Abstract
Formation of primordial follicles occurs when germ cell nests break apart and individual oocytes become surrounded by pregranulosa cells. Why mammalian germ cells develop in germ cell nests is not fully understood but recent work has provided evidence that some oocytes serve as nurse cells supporting other oocytes in the cyst. Headway has also been made in understanding interactions that occur between cyst cells that must change as individual oocytes separate to associate with pregranulosa cells. As germ cell nests undergo breakdown some oocytes are lost by programmed cell death that has been attributed to apoptosis, but newer studies have implicated autophagy in counteracting apoptosis to promote cell survival and maintain the ovarian reserve. Work in the past few years has added to already known pathways regulating primordial follicle formation and has identified new players including signaling molecules, transcription factors and RNA binding proteins.
Keywords: Primordial Follicle Formation, Nest Breakdown, Germ Cell Cysts, Oocyte Survival, Ovarian Reserve
Introduction- The Process of Primordial Follicle Formation
The major events leading up to the formation of primordial follicles in the mouse are displayed in Figure 1. At 10.5 days post coitum (dpc) the primordial germ cells complete their migration and arrive at the genital ridge [1]. Subsequently, the genital ridge undergoes sexual differentiation to adopt an ovarian fate and primordial germ cells become oogonia [2]. Clusters of up to 30 proliferating oogonia are observed connected by intercellular bridges due to incomplete cytokinesis and are referred to as germ cell cysts [3, 4]. During and subsequent to cyst formation, cysts can fragment into smaller cysts and reassociate with unrelated cysts to form nests where some cells are still connected by intercellular bridges with others associated by aggregation [4] and see Figure 2. At 13.5 dpc, oogonia stop mitotic division and transition into meiosis becoming oocytes. Oocytes progress through the substages of meiotic prophase one and arrest in the diplotene stage for an extended period of time [5]. At 17.5 dpc, oocyte apoptosis initiates leading to two-thirds of the pool of oocytes dying [6]. Concurrently oocytes within nests begin to separate from each other either through completion of abscission or due to mechanical stress from the somatic cells invading the nests to surround individual oocytes to form primordial follicles. [7]. Some headway has been made in understanding how primordial follicle formation is regulated [2, 8, 9] but our understanding is far from complete. This review will focus on recent findings in understanding cyst function, the role of autophagy, additional regulatory mechanisms and new ‘omics’ approaches to understand how the ovarian reserve consisting of individual diplotene arrested oocytes contained in primordial follicles becomes established.
Figure 1.
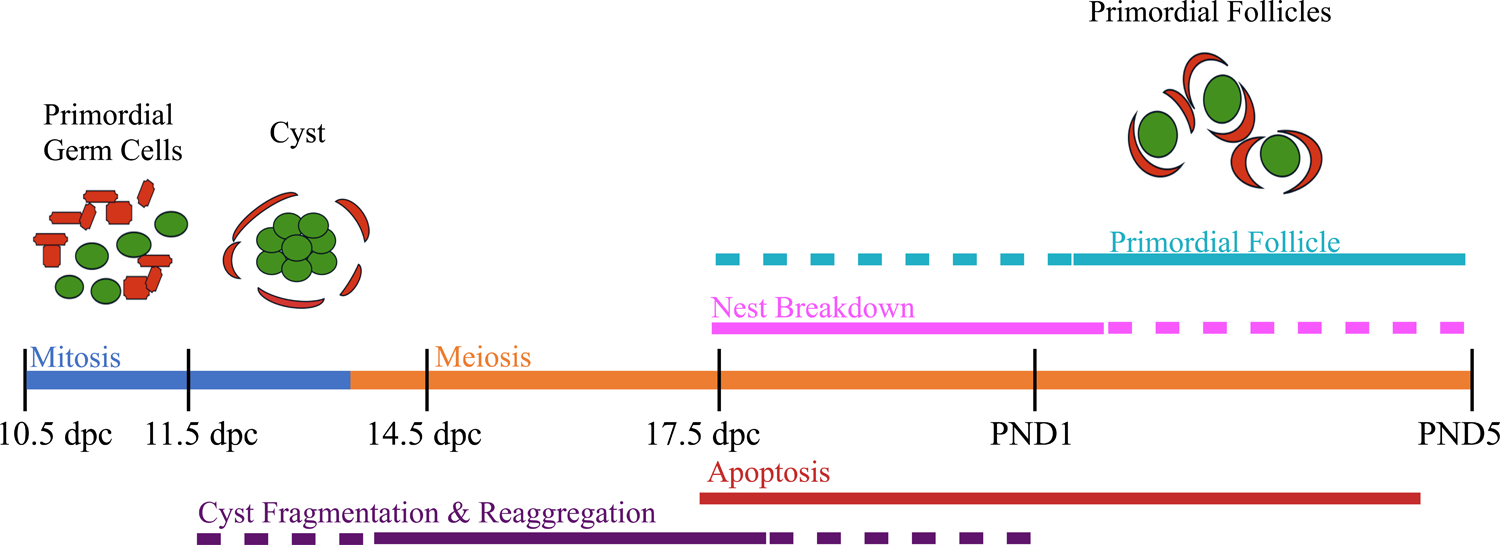
Major events during primordial germ cell differentiation leading to primordial follicle formation in the mouse. Abbreviations: days post coitum (dpc) and postnatal day (PND).
Figure 2.
