Abstract
Pregnant women with heart disease are vulnerable to many adverse cardiovascular events (AE). AEs during and after pregnancy continue to be important causes of maternal mortality and morbidity worldwide, with huge variations in burden in different countries and regions. These AEs are classified as having direct or indirect causes, depending on whether they are directly caused by pregnancy or due to some pre-existing disease and/or non-obstetric cause, respectively.
The risks continue throughout pregnancy and even after childbirth. Apart from immediate complications during pregnancy, there is increasing evidence of a significant link between several events and the risk of cardiovascular disease (CVD) later in life.
A significant number of pregnancy-related deaths caused by cardiovascular disease are preventable. This prevention can be realized through increasing awareness of cardiovascular AE in pregnancy, coupled with the application of strategies for prevention and treatment. Knowledge of the risks associated with CVD and pregnancy is of extreme importance in that regard.
We discuss the global distribution of cardiovascular maternal mortality, adverse events during and after pregnancy, their predictors and risk stratification. In addition, we enumerate possible solutions, particularly the role of cardio-obstetric clinics.
Introduction
Pregnant women with heart disease have pronounced vulnerability to adverse cardiovascular events. Diagnosis and treatment of heart disease in pregnancy is difficult due to similarities between disease manifestations and normal physiological changes.
Cardiovascular adverse events (AEs), during and after pregnancy continue to be important causes of maternal mortality and morbidity worldwide, with huge variations in burden in different countries and regions.1,2 These AEs are classified as having direct or indirect causes, depending on whether they are directly caused by pregnancy or due to some pre-existing disease and/or non-obstetric cause, respectively.3–5
Cardiovascular disease is the single largest cause of indirect maternal mortality,3,6 accounting for over 33% of pregnancy-related maternal deaths.6–11 Additionally, maternal heart disease complicates up to 4% of pregnancies12–14 and up to 16% of pregnancies in women with previous cardiac conditions,13 with risk depending on the underlying cardiac condition.13,15–17
Over 50% of maternal deaths occur post-partum.18 Late maternal mortality is defined as death more than 42 days (and up to one year) after child birth.19 Importantly, the currently cited figures almost certainly constitute an underestimate.19
It is estimated that up to 68% pregnancy-related deaths caused by cardiovascular conditions are preventable.6,20 This can be achieved, through increasing awareness, coupled with applying strategies for prevention and treatment. Knowledge of the risks associated with CVD and pregnancy is of extreme importance in that regard.
We discuss the global distribution of cardiovascular maternal mortality, the adverse events during and after pregnancy, their predictors and risk stratification. In addition, we enumerate possible solutions, particularly the role of cardio-obstetric clinics.
Incidence and global distribution of maternal mortality
According to the World Health Organization (WHO)21 and Global Burden of Disease (GBD),1,2 in 2017 there were up to 295,000 maternal deaths globally, leading to an estimated global maternal mortality rate (MMR) of 211 per 100,000 live births.
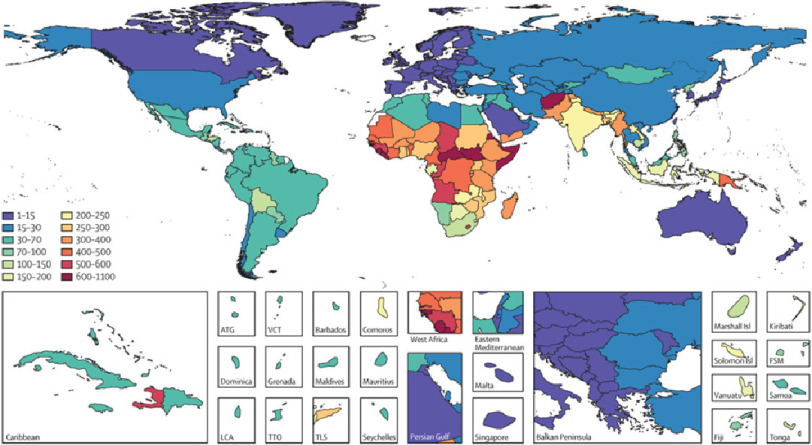
In 2017, every day approximately 810 women died from preventable causes related to pregnancy and childbirth, with 94% of all maternal deaths occurring in low and middle-income countries (LMIC’s).22 Sub-Saharan Africa alone accounted for roughly two-thirds of the estimated global maternal deaths.21 In Egypt the number of maternal deaths was 1,316 in 2017 with a MMR of 62 per 100,000 livebirths.8,23 The regional distribution and pattern of maternal death reported by the GBD varies considerably around the world.23–25 (Figure 1).
Figure 1. Maternal mortality ratio (MMR; number of deaths per 100,000 livebirths) for countries and territories, GBD 2015.23.
Causes of maternal mortality and burden of disease
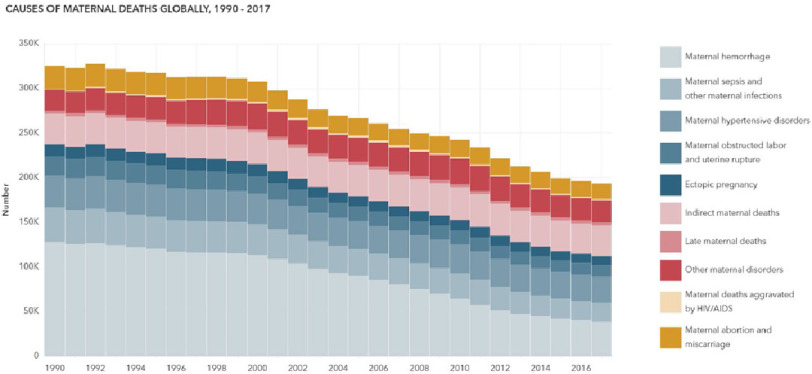
Up to 1990, direct causes accounted for over two-thirds of maternal mortality worldwide. There has been a significant decrease in overall maternal mortality over the years due to progressive drops in direct causes, however, the contribution of indirect and late maternal deaths, as well as maternal hypertensive disorders, has remained unchanged (Figure 2).1,2
Figure 2. Causes of maternal deaths globally, 1990–2017.1,2.
(A) maternal hypertensive disorders: High blood pressure during pregnancy in women who did not already have hypertension, or preeclampsia in women with preexisting hypertension. (B) Indirect maternal deaths: Deaths due to preexisting conditions made worse by physiologic effects of pregnancy. (C) Late maternal deaths: Deaths due to any cause that occurs six weeks to 12 months after pregnancy. (D) Other maternal disorders: All other direct maternal disorders, including anemia in pregnancy, gestational diabetes and embolism.
Regional and national variation in incidence and causes of maternal mortality
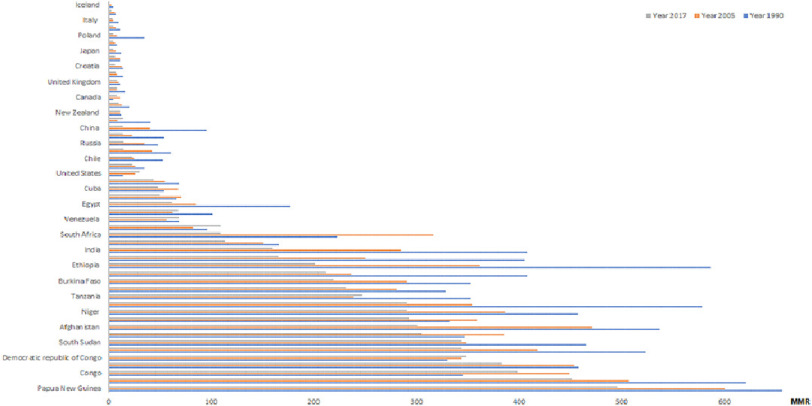
According to the GBD, maternal mortality rate (MMR) varied from 1 to 496 per 100,000 live births (Figure 3) in 2017.1,2 The alarmingly high incidence in MMR in some countries continues, with very few exceptions.
