88 may be due to the sites to which MdBES1 tends to bind under different conditions.
The BR response phenotypes in Arabidopsis mainly include stem elongation and petiole and hypocotyl length. However, in rice, researchers have focused more on plant height and leaf inclination, which are crucial agronomic traits that determine grain yield (Yin et al., 2002; Tian et al., 2017; Xie et al., 2019). Here, we observed similar phenotypes of the BR response in MdBES1 and MdMYB88 OE plants, including increased plant height and enlarged leaf angles (Figure 7; Supplemental Figure S5). BR signaling components repress expression of BR biosynthetic genes by feedback regulation in rice and Arabidopsis, such as DLT, RLA1, and WRKY53. OE of all of these genes decreased the expression of DWF4 compared with wild-type (Tong et al., 2012; Qiao et al., 2017; Tian et al., 2017). We examined the expression of BR biosynthesis genes, including MdDWF4 and MdDET2 in MdBES1 OE plants, and found that expression of both genes was significantly reduced (Supplemental Figure S16), which was consistent with decreased BL levels in MdBES1 OE plants (Supplemental Figure S8E). MdMYB88 acted as a positive regulator of BR content by directly regulating BR biosynthesis genes, which was observed from the accumulated endogenous BR in MdMYB88 OE plants (Figures 8, E and 9, A–C). Similar to other BR biosynthetic genes, the expression of MdMYB88 was also repressed by applying BL (Supplemental Figure S13).
In our research, we found MdBES1 directly bound MdMYB88 promoter (Figure 1) and MdMYB88 transgenic plants showed BR-related response (Figure 8). We then examined the protein interaction between MdBES1 and MdMYB88. Results showed that MdBES1 associates with MdMYB88 in vivo but not in vitro (Supplemental Figure S17, A and B). Previous research showed that BZR1/BES1 interacts with BIN2 and phosphorylated by BIN2 when BR levels are low (He et al., 2019). We also found that MdBES1 interacted with MdBIN2 in apple (Supplemental Figure S17, C and D). We then speculated that MdBIN2 might mediate the association of MdBES1 and MdMYB88 in planta. However, Split luciferase and yeast-two hybrid assays suggested that MdBIN2 did not interact with MdMYB88. Therefore, an unknown protein that mediates the association of MdBES1 and MdMYB88 should be existed.
Previous reports showed that BZR1 and BES1 repress the expression of BR biosynthesis genes by directly binding to the CPD and DWF4 promoters (Sun et al., 2010; Yu et al., 2011). Here, we found that MdBES1 directly bound the MdMYB88 promoter, the latter bound the promoters of MdDET2, MdDWF4, and MdBR6OX2, thus proposing an indirect way for MdBES1 to attenuate the BR responses, which was dependent on MdMYB88.
As leaf angles and plant height are regulated by multiple phytohormones in rice (Luo et al., 2016), we detected several phytohormones and found that the level of endogenous indole acetic acid (IAA) and gibberellic acid 3 (GA3) decreased in MdMYB88/124 RNAi plants compared with GL-3 (Supplemental Figure S18), which might be the reason why exogenous treatment of BR cannot completely rescue the erect leaf and dwarf phenotypes of MdMYB88/124 RNAi plants. Interestingly, we also observed that 3-year-old MdMYB88 OE plants had larger branch angles, which are required to promote flower initiation in some apple cultivars, such as Fuji. However, whether BR controls branch angles in apple and the specific mechanism by which MdMYB88 regulates branch angles needs further research.
Based on the data presented in this work, we proposed a working model for the dynamic and multifaceted regulation of MdBES1 on MdMYB88 during growth and stress response in apple. Under cold or pathogen attack conditions, expression of MdBES1 is induced and MdBES1 preferably binds to the E-box of MdMYB88, resulting in increased expression of MdMYB88 under these two conditions. However, drought stress represses expression of MdBES1 and MdBES1 prefers to bind the BRRE motif of MdMYB88, thereby negatively regulates its expression. Consistently, MdBES1 enhanced cold tolerance and disease resistance but reduced drought tolerance. In addition, MdMYB88 positively regulated the expression of BR biosynthetic genes and the endogenous BR level, resulting in enlarged leaf angles and greater plant heights (Figure 12). Taken together, our results reveal that MdBES1 subtly regulates MdMYB88 during plant growth and diverse stresses.
