Abstract
The circadian clock coordinates the physiological responses of a biological system to day and night rhythms through complex loops of transcriptional/translational regulation. It can respond to external stimuli and adjust generated circadian oscillations accordingly to maintain an endogenous period close to 24 h. However, the interaction between nutritional status and circadian rhythms in plants is poorly understood. Magnesium (Mg) is essential for numerous biological processes in plants, and its homeostasis is crucial to maintain optimal development and growth. Magnesium deficiency in young Arabidopsis thaliana seedlings increased the period of circadian oscillations of the CIRCADIAN CLOCK-ASSOCIATED 1 (CCA1) promoter (pCCA1:LUC) activity and dampened their amplitude under constant light in a dose-dependent manner. Although the circadian period increase caused by Mg deficiency was light dependent, it did not depend on active photosynthesis. Mathematical modeling of the Mg input into the circadian clock reproduced the experimental increase of the circadian period and suggested that Mg is likely to affect global transcription/translation levels rather than a single component of the circadian oscillator. Upon addition of a low dose of cycloheximide to perturb translation, the circadian period increased further under Mg deficiency, which was rescued when sufficient Mg was supplied, supporting the model’s prediction. These findings suggest that sufficient Mg supply is required to support proper timekeeping in plants.
Magnesium maintains the circadian period in Arabidopsis seedlings and interferes with the circadian oscillator, most likely through translational mechanisms.
Introduction
Magnesium (Mg) is one of the most abundant elements in the Earth’s crust (Clark and Washington, 1924; Fleischer, 1954) and in sea water (Culkin and Cox, 1966). It plays many roles in the metabolism of living organisms, such as maintaining ribosome structure (Akanuma et al., 2018), being necessary for the active form of ATP (Fish et al., 1983; Wang et al., 1995) as well as being a co-factor and allosteric modulator for numerous enzymes (Cowan, 1998). In plants, Mg is vital to the photosynthetic machinery (Levitt, 1954) and CO2 assimilation (Hauer-Jákli and Tränkner, 2019). Therefore, imbalances in plant Mg status are likely to cause disorders from cellular to organismal levels. Despite its importance in cellular and organismal metabolic processes across all kingdoms, Mg still does not garner as much attention as other nutrients, such as Ca, N, Zn, or Fe (Hermans et al., 2013).
Plants require usually between 1.5 and 3.5 mg g−1 dry weight for optimal growth (Grzebisz, 2009; Römheld, 2012). A Mg supply below 1–2 mg g−1 leaf dry weight marks the onset of Mg deficiency (Hermans et al. 2004; Hermans and Verbruggen, 2005; Ding et al., 2006). Impaired partitioning of soluble sugars leading to starch accumulation in source leaves are the first sign of Mg deficiency before defects of photosynthetic activity occur (Cakmak et al., 1994; Hermans et al., 2005; Hermans and Verbruggen, 2005). Typical long-term symptoms of Mg starvation in vascular plants are interveinal leaf chlorosis, limited growth, and altered biomass allocation between plant organs (Verbruggen and Hermans, 2013; Hauer-Jákli and Tränkner, 2019). Transcriptomic studies in Arabidopsis thaliana identified CATION EXCHANGER 3 (CAX3) as a suitable molecular marker to monitor the Mg status because it responds to Mg availability before the first visible signs of deficiency or excess occur (Hermans et al., 2010a; Kamiya et al., 2012). Transcriptomic studies further revealed that both early and long-term Mg deficiency altered the expression of genes involved in processes regulated by the circadian oscillator (Hermans et al., 2010a, 2010b).
Daily biological rhythms in plants are regulated by the circadian clock, which runs in a close-to 24-h cycle synchronized to environmental cues such as light and temperature. The circadian clock is maintained by endogenous rhythms of gene expression regulated by transcriptional–translational feedback loops that influence growth, development, flowering time, and responses to biotic and abiotic stresses to promote plant fitness (Green et al., 2002; Harmer, 2009; Greenham and McClung, 2015). The core components of the central oscillator in plants are the dawn-phased genes CIRCADIAN CLOCK-ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY); the morning genes PSEUDO-RESPONSE REGULATOR 9 (PRR9), PRR7, and PRR5; the dusk-phased gene TIMING OF CAB EXPRESSION 1 (TOC1); and the evening-complex composed of EARLY FLOWERING 3 (ELF3), ELF4, and LUX ARRYHTHMO (LUX; McClung, 2006; Webb et al., 2019). Altered expression of one or more of these oscillator components changes the circadian period, amplitude, and phase, and can lead to complete arrhythmia of the endogenous oscillator, which affects plant growth and development (Hicks et al., 1996; Dunlap, 1999; Alabadí et al., 2002; Webb, 2003). Therefore, optimal functioning of the circadian system relies on sensing and integrating internal as well as external signals in order to maintain an endogenous timekeeping mechanism capable of accurately anticipating environmental fluctuations (Dodd et al., 2005; Hotta et al., 2007; Robertson et al., 2009; Hsu and Harmer, 2014). Such external signals can be the nutritional status that cross talks with the circadian clock (Hong et al., 2013) and imbalanced nutritional homeostasis can interfere with circadian timekeeping. Experiments that established the effect of stimuli to change the phase of the circadian oscillator at different times of the day, so-called phase response curve (PRC) experiments, using pulses of nitrogen (N), support the feedback of N status to the circadian oscillator (Gutiérrez et al., 2008). Other studies demonstrated that N deficiency shortened the circadian period in the photosynthetic dinoflagellate Gonyaulax polyedra (Sweeney and Folli, 1984; Haydon et al., 2015). An excess of copper affects the amplitude and phase of CCA1 and LHY expression (Andrés-Colás et al., 2010) while iron deficiency lengthens the circadian period (Chen et al., 2013; Salomé et al., 2013). Furthermore, early- or long-term Mg deficiency alters the expression of Arabidopsis circadian oscillator genes, suggesting a link between Mg homeostasis and the circadian clock (Hermans et al., 2010a, 2010b). While no detailed study describes the interplay between Mg and circadian rhythms in plants, recent findings suggest a key role for Mg in the timekeeping system in Ostreococcus tauri (Feeney et al., 2016), a single-celled alga that shares a common ancestor with vascular plants and diel oscillations of Mg in the plastids of rice (Oryza sativa; Li et al., 2020).
We show that Mg deficiency dose-dependently lengthens the circadian period of core oscillator genes in Arabidopsis, which is independent from fully functional photosynthesis. A comparable period lengthening was reproduced with mathematical modeling when a global impact of Mg on transcription and translation was simulated. Our findings demonstrate that endogenous rhythms in plants strongly depend on nutritional status.
Results
Decreasing external Mg concentrations dose-dependently increase the circadian period under continuous light and impact the phase in light/dark cycles
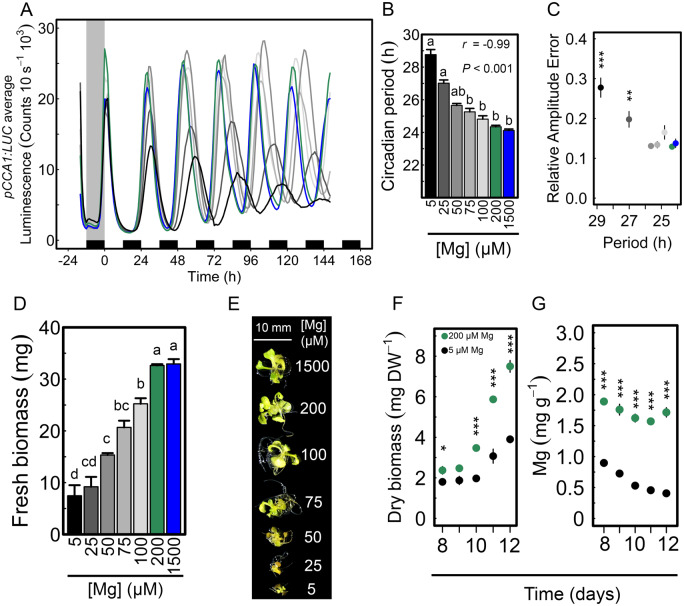
Luciferase (LUC)-based reporters were used to examine circadian rhythms in Arabidopsis seedlings that were germinated and entrained under different Mg concentrations ranging from 5 to 1,500 µM and thereafter released into continuous light (LL). Magnesium depletion lengthened the circadian period of pCCA1:LUC activity by almost 5 h on average when compared with Mg-replete controls: τ = 28.77 versus 24.34 h, respectively, in a dose-dependent manner (r = −0.99, P < 0.001; Figure 1, A and B). The increased circadian period was associated with reduced amplitude of pCCA1:LUC oscillations and an increased relative amplitude error (RAE; Figure 1, A and C). Results were also confirmed with the reporter lines pPRR7:LUC and pTOC:LUC (Supplemental Figure S1, A–C). Also, when no external sucrose was supplied a lengthening of the circadian period due to Mg deficiency was observed (Supplemental Figure S1, D–F). However, the difference between circadian period values of the lowest and highest Mg concentration was greater in the presence of 1% (w/v) sucrose (Supplemental Figure S1) and therefore following experiments were done with external sucrose added.