Figure 3. MMR per country across 1990, 2005 and 2017.1,2.
The regional incidence and cause of maternal mortality varies considerably from one country to another (Table 1). In addition, the representation of maternal hypertensive disorders, indirect maternal deaths as well as the reported late maternal mortality also differs across countries.
Table 1. Global distribution in incidence, causes and timming of maternal mortality by MMR (2017).1,2.
The reported late maternal deaths represented 21.9% of total maternal mortality in Chile, compated to 0.08% in Iceland.
| Country | Number of maternal deaths (2017) | MMR (2017) | Top mortality disorder | Maternal hypertensive disorders(% total maternal deaths) | Indirect maternal deaths(% total maternal deaths) | Late maternal deaths(% total maternal deaths) |
|---|---|---|---|---|---|---|
| Globally | 193,639 | 140 | Other maternal disorders | 15.17 | 17.61 | 1.74 |
| Iceland | 0 | 1 | Other maternal disorders | 0.08 | 0.03 | 0.08 |
| Sweden | 4 | 3 | Other maternal disorders | 7.25 | 2.50 | 7.50 |
| Italy | 17 | 4 | Other maternal disorders | 7.82 | 1.29 | 1.65 |
| Spain | 20 | 5 | Other maternal disorders | 11.40 | 1.20 | 1.50 |
| Poland | 16 | 5 | Other maternal disorders | 6.31 | 0.88 | 6.50 |
| Australia | 15 | 5 | Other maternal disorders | 5.07 | 1.53 | 9.07 |
| Japan | 46 | 5 | Other maternal disorders | 7.87 | 9.93 | 3.50 |
| Netherlands | 11 | 6 | Other maternal disorders | 11.09 | 2.36 | 2.91 |
| Croatia | 2 | 6 | Other maternal disorders | 5.50 | 7.00 | 8.00 |
| France | 52 | 7 | Other maternal disorders | 11.27 | 9.54 | 2.54 |
| United Kingdom | 62 | 8 | Other maternal disorders | 9.11 | 14.56 | 6.27 |
| Portugal | 7 | 8 | Other maternal disorders | 11.14 | 4.29 | 0.86 |
| Canada | 31 | 8 | Other maternal disorders | 6.10 | 12.26 | 16.94 |
| South Korea | 42 | 10 | Other maternal disorders | 10.24 | 6.98 | 2.29 |
| New Zealand | 6 | 11 | Other maternal disorders | 1.33 | 37.00 | 12.17 |
| Latvia | 3 | 14 | Other maternal disorders | 1.67 | 1.67 | 5.33 |
| China | 2241 | 14 | Other maternal disorders | 14.80 | 6.80 | 0.98 |
| Vietnam | 213 | 14 | Maternal hemorrhage | 14.63 | 13.50 | 2.77 |
| Russia | 250 | 15 | Other maternal disorders | 11.33 | 12.35 | 2.08 |
| Kazakhstan | 53 | 15 | Other maternal disorders | 8.32 | 6.53 | 7.66 |
| Chile | 56 | 23 | Late maternal death | 17.27 | 19.84 | 21.91 |
| Iran | 298 | 23 | Indirect maternal disease | 10.58 | 33.20 | 9.95 |
| United States | 1171 | 30 | Other maternal disorders | 10.12 | 15.65 | 17.12 |
| Mexico | 1120 | 44 | Maternal hemorrhage | 23.45 | 19.17 | 7.22 |
| Cuba | 53 | 48 | Indirect maternal disease | 7.09 | 21.43 | 17.06 |
| Colombia | 427 | 50 | Indirect maternal disease | 23.16 | 24.17 | 11.71 |
| Egypt | 1316 | 62 | Other maternal disorders | 15.76 | 19.68 | 3.67 |
| Brazil | 2,054 | 68 | Maternal hypertensive disorders | 20.93 | 18.23 | 6.23 |
| Venezuela | 395 | 69 | Maternal hemorrhage | 26.26 | 22.81 | 2.83 |
| Dominican Republic | 237 | 109 | Hypertensive disorders | 26.21 | 17.34 | 9.92 |
| South Africa | 1191 | 109 | Indirect maternal disease | 15.44 | 35.03 | 1.89 |
| Uganda | 1753 | 113 | Indirect maternal disease | 15.22 | 22.27 | 0.72 |
| India | 39,428 | 160 | Other maternal disorders | 11.50 | 13.16 | 1.78 |
| Indonesia | 6627 | 165 | Maternal hemorrhage | 26.69 | 2.19 | 1.41 |
| Ethiopia | 7451 | 201 | Maternal hemorrhage | 15.56 | 17.38 | 0.73 |
| Mozambique | 2090 | 212 | Indirect maternal disease | 15.07 | 25.13 | 5.29 |
| Burkina Faso | 1863 | 219 | Indirect maternal disease | 12.40 | 28.46 | 0.74 |
| Nigeria | 17982 | 231 | Maternal hemorrhage | 5.22 | 16.79 | 1.68 |
| Tanzania | 4916 | 247 | Maternal hypertensive disorders | 24.61 | 21.65 | 0.79 |
| Mali | 2559 | 291 | Indirect maternal disease | 6.63 | 31.18 | 1.23 |
| Niger | 2930 | 291 | Maternal sepsis and other maternal infections | 2.00 | 26.25 | 0.87 |
| Kenya | 3990 | 292 | Indirect maternal disease | 15.70 | 23.88 | 1.19 |
| Afghanistan | 4095 | 301 | Indirect maternal disease | 9.11 | 38.79 | 0.66 |
| Cote d’Ivoire | 2631 | 305 | Indirect maternal disease | 9.47 | 28.27 | 0.67 |
| South Sudan | 1410 | 344 | Maternal hemorrhage | 5.95 | 8.82 | 0.62 |
| Somalia | 2363 | 344 | Maternal hemorrhage | 9.10 | 16.98 | 0.56 |
| Democratic republic of Congo | 10166 | 349 | Indirect maternal disease | 10.99 | 26.59 | 0.68 |
| Chad | 2745 | 383 | Maternal hemorrhage | 6.29 | 14.41 | 0.78 |
| Congo | 522 | 398 | Indirect maternal disease | 11.60 | 26.63 | 0.80 |
| Guinea | 1916 | 451 | Maternal hemorrhage | 8.35 | 25.25 | 0.82 |
| Papua New Guinea | 1532 | 496 | Indirect maternal disease | 8.86 | 34.92 | 0.59 |
Details of adverse cardiovascular events
Direct maternal adverse cardiovascular events
Hypertensive disorders of pregnancy.
