Abstract
Purpose of Review
Obesity is a significant international public health epidemic with major downstream consequences on morbidity and mortality. While lifestyle factors contribute, there is an evolving understanding of genomic and metabolomic pathways involved with obesity and its relationship with cardiometabolic risk. This review will provide an overview of some of these important findings from both a biologic and clinical perspective.
Recent Findings
Recent studies have identified polygenic risk scores and metabolomic biomarkers of obesity and related outcomes, which have also highlighted biological pathways, such as the branched-chain amino acid (BCAA) pathway that is dysregulated in this disease. These biomarkers may help in personalizing obesity interventions and for mitigation of future cardiometabolic risk.
Summary
A multifaceted approach is necessary to impact the growing epidemic of obesity and related diseases. This will likely include incorporating precision medicine approaches with genomic and metabolomic biomarkers to personalize interventions and improve risk prediction.
Keywords: Obesity, Genomics, Metabolomics, Cardiometabolic, Polygenic risk score, Branched-chain amino acids
Introduction
The epidemic of overweight and obesity is an important contributor to short- and long-term cardiometabolic morbidity and mortality across the world. The negative health consequences of obesity reach across the life span contributing to adverse outcomes for adults, and unfortunately now even in children. While lifestyle factors such as diet and sedentary behavior contribute, novel insights including from genomic and metabolomic studies have highlighted the underlying biology, and, in parallel, have identified clinically relevant biomarkers for personalized medicine approaches to potentially decrease the prevalence of obesity and improve cardiometabolic outcomes.
Obesity: the Scope of the Problem
Epidemiology
The prevalence of overweight (body mass index [BMI] ≥ 25) and obesity (BMI ≥ 30) has reached epidemic proportions in developed countries such as the United States (U.S.), and is starting to increase in prevalence even in the developing world. In the U.S., greater than 40% of adults are obese and Hispanic and Black adults have higher rates of obesity [1]. Internationally, the prevalence of obesity nearly doubled between 1980 and 2014. Sadly, rates of childhood obesity are also rising, creating additional concern for the true extent of long-term health consequences [2]. For example, in the U.S., the 2015–2016 NHANES study reports that 20.6% of children and adolescents 12–19 years old are obese [3]. These rising rates of obesity contribute to significant morbidity and mortality worldwide [4–8]. In 2010, overweight/obesity were estimated to account for 3.4 million deaths per year globally [9].
Cardiometabolic Consequences of Obesity
Obesity is a major risk factor for metabolic diseases including dyslipidemia, insulin resistance, type 2 diabetes mellitus (T2DM), hypertension, and non-alcoholic fatty liver disease (NAFLD) (Fig. 1). A systematic review and meta-analysis of 37 studies noted childhood overweight/obesity to be associated with a higher risk of T2DM in adulthood (odds ratio [OR] 1.70, 95% confidence interval [CI] 1.30–2.22) [10]; a 10-kg increase in body weight is associated with 3.0 mmHg and 2.3 mmHg in systolic and diastolic blood pressure, respectively [11]; and high BMI (75th vs. 25th percentile) is associated with increased risk of NAFLD (OR 1.96, CI 1.51–2.56 for Blacks; OR 2.33, CI 1.70–3.19 for Whites) [12]. Obesity also increases risk of downstream cardiovascular disease (CVD)–related morbidity and mortality and other consequences, partially mediated through these metabolic risk factors. Markers of obesity such as BMI and waist-hip circumference are independent predictors of atherosclerotic CVD and CVD events [13]. These rising rates of obesity contribute to significant morbidity and mortality worldwide [4–8], with each five-unit increase in BMI above 25 kg/m2 associated with a 30% increase in overall mortality, 40% increase in ischemic heart disease and stroke mortality, 120% increase for diabetes-related mortality, 80% increase in hepatic mortality, and a 10% increase for cancer-related mortality [14]. In 2017, overweight/obesity were estimated to account for 4.72 million deaths per year [15]. Even in adolescent obesity, a study found increased risk of death attributable to coronary artery disease in adulthood after adjustment for sex, age, and sociodemographic characteristics (hazard ratio [HR] 4.9, CI 3.9–6.1) [16] and obesity is associated with presence and progression of subclinical atherosclerosis [17–19]. Further, obesity is related to the increasing incidence of heart failure with 9% of cases in males and 14% of cases in females being attributable to obesity [17, 20–22]. Obesity is also an established risk factor for stroke (4% increased risk of ischemic and 6% increased risk of hemorrhagic stroke per 1 unit increase in BMI) [11], atrial fibrillation (RR 1.28, CI 1.20–1.38 per 5 unit increase in BMI) [23], and venous thromboembolism (RR 2.39, CI 1.79–3.17) [24, 25].