Figure 1.
Limiting external Mg availability alters circadian oscillations of CCA1:LUC. (A) Mean circadian oscillations of pCCA1:LUC in LL conditions after being entrained to 12-/12-h light/dark cycles for 8 d on media supplied with different Mg concentrations. (B) pCCA1:LUC period estimates in hours under LL (n = 12; mean ± SEM) (C) RAE of oscillations (mean ± SEM, n = 12) of pCCA1:LUC. (D) Fresh biomass (n = 12; mean ± SEM) and (E) morphological phenotype of seedlings at the end of the experiment. (F) Dry biomass and (G) Mg concentration in plant tissue (mean ± SEM, n = 3 [1 = 15 pooled seedlings]) of 12-d-old seedlings cultivated in light/dark cycles on control (200-µM Mg) and deficient media (5-µM Mg). Significant differences between different Mg concentrations were verified by (B) Spearman’s rho correlation coefficient and Kruskal–Wallis rank sum test followed by Nemenyi post hoc test, (C) One-way ANOVA followed by Tukey’s HSD post hoc test, (D, F, and G) Two-sample Student’s t test 95% CI (different letters indicate significance at the level of P < 0.05. Asterisks represent significance at *P < 0.05, **P < 0.01, and ***P < 0.001).
Magnesium deficiency led to reduced growth; the fresh biomass of 15-d-old seedlings was about 23% less than the biomass of seedlings grown under sufficient Mg supply (200- and 1,500-µM Mg, Figure 1, D and E). Dry biomass was significantly reduced (P < 0.001) and the internal Mg status of seedlings was significantly lower (P < 0.001) when external Mg was limited (Figure 1, F and G).
To test if increased circadian period is linked to growth inhibition provoked by Mg shortage, seedlings were grown under N deficiency, which is also a major macronutrient. Seedlings fully supplied (10 mM) or starved (0.01 mM) with N had a similar free-running circadian period under either condition: τ = 24.3 versus 24.3 h (Supplemental Figure S2, A and B). Yet, the RAE of pCCA1 oscillations was affected by low-N supply (Supplemental Figure S2, D) and seedlings had morphology and size characteristics of severe N deficiency (Supplemental Figure S2, D). These results show that severe growth inhibition induced by nutrient deficiency and circadian period lengthening are not correlated.
The effect of Mg nutrition on the phase was determined during entrainment cycles (12-h light/12-h dark) in seedlings sown on either Mg deficient (5-µM Mg) or sufficient (1,500-µM) medium. In light/dark cycles, CCA1 peak activity is timed to dawn, while TOC1 peak activity is timed to dusk, independent of Mg supply (Figure 2, A). However, when thereafter released into constant light, the entrained circadian phase was delayed under Mg deficiency for both reporters (Supplemental Figure S3), probably due to the missing light/dark signal to which CCA1 as well as TOC1 are strongly responsive. The provoked phase delay manifested for TOC1 at the time of the first subjective dusk and was apparent at the following subjective dawn for CCA1 (Supplemental Figure S3). The phase of pPRR7:LUC peak activity was sensitive to Mg depletion during entrainment cycles and its peak phase was delayed (Figure 2, A and Supplemental Figure S3). To examine this observation further, pPRR7:LUC activity was measured in a 12-/12-h light/dark cycle over the course of 4 d and phase showed a significant delay between deficient and sufficient Mg conditions (Figure 2, B). PRR7 showed sensitivity to Mg deficiency during light/dark cycles among the investigated components of the circadian oscillator, resulting in a lagging phase in response to low Mg during the day. However, it cannot be ruled out that CCA1 and TOC1 do not respond to Mg deficiency during entrainment cycles as measurement of luminescence were only taken hourly and the forcing light/dark cycle might mask the effect.
Figure 2.
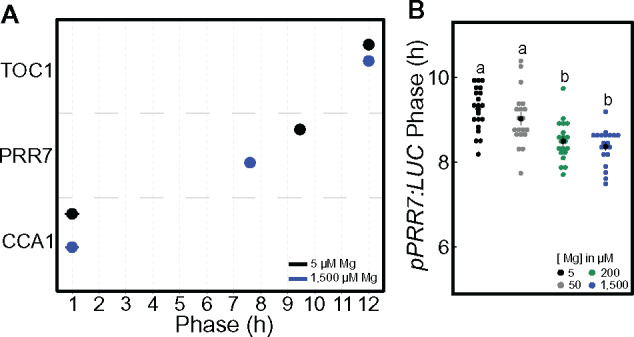
Mg deficiency delays the phase of PRR7 in light/dark cycles. (A) Phase of peak expression (mean ± SEM) in 12-/12-h light/dark cycle of pCCA1:LUC, pPRR7:LUC and pTOC1:LUC in 11-d-old Col-0 WT Arabidopsis seedlings (n = 20). (B) Phase of pPRR7:LUC peak expression in a 4-d 12-/12-h light/dark cycle under different Mg supply in the presence of 1% sucrose. Significant difference between the different Mg concentrations was determined by a Wilcoxon rang sum test. Different letters indicate significance at the level of P ≤ 0.05.
Induction of a Mg-deficiency marker is congruent with circadian clock alteration
Neither a difference in circadian oscillations nor an effect on plant growth was observed when seedlings were supplied with 200-µM Mg or 1,500-µM Mg (Figure 1, A–E). Therefore, we tested whether a more rapid Mg-deficient status in plants could be induced upon pre-cultivation on 200-µM Mg in comparison to the usually used 1,500-µM Mg. In fact, metabolically available Mg concentrations in plant cells are relatively high (15–25 mM) and the vacuolar storage is reported to range from 5 to 80 mM (Hermans et al., 2013). Seedlings were entrained on either 1,500-µM or 200-µM Mg and thereafter transferred to Mg-deficient medium (5 µM) for another 5 d in LL (Supplemental Figure S4, A). As a control, seedlings were transferred to medium supplied with the respective sufficient Mg concentration. Seedlings transferred to 5-µM Mg were pale and produced significantly less biomass only when pre-cultured on 200-µM Mg (Supplemental Figure S4, B and C). When transferred to the respective control medium, CAX3-transcript levels were comparable between 1,500-µM and 200-µM Mg control conditions; however, an increase of expression observed 24 h after the transfer might be a general stress response (Supplemental Figure S4, D). CAX3 and CCA1 expression levels were determined at 24, 48, and 72 h after transfer to deficient or control medium. Expression data were normalized to expression values of the respective control (1,500-µM and 200-µM Mg) at 24 h to account for the observations in Supplemental Figure S4, D. In seedlings transferred to 5-µM Mg after being pre-cultured on 1,500-µM Mg, CAX3 transcript levels significantly increased 72 h after Mg depletion (Figure 3, A). But, in seedlings pre-cultured on 200-µM Mg CAX3 expression was already induced after 48 h of transfer (Figure 3, B), which coincided with a two-fold decrease of CCA1 transcript levels (Figure 3, C). Apparently, supplying seedlings with 1,500-µM Mg provides enough Mg storage to prevent Mg deficiency within the first 3 d of deprivation, whereby entrainment on 200-µM Mg induced a more rapid and severe response toward deficiency stress after transfer to 5-µM Mg (Figure 3, B and C and Supplemental Figure S4, B and C). Therefore, 200-µM Mg was chosen as a new control concentration to entrain seedlings and to investigate the time course of circadian clock alteration and induction of Mg deficiency.
Figure 3.