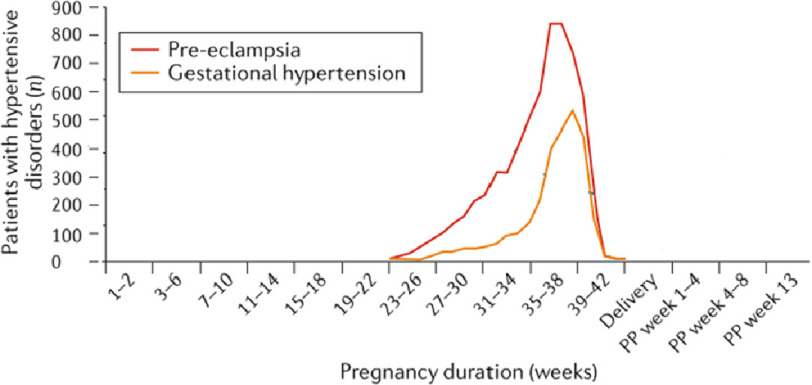
Chronic hypertension, gestational hypertension and preeclampsia26 are important causes of maternal and perinatal morbidity and mortality,9,27–31 particularly toward the end of pregnancy (Figure 4). Novel diagnostic methods and therapies have recently been reviewed.32
Figure 4. Onset of hypertensive disorders at different stages of pregnancy and postpartum (PP) among women without chronic hypertension (adapted from Ramlakhan et al).41,43.
Gestational hypertension is defined as new-onset hypertension arising after 20 weeks of gestation33,34 and occurs in 10% of women.35 It is associated with acute and chronic cardiovascular changes leading to an increased risk for hypertension throughout life,36,37 and a 4-fold increased risk of future maternal cardiovascular events.37–40
Indirect maternal adverse cardiovascular events
Cardiovascular disease is the single largest cause of indirect maternal mortality.3,6 Patients who experience complications during pregnancy may also be at higher risk of cardiac events later in life.44
Arrythmias
The altered cardiac anatomy during pregnancy can elicit new onset arrhythmia or prompt the recurrence of preexisting arrhythmias.45,46 An increased incidence of cardiac arrhythmias has been reported during pregnancy47 in patients with and without identifiable heart disease. Arrhythmias are responsible for complications in 67 per 100,000 pregnancies,49 mainly in the form of atrial fibrillation (27 per 100,000 pregnancies) and supraventricular tachycardia (22 per 100,000 pregnancies).48–50
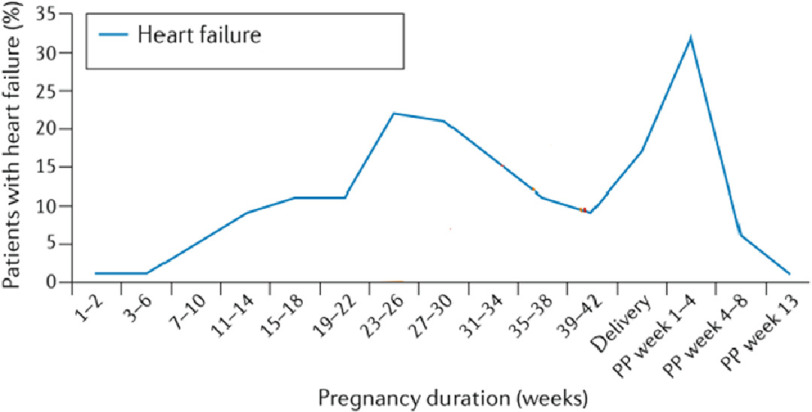
Heart failure
Heart failure (HF) remains the most common complication during pregnancy among all women with heart disease, regardless of the cardiac pathology.42,51 Heart failure can occur during, or immediately after pregnancy (Figure 5). Despite the poor prognosis associated with the diagnosis of HF during pregnancy, and the fact that prevalence of HF among pregnant women has increased over the years - particularly in the post-partum period,52 data in the literature are scarce.
Figure 5. Timing of heart failure in women with structural heart disease at different stages of pregnancy and postpartum (PP) (adapted from Ramlakhan et al).41,42.
Peri-partum cardiomyopathy
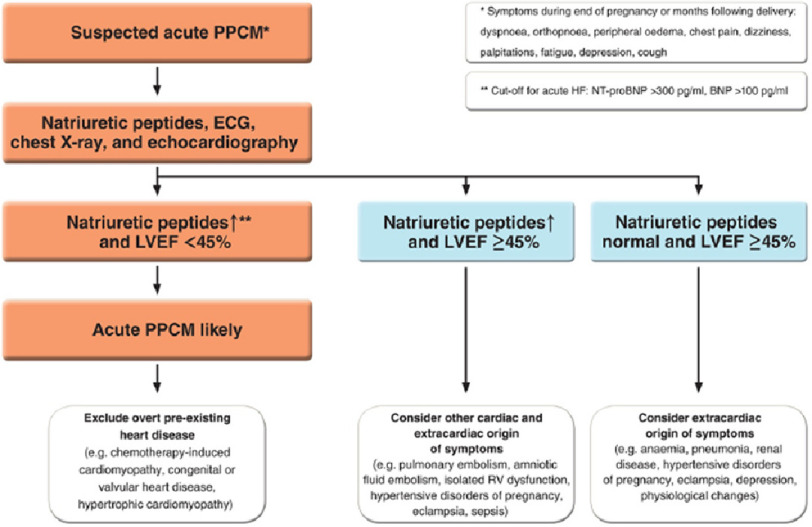
Peri-partum cardiomyopathy (PPCM) is an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricle systolic dysfunction towards the end of pregnancy or in the months following child birth.53–56 At diagnosis, the majority of patients have severe symptoms (NYHA III/IV) and LVEF < 35%, with regional variations in presentation and outcomes57–59 (Figure 6).
Figure 6. Diagnostic pathway in patients with suspected peripartum cardiomyopathy (PPCM).
BNP, B-type natriuretic peptide; ECG, electrocardiogram; HF, heart failure; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RV, right ventricular.60
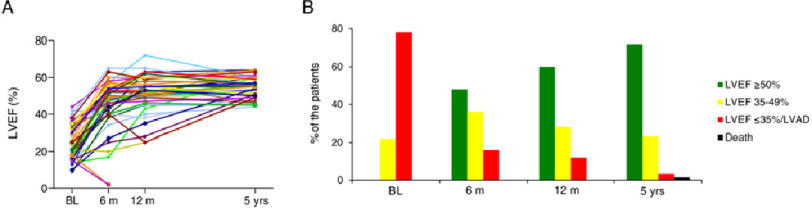
The incidence of PPCM varies markedly from 1–100 per 10,000 live births depending on the region.59,61–63 PPCM has the highest mortality rate in pregnancy, with a worldwide mortality of 2.4%,57 although it may be underdiagnosed. PPCM leads to substantial maternal and neonatal morbidity and mortality, with less than half of all cases recovering full cardiac function58,64–67 (Figure 7). Six-month mortality is around 6%, mainly due to heart failure.58 Neonatal death is around 5%, although with marked regional variation.63
Figure 7. Time course of left ventricular function (adapted from.68).
(A) Changes in left ventricular ejection fraction (LVEF) from baseline (BL) to 5-year follow-up. Remarkably, LVEF further improves even after 1 year. (B) Proportion of patients with full cardiac recovery constantly increases. At 5-year follow-up, 72% had recovered completely and 23% partially. No recovery was observed in 5%. Death occurs up to 5 years.
Management follows the guidelines of HF. In non-responsive patients, other pharmacological agents - such as bromocriptine and prolactin-blocking therapy with dopamine D2 receptor agonists - have been tried with variable results.68
Contributing factors to PPCM include genetic predisposition69 as well as auto-immune responses.70–72 Using such factors, all be it indirectly, to define phenoclusters73 - it could be possible to identify novel therapeutic targets to guide personalized medicine in PPCM.