Fig. 1.
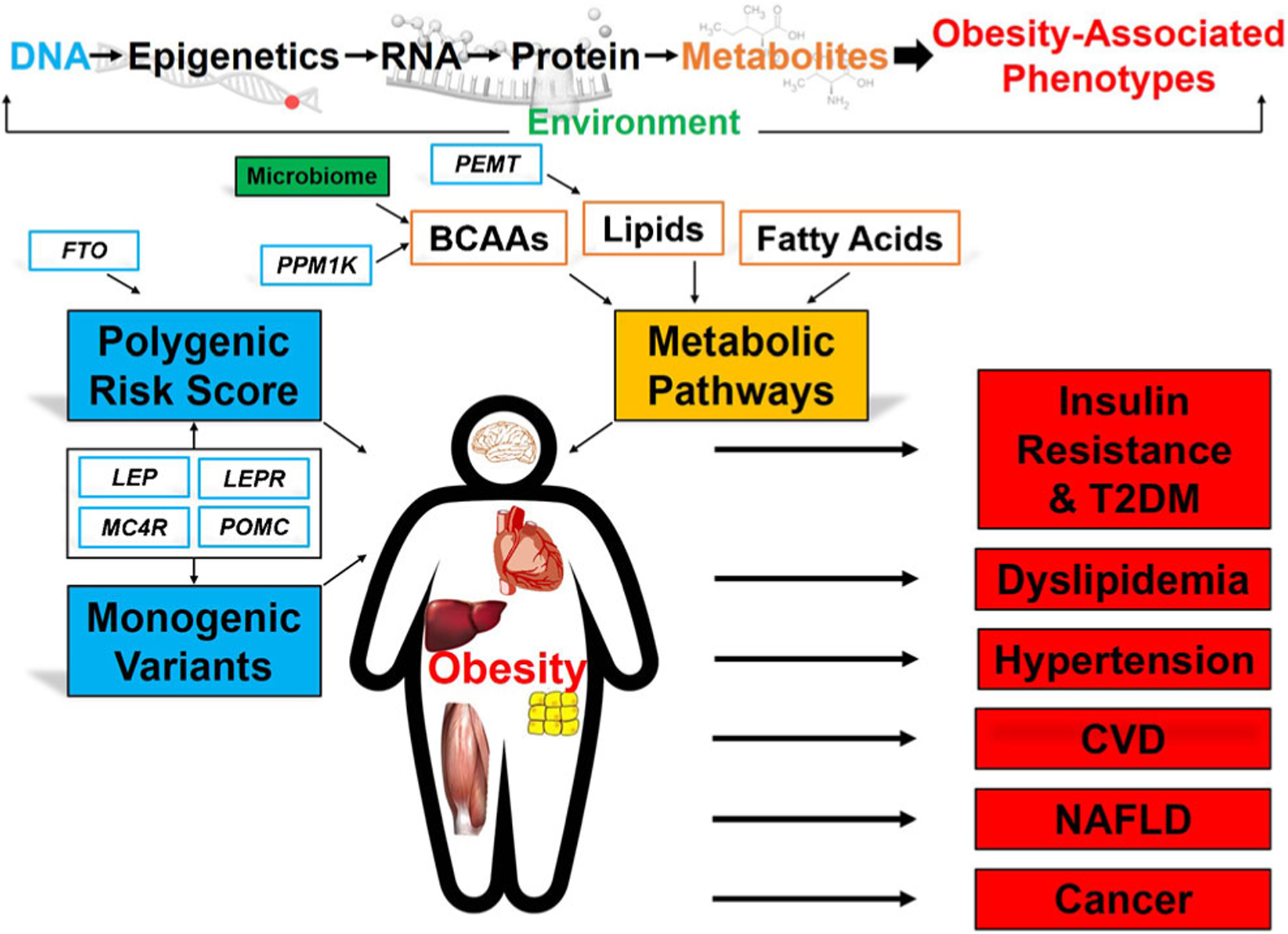
Genomics and metabolic pathways of obesity and associated cardiometabolic risk. Obesity is a systemic disease with impact on the liver, heart, adipose tissue, skeletal muscle, and brain, particularly the hypothalamic-pituitary axis. High-throughput multi-omic profiling (genomic, epigenomic, transcriptomic, proteomic, metabolomic, and microbiome) has advanced the understanding of dysregulated molecular pathways in obesity to improve prediction of cardiometabolic risk. Abbreviations: POMC, pro-opiomelanocortin; LEPR, leptin receptor; LEP, leptin; MC4R, melanocortin 4 receptor; FTO, fat mass and obesity-associated gene; PPM1K, protein phosphatase 1K; PEMT, phosphatidylethanolamine N-methyltransferase; BCAAs, branched-chain amino acids; T2DM, type 2 diabetes mellitus; CVD cardiovascular disease; NAFLD, non-alcoholic fatty liver disease
While these studies provide strong evidence for the association between obesity and cardiometabolic endpoints, such observational studies can be prone to unmeasured confounding. Mendelian randomization leverages genetic variants as instrumental variables for causal inference to help determine potential causality between an intermediate factor and an endpoint. Recent Mendelian randomization studies of obesity have shown that genetic susceptibility to obesity is associated with cardiometabolic diseases, suggesting that obesity has a causal association (i.e., not just due to confounders) with these diseases, including hypertension, hypertriglyceridemia, T2DM, coronary artery disease, atrial fibrillation, venous thromboembolism, aortic stenosis, and heart failure [26–28]. Mendelian randomization analyses of polygenic risk scores for obesity traits including BMI, waist-to-hip ratio (WHR), and BMI-adjusted WHR have identified additional causal associations with stroke, chronic obstructive pulmonary disease, lung cancer, non-alcoholic fatty liver disease, and renal failure, further informing the global impact of obesity on human disease [29].
Heterogeneity in Development of Adverse Cardiometabolic Consequences of Obesity