Circadian oscillator alteration occurs concomitantly with expression of the Mg deficiency marker CAX3. Seedlings entrained for 8 d to 12-/12-h light/dark cycles on medium with 200- or 1,500-µM Mg were transferred to fresh media either deficient in Mg (5 µM) or fully supplied and released in continuous light. CAX3 and CCA1 expression levels were determined at 24, 48, and 72 h after transfer to deficient or control medium. Expression data were normalized to expression values of respective controls (200- or 1,500-µM Mg) at 24 h. (A) CAX3 mRNA expression (mean ± SEM, n = 3 [1 = 30 pooled seedlings]) after transfer from 1,500-µM Mg, (B) and (C) CAX3 mRNA expression and CCA1 mRNA expression (mean ± SEM, n = 3 [1 = 30 pooled seedlings]) after transfer from 200-µM Mg. Seedlings entrained for 8 d to 12-/12-h light/dark cycle on medium supplied with 200-µM Mg were transferred to various Mg concentrations and released into continuous light to determine (D) correlation between estimated circadian period and external Mg concentration, mean ± SEM, n = 12 and (E) respective RAE. Period was calculated on rhythms between 24–120 h in continuous light, mean ± SEM, n = 12, (F) average luminescence traces of pCCA1:LUC activity in continuous light. Significant difference between different Mg concentrations to control conditions was verified by Pearson’s product moment correlation coefficient (P < 0.01) and one-way ANOVA followed by Tukey’s HSD post hoc or two-sample Student’s t test at a 95% CI (asterisks represent significance at **P < 0.01 and ***P < 0.001).
Seedlings entrained on 200-µM Mg were transferred to various Mg concentration and the circadian period of pCCA1:LUC was determined. In accordance with Figure 1, Mg deficiency induced a period lengthening in a Mg-concentration dependent manner as well as an increase of the RAE (Figure 3, D and E) and severe Mg deficiency (5-µM Mg) caused a circadian response already after 1 d in LL following the transfer from 200-µM Mg (Figure 3, F).
Light plays a critical role in the circadian effects of Mg deficiency
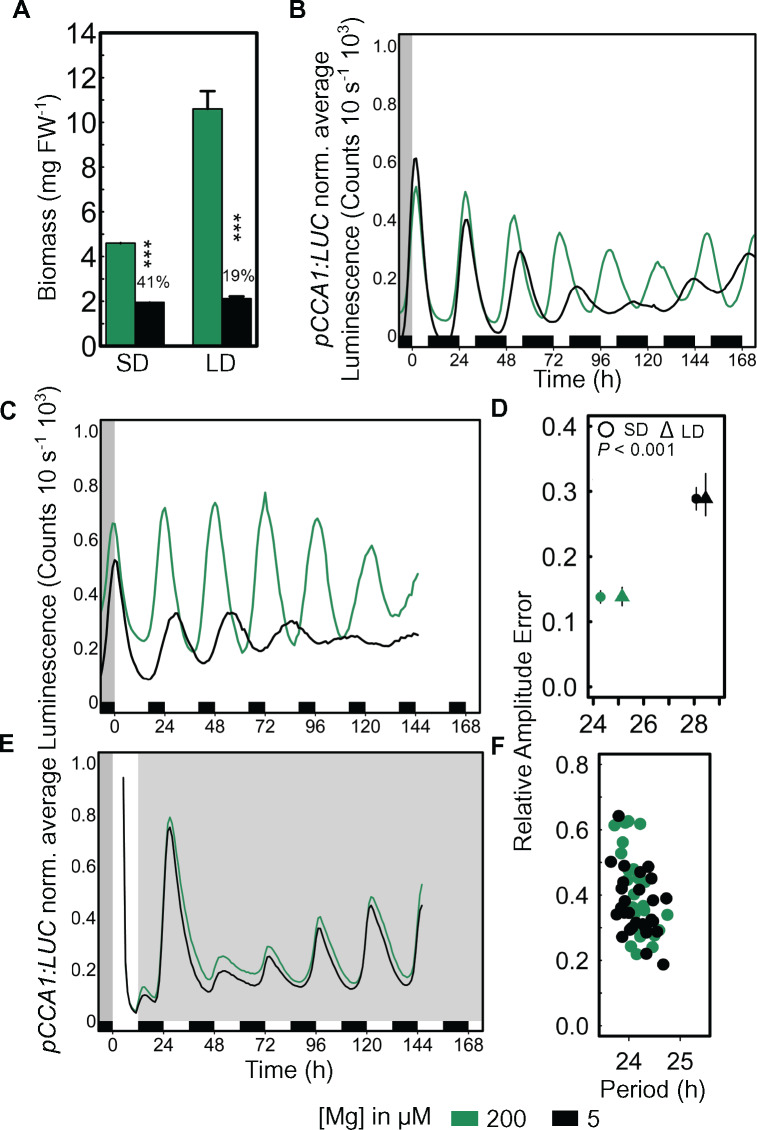
Some circadian alterations are manifested in a light conditional manner (Hicks et al., 1996). To test the role of light in the response of the circadian oscillations in Arabidopsis to Mg, seedlings were entrained to either 8-/16-h light/dark cycles (short days [SDs]) or 16-/8-h light/dark cycles (long days [LDs]). In SDs, the biomass of Mg-deficient seedlings was 59% reduced in comparison to the respective control seedlings, and in LDs the reduction was 81%. However, a longer photoperiod did not lead to a gain in biomass under Mg deficiency compared with SDs as was observed under Mg sufficiency (Figure 4, A). Circadian oscillations of pCCA1:LUC were monitored after release into LL. The effect on the circadian period was visible after 48 h in LL when seedlings were entrained to SDs (Figure 4, B), while period lengthening manifested already after 24 h when seedlings were entrained to LDs (Figure 4, C). Similar to 12-/12-h light/dark entrainment, Mg deficiency significantly increased the circadian period and the corresponding RAE of pCCA1:LUC oscillations for both entrained photoperiods in a comparable manner (Figure 4, D). Magnesium deficiency had no effect on the circadian period after plants were released to constant darkness (DD; Def: τ = 24.16 h versus Ctr: τ = 24.17 h; Figure 4, E and F), which suggests that the provoked effect of Mg deficiency on the circadian oscillator is light dependent. After seedlings were released into DD circadian activity was dampened after 48 h in darkness (Figure 4, E) but rhythms sustained for the duration of the experiment as a result of the presence of 1% sucrose in the culture medium (Supplemental Table S1; Dalchau et al., 2011), which is vital when investigating circadian oscillations under DD.
Figure 4.
Increase of circadian period due to Mg deficiency is light dependent. (A) Fresh weight (FW) biomass (mean ± SEM, n = 3–4 [1 = 4 pooled seedlings]) of seedlings entrained to SD 8-/16-h light/dark cycles and LD 16-/8-h light/dark cycles. pCCA1:LUC average normalized luminescence traces of seedlings entrained for eight days to (B) SD, (C) LD on Mg-sufficient (200 µM) and deficient (5 µM) medium before released into LL, (D) RAE of SD and LD (mean ± SEM, n = 12–48). Circadian period of SD and LD and respective RAE changes significantly between 200-µM and 5-µM Mg (P < 0.001). (E) pCCA1:LUC average normalized luminescence traces of seedlings entrained for 8 d to a 12-/12-h light/dark cycles on 200-µM and 5-µM Mg before released into DD, (F) respective RAE. Significance between Mg concentrations was verified by two-sample Student’s t test at 95% CI (***P < 0.001).
Photosynthesis inhibition does not explain the response of the circadian oscillator to Mg deficiency
The effect of Mg depletion on the circadian oscillator seems to be light dependent (Figure 4) and it is supposable that Mg deficiency hampers photosynthesis, which then gates the signal of Mg deficiency to the oscillator provoking a circadian period lengthening. To investigate whether the effect of Mg depletion was due to an inhibition of photosynthesis, we examined the effect of Mg in the presence or absence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), an inhibitor of the photochemical activity of photosystem II. Experiments were performed in the presence of 1% sucrose as including sucrose in the medium allows us to examine direct effects of photosynthetic inhibition, such as retrograde signaling, rather than effects caused by sugar depletion due to inhibited photosynthesis (Haydon, 2013). If Mg affects the circadian period when photosynthesis is inhibited by DCMU, and sugars are buffered by 1% sucrose in the medium, then the effect must occur through pathways not related to photosynthesis.