Figure 12.

The dynamic and multifaceted regulation of MdBES1 on MdMYB88 during growth and stress in apple. Under cold or pathogen attack conditions, MdBES1 positively regulated MdMYB88. However, under drought conditions, MdBES1 negatively regulated MdMYB88. Consistently, MdBES1 enhanced cold tolerance and disease resistance but reduced drought tolerance. In addition, MdMYB88 positively regulated the expression of BR biosynthetic genes and the endogenous BR level.
In summary, our findings reveal multifaceted regulation of MdBES1 on MdMYB88 in stress tolerance and BR biosynthesis and provide candidate genes for stress tolerance breeding in fruit crops.
Materials and Methods
Plant material and growth conditions
Tissue-cultured GL-3 from progenies of “Gala” apple (Malus × domestica Borkh. cv Royal Gala) was used for stable gene transformation in this study (Dai et al., 2013). Tissue-cultured GL-3 plants were sub-cultured for 4 weeks under subculture medium (4.43 g/L MS salts, 30 g/L sucrose, 0.2 mg/L 6-BA, 0.2 mg/L IAA, and 7.5 g/L agar, pH = 5.8) and then transferred to rooting medium (2.215 g/L MS salts, 20 g/L sucrose, 0.5 mg/L IBA, 0.5 mg/L IAA, and 7.5 g/L agar, pH = 5.8) for 8 weeks. Plants were then grown in a growth chamber under a long-day condition (16-h light/8-h dark) at 22°C for various stress treatments. The MdMYB88 OE and RNAi plants were generated previously (Xie et al., 2018). Because of the sequence similarity between MdMYB88 and MdMYB124, it is not possible to knock down each individual gene; hence, we knocked down both MdMYB88 and MdMYB124 simultaneously with an RNAi approach, and named the RNAi plants as MdMYB88/124 RNAi plants (Xie et al., 2018).
Generation of transgenic apple plants
To generate stable transgenic plants, the full-length coding sequence (CDS) of MdBES1 was cloned and inserted into the pK2GW7 vector for OE by a gateway recombination technology (Invitrogen). A fragment (185 bp) of MdBES1 was cloned and introduced into the pK7GWIWG2D vector for RNA silencing. The resulting vectors were transformed into Agrobacterium tumefaciens (strain EHA105), and MdBES1 OE and RNAi plants were generated through the Agrobacterium-mediated transformation with GL-3 as the background.
Y1H screening assay
Y1H screening assay was conducted using Matchmaker® Gold Yeast One-Hybrid Library Screening System (Clontech, USA). MdMYB88 promoter (–1401 to –933 bp) was cloned and cloned to pAbAi vector, which was then linearized and transformed into yeast strain Y1H Gold, followed by growth selection on Synthetic Dropout (SD) medium (Clontech, USA) without uracil for 3–5 d under 28°C. Positive clones were selected and used to determine the Aureobasidin A (AbA) concentration. The cDNA library of apple was then transformed into Y1H Gold competent cells (MdMYB88-pAbAi) and selected on SD medium without Leu supplemented with AbA. Positive clones were collected and sequenced. pGADT7 empty vector served as a negative control.
A point-to-point Y1H assay was performed to verify the potential protein–DNA interaction. Briefly, MdMYB88 promoter regions (P1, P2, and P3) were cloned into pAbAi vector, and full-length CDS of MdBES1 was cloned into pGADT7 vector, resulting in MdBES1-pGADT7. Yeast cells carried MdMYB88 promoters-PAbAi were selected on SD-Ura medium and used to determine the AbA concentration. The MdBES1-pGADT7 vector then was transformed into Y1H Gold competent cells (MdMYB88-P1-pAbAi, MdMYB88-P2-pAbAi, and MdMYB88-P3-pAbAi). Cell growth was observed on SD-Leu/AbA. The experiments were repeated at least three times.