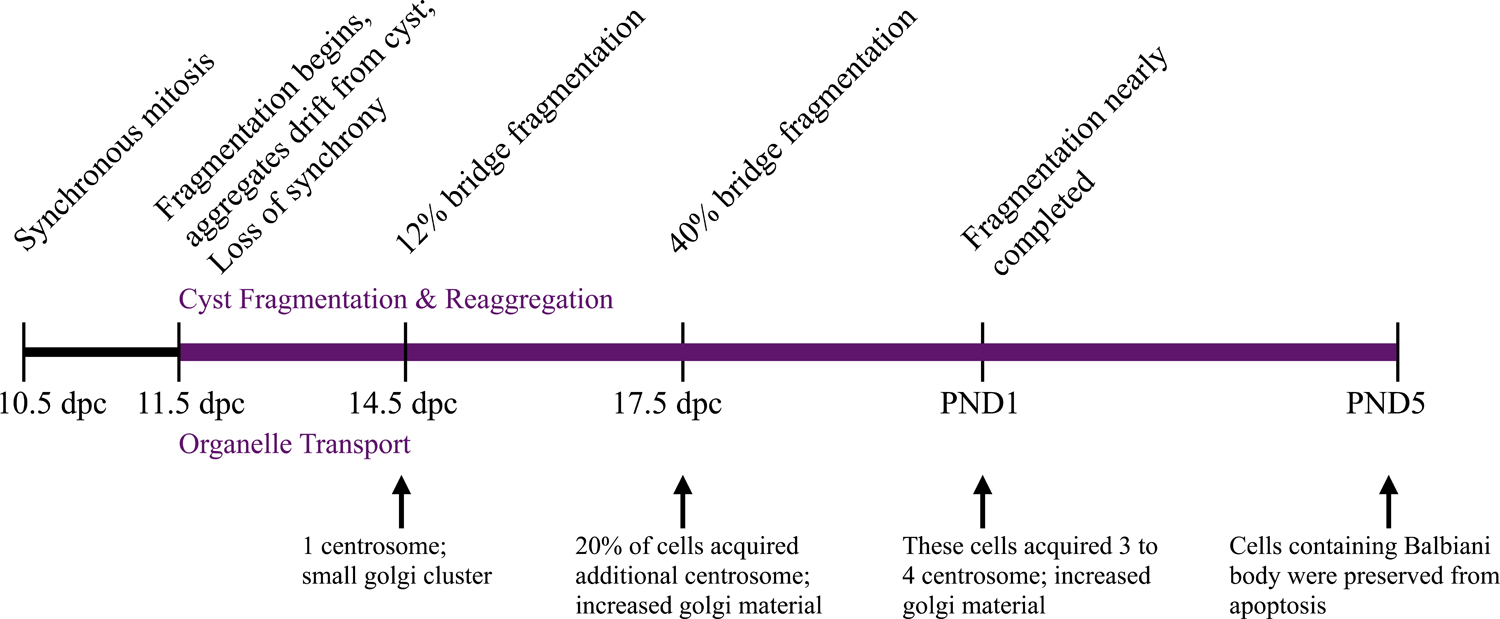
Cyst Fragmentation, Reaggregation and Organelle Transport in Female Germ Cells. Timing of cyst fragmentation and reaggregation shown above timeline. Timing of organelle movement through intercellular bridges shown below timeline.
Function and Maintenance of Germ Cell Cysts
The formation of germ cell cysts is a prominent mechanism in oogenesis and though several potential functions for cysts have been proposed their importance in mouse germ cell development is unclear [7, 10]. Most of our knowledge regarding germ cell cysts stems from studies in Drosophila where the precursor germ cell termed cystoblast proliferates to form a 16 cell cyst of interconnected cells [11]. One cell in the cyst becomes the oocyte while the remaining 15 cells sacrifice their organelles, mRNAs and proteins to the oocyte and eventually undergo programmed cell death. Recently, with organelle labeling and cell lineage tracing, Lei and Spradling have provided evidence that some of the germ cells in each cyst serve as nurse cells providing organelles to their sister germ cells [12]. Specifically, Golgi, centrosomes and mitochondria were found to accumulate in only some of the oocytes within a cyst. Eventually, these oocytes became larger, did not express cell death markers, and organelles coalesced to form a Balbiani body (a structure indicative of oocyte differentiation). In addition, inhibition of microtubules in fetal ovary organ culture by colchicine or inhibition of the microtubule motor protein dynein resulted in reduced organelle transport supporting the idea of transport through intercellular bridges occurs across microtubules mediated by associated motor proteins [7, 12, 13]. Their work has not only shown evidence of dying oocytes acting as nurse cells but has given use a detailed timeline of cyst fragmentation, reaggregation and organelle transport as seen in Figure 2. For further discussion of mouse nurse cells and organelle transport see [14] and [15].
Growing evidence in the past few years has revealed the importance of cell adhesion molecules both in maintaining cyst architecture as well as primordial follicle structure. Recent studies in the mouse show down regulation of endogenous E-cadherin protein expression during primordial follicle formation [16]. The same study also revealed the connection between c-Jun amino-terminal kinase (JNK) signaling and E-cadherin showing E-cadherin protein levels remained high when JNK signaling was inhibited causing cyst breakdown to be impeded. They found this was due to JNK increasing the expression of Mouse double minute 2 (MDM2), a RING finger-containing E3 enzyme that has been reported to regulate E-cadherin protein levels during follicle formation. Additionally, E-cadherin has been reported to play a role in maintaining primordial follicle structure by facilitating cell-cell adhesion with pregranulosa cells [17]. On top of supporting cyst and primordial follicle formation, E-cadherin has displayed an importance in maintaining the survival of oocytes. Specifically, knockdown of E-cadherin in neonatal ovaries caused oocyte apoptosis in turn decreasing the pool of primordial follicles [18]. They further found that this was through E-cadherin regulating NOBOX, a transcription factor required for regulating oocyte specific gene expression. Another study also addressed the importance of E-cadherin in maintaining oocyte survival using Cre-recombinase techniques. Somatic cell and oocyte specific knockouts of E- cadherin were generated using OCT4 and SF1 driven Cre recombinases that are active starting at 11.5 dpc [18]. Both strains lost a significant number of oocytes starting at 16.5 dpc and oocytes that remained were not assembled into primordial follicles. Oocyte and somatic cell specific knockouts of N-cadherin were also generated and had similar phenotypes to the E-cadherin cell specific knockouts [19]. The phenotypes of these mouse lines suggest that both E-cadherin and N-cadherin are required for oocyte survival prior to primordial follicle formation and for interactions between the oocyte and the granulosa cells when the follicles are assembled. However, effects on germ cell cysts before primordial follicle formation were not reported. In addition, the effects of E-cadherin or N-cadherin on follicle formation may be indirect and generation of mutants that remove E-cadherin or N-cadherin just before primordial follicle formation would be telling.