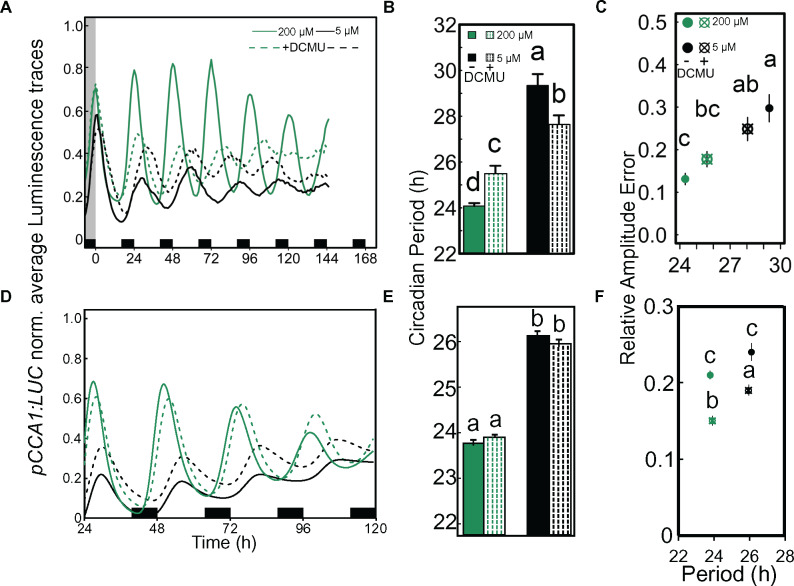
In the presence of sucrose, DCMU has little or no effect on the circadian period (Figure 5) as reported previously (Haydon et al., 2013; Takahashi et al., 2015). Magnesium deficiency profoundly affected the circadian period in the presence or absence of DCMU when sucrose was added to the medium. Hence, the effect of Mg on the oscillator might not be due to the inhibition of photosynthesis and associated downstream processes (Figure 5, B and E). The attempt to examine the effect of DCMU combined with Mg deficiency in the absence of added sucrose resulted in a strong effect of DCMU on plant performance and health making it impossible to detect circadian rhythms (Supplemental Figure S5).
Figure 5.
An active photosynthetic system is not required to detect Mg deficiency-dependent circadian alterations. Seedlings entrained for 8 d to 16-/8-h light/dark cycles on either 200-µM or 5-µM Mg media were released into LL in the presence or absence of 20-µM DCMU. The experiment was independently repeated [Experiment 1 A–C; Experiment 2 D–F]. (A) and (D) Normalized luminescence traces of pCCA1:LUC in LL, (B) and (E) Estimated circadian period (h) of pCCA1:LUC activity (mean ± SEM, n = 12), (C) and (F) RAE of pCCA1:LUC oscillations (mean ± SEM, n = 12). Statistical significance between Mg concentrations was verified by factorial ANOVA followed by Tukey’s HSD post hoc (different letters indicate significance at the level of P < 0.05). Experiments were undertaken in the laboratories at Université libre de Bruxelles (A–C) and Cambridge University (D–F).
External Mg supply is unlikely to be a zeitgeber
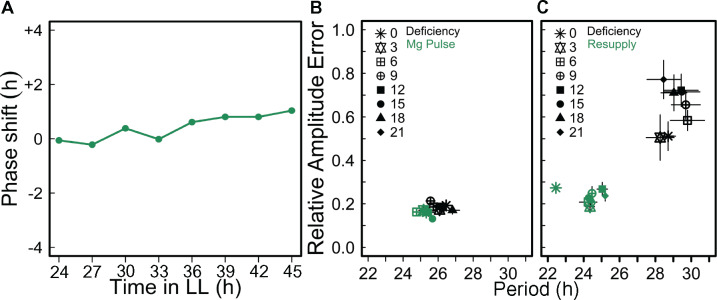
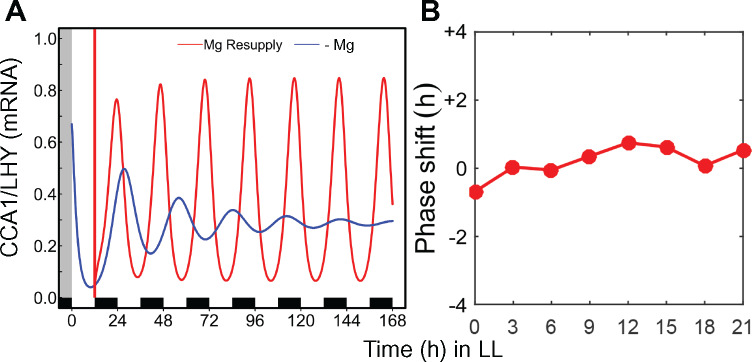
Magnesium deficiency has an effect on the circadian period (Figure 1), which can suggest an effect on entrainment. Because there are diel oscillations of Mg in green algae and in rice plastids (Feeney et al., 2016; Li et al., 2020) we tested whether Mg could act as a clock regulator in plants. A 4-h long pulse was applied with 10-mM Mg in intervals of 3 h to seedlings that had been entrained with 50-µM Mg (insufficient concentration, see Figure 1) for 8 d. Under those conditions, a Mg pulse did not cause a phase shift of pCCA1:LUC peak expression at any time point (Figure 6, A). It can be concluded that external Mg is unlikely to be a zeitgeber for the circadian clock in Arabidopsis. However, the applied Mg pulse decreased the RAE at all the time points (Figure 6, B) and full resupply of Mg to deficient seedlings restored rhythmicity independent of the time of day Mg was resupplied (Figure 6, C). When seedlings were grown on medium overly supplied with Mg, there was no effect on circadian period (Supplementary Figure S6). Thus, sufficient external Mg supply seems necessary to maintain proper functioning of the circadian oscillator but is not associated with entrainment.
Figure 6.
External applied Mg is unlikely to be a zeitgeber to set circadian time. Seedlings were entrained for 7 d to 12-/12-h light/dark cycles on medium supplied with 50-µM Mg before released into LL. A pulse of 10-mM Mg was applied during 4 h in 3-h intervals from ZT24 in LL along one circadian cycle. (A) Phase response of pCCA1:LUC activity rhythms to pulses of Mg at different time points in LL. (B) RAE of the oscillations (mean ± SEM, n = 6). (C) RAE of pCCA1:LUC oscillations of seedlings entrained on medium containing 5-µM Mg that were resupplied with 3-mM Mg every 3 h under LL conditions (means ± SEM, n = 6).
Simulation of a model of the Arabidopsis circadian oscillator predicts that Mg globally affects the kinetics of the circadian oscillator
A mathematical model (De Caluwé et al., 2016) was used to gain further insight concerning the mechanism underlying the impact of Mg nutrition on the circadian oscillator. With the default parameter values (kinetics rates), the model simulates the behavior of wild-type plants in control conditions assuming sufficient supply with Mg. First, single parameters were changed to see whether the predicted circadian period was comparable to what was observed under Mg deficiency in free-running conditions. When modeling reduced PRR5/TOC1 protein degradation rate (Supplemental Figure S7, A) or reduced PRR7/PRR9 RNA synthesis rate (Supplemental Figure S7, B), higher expression of CCA1/LHY was predicted by the model while experimentally, Mg deficiency decreased CCA1 transcript levels (Figure 3, C). Reduced RNA synthesis rate of the ELF4/LUX evening complex lowered the amplitude of CCA1/LHY mRNA oscillations but did not predict an increase in the circadian period (Supplemental Figure S7, C). These results, together with other simulations of single parameter changes did not simultaneously reproduce the decreased amplitude of CCA1 and the increased period observed experimentally. We then tested if alterations in overall kinetic parameters such as rates of transcription, translation, and protein degradation could lead to the observed tendencies. The model predicted very long free-running periods and damped oscillations in response to reduced global rates of mRNA and protein synthesis as well as protein degradation rates (Figure 7, A) resembling the experimental observations under Mg deficiency (Figure 1, A). The model predicted a restoration of amplitude and a decrease in the circadian period of CCA1/LHY activity rhythms when Mg was reintroduced (Figure 7, A), which is in accordance with experimental data (Figure 6, C) and confirms the necessity of Mg to maintain the circadian period in Arabidopsis. Thus, global rates of transcription, translation, and protein degradation are likely to be affected by Mg deficiency, provoking an increase in the circadian period.
Figure 7.
Simulation of global effect of Mg deficiency on transcription/translation rate increases the circadian period of CCA1 activity rhythms. (A) CCA1/LHY oscillations under Mg deficiency (blue) and when Mg was resupplied (red) under the assumption that Mg deficiency affects global rates of transcription and translation. Vertical red bar represents the time of simulated Mg resupply. (B) response curve to Mg pulse.