For a small proportion of patients with rapidly-progressive PPCM resistant to conventional therapy, the use of the current generation of left ventricular assist devices can give long-lasting “cures” in some patients (personal experience in Harefield and Aswan series).74,75
Registries on the condition will provide fundamental data on predisposing factors, potential aetiologia and regional variations.57,63,76,77
Mechanical valve thrombosis
Even with the right care, the incidence of thromboembolism during pregnancy is estimated from 7–23% with half of these cases being mechanical valve thrombosis (MVT),78 which is associated with 20% mortality.79 Thrombosis is the most life-threatening complication for women with prosthetic heart valve, during pregnancy.78,80 The chance of a successful uncomplicated pregnancy, which depends on the balance between the thrombotic and bleeding risks, is around 57%.
Acute coronary syndromes
The incidence of coronary artery disease (CAD) in women of childbearing age is unclear and varies between countries.81,82 CAD, is a major cause of maternal death and accounts for over 20% of maternal cardiac deaths,83 especially in the form of acute coronary syndromes (ACSs).84 The estimated incidence of 6.2 per 100,000 deliveries85–88 nearly 4 times higher than in non-pregnant women89 and reflects the growing prevalence of cardiovascular risk factors in the pregnant population.90
Risk stratification
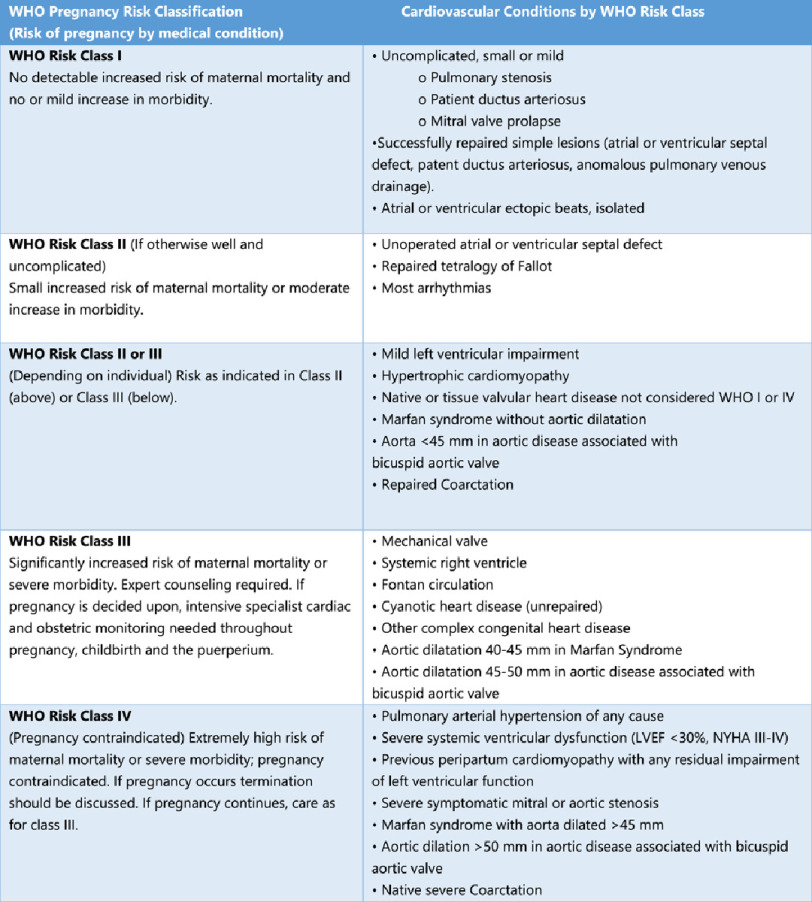
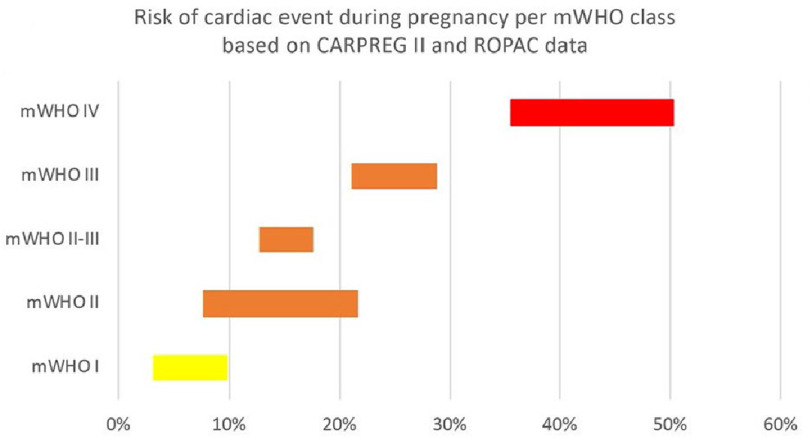
Several tools have been developed to estimate morbidity and mortality risk in pregnant women with cardiac disease, such as CARPREG13 and ZAHARA.12 However, the best estimate of the risk of cardiovascular events during pregnancy in pre-existing heart disease is the WHO’s91 (Figure 8), as it integrates congenital and acquired heart disease.
Figure 8. Modified WHO classification of maternal cardiovascular risk: application.92.
The risk of cardiac adverse events during pregnancy are significantly increased in mWHO IV compared to mWHO I (Figure 9).
Figure 9. Risk of cardiac event during pregnancy per mWHO class based on CARPREG II and ROPAC data.
12,13,93 adapted from.94
Based on the modified WHO risk classification for milder conditions, where the risk of pregnancy is very low to moderate, the needed care might be limited to a few visits during pregnancy, while in case of high risk of complications, a more frequent follow-up schedule is recommended. Women in the highest risk group (WHO IV) should be advised against pregnancy. In case of pregnancy, strict monitoring is required.92 More comparative studies should be performed in order to define the most accurate risk index for pregnant women with heart disease.91,95
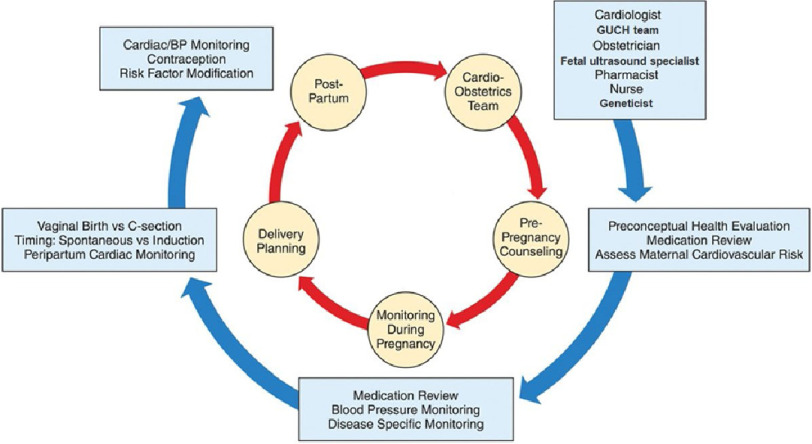
The role of comprehensive cardio-obstetric clinics
The concept of multidisciplinary cardio-obstetric clinics (Figure 10) has evolved and been applied in several countries, with extremely encouraging results96–114 and excellent survival rates of mothers even with complex diseases, and their offspring.24
Figure 10. Cardio-obstetrics team in the management of women before pregnancy, during pregnancy, and postpartum (GUCH: grown-up congenital heart disease) Adapted from.115.