EMSA assay
To purify recombinant protein, CDS of MdBES1 was amplified by PCR and cloned into the pMAL-c5x vector. The resulting plasmid was then transformed into Escherichia coli (BL21 Rosetta-gami). The proteins were expressed at 37°C for 3 h using 0.5 mM isopropyl b-d-1-thiogalactopyranoside (IPTG) and purified using Amylose Resin (New England Biolabs). Recombinant MdMYB88-His protein was expressed and purified according to Xie et al. (2018). The primers, restriction sites, and vectors used for plasmid construction are listed in Supplemental Table S1.
EMSA was performed as previously described (Xie et al., 2018). Sequences of probes and mutated probes used for EMSA are listed in Supplemental Table S1. The experiments were repeated at least three times.
Chromatin immunoprecipitation (ChIP)-qPCR assay
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (Xie et al., 2018). In brief, leaves were collected from GL-3 plants grown under normal condition, cold (4°C) for 8 h, or short-term drought treatment before cross-link in 1% (w/v) formaldehyde. Anti-MdBES1 antibody was used to immunoprecipitated chromatin. The recovered DNA was performed for real-time qPCR using the primers listed in Supplemental Table S1. The experiments were repeated three times.
Subcellular localization assays in Nicotiana benthamiana leaves
Subcellular localization assays were performed as previously described (Xie et al., 2018). In brief, full-length CDS of MdBES1 was introduced into pEarleyGate104, resulting in MdBES1-pEarleyGate104. Agrobacterium strain C58C1 carried MdBES1-pEarleyGate104 together was used to co-infiltrate 5-week-old leaves of tobacco (Nicotiana benthamiana) plants with P19. The infected tobacco plants were grown under long-day photoperiod at 21°C for 3 d. Finally, Nikon A1R/A1 confocal microscope (Nikon, Tokyo, Japan) was used to detect fluorescence signals with two channel detection wavelengths: excitation wavelengths 488 nm for GFP and 405 nm for dihydrochloride (DAPI). Empty vector pEarleyGate104 was used as a control for Green Fluorescent Protein (GFP). The experiments were repeated three times.
Detection of CS and phenylalanine content assays
For CS detection, the extraction procedure of hormone was as follows: (1) grind mature leaves of 2-month-old plants into fine powder in lipid nitrogen and accurately weigh 2 g frozen leaf powder (three duplications) by 1/10,000 balance; (2) ultrasonically extract the samples with 10 mL 80% (v/v) methanol for 2 h at 4°C; (3) centrifuge at 10,000 rpm/min, 4°C for 5 min, then take the supernatant, pass through a Bond Elut pre-packed column, and elute with 3 mL methanol; (4) pass through strata-X cartridge and elute with 3 mL methanol; (5) volatilize methanol with nitrogen, and add 200-µL methanol to dissolve; and (6) filter with 0.22-µm organic membrane (New Asia, Shanghai) into sample vial for High Performance Liquid Chromatography massspectrometry (HPLC-MS)/MS analysis (Agilent, USA). Three biological replicates were applied. Eighteen plants were used in total, and six plants were used as one biological replicate.
For HPLC–MS/MS analysis: (1) prepare standard solution using methanol as the solvent for gradients of 0.5 ng/mL, 1 ng/mL, 2 ng/mL, 5 ng/mL, 10 ng/mL, 20 ng/mL, 50 ng/mL CS (Sigma, USA); (2) the Liquid Chromatography was equipped with a ZORBAX SB-C18 column (2.1 × 150, 3.5-μm film thickness, Agilent, USA) and column temperature was 35°C; (3) mobile phase: A:B=[acetonitrile/0.1% (v/v) formic acid]: [water/0.1% (v/v) formic acid] and flow rate: 0.35 mL/min; (4) elution gradient: 0–2 min, A = 80%; 2–3.5 min, an increase to 95%; 3.5–6 min, A = 95%; 6–6.1 min, A decrease to 80%; 6.1–10 min, A = 80%. (5) The volume1 of injection: 5 μL; (6) mass spectrometry conditions: curtain gas: 15 psi; ionspray voltage: 5500 v; Gas1: 65 psi; Gas2: 70 psi; atomization temperature: 350°C.