The simulated PRC based on global changes in transcription and translation rate does not predict a substantial phase shift in response to a Mg pulse (Figure 7, B), which rules out Mg as a zeitgeber for the circadian oscillator and is in line with experimental data (Figure 6, A).
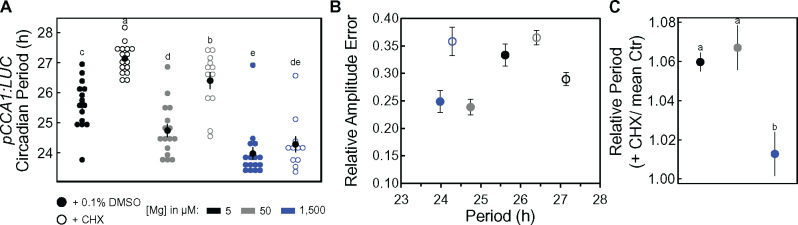
A translation perturbation assay using cycloheximide (CHX) was performed combined with different Mg concentrations to test the model’s prediction that a global decrease in translation could account for the experimental results. Thereby, the intention was to apply a mild concentration of CHX to perturb translation but not completely block it. The application of a low dose of CHX (0.5 µg mL−1) increased the circadian period of pCCA1:LUC and its respective RAE, thereby, translation perturbation had a significant effect on the circadian period under insufficient Mg supply (5- and 50-µM Mg; Figure 8, A and B). When 1,500-µM Mg was supplied, the effect of CHX on the circadian period was abolished (Figure 8, A). The relative change in the circadian period between CHX-treated and the respective control (+ dimethyl sulfoxide, DMSO) increased with low Mg concentrations but was significant only between 5- und 1,500-µM Mg (Figure 8, C). Sufficient Mg supply seems to compensate for the perturbed translation by CHX, which would support the model’s prediction that Mg deficiency has a global effect on translation, hampering the circadian oscillator.
Figure 8.
Perturbing translation increases circadian period of pCCA1:LUC. pCCA1:LUC Col-0 seedlings were entrained for 11 d to a 12-h light/dark cycle on media supplied with different Mg concentrations before released into LL. 0.5 µg mL−1 CHX (+ CHX) or 0.1% DMSO as a control (Ctr) was applied after 24 h in LL. (A) Estimated circadian period in hours (h), error bars represent SEM, and (B) RAE of pCCA1:LUC activity under LL after treatment. (C) Circadian period of +CHX relative to their respective controls (+ CHX/mean Ctr). (B) and (C): mean ± SEM, n = 12–16. Significant differences between treatments were determined by a Wilcoxon rank sum test. Different letters indicate significance at the level of P ≤ 0.05.
Discussion
Magnesium is essential for multiple processes in plants. It is highly important for photosynthesis where it is bound in the chloroplast as a key compound of the energy transfer in chlorophyll (Lilley et al., 1974; Strasser and Butler, 1977; Walker and Weinstein, 1991). Additionally, it is crucial for sucrose loading into the phloem and its partitioning from source leaves to sink plant organs. Magnesium is vital to the cellular energy metabolism sustaining the ribosome structure and is therefore important for protein translation (Chen et al., 2017). In this study, we showed that Mg deficiency increased the circadian period of pCCA1:LUC, pPRR7:LUC, and pTOC1:LUC in a dose-dependent manner in Arabidopsis seedlings under constant light and the effect on period was greater when 1% exogenous sucrose was supplied (Figure 1, A and Supplemental Figure S1). The circadian period increase due to Mg deficiency manifested already after 24 h after release into continuous light when seedlings were entrained to LD conditions (Figure 4, C). Within the experimental set up Mg was supplied as MgSO4. Under deficient conditions, Na2SO4 or K2SO4 was added to the medium to avoid sulfur deficiency following MgSO4 restriction. To exclude higher Na+ or K+ concentrations as being the cause for the observed period increase, MgCl2 was used as the Mg2+ source, which resulted in an increase of the circadian period (Supplemental Figure S8) confirming Mg depletion being the cause of the circadian period increase.
An increase of the circadian period caused by iron deficiency was shown to result from disordered photosynthetic functioning (Chen et al., 2013; Salomé et al., 2013). Sugars deriving from photosynthesis feed into the circadian clock, defining a metabolic dawn and adjusting the phase of CCA1 expression (Haydon et al., 2013). Inhibition of photosynthesis lengthens the circadian period as does constant dim light (Haydon et al., 2013). Here, circadian period lengthening caused by Mg deficiency was dependent on light signaling (Figure 4) but the observed effects were not due to direct effects of Mg deficiency on photosynthesis. In the presence of DCMU to inhibit photosynthesis and 1% sucrose to buffer changes in associated sugar production, we found that Mg deficiency had a profound effect on the circadian period of pCCA1:LUC oscillations (Figure 5). Because Mg deficiency could affect the oscillator when photosynthesis was impaired and when changes in the sugar production are buffered, we conclude that Mg might affect the oscillator through other pathways. That the effect of Mg is greater in the presence of added sucrose suggests an association with some energy dependent mechanism.
The phase of the circadian oscillator can be entrained by signals that regulate its individual components based on their temporal availability. Thereby, the individual components of the circadian oscillator are not tightly linked and the relative timing of peak expression between individual components can be plastic up to a certain degree in response to a stimulus. Such dynamic plasticity of oscillator period and phase to environmental signals enables the oscillator to keep internal time in synchrony with its environment (Webb et al., 2019). Magnesium deficiency induced a lagging in the phase of pPRR7:LUC peak expression in a 12-/12-h light/dark cycle (Figure 2 and Supplemental Figure S3), while for pCCA1:LUC and pTOC1:LUC we did not observe a Mg-sensitive response and they had a locked peak phase at dawn or dusk, respectively. Interpretation of the timing of LUC signals must be treated with caution as the peak of expression is a product of both promoter activity as well as the rate of LUC translation and protein folding. The effect of Mg on the timing of PRR7 expression is notable because prr7-11 loss of function mutants abolish the effects of nicotinamide, sugars, and light on circadian period (Farré et al., 2005; Haydon et al., 2013; Mombaerts et al., 2019). As the oscillator components are not locked to each other, this might assign PRR7 as the sensitive component during the day in the central oscillator that can respond to environmental and internal cues to adjust phase because it is not locked to the dawn and dusk zeitgebers.
Markedly, at the first time point of subjective dusk after release from continuous light, the TOC1 phase was delayed under Mg-deficient conditions (Supplemental Figure S3) probably upon the missing light-offset signal. It seems that the effect of Mg deficiency evolves over the course of the day visible in the sensitivity of the PRR7 phase in a light/dark cycle (Figure 2). During subjective night under constant light, the phase delay is carried over to the following subjective day and provokes a phase delay of CCA1 peak expression (Supplemental Figure S3). That the effect of Mg deficiency on the circadian period is light dependent and hence manifests during the day was also demonstrated in Figure 4, E, when no effect of Mg deficiency on the circadian period occurred under constant darkness clearly indicating a day dependence. Circadian oscillations in plants are generated through transcriptional/translational feedback loops whereby sucrose increases translation rates and global protein abundance (Osuna et al., 2007). Proper function of the clock does rely on a diel cycle of transcriptional control (Flis et al., 2016) plus the level of ribosomal loading driven by the circadian clock (Missra et al., 2015). Light induces proteome-wide changes in protein abundance in correlation with their transcript abundance depending on the length of the photoperiod whereby LDs increase the abundance of several photosynthetic proteins that further affected protein abundances of downstream processes (Seaton et al., 2018). Magnesium is a very important co-factor required for translation/protein synthesis (Chen et al., 2017). It stabilizes ribosomal structure (Klein et al., 2004) and is required for ribosome activity and translation (Weiss and Morris, 1973; Sperrazza and Spremulli, 1983). Magnesium deficiency might limit translation efficiency during the day, when normally the translation rate in plants is high, thereby hampering transcriptional/translational feedback loops on which the circadian oscillator depends and is visible in a PRR7 phase delay (Figure 2). However, the effect of Mg deficiency is absent under constant darkness and it is thinkable, as translation efficiency at night is lower, that limited Mg does not provoke any further visible effect. Inhibiting translation increases the circadian period of CCA1:LUC in Ostreococcus (Feeney et al., 2016) similar to the effect of Mg depletion reported here under continuous light. Feeney et al. (2016) demonstrated that a high endogenous Mg level increased translation rates in Ostreococcus and circadian oscillations of Mg levels in those cells correlate with circadian-dependent translation rates. Light signaling contributes to photoperiod-dependent changes in gene expression at dawn because of the impact of light on transcript abundance (Flis et al., 2016). An interference of transcriptional and translational processes by Mg deficiency might lengthen the period of the circadian oscillator in Arabidopsis in free-running conditions where dawn and dusk are absent as strong entrainment signals. This is in accordance with a simulated increase in the circadian period obtained by a mathematical model assuming that Mg deficiency impacts overall kinetic parameters like translation rate, transcription rate, and rates of protein degradation (Figure 7, A). In support of the model’s prediction, application of a low dose of the translation inhibitor CHX to perturb translation in Arabidopsis seedlings increased the circadian period of pCCA1:LUC activity under Mg deficiency, while sufficient supply seems to compensate for the effect of perturbed translation on the circadian period (Figure 8). Indeed, hampered translation could account for a period increase and is in accordance with results obtained in Ostreococcus and human cells (Feeney et al., 2016). Modeling Mg resupply to deficient seedlings decreased the circadian period as well as restored rhythmicity (Figure 7, A) and was also shown experimentally (Figure 6, C). Under excess Mg supply a period length of nearly 24 h was maintained (Supplemental Figure S6). Both underline the importance of Mg as a crucial cofactor for processes related to circadian timekeeping such as transcriptional/translational control.