However, the need for cardio-obstetric clinics remains un-met, with most programs to be found in developed countries and with only a few centers continuing follow-up long term.24,101,102
Although these initiatives might be feasible in large metropolitan areas, smaller towns, rural communities and remote regions are completely neglected. There is an urgent need for action and worldwide implementation of cardio-obstetric clinics, strategically placed, in order to reach the majority of those in need.
Integrated, tailored and dynamic healthcare services responding to current state of disease burden and initiatives are essential. Cardio-obstetric care clinics (COcare) have been initiated in the metropolitan Aswan Heart Center as well as its rural branch in Ballana.116
Conclusions
Preventable maternal cardiovascular adverse events continue to be a global problem with an unacceptably high burden of disease. This requires urgent concerted efforts from governments, individuals and professionals, who have first-hand experience of the magnitude of the problem. Multidisciplinary cardio-obstetric care should also play an important role.
References
- 1.IHME . Inst Heal Metrics Eval (IHME) Seattle. WA IHME; 2018. Findings from the Global Burden of Disease Study 2017 (GBD 2017) [Google Scholar]
- 2.Maternal Health Atlas [4 December 2020]. https://maternalhealthatlas.org/
- 3.Nair M, Nelson-Piercy C, Knight M. Indirect maternal deaths: UK and global perspectives. Obstet Med. 2017;10(1):10–15. doi: 10.1177/1753495X16689444. doi: 10.1177/1753495X16689444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storm F, Agampodi S, Eddleston M, Sørensen JB, Konradsen F, Rheinländer T. Indirect causes of maternal death. Lancet Glob Heal. 2014;2(10):e566. doi: 10.1016/S2214-109X(14)70297-9. doi: 10.1016/S2214-109X(14)70297-9. [DOI] [PubMed] [Google Scholar]
- 5.2012. [13 January 2021]. The WHO Application of ICD-10 to Deaths during Pregnancy, Childbirth and the Puerperium: ICD-MM. http://www.who.int/about/licensing/
- 6.Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakepeare J, Kurinczuk JJ. Saving Lives, Improving Mothers’ Care Lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Published online. 2012;2012:1–120. [Google Scholar]
- 7.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy. Eur Heart J. 2011;32(24):3147–3197. doi: 10.1093/eurheartj/ehr218. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 8.Alkema L, Chou D, Hogan D, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the un maternal mortality estimation inter-agency group. Lancet. 2016;387(10017):462–474. doi: 10.1016/S0140-6736(15)00838-7. doi: 10.1016/S0140-6736(15)00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–1074. doi: 10.1016/S0140-6736(06)68397-9. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 10.Macdorman MF, Declercq E, Cabral H, Morton C. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128(3):447–455. doi: 10.1097/AOG.0000000000001556. doi: 10.1097/AOG.0000000000001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferranti EP, Jones EJ, Hernandez TL. Pregnancy reveals evolving risk for cardiometabolic disease in women. JOGNN - J Obstet Gynecol Neonatal Nurs. 2016;45(3):413–425. doi: 10.1016/j.jogn.2016.02.004. doi: 10.1016/j.jogn.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31(17):2124–2132. doi: 10.1093/eurheartj/ehq200. doi: 10.1093/eurheartj/ehq200. [DOI] [PubMed] [Google Scholar]
- 13.Silversides CK, Grewal J, Mason J, et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. 2018;71(21):2419–2430. doi: 10.1016/j.jacc.2018.02.076. doi: 10.1016/j.jacc.2018.02.076. [DOI] [PubMed] [Google Scholar]
- 14.Roos-Hesselink JW, Ruys TPE, Stein JI, et al. Outcome of pregnancy in patients with structural or ischaemic heart disease: results of a registry of the european society of cardiology. Eur Heart J. 2013;34(9):657–665. doi: 10.1093/eurheartj/ehs270. doi: 10.1093/eurheartj/ehs270. [DOI] [PubMed] [Google Scholar]
- 15.Khursheed R, Tabasum AZB. Maternal and fetal outcome in pregnancies [9]complicated with maternal cardiac diseases: experience at tertiary care hospital. Internet J Gynecol Obstet. 2015;19(1):01–06. [Google Scholar]
- 16.McFaul PB, Dornan JC, Lamki HBD. Pregnancy complicated by maternal [10]heart disease. A review of 519 women. BJOG An Int J Obstet Gynaecol. 1988;95(9):861–67. doi: 10.1111/j.1471-0528.1988.tb06570.x. [DOI] [PubMed] [Google Scholar]
- 17.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 18.Lameijer H, Schutte JM, Pieper PG, Van Roosmalen JJM, Schuitemaker NWE. Maternal mortality due to cardiovascular disease in the Netherlands: a 21-year experience for Dutch Maternal Mortality and Morbidity Committee. Netherlands Hear J. 2020;28:27–36. doi: 10.1007/s12471-019-01340-w. doi: 10.1007/s12471-019-01340-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sliwa K, Anthony J. Late maternal deaths: a neglected responsibility. Published online 2016. 2016 doi: 10.1016/S0140-6736(16)00651-6. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PubMed] [Google Scholar]
- 20.Building U.S. Capacity to review and prevent maternal deaths: report from nine maternal mortality review committees. https://reviewtoaction.org/Report_fro .
- 21.WHO . Trends in maternal mortality: 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization; WHO 2019; 2019. [Google Scholar]
- 22.WHO 2021. [21 January 2021]. Maternal mortality. https://www.who.int/news-room/fact-sheets/detail/maternal-mortality .