There have been major milestones in our understanding of the purpose, function and maintenance of mammalian germ cell cysts and nests in the ovary. In the past few years, we have learned more about organelle transport between germ cells in cysts supporting the idea that some of the cysts cells serve as nurse cells. We have also gained a better understanding of the role of cell adhesion in maintaining the structure of both germ cells cysts and primordial follicles.
Autophagy During Primordial Follicle Formation
Findings supporting the role of cadherin junctions in follicle formation and oocyte loss show how intertwined each process is. On top of errors in signaling or possible meiotic damage during primordial follicle formation, the pool of oocyte quantity plummets to 20% of the original pool. Apoptosis is thought to be the prime mechanism of oocyte death during this transition [20]. The BCL2 family of apoptotic regulators, specifically BAX and MCL1, have been implicated in regulating apoptosis during cyst breakdown [21, 22]. Besides apoptosis, recent reports in the past decade have revealed the function of autophagy to promote oocyte survival during this period [23]. The function of autophagy is to remove defective organelles from the cell. The organelles become enclosed in an autophagosome which then fuses with a lysosome and the contents are degraded. In some contexts, autophagy promotes cell survival, while in others, this mechanism can promote cell death by apoptosis.
Autophagy was first implicated as a mechanism of oocyte death during primordial follicle formation when it was found that germ cell survival was affected in autophagy gene mutants. A null mutation in Atg7, an autophagy gene, resulted in almost 100% loss of germ cells, perinatally while heterozygous mutants of another autophagy gene, Becn1 lost approximately 50% of their oocytes, however, effects on the process of follicle formation were not addressed [24, 25]. In the past few years, several reports have inferred that autophagy regulates oocyte survival and primordial follicle formation though different groups report different or conflicting results that appear to depend on several variables including the mouse strain used, the method of inducing or inhibiting autophagy, the concentration of the agent used or the time of exposure (Table 1).
Table 1.
Studies reporting varying effects of autophagy on primordial follicle formation.
| Author | Mouse Strain | Autophagy | System | Treatment | Time | Primordial Follicle Formation | Oocyte Number |
|---|---|---|---|---|---|---|---|
| Sun et al., 2020 | CD1 | activate | Organ culture | 100 uM Rapamycin | PND 0–3 | increased | no change |
| Sun et al., 2018 | CD1 | activate | Organ culture | 300 nM Rapamycin | PND* 0–3 | reduced | increased |
| Zhang et al., 2017 | CD1 | activate | Organ culture | 250 nM Rapamycin | 18.5 – 19.5 dpc | reduced | no change |
| Zhihan et al., 2019 | K | activate | Organ culture | 100 nM Rapamycin | 17.5 dpc-PND 3 | no change | no change |
| Sun et al., 2018 | CD1 | inhibit | Organ culture | 10 mM 3-MA | PND* 0–3 | increased | reduced |
| Sun et al., 2020 | CD1 | inhibit | Organ culture | 1 mM 3-MA | PND 0–3 | reduced | no change |
| Zhihan et al., 2019 | K | inhibit | Organ culture | 1 mM 3-MA | 17.5 dpc-PND 3 | reduced | no change |
| Zhihan et al., 2019 | K | inhibit | In vivo injection | 15ug/gram body weight/ day 3-MA | PND 0–3 | reduced | no change |
| Sun et al., 2020 | CD1 | activate | In vivo | starvation | PND 0–1.5 | increased | increased (but n=1) |
| Watanabe & Kimura, 2018 | B6 | activate | In vivo | starvation | PND 0.5–1.5 | increased | not reported |
| Wang et al 2017 | CD1 | activate | In vivo | starvation | PND 1.5–3 | reduced | no change |
| Sun et al., 2020 | CD1 | activate | In vivo | starvation | PND 0–2 | no change | no change |
| Sun et al., 2020 | CD1 | activate | Organ culture | miR378–3 overexpression | PND 0–3 | increased | no change |
| Watanabe et al., 2020 | B6 | activate | In vivo injection | TAT-Beclin 1 D11 peptide | PND 0–2.5 | increased | no change |
| Sun et al., 2020 | CD1 | inhibit | Organ culture | miR378–3 inhibition | PND 0–3 | reduced | no change |
started culture at 17.5 dpc for 2 days then treated for the following 3 days to the equivalent of PND 3.
The immunosuppressant drug, Rapamycin is a known inducer of autophagy while 3-methyadenosine (3-MA) inhibits autophagy. These compounds have been tested in perinatal ovarian organ culture to examine effects on primordial follicle formation. For both compounds, effects vary depending on the concentration used. A relatively high concentration of Rapamycin (100 μM) promotes primordial follicle formation [26] while lower concentrations (300 nM, 250 nM) have the opposite effect [27, 28] and an even lower concentration (100 nM) has no effect [29]. When high concentrations of the autophagy inhibitor 3-MA (10 mM) were used primordial follicle formation was increased [27] while lower concentrations (1 mM) or in vivo injection reduced primordial follicle formation [26, 29].
As the developing mouse undergoes a transition from the fetal to the neonatal stage, there is a period of severe starvation due to loss of the placental nutrient supply and the conversion to lactation nutrient supply. During this time, starvation upregulates autophagy which is thought to compensate for the loss of nutrients [30]. Several studies have investigated effects of this phenomenon and of the extension of starvation for up to 36 hours after birth with a focus on primordial follicle formation. When the normal starvation period is extended to PND 1, autophagy continues to be upregulated and primordial follicle formation is promoted [26, 31]. However, if the mice are starved from PND 1.5 to 3 primordial follicle formation is reduced [32] or if from PND 0 to 2 there is no effect [26]. Additionally, Sun and colleagues found that at first autophagy was upregulated up to 1.5 days of starvation but when continued to 2 days (the limit for neonatal survival) apoptosis was induced in the ovary. This suggests there is a delicate balance between survival and death regulated by autophagy and apoptosis. Further investigation is warranted to fully understand the role of autophagy during primordial follicle formation.