Conclusion
Magnesium is essential for proper timekeeping in Arabidopsis. We demonstrated here that insufficient Mg supply increases the circadian period and causes a phase delay in light/dark cycles. Our data suggest that one mechanism by which Mg deficiency can affect the oscillator might be through interference with global translational/transcriptional processes. While Mg deficiency can affect circadian function we obtained no evidence that changes in Mg levels can act as zeitgeber setting circadian time.
Material and methods
Plant material and growth conditions
Arabidopsis thaliana wild-type seeds and reporter lines pCCA1:LUC, pPRR7:LUC, and pTOC1:LUC were all in the Columbia-0 ecotype background. A detailed description about the luciferase-expressing constructs is available elsewhere (Salomé and McClung, 2005). Seeds were sterilized as previously described (De Caluwé et al., 2017), individually plated on self-prepared Murashige and Skoog medium adapted from Hermans et al. (2010a), solidified with 0.5% w/v Mg-free high gel strength agar (Sigma–Aldrich, Germany), and supplied with different concentrations of Mg (Supplemental Table S1). Thereby, 1% w/v sucrose was added to the medium unless stated otherwise. After stratification for 2 d in the dark at 4°C, seedlings were entrained for 8–11 d to different photoperiods with white light (∼100 µmol photons m2 s−1) under constant temperature of 19°C (Panasonic MLR-352-PE, The Netherlands). Seedlings were entrained to the respective Mg concentration from germination on unless stated otherwise. Treatment with DCMU was done as described elsewhere (Haydon and Webb, 2016). For PRC, Mg-deprived seedlings (50 µM) were transferred to medium containing 10-mM Mg for 4 h in 3-h intervals and subsequently returned to entrainment medium (Supplemental Figure S9). For the Mg resupply assay, Mg-deprived seedlings were resupplied in 3-h intervals by transferring seedlings to medium containing 3-mM Mg. The PRC was calculated as described previously (Johnson, 1992).
Ionome profiling
Arabidopsis Col-0 seedlings were cultivated, as described above, on media supplemented with either 200-µM or 5-µM Mg from germination on. Fifteen pooled seedlings were harvested in the morning (ZT4) on days 8–12, rinsed three times in deionized water, carefully cleaned for left-over agar medium and dried at 70°C for 72 h. Dried plant material was digested in 500-µL 35% (v/v) HNO3 at 90°C for 1 h. From the digest, 200 µL was diluted in 7-mL Milli-Q water and filtered. The mineral concentrations were determined by NexION 350S ICP-MS (PerkinElmer). Indium was used as an internal standard to correct for instrument instability and oxide interferences.
Luciferase experiments and rhythms analysis
Seedlings were sprayed with 2-mM Luciferin (VivoGlo Luciferin, In Vivo Grade, Promega, The Netherlands) 1 d before they were released either into continuous light or dark. Following the dosing, seedlings were individually transferred to 96-well opaque white microplates (23,300, Berthold, Germany) containing 150 µL of the respective liquid growth medium plus 2-mM d-Luciferin. Microplates were sealed with transparent EASYseal sealing film 120 × 80 mm (Greiner bio-one, Germany) that was punctured (∼1.0-mm Ø) to allow gas exchange. Luminescence was detected at 590 nm for 5–10 s hourly after 120 s in darkness using a multimode microplate reader (TriStar2 LB942 Berthold, Germany). Data were processed with MikroWin software v. 5.21 (Labsis Laborsysteme, Germany) and rhythms of LUC activity were analyzed with BioDare2 beta (https://biodare2.ed.ac.uk/; Zielinski et al., 2014). Luminescence traces were normalized to the highest value. Period and RAE estimates were calculated on rhythms between 24 and 120 h in continuous light on non-normalized data using Fast-527 Fourier Transformed Non-Linear Least Squares (FFT-NLLS) after linear detrending.
RNA isolation and reverse transcription quantitative PCR (RT-qPCR)
Total RNA was isolated from 100 mg frozen ground tissues of whole seedlings. RNA was purified with Maxwell 16 LEV Plant RNA Kit (Promega, Benelux BV) using Maxwell 16 AS2000 Instrument (Promega) according to the manufacturer’s recommendation. Quality and purity of the samples were verified with a NanoDrop 2000 UV–Vis Spectrophotometer (Thermo Scientific, Loughborough, UK). cDNA was synthesized from 1-µg RNA with GoScript Reverse Transcription System (Promega) and thereafter diluted to 1:30 (v/v) with autoclaved nuclease-free water for quantitative real-time PCR (qPCR). qPCR was carried out in 96-well microplates in the PikoReal real-time PCR system (Thermo Scientific). Each reaction contained 5 µL of 2× SYBR Green mastermix (Promega), 2.5 µL of primer mix (forward and reverse, 2.5 µM each), and 2.5-µL of 1:30 diluted cDNA. Thermocycles were as follows: pre-incubation at 95°C for 3 min, 40 cycles at 95°C for 30 s, and 60°C for 1 min. ELONGATION FACTOR 1α (EF1α, At5g60390), UBIQUITIN 10 (UBQ10, At4g05320), and CYCLIN-DEPENDENT KINASE A;1 (CDKA, At3g48750) were used as reference genes. Oligo nucleotide sequences and their respective efficiencies are given in Supplemental Table S2.
Mathematical modeling
A previously developed model (De Caluwé et al., 2016) of the core oscillator was used to investigate the mechanisms of Mg input to the clock. The model consists of eight differential equations describing the evolution of mRNA and protein concentrations of four pairs of circadian clock genes: CCA1/LHY, PRR9/PRR7, PRR5/TOC1, and ELF4/LUX. The kinetic parameters represent synthesis rates, degradation rates, and enzymatic constants. The full equations and parameter values for the model are described elsewhere in detail, along with a description of the building and optimization process (De Caluwé et al., 2016). We used the original parameter values to represent plants fully supplied with Mg. The intermediate- and low-Mg conditions were modeled by simulating by reducing the mRNA and protein synthesis rates by a factor of 0.5 (intermediate Mg, Figure 7, B) or 0.35 (low Mg, Figure 7, A blue curve), and the protein degradation rates by 0.7 (intermediate Mg, Figure 7, B) or 0.65 (low Mg, Figure 7, A blue curve). The periods of the Mg-deficient clock were around 26 (intermediate Mg) or 28.5 h (low Mg). For modeling single parameters as a Mg input, we multiplied specific kinetic parameters by 0.2 (representing a reduction of 80% of their value).
To construct the PRC, entrainment cycles were simulated on either intermediate-Mg or low-Mg conditions and, thereafter, Mg resupply was simulated in continuous light by restoring all parameters to their initial values. The circadian phase was calculated as previously described (Johnson 1992). Phase shifts were defined as the difference in circadian phase between the first peaks of the treated and control simulations. All simulations were performed in MATLAB (Mathworks, Cambridge). The integration of the differential equations used the external CVODES solver.