- 23.Kassebaum NJ, Barber RM, Dandona L, et al. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812. doi: 10.1016/S0140-6736(16)31470-2. doi: 10.1016/S0140-6736(16)31470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sliwa K, Libhaber E, Elliott C, et al. Spectrum of cardiac disease in maternity in a low-resource cohort in South Africa. Heart. 2014;100(24):1967–1974. doi: 10.1136/heartjnl-2014-306199. doi: 10.1136/heartjnl-2014-306199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rana S, Lemoine E, Granger J, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 27.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Steegers EAP, Von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. The Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 29.Sammour MB, El-Kabarity H, Fawzy MM, Schindler AE. 2011. [6 November 2011]. WHO recommendations for prevention and treatment of Pre-Eclampsia and Eclampsia. Vol 97. World Health Organization; 2011. https://www.ncbi.nlm.nih.gov/books/NBK140561/
- 30.Peters RM, Flack JM. Hypertensive disorders of pregnancy. JOGNN - J Obstet Gynecol Neonatal Nurs. 2004;33(2):209–220. doi: 10.1177/0884217504262970. doi: 10.1177/0884217504262970. [DOI] [PubMed] [Google Scholar]
- 31.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373. doi: 10.1097/AOG.0000000000002114. doi: 10.1097/AOG.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275–289. doi: 10.1038/s41581-019-0119-6. doi: 10.1038/s41581-019-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: A systematic overview. Epidemiology. 2003;14(3):368–374. doi: 10.1097/00001648-200305000-00020. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Robertson RM. Women and cardiovascular disease. Circulation. 2001;103(19):2318–2320. doi: 10.1161/01.CIR.103.19.2318. doi: 10.1161/01.CIR.103.19.2318. [DOI] [PubMed] [Google Scholar]
- 35.Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol. 1998;147(11):1062–1070. doi: 10.1093/oxfordjournals.aje.a009400. doi: 10.1093/oxfordjournals.aje.a009400. [DOI] [PubMed] [Google Scholar]
- 36.Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: Results from cohort study. Br Med J. 2003;326(7394):845–849. doi: 10.1136/bmj.326.7394.845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garovic VD, Bailey KR, Boerwinkle E, et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens. 2010;28(4):826–833. doi: 10.1097/HJH.0b013e328335c29a. doi: 10.1097/HJH.0b013e328335c29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): Population-based retrospective cohort study. Lancet. 2005;366(9499):1797–1803. doi: 10.1016/S0140-6736(05)67726-4. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 39.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. Br Med J. 2007;335(7627):974–977. doi: 10.1136/bmj.39335.385301.BE. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roccella EJ. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):s1–s22. doi: 10.1067/mob.2000.107928. doi: 10.1067/mob.2000.107928. [DOI] [PubMed] [Google Scholar]
- 41.Ramlakhan KP, Johnson MR, Roos-Hesselink JW. Pregnancy and cardiovascular disease. Nat Rev Cardiol. 2020;17(11):718–731. doi: 10.1038/s41569-020-0390-z. doi: 10.1038/s41569-020-0390-z. [DOI] [PubMed] [Google Scholar]
- 42.Ruys TEP, Roos-Hesselink JW, Hall R, et al. Heart failure in pregnant women with cardiac disease: Data from the ROPAC. Heart. 2014;100(3):231–238. doi: 10.1136/heartjnl-2013-304888. doi: 10.1136/heartjnl-2013-304888. [DOI] [PubMed] [Google Scholar]
- 43.Shiozaki A, Matsuda Y, Satoh S, Saito S. Comparison of risk factors for gestational hypertension and preeclampsia in Japanese singleton pregnancies. J Obstet Gynaecol Res. 2013;39(2):492–499. doi: 10.1111/j.1447-0756.2012.01990.x. doi: 10.1111/j.1447-0756.2012.01990.x. [DOI] [PubMed] [Google Scholar]
- 44.Balint OH, Siu SC, Mason J, et al. Cardiac outcomes after pregnancy in women with congenital heart disease. Heart. 2010;96(20):1656–1661. doi: 10.1136/hrt.2010.202838. doi: 10.1136/hrt.2010.202838. [DOI] [PubMed] [Google Scholar]
- 45.Brodsky M, Doria R, Allen B, Sato D, Thomas G, Sada M. New-onset ventricular tachycardia during pregnancy. Am Heart J. 1992;123(4 PART 1):933–941. doi: 10.1016/0002-8703(92)90699-V. doi: 10.1016/0002-8703(92)90699-V. [DOI] [PubMed] [Google Scholar]
- 46.Meah VL, Cockcroft JR, Backx K, Shave R, Stöhr EJ. Cardiac output and related haemodynamics during pregnancy: a series of meta-analyses. Heart. 2016;102(7):518–526. doi: 10.1136/heartjnl-2015-308476. doi: 10.1136/heartjnl-2015-308476. [DOI] [PubMed] [Google Scholar]
- 47.CK S. Physiological changes in pregnancy. In: Warn CA, Oakley C, editors. Hear Dis pregnancy Massachussetts Blackwell Publ 2007. [Google Scholar]
- 48.Shotan A, Ostrzega E, Mehra A, Johnson JV, Elkayam U. Incidence of arrhythmias in normal pregnancy and relation to palpitations, dizziness, and syncope. Am J Cardiol. 1997;79(8):1061–1064. doi: 10.1016/S0002-9149(97)00047-7. doi: 10.1016/S0002-9149(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 49.Vaidya VR, Arora S, Patel N, et al. Burden of Arrhythmia in Pregnancy. Circulation. 2017;135(6):619–621. doi: 10.1161/CIRCULATIONAHA.116.026681. doi: 10.1161/CIRCULATIONAHA.116.026681. [DOI] [PubMed] [Google Scholar]
- 50.Li JM, Nguyen C, Joglar JA, Hamdan MH, Page RL. Frequency and outcome of arrhythmias complicating admission during pregnancy: experience from a high-volume and ethnically-diverse obstetric service. Clin Cardiol. 2008;31(11):538–541. doi: 10.1002/clc.20326. doi: 10.1002/clc.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sliwa K, Anthony J. Decompensated heart failure in pregnancy. Card Fail Rev. 2016;2(1):20. doi: 10.15420/cfr.2015:24:2. doi: 10.15420/cfr.2015:24:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mogos MF, Piano MR, McFarlin BL, Salemi JL, Liese KL, Briller JE. Heart failure in pregnant women: a concern across the pregnancy continuum. Circ Heart Fail. 2018;11(1) doi: 10.1161/CIRCHEARTFAILURE.117.004005. doi: 10.1161/CIRCHEARTFAILURE.117.004005. [DOI] [PubMed] [Google Scholar]
- 53.Hilfiker-Kleiner D, Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol. 2014;11(6):364–370. doi: 10.1038/nrcardio.2014.37. doi: 10.1038/nrcardio.2014.37. [DOI] [PubMed] [Google Scholar]
- 54.Azibani F, Sliwa K. Peripartum cardiomyopathy: an update. Curr Heart Fail Rep. 2018;15(5):297–306. doi: 10.1007/s11897-018-0404-x. doi: 10.1007/s11897-018-0404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricke-Hoch M, Pfeffer TJ, Hilfiker-Kleiner D. Peripartum cardiomyopathy: Basic mechanisms and hope for new therapies. Cardiovasc Res. 2020;116(3):520–531. doi: 10.1093/cvr/cvz252. doi: 10.1093/cvr/cvz252. [DOI] [PubMed] [Google Scholar]
- 56.Jackson AM, Dalzell JR, Walker NL, Coats CJ, Jhund PS, Petrie MC. Peripartum cardiomyopathy: diagnosis and management. Heart. 2018;104(9):779–786. doi: 10.1136/heartjnl-2016-310599. doi: 10.1136/heartjnl-2016-310599. [DOI] [PubMed] [Google Scholar]
- 57.Sliwa K, Mebazaa A, Hilfiker-Kleiner D, et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur J Heart Fail. 2017;19(9):1131–1141. doi: 10.1002/ejhf.780. doi: 10.1002/ejhf.780. [DOI] [PubMed] [Google Scholar]