Phenylalanine content assay was performed as previously described (Geng et al., 2020). Three biological replicates were applied. Eighteen plants were used in total, and six plants were used as one biological replicate. The experiments were repeated three times.
Measurement of drought and cold tolerance, and disease resistance of MdBES1 transgenic plants
Three-month-old plants were used for the short-term drought treatment. Volumetric water content (VWC) was detected with the soil moisture meter TDR350 (Spectrum, USA). An initial VWC of 50% was required before the treatment. Plants were then withheld with water for 24 d until VWC decreased to 0. During the treatment, the GL-3 and MdBES1 OE plants were supplemented with water to maintain the same VWC with MdBES1 RNAi plants. After 2 d of drought treatment, the status of intact plants was photographed, and plants were recovered under normal conditions for 7 d and survival rates were then calculated. Three biological replicates were applied. Eighteen plants were used in total, and six plants were used as one biological replicate.
For the long-term drought treatment, 2-month-old GL-3 and MdBES1 transgenic plants were transferred to pots (30 cm × 18 cm) for an additional month in a greenhouse. Then, plants of each line were divided into a well-watered group and long-term drought group. Plants of the well-watered group were irrigated normally to maintain field capacity of 75%–85%; plants of the long-term drought group were daily irrigated to maintain a field capacity of 35%–45%. The long-term drought stress treatment lasted for 3 months. Twenty plants were used in total. At the end of treatment, root hydraulic conductivity was measured using an HPFM (Dynamax, Houston) as described by Geng et al. (2018).
Cold tolerance assays were performed according to the method described by Xie et al. (2018). Three biological replicates were applied. Eighteen plants were used in total, and six plants were used as one biological replicate.
For disease tolerance assay, leaves of 3-month-old MdBES1 transgenic plants were detached and incubated with V. mali for 7 d. Stems of 3-year-old MdBES1 transgenic plants were detached and incubated with V. mali for 9 d. Three biological replicates were applied. Eighteen plants were used in total, and six plants were used as one biological replicate. The experiments were repeated at least three times. The severity of symptoms was determined by lesion areas using Image J software.
RNA extraction and RT-qPCR analysis
RNA extraction and RT-qPCR analysis were performed as previously described (Xie et al., 2018). Primers used are listed in Supplemental Table S1.
Application of BL treatment
Six-week-old plants were pretreated with 250 nmol/l BL (Sigma, USA) once every 2 d and lasted for 2 months. The plant height and leaf angles were, respectively, measured by meter ruler and protractor. After that, the tolerances of these plants to drought, cold, and disease were then examined. The experiments were repeated three times.
Transient expression assay
To transiently overexpress MdMYB88 in MdBES1 OE plants, the full-length CDS of MdMYB88 was cloned into pCambia1305 vector. To transiently silence MdMYB88 in MdBES1 OE plants, a 500-bp fragment of MdMYB88 was introduced into the pHellsgate2 vector. The resulting vectors were transformed into Agrobacterium strain EH105 and infiltrated into the leaves of 1-month-old tissue-cultured MdBES1 OE plants under vacuum conditions. The infiltrated apple plants were grown for an additional 3 d at 22°C before various stress treatments.
Transient double transgenic plants were verified by RT-qPCR analysis. Cold treatment of MdBES1 OE MdMYB88124 RNAi plants was carried out by a 4°C treatment for 8 h, and the drought treatment of MdBES1 OE MdMYB88 OE was performed by a waterloss treatment for 1 h. The disease resistance of MdBES1 OE MdMYB88/124 RNAi plants was examined by V. mali incubation for 3 d. Three biological replicates were applied. Eighteen leaves were used, and six leaves were used as one replicate. The experiments were repeated three times.
Statistical analysis
Statistical analysis was carried out by one-way Analysis of Variance (ANOVA) (Tukey’s test) analysis (SPSS software version 21.0, USA). Significance level of variation was represented by asterisks: *P < 0.05, **P < 0.01, ***P < 0.001.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: MdBES1 (XM_008339943.2), MdMYB88 (KY569647), MdMYB124 (KY569648) MdDET2 (XM_008357488.3), MdBR6OX2 (XM_008381225.3), MdDWF4 (XM_008339876.2).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Protein alignment of MdBES1 and BES1.