Although it is clear that obesity is a strong risk factor for these cardiometabolic consequences, there is marked heterogeneity in their development, complicating risk prediction models [30]. For example, a 2008 NHANES study examined the prevalence of poor metabolic health in obesity and found that a surprising one-third of obese individuals were metabolically healthy despite being obese when evaluating the prevalence of cardiometabolic abnormalities (hypertension, elevated levels of triglycerides, fasting plasma glucose, C-reactive protein, insulin resistance, and low high-density lipoprotein [HDL]) [31]. Younger age, non-Hispanic Black ethnicity, and higher physical activity were independent correlates of metabolic health in overweight and obese individuals. Similar heterogeneity is seen in development of CVD outcomes in individuals with obesity: a systematic review of all-cause mortality for obesity demonstrated that while obesity was a consistent risk factor, hazard ratios varied from 0.95 to 1.29 across BMI categories with the majority of patients with obesity not suffering from the endpoint [32]. Given this heterogeneity, additional measures of obesity and body fat distribution, such as WHR, may be better markers than BMI for certain cardiometabolic comorbidities. For example, a polygenic risk score for waist-to-hip ratio was associated with T2DM and coronary heart disease even after adjustment for BMI [26, 33].
This disconnect between obesity measures and cardiometabolic consequences leads to incomplete risk prediction models and difficulty in identifying obese individuals with the greatest need for therapeutic interventions to prevent future events. While lifestyle factors such as diet and sedentary behavior are important risk factors for overweight and obesity, and public health measures aimed at the prevention of obesity nationally and internationally through targeting these lifestyle factors are key, it is also imperative to understand the biology and related biomarkers that could help prevent and treat overweight and obesity to prevent downstream consequences.