- 58.Sliwa K, Petrie MC, Meer PVanDer, et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: An ESC EORP registry. Eur Heart J. 2020;41(39):3787–3797. doi: 10.1093/eurheartj/ehaa455. doi: 10.1093/eurheartj/ehaa455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bauersachs J, König T, van der Meer P, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019;21(7):827–843. doi: 10.1002/ejhf.1493. doi: 10.1002/ejhf.1493. [DOI] [PubMed] [Google Scholar]
- 60.Bauersachs J, König T, Meer P, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the heart failure association of the european society of cardiology study group on peripartum cardiomyopathy. Eur J Heart Fail. 2019;21(7):827–843. doi: 10.1002/ejhf.1493. doi: 10.1002/ejhf.1493. [DOI] [PubMed] [Google Scholar]
- 61.Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the heart failure association of the european society of cardiology working group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi: 10.1093/eurjhf/hfq120. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 62.Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44(5):964–968. doi: 10.1161/01.CIR.44.5.964. doi: 10.1161/01.CIR.44.5.964. [DOI] [PubMed] [Google Scholar]
- 63.Sliwa K, Petrie MC, van der Meer P, et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J. 2020;41(39):3787–3797. doi: 10.1093/eurheartj/ehaa455. doi: 10.1093/eurheartj/ehaa455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blauwet LA, Libhaber E, Forster O, Tibazarwa K, Mebazaa A, Hilfiker-Kleiner DKS. Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Hear. 2013:99308–313. doi: 10.1136/heartjnl-2012-302760. [DOI] [PubMed] [Google Scholar]
- 65.Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen PTD, Jordan J, Lichtinghagen R, Kaisenberg CS, Struman I, Bovy N, Sliwa KB, H-KD J. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;2013:108366. doi: 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irizarry OC, Levine LD, Lewey J, Boyer T, Riis V, Elovitz MAAZ. Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between African American and non-African American women. JAMA Cardiol. 2017:21256. doi: 10.1001/jamacardio.2017.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNamara DM, Elkayam U, Alharethi R, et al. Clinical outcomes for peripartum cardiomyopathy in North America: Results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy) J Am Coll Cardiol. 2015;66(8):905–914. doi: 10.1016/j.jacc.2015.06.1309. doi: 10.1016/j.jacc.2015.06.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moulig V, Pfeffer TJ, Ricke-Hoch M, et al. Long-term follow-up in peripartum cardiomyopathy patients with contemporary treatment: low mortality, high cardiac recovery, but significant cardiovascular co-morbidities. Eur J Heart Fail. 2019;21(12):1534–1542. doi: 10.1002/ejhf.1624. doi: 10.1002/ejhf.1624. [DOI] [PubMed] [Google Scholar]
- 69.Ware JS, Li J, Mazaika E, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374(3):233–241. doi: 10.1056/nejmoa1505517. doi: 10.1056/nejmoa1505517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caforio ALP, Adler Y, Agostini C, Allanore Y, Anastasakis A, Arad MBM, Charron P, Elliott PM, Eriksson U, Felix SB, Garcia-Pavia P, Hachulla E, Heymans S, Imazio M, Klingel K, Marcolongo R, Cerinic MM, Pantazis A, Plein S, Poli V, Rigopoulos A, Seferovic P, Shoenfeld Y, Zamorano JLLA. Diagnosis and management of myocardial involvement in systemic immune- mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Hear J. 2017:382649–2662. doi: 10.1093/eurheartj/ehx321. [DOI] [PubMed] [Google Scholar]
- 71.Warraich RS, Sliwa K, Damasceno A, et al. Impact of pregnancy-related heart failure on humoral immunity: Clinical relevance of G3-subclass immunoglobulins in peripartum cardiomyopathy. Am Heart J. 2005;150(2):263–269. doi: 10.1016/j.ahj.2004.09.008. doi: 10.1016/j.ahj.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Ansari AA, Fett JD, Carraway RE, Mayne AE, Onlamoon N, Sundstrom JB. Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol. 2002;23(3):301–324. doi: 10.1385/CRIAI:23:3:301. doi: 10.1385/CRIAI:23:3:301. [DOI] [PubMed] [Google Scholar]
- 73.Verdonschot JAJ, Merlo M, Dominguez F, et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur Heart J. 2021;42(2):162–174. doi: 10.1093/eurheartj/ehaa841. doi: 10.1093/eurheartj/ehaa841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oosterom L, Jonge NDe, Kirkels JH, Klöpping C, Lahpor JR. Left ventricular assist device as a bridge to recovery in a young woman admitted with peripartum cardiomyopathy. Netherlands Hear J. 2008;16(12):426–428. doi: 10.1007/BF03086192. doi: 10.1007/BF03086192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayworth R. From labor and delivery to left ventricular assist device: A peripartum cardiomyopathy case report. Hear Lung. 2020;49(1):54–57. doi: 10.1016/j.hrtlng.2019.06.005. doi: 10.1016/j.hrtlng.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 76.Sliwa K, Hilfiker-Kleiner D, Mebazaa A, et al. EURObservational research programme: A worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the heart failure association of the european society of cardiology working group on PPCM. Eur J Heart Fail. 2014;16(5):583–591. doi: 10.1002/ejhf.68. doi: 10.1002/ejhf.68. [DOI] [PubMed] [Google Scholar]
- 77. [26 January 2021]. PeriPartum CardioMyopathy (PPCM) Registry. https://www.escardio.org/Research/Registries-&-surveys/Observational-researchprogramme/PeriPartum-CardioMyopathy-PPCM-Registry .
- 78.Elkayam U, Bitar F. Valvular heart disease and pregnancy - Part II: Prosthetic valves. J Am Coll Cardiol. 2005;46(3):403–410. doi: 10.1016/j.jacc.2005.02.087. doi: 10.1016/j.jacc.2005.02.087. [DOI] [PubMed] [Google Scholar]
- 79.Van Hagen IM, Roos-Hesselink JW, Ruys TPE, et al. Pregnancy in women with a mechanical heart valve. Circulation. 2015;132(2):132–142. doi: 10.1161/CIRCULATIONAHA.115.015242. doi: 10.1161/CIRCULATIONAHA.115.015242. [DOI] [PubMed] [Google Scholar]
- 80.Mahgoub A, Kotit S, Bakry K, Magdy A, Hosny H, Yacoub M. Thrombosis of mechanical mitral valve prosthesis during pregnancy: An ongoing saga in need of comprehensive solutions. Glob Cardiol Sci Pract. 2020;2020(3) doi: 10.21542/gcsp.2020.32. doi: 10.21542/gcsp.2020.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petitti DB, Sidney S, Quesenberry CP, Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke. 1997;28(2):280–283. doi: 10.1161/01.STR.28.2.280. doi: 10.1161/01.STR.28.2.280. [DOI] [PubMed] [Google Scholar]
- 82.Etchill E, Clarke N, Giuliano K, Lawton J, Choi CW. Post-partum spontaneous coronary artery dissection refractory to conservative management. Glob Cardiol Sci Pract. 2020;2020(3) doi: 10.21542/gcsp.2020.34. doi: 10.21542/gcsp.2020.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knight M, Nair M, Tuffnell D, et al. Saving Lives, Improving Mothers’ Care Maternal, Newborn and Infant Clinical Outcome Review Programme. 2009. [Google Scholar]
- 84.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. BJOG An Int J Obstet Gynaecol. 2011;118:1–203. doi: 10.1111/j.1471-0528.2010.02847.x. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 85.Ginz B. MYOCARDIAL INFARCTION IN PREGNANCY. BJOG An Int J Obstet Gynaecol. 1970;77(7):610–615. doi: 10.1111/j.1471-0528.1970.tb03578.x. doi: 10.1111/j.1471-0528.1970.tb03578.x. [DOI] [PubMed] [Google Scholar]