Supplemental Figure S2. Promoter analysis of MdMYB88.
Supplemental Figure S3. MdBES1 failed to bind the promoter of MdMYB124 by yeast one-hybrid assay (Y1H).
Supplemental Figure S4. MdBES1 is localized in the nucleus of tobacco leaf epidermal cells.
Supplemental Figure S5. MdBES1 expression pattern in the GL-3 under cold, drought, and disease conditions by RT-qPCR.
Supplemental Figure S6. The expression level of MdBES1 in MdBES1 transgenic plants.
Supplemental Figure S7. Root dry weight of GL-3 and MdBES1 transgenic plants after long-term drought stress.
Supplemental Figure S8. MdBES1 enlarges leaf and branch angles.
Supplemental Figure S9. Cs content of GL-3 and MdMYB88/124 RNAi plants under control, drought, and cold stress conditions.
Supplemental Figure S10. Promoter analysis of MdDET2.
Supplemental Figure S11. Promoter analysis of MdDWF4.
Supplemental Figure S12. Promoter analysis of MdBR6OX2.
Supplemental Figure S13. The expression pattern of MdMYB88 under control and BL treatment.
Supplemental Figure S14. The expression of MdMYB88 in MdBES1 OE #4, MdBES1 OE #4 MdMYB88/124 RNAi, and MdBES1 OE #4 MdMYB88 OE transgenic plants.
Supplemental Figure S15. The enrichment of MdBES1 in different fragments of MdMYB88 promoter under drought (short-term drought treatment for 6 d) and cold (4°C 8 h) conditions.
Supplemental Figure S16. The expression of MdDET2 and MdDWF4 in MdBES1 transgenic plants and GL-3.
Supplemental Figure S17. MdBES1 interacts with MdMYB88 in vivo but not in vitro.
Supplemental Figure S18. The content of indole acetic acid (IAA) and gibberellic acid 3 (GA3) in MdMYB88 transgenic plants.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgment
We thank Dr Zhihong Zhang from Shenyang Agricultural University for providing tissue-cultured GL-3 plants.
Funding
This work was supported by the National Natural Science Foundation of China (31872080) and National Key Research and Development Program of China (2018YFD1000100).
Conflict of interest statement. The authors declare that they have no conflicts of interest.
Q.G. and H.F. conceived experiments; P.C. carried out EMSA; Y.G. performed disease resistance of transgenic plants; C.Z. and Y.X. performed short-term drought treatment; S.W. performed BL treatment experiments; Y.X. provided transgenic plants; P.Y. provided a greenhouse; X.W. and F.M. analyzed data; X.L., C.Z., and Q.G. wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Qingmei Guan qguan@nwafu.edu.cn.
References
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Min YK, Nagata N, Yamagishi K, Takatsuto S, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S (2000) Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol 123: 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai MY, Zhang LY, Gampala SS, Zhu SW, Song WY, Chong K, Wang ZY (2007) Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Natl Acad Sci U S A 104: 13839–13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y (2017) Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 29: 1425–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD (1996) Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J 10: 1–8 [DOI] [PubMed] [Google Scholar]
- Cui XY, Gao Y, Guo J, Yu TF, Zheng WJ, Liu YW, Chen J, Xu ZS, Ma YZ (2019) BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol 180: 605–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai HY, Li WR, Han GF, Yang Y, Ma Y, Li H, Zhang ZH (2013) Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci Hortic 164: 202–208 [Google Scholar]
- Denekamp M, Smeekens SC (2003) Integration of wounding and osmotic stress signals determines the expression of the AtMYB102 transcription factor gene. Plant Physiol 132: 1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2: 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremina M, Unterholzner SJ, Rathnayake AI, Castellanos M, Khan M, Kugler KG, May ST, Mayer KF, Rozhon W, Poppenberger B (2016) Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc Natl Acad Sci U S A 113: E5982–E5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabregas N, Lozano-Elena F, Blasco-Escamez D, Tohge T, Martinez-Andujar C, Albacete A, Osorio S, Bustamante