Specific molecules that regulate autophagy have begun to be examined in primordial follicle formation. The microRNA, miR378-3 is upregulated during primordial follicle formation and increases autophagy. Overexpression of miR378-3 in neonatal ovary organ culture increases primordial follicle formation while knockdown decreases primordial follicle formation [26]. A strikingly similar result was observed using another microRNA, miR92b-3 [33]. Another group used an autophagy inducer called Tat-beclin 1 D-11 which contains a peptide from the autophagy activating region of BECLIN 1 [34]. This inducer also upregulated primordial follicle formation when injected into neonatal female mice. Studies have overall inferred that autophagy is important for cell survival during follicle formation but recent conflicting reports question to what extent is autophagy a cell survival mechanism. Future work investigating specific autophagic regulators are needed to complete our understanding of the function of autophagy.
Signaling Pathways Regulating the Formation of Primordial Follicles
Many signaling pathways like the JNK pathway discussed previously, have now been associated with the regulation of primordial follicle formation. Multiple reviews have summarized these pathways [2, 8, 9, 35] and will not be discussed here. However, three signaling pathways, Retinoic acid (RA), Receptor tyrosine kinases (RTKs) and Notch have been the focus of several recent reports.
RA signaling is known to initiate meiosis in female germ cells, but less is known about its role in meiotic progression through prophase I and primordial follicle formation. Lanosterol 14 α-demethylase (CYP51) is required for meiotic resumption prior to ovulation by catalyzing the synthesis of meiosis activating sterol (MAS). Recently, Mu and colleagues investigated the function of CYP51 using cultured CD1 mouse ovaries and showed evidence supporting the idea that RA promoted the change in CYP51 localization from the cytoplasm to the nucleus and that CYP51 is important for meiotic progression [36]. Specifically, inhibition of CYP51 in cultured fetal ovaries delayed meiotic progression at the transition from the zygotene to the pachytene stage and reduced primordial follicle assembly. In 2020, another group used an inhibitor of RA synthesis to investigate meiotic progression and primordial follicle assembly in CD1 mice again using fetal ovary organ culture [37]. However, in this case the opposite effect was found with accelerated meiotic progression and primordial follicle formation even though in both studies RA signaling was reduced. The same mouse strain, CD1, was used for both studies but in the 2020 report, ovary culture was started one day earlier than the Mu et al. report (13.5 dpc vs. 14.5 dpc) so perhaps the difference in results is an issue of timing.
CYP51 was originally identified for its role in meiotic resumption prior to ovulation in adult ovaries. Studies identifying new roles for essential proteins such as CYP51, supports the notion that additional analysis of other critical proteins during primordial follicle formation is needed. For example, signaling through RTKs is known to play a role in primordial follicle formation and activation. The RTK, KIT when modulated in organ culture can affect primordial follicle formation [38]. Additional studies injecting the pharmacological inhibitor, Imatinab into neonatal rats resulted in a reduction in primordial follicle formation as well as activation. Imatinib blocks not only KIT but several other receptors as well including c-ABL and PDGFR suggesting other RTKs in addition to KIT signaling play roles during this phase [39].
Aside from focusing on signaling in oocyte, Notch signaling is specifically active in the pregranulosa cells and has been found to regulate primordial follicle formation. Somatic cell knockouts of Notch2 possess large numbers of germ cell nests and only few primordial follicles. [40]. Two proteins upstream of NOTCH2 have been identified in primordial follicle formation. Caveolin 1 (CAV1), a protein important for vesicle trafficking was detected in the pregranulosa cells of fetal ovaries [41]. Further, knockdown of Cav1 in ovary organ culture resulted in decreased primordial follicle formation, decreased somatic cell proliferation and downregulation of NOTCH2. Additional studies by the same group when investigating signaling through the JAK pathway found that pharmacological inhibition of JAK2 or JAK3 using organ culture, reduced primordial follicle formation [42]. In addition, JAK3 inhibition reduced somatic cell proliferation and downregulated NOTCH2. The existing heterogeneity of the mechanisms surrounding follicle formation are clearly intertwined. Recent studies such as the signaling pathways described above support the idea of revisiting already identified essential proteins and re-evaluating their roles during primordial follicle formation.
Transcriptional and Translational Regulation Controlling Assembly of Follicles
Multiple transcription factors and RNA binding proteins have recently been identified in the regulation of primordial follicle formation (Table 2). Oocyte specific transcriptional regulators, FIGLA, LHX8 and SOHLH1 play pivotal roles in primordial follicle formation and are summarized in several reviews [2, 8, 9, 35]. Global gene expression profiles of perinatal ovaries obtained through RNA-sequencing analysis was employed to assess FIGLA and LHX8 targets by comparing transcripts from Figla and Lhx8 null mutant mice to wild type animals and [43] to Sohlh1 mutant transcripts reported from a different study [44]. A striking overlap of transcripts down regulated in mutants for each of these genes suggested they might be part of a regulatory network controlling oogenesis. Using immunohistochemistry and co-IP, Wang and colleagues found FIGLA, LHX8 and SOHLH1 were coexpressed and physically interact with each other in oocytes. They put forth a model of the three proteins forming a nuclear complex that coordinates the program of oocyte development.
Table 2.