The Genetics of Obesity: a Prototypical Common Complex Genetic Disease
Monogenic Forms of Obesity
Monogenic forms of obesity identified through linkage analyses of families with obesity account for an extremely small proportion of obesity. These include syndromic and nonsyndromic forms of obesity and usually result in severe obesity that manifests in childhood. Syndromic monogenic obesity disorders are rare (1 in 565 to < 1 in 1,000,000) [34] and include Bardet-Biedl syndrome, an autosomal recessive disorder accompanied by retinal defects and caused by one of 21 genes that encode structure and function of cilia [35–37], and Prader-Willi syndrome, caused by loss of imprinted genes that are paternally expressed from the chromosome 15q11-q13 region and accompanied by hypogonadism, short stature, and mild learning and behavioral problems [38]. Nonsyndromic monogenic forms of obesity are caused by loss-of-function variants in the leptin (LEP), the leptin receptor (LEPR), pro-opiomelanocortin (POMC), or melanocortin 4 receptor (MC4R) genes which impact obesity susceptibility usually through dysregulated food intake [39–42].
Polygenic Common Complex Obesity: Common Variants, Common Disease
Although obesity is influenced by lifestyle and environmental factors, heritable genetic determinants that contribute to obesity risk and the underlying heritability of obesity have been identified. Heritability estimates for obesity calculated from studies of families range from 59 to 77% [43, 44]. Overweight and obesity in the majority of the population operates as a prototypical common complex disease. Early genome-wide studies that attempted discovery beyond candidate gene studies utilized linkage analysis in families with non-monogenic obesity, operating under the assumption that genetic discovery techniques for monogenic diseases would also be useful in common complex diseases. These first genome-wide linkage analyses of obesity identified a locus on chromosome 10p [45] that was replicated in two additional cohorts [46, 47]. Fine-mapping of this locus identified association of BMI with common variants in glutamate decarboxylase 2 (GAD2) [48]; however, these results were not widely replicated [49, 50]. The first genome-wide association studies (GWAS) of BMI and childhood and adult obesity identified common intronic single nucleotide polymorphisms (SNPs) in the FTO (fat mass and obesity associated) gene as the most strongly associated gene, with each risk allele increasing obesity risk 1.2 times compared with no risk alleles [51–53]. Common SNPs in FTO have remained the most consistent and strongest GWAS locus. As GWAS expanded in sample sizes through collaborative consortia studies and meta-analyses, additional loci were identified albeit less significant than FTO, and included common variants near MC4R (a monogenic cause of obesity) [54], coding missense variants in NPC1 (endosomal/lysosomal Niemann-Pick C1 gene), and noncoding SNPs near MAF (encoding the transcription factor c-MAF), PTER (phosphotriesterase-related gene), and PRL (prolactin) [55]. In fact, greater than 200 loci associated with BMI and obesity have been identified by GWAS to date and have estimated > 20% of BMI heritability can be attributed to inheritance of common variants [56, 57••].
Rare Variants, Common Disease?
Unfortunately, these common variants identified from GWAS individually have small effect sizes and in aggregate, loci from these initial GWAS studies only explain 2–3% of the variance in BMI; even FTO with high population frequency and the largest effect size only explains 0.34% of inter-individual BMI variation [56, 58–60]. As such, as high-throughput next-generation sequencing technologies evolved, the genomics community looked to analysis of rare variation as a solution to the conundrum of the “missing heritability” in common complex diseases, in line with the “rare variant, common disease” hypothesis where variants albeit rare exert larger effect sizes and in aggregate can thus account for a common disease. Interestingly, the first whole-exome sequencing (WES) studies of obesity identified rare coding variants in the known monogenic obesity gene LEPR [61, 62], suggesting that this gene contributes to both monogenic and more common later-onset forms of obesity. These WES also identified new genes such as density lipoprotein receptor–related protein 2 (LRP2), uncoupling protein 2 (UCP2), dynein axonemal assembly factor 1 (DNAAF1) [63], and laminin subunit beta 3 (LAMB3) [64]. A more recent study of 2737 severely obese cases used exome and targeted sequencing to identify this missing heritability and found rare variants in Pleckstrin homology domain–interacting protein (PHIP), diacylglycerol kinase iota (DGKI), and zinc-finger-MYM-type-containing 4 (ZMYM4) [65•]. A large study using a hybrid approach of whole-genome sequencing (WGS) and GWAS confirmed considerable overlap between monogenic and polygenic contributors to BMI and other anthropometric traits, compromised of mostly common variants with small effect sizes [66]. Overall, these studies suggest that a combination of common and rare variants contributes to the burden of common obesity, as the scientific community is finding to be the case for many common complex diseases. A summary of genes identified through genomic studies of obesity is provided in Table 1.