M, Riechmann JL, Nomura T, et al. (2018) Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nature Commun 9: 4680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng D, Chen P, Shen X, Zhang Y, Li X, Jiang L, Xie Y, Niu C, Zhang J, Huang X, et al. (2018) MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol 178: 1296–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng D, Shen X, Xie Y, Yang Y, Bian R, Gao Y, Li P, Sun L, Feng H, Ma F, et al. (2020) Regulation of phenylpropanoid biosynthesis by MdMYB88 and MdMYB124 contributes to pathogen and drought resistance in apple. Hortic Res 7: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Li L, Aluru M, Aluru S, Yin Y (2013) Mechanisms and networks for brassinosteroid regulated gene expression. Curr Opin Plant Biol 16: 545–553 [DOI] [PubMed] [Google Scholar]
- He G, Liu J, Dong H, Sun J (2019) The blue-light receptor CRY1 interacts with BZR1 and BIN2 to modulate the phosphorylation and nuclear function of BZR1 in repressing BR signaling in Arabidopsis. Mol Plant 12: 689–703 [DOI] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SSL, Gendron N, Sun CQ, Wang ZY (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288: 2360–2363 [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Matsuoka M (2004) Brassinosteroids and rice architecture. J Pestic Sci 29: 184–188 [Google Scholar]
- Jang S, An G, Li HY (2017) Rice leaf angle and grain size are affected by the OsBUL1 transcriptional activator complex. Plant Physiol 173: 688–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P (2007) Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225: 353–364 [DOI] [PubMed] [Google Scholar]
- Li H, Ye K, Shi Y, Cheng J, Zhang X, Yang S (2017) BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol Plant 10: 545–559 [DOI] [PubMed] [Google Scholar]
- Li JM, Chory J (1999) Brassinosteroid actions in plants. J Exp Bot 50: 275–282 [Google Scholar]
- Luo X, Zheng J, Huang R, Huang Y, Wang H, Jiang L, Fang X (2016) Phytohormones signaling and crosstalk regulating leaf angle in rice. Plant Cell Rep 35: 2423–2433 [DOI] [PubMed] [Google Scholar]
- Marcos R, Izquierdo Y, Vellosillo T, Kulasekaran S, Cascon T, Hamberg M, Castresana C (2015) 9-Lipoxygenase-derived oxylipins activate brassinosteroid signaling to promote cell wall-based defense and limit pathogen infection. Plant Physiol 169: 2324–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Schmitz G, Theres K (2006) Blind homologous R2R3 Myb genes control the pattern of lateral meristem initiation in Arabidopsis. Plant Cell 18: 586–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie SM, Huang SH, Wang SF, Mao YJ, Liu JW, Ma RL, Wang XF (2019) Enhanced brassinosteroid signaling intensity via SlBRI1 overexpression negatively regulates drought resistance in a manner opposite of that via exogenous BR application in tomato. Plant Physiol Biochem 138: 36–47 [DOI] [PubMed] [Google Scholar]
- Nolan T, Chen J, Yin Y (2017) Cross-talk of Brassinosteroid signaling in controlling growth and stress responses. Biochem J 474: 2641–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TM, Brennan B, Yang M, Chen J, Zhang M, Li Z, Wang X, Bassham DC, Walley J, Yin Y (2017) Selective autophagy of BES1 mediated by DSK2 balances plant growth and survival. Dev Cell 41: 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TM, Vukasinovic N, Liu D, Russinova E, Yin Y (2020) Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32: 295–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Kanei-Ishii C, Sasaki M, Hatanaka H, Nagadoi A, Enari M, Nakamura H, Nishimura Y, Ishii S, Sarai A (1996) The cavity in the hydrophobic core of Myb DNA-binding domain is reserved for DNA recognition and trans-activation. Nat Struct Biol 3: 178–187 [DOI] [PubMed] [Google Scholar]
- Planas-Riverola A, Gupta A, Betegon-Putze I, Bosch N, Ibanes M, Cano-Delgado AI (2019) Brassinosteroid signaling in plant development and adaptation to stress. Development 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao S, Sun S, Wang L, Wu Z, Li C, Li X, Wang T, Leng L, Tian W, Lu T, et al. (2017) The RLA1/SMOS1 transcription factor functions with OsBZR1 to regulate brassinosteroid signaling and rice architecture. Plant Cell 29: 292–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam RK (1994) Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul 14: 173–181 [Google Scholar]