RNA Binding Proteins and Transcription Factors recently implicated in Primordial Follicle Formation
| Protein | Location | Known Function | Findings | Reference |
|---|---|---|---|---|
| DAZL | Oocytes | RBP essential for meiosis entry and progression |
|
[48] |
| DDX6 | Oocytes | RNA Helicase |
|
[49] |
| ELAVL2 | Oocytes | RBP, targets DDX6 |
|
[49] |
| FIGLA | Oocytes | Transcription Factor |
|
[43] |
| IRX3 |
Somatic Cells- during nest stage Oocytes- nest breakdown becomes oocyte specific post follicle formation |
Transcription Factor |
|
[45, 46] |
| IRX5 |
Somatic Cells- during nest stage and post follicle formation, extinguishes before onset of puberty Oocytes- during nest breakdown |
Transcription Factor |
|
[45, 46] |
| LHX8 | Oocytes | Transcription Factor |
|
[43] |
| SOHLH1 | Oocytes | Transcription Factor |
|
[43] |
| SP1 | Oocytes and Somatic Cells | Transcription Factor |
|
[47] |
Numerous reports have revealed several transcription factors not only in oocytes but pregranulosa cells are important for primordial follicle formation. Iroquois homeobox transcription factor family members IRX3 and IRX5 have also been proposed to be crucial for establishing interactions between the oocyte and surrounding pregranulosa cells as follicles form. Prior to primordial follicle formation, IRX3 and IRX5 localize specifically to the pregranulosa cells surrounding germ cell cysts and are upregulated in oocytes during primordial follicle formation [45]. Eventually, IRX5 expression is lost completely and IRX3 continues to be expressed only in oocytes. Double mutants still generate primordial follicles that can develop into primary follicles, but there are fewer oocyte-granulosa cell gap junctions and granulosa cells extend fewer transzonal projections suggesting a loss of oocyte-granulosa cell communication. In a follow up study, Fu and colleagues assessed the role the somatic cell contribution of IRX3 and IRX5 and found reduced follicle development, ovulation and fertility [46]. The authors suggest that the expression of IRX3 and IRX5 during primordial follicle formation is important to set up oocyte-granulosa cells interactions later in the adult to support fertility.
Another transcription factor, SP1, a specific protein/ Krüppel-like factor (Sp/KLF) family member, is expressed in both oocytes and pregranulosa cells during primordial follicle formation. Utilizing a lentivirus shRNA to knockdown Sp1 specifically in pregranulosa cells, primordial follicle formation was significantly reduced and the number of FOXL2+ pregranulosa was decreased [47]. Thus, in addition to oocyte specific transcription regulators, transcription factors expressed in somatic cells such as SP1 or IRX 3 and IRX5 are important for the formation of follicles.
Post transcriptional RNA regulation is another mechanism used to control gene expression in the ovary. RNA binding proteins (RBPs) can bind to specific mRNAs and promote their translation. Deleted in azoospermia-like (DAZL) is a germ cell specific RBP that has been shown to be necessary for entry and progression through meiosis. Mutations in Dazl result in the loss of germ cells during fetal development precluding analysis of primordial follicle formation. However, recently, knockdown of Dazl after the onset of meiosis utilizing siRNA in ovary organ culture exhibited a reduction of primordial follicle formation [48]. In addition, expression of Testis-expressed protein (TEX) 14, a component of the intercellular bridges connecting oocytes within germ cell cysts, was reduced in the Dazl knockdown ovaries. Furthermore, DAZL protein was found to bind to the 3’ UTR of Tex14 mRNA and promote its translation suggesting that siRNA knockdown of Dazl caused a loss of TEX14 protein and impaired function of intercellular bridges necessary to promote primordial follicle formation.
Two additional RBPs, Embryonic Lethal and Abnormal Vision-Like 2 (ELAVL2) and DDX6 were found to be important for primordial follicle formation [49]. ELAVL2 was expressed in perinatal oocytes and assembly of primordial follicles was severely reduced in Elavl2 mutants. ELAVL2 associated with and was able to promote Ddx6 mRNA translation. DDX6 is a component of P-bodies (cytoplasmic granules important for mRNA processing and decay) in somatic cells. Kato and colleagues detected DDX6 in P-body-like granules in oocytes both from nests and primordial follicles. Oocyte specific deletion of Ddx6 reduced primordial follicle formation and P-body-like granule assembly. The authors propose that ELAVL2 directs the assembly of these granules and that the granules play an important role during primordial follicle formation. Previous studies have identified a cytoplasmic structure called the Balbiani body [12, 50] but the relationship between the Balbiani body and the P-body-like structure is unknown. Discovery of several RBPs with roles in follicle formation highlights the importance of translational regulation during this stage.
“Omics” Approaches to Understanding how Primordial Follicles Are Made
The study described in the previous section investigating the oocyte specific proteins, FIGLA, LHX8 and SOHLH1 [43] illustrates the increasing importance of utilizing transcriptomic and proteomic approaches to understand developmental processes. Sophisticated approaches have been used in the past few years and are summarized in Table 3. For example, the developmental differences between the medullary region verses the cortical region of the ovary are unclear. To address this question, Niu and Spradling employed Single Cell RNA-sequencing (SC RNA-seq) to compare the pregranulosa cell transcriptome of primordial follicles in the medullary region of the developing ovary to the cortical region from 11.5 dpc to PND6 [51]. This approach indicated the two populations of pregranulosa cells were similar but not identical and gave an insight as to how their differentially expressed genes may be candidates to control wave one and wave two follicle progression in the mouse ovary. Bipotential derived pregranulosa cells (BPG) were located in the medullar region and are thought to assist in rapid direct follicle development. BPG cells were shown to express more androgen degrading enzymes which could be how they promote direct development of the medullar follicles before the follicles in the cortex. The follicles in the cortex contain epithelial derived pregranulosa (EPG) cells that begin to differentiate around 14.5 dpc. After birth, Lgr5 and Aldh1a2 RNA levels are higher in EPG cells compared to BPG cells. Interestingly, Aldh1a2 encodes an RA synthase which stimulates gonadal cells to produce FOXL2, ESR2 and WNT4.
Table 3.