- 86.Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: A population-based study. Obstet Gynecol. 2005;105(3):480–484. doi: 10.1097/01.AOG.0000151998.50852.31. doi: 10.1097/01.AOG.0000151998.50852.31. [DOI] [PubMed] [Google Scholar]
- 87.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: A United States population-based study. Circulation. 2006;113(12):1564–1571. doi: 10.1161/CIRCULATIONAHA.105.576751. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 88.Bush N, Nelson-Piercy C, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Myocardial infarction in pregnancy and postpartum in the UK. Eur J Prev Cardiol. 2013;20(1):12–20. doi: 10.1177/1741826711432117. doi: 10.1177/1741826711432117. [DOI] [PubMed] [Google Scholar]
- 89.Caspi J, Pettitt TW, Sperrazza C, Mulder TSA. Reimplantation of anomalous left coronary artery from the pulmonary artery without mitral valve repair. Ann Thorac Surg. 2007:84619–23. doi: 10.1016/j.athoracsur.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 90.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: A United States population-based study. Circulation. 2006;113(12):1564–1571. doi: 10.1161/CIRCULATIONAHA.105.576751. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 91.Balci A, Sollie-Szarynska KM, Van Der Bijl AGL, et al. Prospective validation and assessment of cardiovascular and offspring risk models for pregnant women with congenital heart disease. Heart. 2014;100(17):1373–1381. doi: 10.1136/heartjnl-2014-305597. doi: 10.1136/heartjnl-2014-305597. [DOI] [PubMed] [Google Scholar]
- 92.European ES of G (ESG); A for, Medicine PC (AEPC); GS for G, (DGesGM) Regitz-Zagrosek V, Blomstrom Lundqvist C, Al Borghi CE. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC) Eur Hear J. 2011;32(24):3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 93.van Hagen IM, Boersma E, Johnson MR, et al. Global cardiac risk assessment in the registry of pregnancy and cardiac disease: results of a registry from the European Society of Cardiology. Eur J Heart Fail. 2016;18(5):523–533. doi: 10.1002/ejhf.501. doi: 10.1002/ejhf.501. [DOI] [PubMed] [Google Scholar]
- 94.Van Hagen IM, Roos-Hesselink JW. Pregnancy in congenital heart disease: Risk prediction and counselling. Heart. 2020;106(23):1853–1861. doi: 10.1136/heartjnl-2019-314702. doi: 10.1136/heartjnl-2019-314702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu CW, Shih JC, Chen SY, et al. Comparison of 3 risk estimation methods for predicting cardiac outcomes in pregnant women with congenital heart disease. Circ J. 2015;79(7):1609–1617. doi: 10.1253/circj.CJ-14-1368. doi: 10.1253/circj.CJ-14-1368. [DOI] [PubMed] [Google Scholar]
- 96.Davis MB, Walsh MN. Cardio-obstetrics: Team-based care to improve maternal outcomes. Circ Cardiovasc Qual Outcomes. 2019;1(12) doi: 10.1161/CIRCOUTCOMES.118.005417. doi: 10.1161/CIRCOUTCOMES.118.005417. [DOI] [PubMed] [Google Scholar]
- 97.Magun E, DeFilippis EM, Noble S, et al. Cardiovascular Care for Pregnant Women With Cardiovascular Disease. J Am Coll Cardiol. 2020;76(18):2102–2113. doi: 10.1016/j.jacc.2020.08.071. doi: 10.1016/j.jacc.2020.08.071. [DOI] [PubMed] [Google Scholar]
- 98. [17 January 2021]. UCSF Pregnancy and Cardiac Treatment (PACT) Program — Obstetrics, Gynecology & Reproductive Sciences. https://obgyn.ucsf.edu/maternal-fetal-medicine/ucsf-pregnancy-and-cardiac-treatment-pact-program .
- 99. [17 January 2021]. Penn Pregnancy and Heart Disease Program—Penn Medicine. https://www.pennmedicine.org/for-patients-and-visitors/find-a-program-or-service/obstetrics/pregnancy-and-heart-disease .
- 100. [17 January 2021]. Heart Disease in Pregnancy Program — Cardiovascular Medicine — Medical College of Wisconsin. https://www.mcw.edu/departments/medicine/divisions/cardiovascularmedicine/patient-care/heart-disease-in-pregnancy-program .
- 101. [17 January 2021]. Perinatology Maternal Cardiac Program — Center for Women’s Health — OHSU. https://www.ohsu.edu/womens-health/perinatology-maternalcardiac-program .
- 102. [17 January 2021]. Pregnancy and Cardiac Treatment (PACT) Program — Clinics — UCSF Health. https://www.ucsfhealth.org/clinics/pregnancy-and-cardiactreatment-pact-program .
- 103. [17 January 2021]. Cardiac Obstetrics — Heart Centre. https://heartcentre.ca/services/cardiac-obstetrics .
- 104. [17 January 2021]. Pregnancy and heart disease centre — Royal Brompton & Harefield NHS Foundation Trust. https://www.rbht.nhs.uk/our-services/heart/inherited-cardiovascular-conditions/pregnancy-and-heart-disease-centre .
- 105. [17 January 2021]. Cardiac antenatal clinic — University Hospitals Bristol NHS Foundation Trust. http://www.uhbristol.nhs.uk/patients-and-visitors/yourhospitals/bristol-heart-institute-clinical-services/adult-congenital-heartdisease/cardiac-antenatal-clinic/
- 106.Sliwa K, Libhaber E, Elliott C, et al. Spectrum of cardiac disease in maternity in a low-resource cohort in South Africa. Hear. 2014:1001967–1974. doi: 10.1136/heartjnl-2014-306199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma G, Zakaria S, Michos ED, et al. Improving cardiovascular workforce competencies in cardio-obstetrics: current challenges and future directions. J Am Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.119.015569. doi: 10.1161/JAHA.119.015569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Easter SR, Valente AM, Economy KE. Creating a multidisciplinary pregnancy heart team. Curr Treat Options Cardiovasc Med. 2020;22(1) doi: 10.1007/s11936-020-0800-x. doi: 10.1007/s11936-020-0800-x. [DOI] [PubMed] [Google Scholar]
- 109.Ouyang P, Sharma G. The potential for pregnancy heart teams to reduce maternal mortality in women with cardiovascular disease. J Am Coll Cardiol. 2020;76(18):2114–2116. doi: 10.1016/j.jacc.2020.09.007. doi: 10.1016/j.jacc.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 110.Brown HL, Warner JJ, Gianos E, et al. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration with Obstetricians and Gynecologists: A Presidential Advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol Surv. 2018;73(10):574–576. doi: 10.1097/OGX.0000000000000600. doi: 10.1097/OGX.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 111.Khairy P, Ouyang DW, Fernandes SM, Lee-Parritz A, Economy KE, Landzberg MJ. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006;113(4):517–524. doi: 10.1161/CIRCULATIONAHA.105.589655. doi: 10.1161/CIRCULATIONAHA.105.589655. [DOI] [PubMed] [Google Scholar]
- 112. [17 January 2021]. Cardio-Obstetrics Program — Von Voigtlander Women’s Hospital — Michigan Medicine. https://www.umwomenshealth.org/conditions-treatments/cardio-obstetrics-program .
- 113. [17 January 2021]. Cardio-Obstetrics Program — NorthShore. https://www.northshore.org/cardiovascular-institute/our-programs/cardio-obstetrics-program/
- 114. [17 January 2021]. Center for Maternal Cardiac Care — TriHealth. https://www.trihealth.com/institutes-and-services/womens-services/high-risk-pregnancy/center-for-maternal-cardiac-care .
- 115.Mehta LS, Warnes CA, Bradley E, et al. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the american heart association. Circulation. 2020;141(23):e884–e903. doi: 10.1161/CIR.0000000000000772. doi: 10.1161/CIR.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 116. Ballana Heart Study. https://myglobalheart.org/wp-content/uploads/2019/03/Ballana-Heart-Study-022819.pdf .