Translation perturbation assay
For the assay, clusters of 8–10 pCCA1:LUC Col-0 seeds were sown in plastic rings sealed at the base with 0.5-µm nylon mesh on solid media (0.5% w/v Mg-free agar) supplemented with 1% w/v sucrose containing different concentrations of Mg. Growth conditions were the same as described earlier. Eleven-day-old seedlings were dosed with 2-mM luciferin and luminescence was detected for 800 s hourly under LL with a Nightshade CCD camera and imaging chamber (Berthold). The translation inhibitor was applied just before subjective dawn of the second day of LL by transferring seedlings to liquid media with the respective Mg supply containing 0.5 µg mL−1 CHX (dissolved in DMSO) or 0.1% w/v DMSO as solvent control. Captured data were processed with IndiGO software (Berthold) and rhythms of pCCA1:LUC were analyzed with BioDare2 beta (https://biodare2.ed.ac.uk/) as described earlier. Replicates with a RAE ≥0.5 were excluded from statistical analysis. A two-way analysis of variance (ANOVA) was performed followed by Wilcoxon Rank Sum test to determine whether CHX and/or Mg deficiency significantly alter the circadian period with a confidence interval (CI) of 95%.
Statistical analysis
All statistical analyses performed in this study were carried out in R software version 3.4.1. First, the distribution of the residuals was checked using the functions hist and shapiro.test (packages graphics and stats, respectively). Homogeneity of variances across groups was tested with Levene’s test (leveneTest from package car). Parametric statistics were performed (in experiments with normally distributed and homoscedastic residuals) by using two-sample Student’s t test with 95% CI (t.test function from package stats) to compare the means between two groups of values. One-way ANOVA or factorial ANOVA were used to compare the means between more than two groups (aov function from package stats). Tukey’s “Honest Significant Difference” (HSD) method was performed to generate a CI on the differences between multiple means being compared with either ANOVA test (TukeyHSD function from package stats). For non-parametric statistics, Kruskal–Wallis Rank Sum test followed by Nemenyi post hoc test were performed (kruskal.test and posthoc.kruskal.nemenyi.test functions from packages stats and “PMCMR,” respectively).
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database or the GenBank/EMBL libraries under the following accession numbers: CCA1 (AT2G46830); LHY1 (AT1G01060); PRR9 (AT2G46790); PRR7 (AT5G02810); PRR5 (AT5G24470); TOC1 (AT5G61380); ELF4 (AT2G40080); LUX (AT3G46640); and CAX3 (AT3G51860).
Supplemental data
Supplemental Figure S1. Limiting Mg availability increases the circadian period in A. thaliana seedlings.
Supplemental Figure S2. Limited external N availability does not alter the circadian period.
Supplemental Figure S3. Mg deficiency induced a lagging phase of PRR7 during entrainment.
Supplemental Figure S4. Young Arabidopsis seedlings successfully cope with lower Mg concentrations than the usually supplied in the growth medium.
Supplemental Figure S5. DCMU treatment without external supply of sucrose hampers circadian oscillations of pCCA1:LUC.
Supplemental Figure S6. Excess Mg supply maintains the circadian period in A. thaliana seedlings.
Supplemental Figure S7. Modeling predictions and translation inhibition assay.
Supplemental Figure S8. Adding MgCl2 as external Mg source increased the circadian period of pCCA1:LUC.
Supplemental Figure S9. Experimental design of PRC.
Supplemental Table S1. Composition of modified MS medium for in vitro plant culture
Supplemental Table S2. List of primers used for quantification of transcript levels
Supplementary Material
Acknowledgments
The authors thank Dr. C. Robertson McClung (Dartmouth College) for providing the luciferase reporter seeds. They also thank Dr. Natsuko Kobayashi and Keitaro Tanoi (University of Tokyo) for the ICP-MS facility. Finally, they thank Dr. Michael Haydon (University of Melbourne) and Dr. John O’Neill, for insightful discussions about luminescence and PRC data.
Funding
This research was funded by the Actions de Recherche Concertée (ARC 2012–2017), French community of Belgium, Fonds national de la Recherche scientifique (F.R.S.-FNRS) (PDR T 0085.16 2016–2020; CH is F.R.S.-FNRS research associate), ‘Fonds Emile DEFAY and Fonds d’Encouragement à la Recherche as well as Foundation Wiener-Anspach at ULB.
Conflict of interest statement. None declared.
J.-C.L., C.H., A.A.R.W., and N.V. conceived the project and acquired the funding. D.G., C.H., N.V., A.A.R.W., A.G., and J.R.F.d.M. designed the experiments. J.R.F.d.M. and A.G. performed the experiments and analyzed the data, and wrote the manuscript to which all co-authors contributed. T.D.C., D.G., and J.R.F.d.M. designed and analyzed the modeling data. T.D.C. carried out the numerical simulations. All authors participated in the data analysis discussions.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys) is: Nathalie Verbruggen (nverbru@ulb.ac.be).
References
- Akanuma G, Yamazaki K, Yagishi Y, Iizuka Y, Ishizuka M, Kawamura F, Kato-Yamada Y (2018) Magnesium suppresses defects in the formation of 70S ribosomes as well as in sporulation caused by lack of several individual ribosomal proteins. J Bacteriol 200:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabadí D, Yanovsky MJ, Más P, Harmer SL, Kay SA (2002) Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr Biol 12:757–761 [DOI] [PubMed] [Google Scholar]
- Andrés-Colás N, Perea-García A, Puig S, Peñarrubia L (2010) Deregulated copper transport affects Arabidopsis development especially in the absence of environmental cycles. Plant Physiol 153:170–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I, Hengeler C, Marschner H (1994) Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J Exp Bot 45:1251–1257 [Google Scholar]
- De Caluwé J, de Melo JRF, Tosenberger A, Hermans C, Verbruggen N, Leloup JC, Gonze D (2017) Modeling the photoperiodic entrainment of the plant circadian clock. J Theor Biol 420:220–231 [DOI] [PubMed] [Google Scholar]
- De Caluwé J, Xiao Q, Hermans C, Verbruggen N, Leloup JC, Gonze D (2016) A compact model for the complex plant circadian clock. Front Plant Sci 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-Y, Wang Y, Shin L-J, Wu J-F, Shanmugam V, Tsednee M, Lo J-C, Chen C-C, Wu S-H, Yeh K-C (2013) Iron is involved in the maintenance of circadian period length in Arabidopsis. Plant Physiol 161:1409–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZC, Peng WT, Li J, Liao H (2017) Functional dissection and transport mechanism of magnesium in plants. Semin Cell Dev Biol 74:142–152 [DOI] [PubMed] [Google Scholar]
- Clark FW, Washington HS (1924) The Composition of the Earth’s Crust. U.S. Government Printing Office, Washington [Google Scholar]
- Cowan JA (1998) Metal activation of enzymes in nucleic acid biochemistry. Chem Rev 98:1067–1087 [DOI] [PubMed] [Google Scholar]
- Culkin F, Cox RA (1966) Sodium, potassium, magnesium, calcium and strontium in sea water. Deep Res 13:789–804 [Google Scholar]
- Dalchau N, Baek SJ, Briggs HM, Robertson FC, Dodd AN, Gardner MJ, Stancombe MA, Haydon MJ, Stan GB, Gonçalves JM, et al. (2011) The circadian oscillator gene GIGANTEA mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proc Natl Acad Sci U S A 108:5104–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Luo W, Xu G (2006) Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann Appl Biol 149:111–123 [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AA (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309:630–633 [DOI] [PubMed] [Google Scholar]
- Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96:271–290 [DOI] [PubMed] [Google Scholar]
- Farré EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA (2005) Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr Biol 15:47–54 [DOI] [PubMed] [Google Scholar]
- Feeney KA, Hansen LL, Putker M, Olivares-Yañez C, Day J, Eades LJ, Larrondo LF, Hoyle NP, O’Neill JS, van Ooijen G (2016) Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 532:375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish LE, Deshaies R, Jagendorf AT, Section PB (1983) A Mg2+ requirement for rapid ATP-driven protein synthesis by intact pea chloroplasts. Plant Sci Lett 31:139–146 [Google Scholar]
- Fleischer M (1954) The abundance and distribution of the chemical elements in the Earth’s crust. J Chem Educ 31:446–455 [Google Scholar]