Recent Transcriptomic and Proteomic Studies providing insight into Primordial Follicle Formation
| Transcriptomics | Proteomics |
|---|---|
|
Method: RNA sequencing on mouse ovaries |
Method: LCMS/MS, GO and KEGG pathway analysis on mouse ovaries |
Results:
|
Results:
|
| Reference: [43] | Reference: [52] |
|
Method: SC-RNA sequencing on mouse pre-granulosa cells. |
Method: 2D-DIGE and MALDI-TOF MS on pig ovaries |
Results:
|
Results:
|
| Reference: [51] | Reference: [53] |
Tandem mass tags (TMT) labeling with 2D Liquid Chromatography Mass Spectroscopy (LCMS/MS) was utilized in a recent analysis of proteomic profiling on whole ovaries during primordial follicle formation and activation [52]. Proteins that are expressed in oocytes, specifically ZP2, ZP3, PAD16, FIGLA and OOEP were less abundant at PND2, during primordial follicle formation compared to PND8, when primordial follicle formation is completed suggesting significant differences in regulation during formation and activation. Additionally, analyzing cellular metabolism during primordial follicle formation and activation using KEGG pathway analysis showed pyruvate metabolism, glycolysis/gluconeogenesis, and fat digestion and absorption involved in energy metabolism were upregulated at both PND2 and PND8, suggesting energy metabolism may be related to primordial follicle formation and activation. Finally, pathway analysis suggested that protein degradation was inhibited and protein synthesis was increased during both primordial follicle formation and activation.
Another interesting proteomic analysis comparing primordial follicle formation and activation was performed via two-dimensional difference in-gel electrophoresis (2D-DIGE) and Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) on porcine ovaries [53]. Their results showed members of the CREC family as well as reticulocalbin-1 (RNC1), and RCN3 are regulators of primordial follicle formation. Actin along with heterogeneous nuclear ribonucleoprotein K (HNRNPK) which is a protein involved in the mRNA processing, mRNA transport and chromatin remodeling were also shown to be expressed in the ovaries during primordial follicle formation. As for primordial follicle activation, heat shock protein (HSPA2) and gelsolin, an actin binding protein involved in cell growth and other functions, are upregulated during this time. Interestingly, gelsolin has also been shown to negatively regulate apoptotic expression when over expressed and promote apoptosis when down regulated. The advances in sophisticated techniques to analyze the transcriptome and proteome allows us to quickly elucidate regional and temporal differences that occur in the ovary as follicles form.
Concluding Remarks
Primordial follicle formation is essential to establish the ovarian reserve needed for fertility. Understanding why germ cells develop in structures such as cysts or nests will improve our understanding of prerequisites needed for primordial follicle formation. Revisiting signaling pathways, genes and proteins known to be essential in many developmental processes will lead to discovery of new roles in the assembly of primordial follicles. Continuing to uncover crucial molecules using state of the art ‘omic’ techniques will improve our knowledge of regulatory pathways important for formation of primordial follicles that make up the ovarian reserve.
Acknowledgments
The authors thank Suzanne Getman and Joshua Burton for critical proofreading of the manuscript.
Funding:
Our research is supported by the National Institutes of Health (R15 HD 099859).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None.
References
- [1].Molyneaux KA, Stallock J, Schaible K, Wylie C, Time-lapse analysis of living mouse germ cell migration, Dev Biol 240(2) (2001) 488–98 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- [2].Pepling ME, Follicular assembly: mechanisms of action, Reproduction 143(2) (2012) 139–49 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- [3].Pepling ME, Spradling AC, Female mouse germ cells form synchronously dividing cysts, Development 125(17) (1998) 3323–8. [DOI] [PubMed] [Google Scholar]
- [4].Lei L, Spradling AC, Mouse primordial germ cells produce cysts that partially fragment prior to meiosis, Development 140(10) (2013) 2075–81 10.1242/dev.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cohen PE, Pollack SE, Pollard JW, Genetic analysis of chromosome pairing, recombination, and cell cycle control during first meiotic prophase in mammals, Endocr Rev 27(4) (2006) 398–426 10.1210/er.2005-0017. [DOI] [PubMed] [Google Scholar]
- [6].Pepling ME, Sundman EA, Patterson NL, Gephardt GW, Medico L Jr., Wilson KI, Differences in oocyte development and estradiol sensitivity among mouse strains, Reproduction 139(2) (2010) 349–57 REP-09-0392. [DOI] [PubMed] [Google Scholar]
- [7].Pepling ME, Spradling AC, Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles, Dev Biol 234(2) (2001) 339–51 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- [8].Grive KJ, Freiman RN, The developmental origins of the mammalian ovarian reserve, Development 142(15) (2015) 2554–63 10.1242/dev.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pepling ME, From primordial germ cell to primordial follicle: mammalian female germ cell development, Genesis 44(12) (2006) 622–32 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- [10].Pepling ME, de Cuevas M, Spradling AC, Germline cysts: a conserved phase of germ cell development?, Trends Cell Biol 9(7) (1999) 257–62. [DOI] [PubMed] [Google Scholar]
- [11].de Cuevas M, Lilly MA, Spradling AC, Germline cyst formation in Drosophila, Annu Rev Genet 31 (1997) 405–28 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- [12].Lei L, Spradling AC, Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells, Science 352(6281) (2016) 95–9 10.1126/science.aad2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cox RT, Spradling AC, A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis, Development 130(8) (2003) 1579–90 10.1242/dev.00365. [DOI] [PubMed] [Google Scholar]
- [14].Ikami K, Nuzhat N, Lei L, Organelle transport during mouse oocyte differentiation in germline cysts, Curr Opin Cell Biol 44 (2017) 14–19 10.1016/j.ceb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- [15].Pepling M, Lei L, Oocyte Nests/Germline Cysts, in: Yan W, McCarrey J (Eds.), Encyclopedia of Reproduction, Elsevier; 2018. [Google Scholar]
- [16].Niu W, Wang Y, Wang Z, Xin Q, Wang Y, Feng L, Zhao L, Wen J, Zhang H, Wang C, Xia G, JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice, Development 143(10) (2016) 1778–87 10.1242/dev.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Piprek RP, Kolasa M, Podkowa D, Kloc M, Kubiak JZ, Tissue-specific knockout of E-cadherin (Cdh1) in developing mouse gonads causes germ cells loss, Reproduction 158(2) (2019) 147–157 10.1530/REP-18-0621. [DOI] [PubMed] [Google Scholar]
- [18].Yan H, Wen J, Zhang T, Zheng W, He M, Huang K, Guo Q, Chen Q, Yang Y, Deng G, Xu J, Wei Z, Zhang H, Xia G, Wang C, Oocyte-derived E-cadherin acts as a multiple functional factor maintaining the primordial follicle pool in mice, Cell Death Dis 10(3) (2019) 160 10.1038/s41419-018-1208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors show that knockdown of E-cadherin results in loss of primordial follicles and a reduction of cell contacts between the oocyte and surrounding pre-granulosa cells.