- Stracke R, Ishihara H, Barsch GHA, Mehrtens F, Niehaus K, Weisshaar B (2007) Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J 50: 660–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Yu H, Qu J, Cao Y, Ding L, Feng W, Khalid MHB, Li W, Fu F (2020) Maize ZmBES1/BZR1-5 decreases ABA sensitivity and confers tolerance to osmotic stress in transgenic Arabidopsis. Int J Mol Sci 21: 996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Chen D, Li X, Qiao S, Shi C, Li C, Shen H, Wang X (2015) Brassinosteroid signaling regulates leaf erectness in Oryza sativa via the control of a specific U-type cyclin and cell proliferation. Dev Cell 34: 220–228 [DOI] [PubMed] [Google Scholar]
- Sun Y, Fan XY, Cao DM, Tang W,, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E, et al. (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19: 765–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Nakagawa H, Tomita C, Shimatani Z, Ohtake M, Nomura T, Jiang CJ, Dubouzet JG, Kikuchi S, Sekimoto H, et al. (2009) BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol 151: 669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Li X, Zhou W, Ren Y, Wang Z, Liu Z, Tang J, Tong H, Fang J, Bu Q (2017) Transcription factor OsWRKY53 positively regulates brassinosteroid signaling and plant architecture. Plant Physiol 175: 1337–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong HN, Liu LC, Jin Y, Du L, Yin YH, Qian Q, Zhu LH, Chu CC (2012) DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24: 2562–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang J, Liu J, Hu J, Liu J, Chen Y, Cai Z, Wang X (2018) Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Mol Plant 11: 315–325 [DOI] [PubMed] [Google Scholar]
- Wang L, Xu Y, Zhang C, Ma Q, Joo SH, Kim SK, Xu Z, Chong K (2008) OsLIC, a novel CCCH-type zinc finger protein with transcription activation, mediates rice architecture via brassinosteroids signaling. PLoS One 3: e3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Lim JH, Kim SS,, Cho SH, Yoo SC, Koh HJ, Sakuraba Y, Paek NC (2015) Mutation of SPOTTED LEAF3 (SPL3) impairs abscisic acid-responsive signalling and delays leaf senescence in rice. J Exp Bot 66: 7045–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YH, Liu DP, Zhang GX, Tong HN, Chu CC (2017) Brassinosteroids regulate OFP1, a DLT interacting protein, to modulate plant architecture and grain morphology in rice. Front Plant Sci 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YP, Chen PX, Yan Y, Bao CN, Li XW, Wang LP, Shen XX, Li HY, Liu XF, Niu CD, et al. (2018) An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol 218: 201–218 [DOI] [PubMed] [Google Scholar]
- Xie Z, Nolan T, Jiang H, Tang B, Zhang M, Li Z, Yin Y (2019) The AP2/ERF transcription factor TINY modulates brassinosteroid-regulated plant growth and drought responses in Arabidopsis. Plant Cell 31: 1788–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Liu S, Tang B, Chen J, Xie Z, Nolan TM, Jiang H, Guo H, Lin HY, Li L, et al. (2017) RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat Commun 8: 14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109: 181–191 [DOI] [PubMed] [Google Scholar]
- Youn JH, Kim TW (2015) Functional insights of plant GSK3-like kinases: multi-taskers in diverse cellular signal transduction pathways. Mol Plant 8: 552–565 [DOI] [PubMed] [Google Scholar]
- Yu X, Li L, Zola J, Aluru M, Ye H, Foudree A, Guo H, Anderson S, Aluru S, Liu P, et al. (2011) A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J 65: 634–646 [DOI] [PubMed] [Google Scholar]
- Zhang YL, Zhang CL, Wang GL, Wang YX, Qi CH, Zhao Q, You CX, Li YY, Hao YJ (2019) The R2R3 MYB transcription factor MdMYB30 modulates plant resistance against pathogens by regulating cuticular wax biosynthesis. BMC Plant Biol 19: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ (2014) H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot 65: 4371–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.