- Flis A, Sulpice R, Seaton DD, Ivakov AA, Liput M, Abel C, Millar AJ, Stitt M (2016) Photoperiod-dependent changes in the phase of core clock transcripts and global transcriptional outputs at dawn and dusk in Arabidopsis. Plant Cell Environ 39:1955–1981 [DOI] [PubMed] [Google Scholar]
- Green RM, Tingay S, Wang ZY, Tobin EM (2002) Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol 129:576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenham K, McClung CR (2015) Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet 16:598–610 [DOI] [PubMed] [Google Scholar]
- Grzebisz W (2009) Magnesium—food and human health. J Elem 16: 299–323 [Google Scholar]
- Gutiérrez RA, Stokes TL, Thum K, Xu X, Obertello M, Katari MS, Tanurdzic M, Dean A, Nero DC, McClung CR, et al. (2008) Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc Natl Acad Sci U S A 105:4939–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL (2009) The circadian system in higher plants. Annu Rev Plant Biol 60:357–377 [DOI] [PubMed] [Google Scholar]
- Hauer-Jákli M, Tränkner M (2019) Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: a systematic review and meta-analysis from 70 years of research. Front Plant Sci 10:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AAR (2013) Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502:689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon MJ, Román Á, Arshad W (2015) Nutrient homeostasis within the plant circadian network. Front Plant Sci 6:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon MJ, Webb AAR (2016) Assessing the impact of photosynthetic sugars on the Arabidopsis circadian clock. Environ Responses Plants Methods Mol Biol 1398: 133–140. (doi: 10.1007/978-1-4939-3356-3_12) [DOI] [PubMed] [Google Scholar]
- Hermans C, Johnson GN, Strasser RJ, Verbruggen N (2004) Physiological characterisation of magnesium deficiency in sugar beet: acclimation to low magnesium differentially affects photosystems I and II. Planta 220:344–355 [DOI] [PubMed] [Google Scholar]
- Hermans C, Verbruggen N (2005) Physiological characterization of magnesium deficiency in Arabidopsis thaliana. J Exp Bot 56:2153–2161 [DOI] [PubMed] [Google Scholar]
- Hermans C, Bourgis F, Faucher M, Strasser RJ, Delrot S, Verbruggen N (2005) Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 220:541–549 [DOI] [PubMed] [Google Scholar]
- Hermans C, Conn SJ, Chen J, Xiao Q, Verbruggen N (2013) An update on magnesium homeostasis mechanisms in plants. Metallomics 5:1170. [DOI] [PubMed] [Google Scholar]
- Hermans C, Vuylsteke M, Coppens F, Craciun A, Inzé D, Verbruggen N (2010a) Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol 187:119–131 [DOI] [PubMed] [Google Scholar]
- Hermans C, Vuylsteke M, Coppens F, Cristescu SM, Harren FJM, Inzé D, Verbruggen N (2010b) Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol 187:132–144 [DOI] [PubMed] [Google Scholar]
- Hicks KA, Millar AJ, Carré IA, Somers DE, Straume M, Meeks-Wagner DR, Kay SA (1996) Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science 274:790–792 [DOI] [PubMed] [Google Scholar]
- Hong S, Kim SA, Guerinot M Lou, Robertson McClung C (2013) Reciprocal interaction of the circadian clock with the iron homeostasis network in Arabidopsis. Plant Physiol 161:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AAR (2007) Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ 30:333–349 [DOI] [PubMed] [Google Scholar]
- Hsu PY, Harmer SL (2014) Wheels within wheels: the plant circadian system. Trends Plant Sci 19:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH (1992) Phase response curves: what do they tell use about circadian clocks?, In: T Hiroshige, K Honma, eds, Circadian Clocks from Cell to Human, Hokkaido University Press, Japan, pp 209–249 [Google Scholar]
- Kamiya T, Yamagami M, Hirai MY, Fujiwara T (2012) Establishment of an in planta magnesium monitoring system using CAX3 promoter-luciferase in Arabidopsis. J Exp Bot 63:355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DJ, Moore PB, Steitz TA (2004) The contribution of metal ions to the structural stability of the large ribosomal subunit. RNA 10:1366–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt LS (1954) The role of magnesium in photosynthesis. Science 120:33–35 [DOI] [PubMed] [Google Scholar]
- Li J, Yokosho K, Liu S, Cao HR, Yamaji N, Zhu XG, Liao H, Ma JF, Chen ZC (2020) Diel magnesium fluctuations in chloroplasts contribute to photosynthesis in rice. Nat Plants 6:848–859 [DOI] [PubMed] [Google Scholar]
- Lilley RM, Holborow K, Walker DA (1974) Magnesium activation of photosynthetic CO2-fixation in a reconstituted chloroplast system. New Phytol 73:657–662 [Google Scholar]
- McClung CR (2006) Plant circadian rhythms. Plant Cell 18:792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missra A, Ernest B, Lohoff T, Jia Q, Satterlee J, Ke K, Von Arnim AG (2015) The circadian clock modulates global daily cycles of mRNA ribosome loading. Plant Cell 27:2582–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts L, Carignano A, Robertson FC, Hearn TJ, Junyang J, Hayden D, Rutterford Z, Hotta CT, Hubbard KE, Maria MRC, et al. (2019) Dynamical differential expression (DyDE) reveals the period control mechanisms of the Arabidopsis circadian oscillator. PLOS Comput Biol 15:e1006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Gibon Y, Bläsing OE, Höhne M, Günter M, Kamlage B, Trethewey R, Scheible W-R, et al. (2007) Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J 49:463–491 [DOI] [PubMed] [Google Scholar]
- Robertson FC, Skeffington AW, Gardner MJ, Webb AAR (2009) Interactions between circadian and hormonal signalling in plants. Plant Mol Biol 69:419–427 [DOI] [PubMed] [Google Scholar]
- Römheld V (2012) Diagnosis of deficiency and toxicity of nutrients. In: Marschner P, ed., Mineral Nutrition of Higher Plants, 3rd edn, Academic Press, London, UK , pp 299–311 [Google Scholar]
- Salomé PA, McClung CR (2005) Pseudo-response regulator 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell 17:791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé PA, Oliva M, Weigel D, Krämer U (2013) Circadian clock adjustment to plant iron status depends on chloroplast and phytochrome function. EMBO J 32:511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton DD, Graf A, Baerenfaller K, Stitt M, Millar AJ, Gruissem W (2018) Photoperiodic control of the Arabidopsis proteome reveals a translational coincidence mechanism. Mol Syst Biol 14:e7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperrazza JM, Spremulli LL (1983) Quantitation of cation binding to wheat germ ribosomes: influences on submit association equilibria and ribosome activity. Nucleic Acids Res 11:2665–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser RJ, Butler WL (1977) Energy transfer and the distribution of excitation energy in the photosynthetic apparatus of spinach chloroplasts. Biochim Biophys Acta 460:230–238 [DOI] [PubMed] [Google Scholar]
- Sweeney BM, Folli SI (1984) Nitrate deficiency shortens the circadian period in Gonyaulax. Plant Physiol 75:242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Hirata Y, Aihara K, Mas P (2015) A hierarchical multi-oscillator network orchestrates the Arabidopsis circadian system. Cell 163:148–159 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C (2013) Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 368:87–99 [Google Scholar]
- Walker CJ, Weinstein JD (1991) Further characterization of the magnesium chelatase in isolated developing cucumber chloroplasts. Plant Physiol 95:1189–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Guan KL, Roach PJ, DePaoli-Roach AA (1995) Phosphorylation and activation of the ATP-Mg-dependent protein phosphatase by the mitogen-activated protein kinase. J Biol Chem 270:18352–18358 [DOI] [PubMed] [Google Scholar]
- Webb AAR (2003) The physiology of circadian rhythms in plants. New Phytol 160:281–303 [DOI] [PubMed] [Google Scholar]
- Webb AAR, Seki M, Satake A, Caldana C (2019) Continuous dynamic adjustment of the plant circadian oscillator. Nat Commun 10:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RL, Morris DR (1973) Cations and ribosome structure. I. Effects of the 30S subunit of substituting polyamines for magnesium ion. Biochemistry 12:435–441 [DOI] [PubMed] [Google Scholar]
- Zielinski T, Moore AM, Troup E, Halliday KJ, Millar AJ (2014) Strengths and limitations of period estimation methods for circadian data. PLoS ONE 9:e96462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.