- [19].Piprek RP, Kolasa M, Podkowa D, Kloc M, Kubiak JZ, N-Cadherin Is Critical for the Survival of Germ Cells, the Formation of Steroidogenic Cells, and the Architecture of Developing Mouse Gonads, Cells 8(12) (2019) 10.3390/cells8121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grive KJ, Pathways coordinating oocyte attrition and abundance during mammalian ovarian reserve establishment, Mol Reprod Dev 87(8) (2020) 843–856 10.1002/mrd.23401. [DOI] [PubMed] [Google Scholar]
- [21].Jones RL, Pepling ME, Role of the antiapoptotic proteins BCL2 and MCL1 in the neonatal mouse ovary, Biol Reprod 88(2) (2013) 46 10.1095/biolreprod.112.103028. [DOI] [PubMed] [Google Scholar]
- [22].Greenfeld CR, Pepling ME, Babus JK, Furth PA, Flaws JA, BAX regulates follicular endowment in mice, Reproduction 133(5) (2007) 865–76 133/5/865. [DOI] [PubMed] [Google Scholar]
- [23].Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF, Multiple mechanisms of germ cell loss in the perinatal mouse ovary, Reproduction 137(4) (2009) 709–20 10.1530/REP-08-0203. [DOI] [PubMed] [Google Scholar]
- [24].Gawriluk TR, Hale AN, Flaws JA, Dillon CP, Green DR, Rucker EB 3rd, Autophagy is a cell survival program for female germ cells in the murine ovary, Reproduction 141(6) (2011) 759–65 10.1530/REP-10-0489. [DOI] [PubMed] [Google Scholar]
- [25].Song ZH, Yu HY, Wang P, Mao GK, Liu WX, Li MN, Wang HN, Shang YL, Liu C, Xu ZL, Sun QY, Li W, Germ cell-specific Atg7 knockout results in primary ovarian insufficiency in female mice, Cell Death Dis 6 (2015) e1589 10.1038/cddis.2014.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sun X, Klinger FG, Liu J, De Felici M, Shen W, Sun X, miR-378-3p maintains the size of mouse primordial follicle pool by regulating cell autophagy and apoptosis, Cell Death Dis 11(9) (2020) 737 10.1038/s41419-020-02965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; *A starvation model is used to induce autophagy for one and a half days and leads to increased follicle formation. Extention of starvation to two days lead to a switch from autophagy to apoptosis, in part explaining conflicting results from previous studies.
- [27].Sun YC, Wang YY, Sun XF, Cheng SF, Li L, Zhao Y, Shen W, Chen H, The role of autophagy during murine primordial follicle assembly, Aging (Albany NY) 10(2) (2018) 197–211 10.18632/aging.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang J, Liu W, Sun X, Kong F, Zhu Y, Lei Y, Su Y, Su Y, Li J, Inhibition of mTOR Signaling Pathway Delays Follicle Formation in Mice, J Cell Physiol 232(3) (2017) 585–595 10.1002/jcp.25456. [DOI] [PubMed] [Google Scholar]
- [29].Zhihan T, Xinyi M, Qingying L, Rufei G, Yan Z, Xuemei C, Yanqing G, Yingxiong W, Junlin H, Autophagy participates in cyst breakdown and primordial folliculogenesis by reducing reactive oxygen species levels in perinatal mouse ovaries, J Cell Physiol 234(5) (2019) 6125–6135 10.1002/jcp.27367. [DOI] [PubMed] [Google Scholar]
- [30].Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N, The role of autophagy during the early neonatal starvation period, Nature 432(7020) (2004) 1032–6 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- [31].Watanabe R, Kimura N, Non-suckling starvation of neonatal mice promotes primordial follicle formation with activation of ovarian autophagy, J Reprod Dev 64(1) (2018) 89–94 10.1262/jrd.2017-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang YY, Sun YC, Sun XF, Cheng SF, Li B, Zhang XF, De Felici M, Shen W, Starvation at birth impairs germ cell cyst breakdown and increases autophagy and apoptosis in mouse oocytes, Cell Death Dis 8(2) (2017) e2613 10.1038/cddis.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li T, Liu X, Gong X, Q. E, X. Zhang, X. Zhang, microRNA 92b-3p regulates primordial follicle assembly by targeting TSC1 in neonatal mouse ovaries, Cell Cycle 18(8) (2019) 824–833 10.1080/15384101.2019.1593648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Watanabe R, Sasaki S, Kimura N, Activation of autophagy in early neonatal mice increases primordial follicle number and improves lifelong fertilitydagger, Biol Reprod 102(2) (2020) 399–411 10.1093/biolre/ioz179. [DOI] [PubMed] [Google Scholar]
- [35].Wang C, Zhou B, Xia G, Mechanisms controlling germline cyst breakdown and primordial follicle formation, Cell Mol Life Sci 74(14) (2017) 2547–2566 10.1007/s00018-017-2480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mu X, Wen J, Chen Q, Wang Z, Wang Y, Guo M, Yang Y, Xu J, Wei Z, Xia G, Yang M, Wang C, Retinoic acid-induced CYP51 nuclear translocation promotes meiosis prophase I process and is correlated to the expression of REC8 and STAG3 in mice, Biol Open 7(11) (2018) 10.1242/bio.035626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rosario R, Stewart HL, Walshe E, Anderson RA, Reduced retinoic acid synthesis accelerates prophase I and follicle activation, Reproduction 160(3) (2020) 331–341 10.1530/REP-20-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Inhibition of retinoid acid synthesis in fetal ovary organ culture promoted primordial follicle formation and enhanced meiotic prophase I progression.
- [38].Jones RL, Pepling ME, KIT signaling regulates primordial follicle formation in the neonatal mouse ovary, Dev Biol 382(1) (2013) 186–97 10.1016/j.ydbio.2013.06.030. [DOI] [PubMed] [Google Scholar]
- [39].Asadi-Azarbaijani B, Santos RR, Jahnukainen K, Braber S, van Duursen MBM, Toppari J, Saugstad OD, Nurmio M, Oskam IC, Developmental effects of imatinib mesylate on follicle assembly and early activation of primordial follicle pool in postnatal rat ovary, Reprod Biol 17(1) (2017) 25–33 10.1016/j.repbio.2016.11.003. [DOI] [PubMed] [Google Scholar]
- [40].Xu J, Gridley T, Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles, BMC biology 11 (2013) 13 10.1186/1741-7007-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huang K, Dang Y, Zhang P, Shen C, Sui X, Xia G, Qin Y, Jiao X, Wang C, Huo R, Chen ZJ, CAV1 regulates primordial follicle formation via the Notch2 signalling pathway and is associated with premature ovarian insufficiency in humans, Hum Reprod 33(11) (2018) 2087–2095 10.1093/humrep/dey299. [DOI] [PubMed] [Google Scholar]
- [42].Huang K, Wang Y, Zhang T, He M, Sun G, Wen J, Yan H, Cai H, Yong C, Xia G, Wang C, JAK signaling regulates germline cyst breakdown and primordial follicle formation in mice, Biol Open 7(1) (2018) 10.1242/bio.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang Z, Liu CY, Zhao Y, Dean J, FIGLA, LHX8 and SOHLH1 transcription factor networks regulate mouse oocyte growth and differentiation, Nucleic Acids Res 48(7) (2020) 3525–3541 10.1093/nar/gkaa101. [DOI] [PMC free article] [PubMed] [Google Scholar]; *RNA-seq analysis was used to compare transcripts from wild type mice and Figla. Lhx8 and Sohlh1 mutants and overlapping sets of mRNAs were downregulated in each mutant. The proteins encoded by these three genes were coexpressed in oocytes and could physically interact.
- [44].Shin YH, Ren Y, Suzuki H, Golnoski KJ, Ahn HW, Mico V, Rajkovic A, Transcription factors SOHLH1 and SOHLH2 coordinate oocyte differentiation without affecting meiosis I, J Clin Invest 127(6) (2017) 2106–2117 10.1172/JCI90281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fu A, Oberholtzer SM, Bagheri-Fam S, Rastetter RH, Holdreith C, Caceres VL, John SV, Shaw SA, Krentz KJ, Zhang X, Hui CC, Wilhelm D, Jorgensen JS, Dynamic expression patterns of Irx3 and Irx5 during germline nest breakdown and primordial follicle formation promote follicle survival in mouse ovaries, PLoS Genet 14(8) (2018) e1007488 10.1371/journal.pgen.1007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fu A, Koth ML, Brown RM, Shaw SA, Wang L, Krentz KJ, Zhang X, Hui CC, Jorgensen JS, IRX3 and IRX5 collaborate during ovary development and follicle formation to establish responsive granulosa cells in the adult mousedagger, Biol Reprod 103(3) (2020) 620–629 10.1093/biolre/ioaa100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cai H, Liu B, Wang H, Sun G, Feng L, Chen Z, Zhou J, Zhang J, Zhang T, He M, Yang T, Guo Q, Teng Z, Xin Q, Zhou B, Zhang H, Xia G, Wang C, SP1 governs primordial folliculogenesis by regulating pregranulosa cell development in mice, J Mol Cell Biol 12(3) (2020) 230–244 10.1093/jmcb/mjz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rosario R, Crichton JH, Stewart HL, Childs AJ, Adams IR, Anderson RA, Dazl determines primordial follicle formation through the translational regulation of Tex14, FASEB J 33(12) (2019) 14221–14233 10.1096/fj.201901247R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kato Y, Iwamori T, Ninomiya Y, Kohda T, Miyashita J, Sato M, Saga Y, ELAVL2-directed RNA regulatory network drives the formation of quiescent primordial follicles, EMBO Rep 20(12) (2019) e48251 10.15252/embr.201948251. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study demonstrates that the RNA binding protein, ELAVL2 promotes primordial follicle formation and regulates the translation of DDX6, a P-body component in somatic cells. Further, DDX6 is localized to P-body-like granules in oocytes which may play an important role in follicle formation.
- [50].Pepling ME, Wilhelm JE, O’Hara AL, Gephardt GW, Spradling AC, Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body, Proc Natl Acad Sci U S A 104(1) (2007) 187–92 10.1073/pnas.0609923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Niu W, Spradling AC, Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary, Proc Natl Acad Sci U S A 117(33) (2020) 20015–20026 10.1073/pnas.2005570117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Xiong J, Wu M, Zhang Q, Zhang C, Xiong G, Ma L, Lu Z, Wang S, Proteomic analysis of mouse ovaries during the prepubertal stages, Exp Cell Res 377(1–2) (2019) 36–46 10.1016/j.yexcr.2019.02.016. [DOI] [PubMed] [Google Scholar]
- [53].Xu M, Che L, Yang Z, Zhang P, Shi J, Li J, Lin Y, Fang Z, Che L, Feng B, Wu S Xu, Proteomic Analysis of Fetal Ovaries Reveals That Primordial Follicle Formation and Transition Are Differentially Regulated, Biomed Res Int 2017 (2017) 6972030 10.1155/2017/6972030. [DOI] [PMC free article] [PubMed] [Google Scholar]