Table 1.
Overview of key genes identified from studies of obesity
| Gene | Biology | Other Associated Phenotypes |
|---|---|---|
| Identified from genome-wide association studies (GWAS) | ||
| Fat mass and obesity-associated gene (FTO) [48–50, 71, 64, 66, 68, 69] |
|
|
| Melanocortin 4 receptor (MC4R) [38, 40, 41, 51] |
|
|
| Endosomal/lysosomal Niemann-Pick C1 gene (NPC1) [52, 73–75] |
|
|
| c-MAF proto-oncogene (MAF) [52, 76, 77, 181] |
|
|
| Phosphotriesterase-related gene (PTER) [52] |
|
|
| Prolactin (PRL) [52] |
|
|
| Identified from whole-exome sequencing (WES) | ||
| Leptin receptor (LEPR) [38, 41, 57, 58] |
|
|
| Lipoprotein receptor-related protein 2 (LRP2) [59, 182] |
|
|
| Uncoupling protein 2 (UCP2) [59, 78, 174, 175] |
|
|
| Dynein axonemal assembly factor 1 (DNAAF1) [59, 183, 184] |
|
|
| Laminin subunit beta 3 (LAMB3) [60, 185] |
|
|
| Pleckstrin homology domain interacting protein (PHIP) [61] |
|
|
| Diacylglycerol kinase iota (DGKI) [61] |
|
|
| Zinc-finger-MYM-type-containing 4 (ZMYM4) [61] |
|
|
Insight into the Biology of Obesity Afforded by Genomic Studies
While genomic studies have highlighted potential genetic risk markers of obesity, they have also highlighted the underlying biology of obesity risk. For example, genetic variants in the leptin-melanocortin pathways (LEP, LEPR, LRP2, POMC, and MC4R) contribute to obesity, at least in part, through hypothalamic control of energy balance and susceptibility to increased food intake. Leptin, secreted from adipose tissue, acts in the arcuate nucleus of hypothalamic satiety center where melanocortin neurons produce POMC that ultimately stimulate MC4R to reduce feeding [68]. PHIP enhances transcription of POMC, lending further evidence to the biologic pathways affected by genetic variants associated with obesity. When GWAS studies first identified the association between FTO and obesity, the biologic role of FTO was unknown, but mouse models have subsequently shown the importance of FTO in regulation of fat mass and adipogenesis [69]. FTO is highly expressed in the hypothalamus and pituitary and adrenal glands [83]; it has a role in development, as postnatal mortality and growth retardation have been observed in FTO deficiency [67]. Data has suggested that the influence of FTO SNPs on obesity may be due to their impact on expression of neighboring genes [70, 71, 84]; however, work in human fibroblasts and blood cells have confirmed the link between SNP risk genotype and FTO expression [72–74]. From studies of NPC1 mutations in the lipid storage disease Niemann-Pick type C, the NPC1 protein is known to be involved in endosomal cholesterol transport in the central nervous system, liver, and macrophages [77, 85, 86], but further studies of the specific NPC1 variants associated with obesity are warranted. The c-MAF transcription factor is involved in cellular differentiation in the pancreas and adipose tissue as well as tissue-specific gene expression, including insulin and glucagon [87, 88]. At a molecular level, UCP2 functions in oxidative phosphorylation and mitochondrial membrane transport to regulate energy balance and ultimately body weight [89]. In the same study that used WES to identify LAMB3 to be associated with BMI, the investigators found LAMB3 mRNA levels to be correlated with BMI and adipose morphology and in vitro knockdown of LAMB3 inhibited adipogenesis [64]. Additional loci identified by GWAS in association with obesity have replicated genes implicated in neuronal processes, hypothalamic function, and energy homeostasis. Little is known about the biology of DGK1 and ZMYM4.
Transethnic Genetic Studies of Obesity
The majority of the initial GWAS and WES studies was done in primarily European cohorts. More recent studies have evaluated transethnic genetic analyses (African, Hispanic/Latino, Asian, and European descent) of BMI and obesity and found consistency with previously reported SNPs like in FTO (lead SNP effect 1.34%, standard error 0.10%, p = 2.3E−42); nearly a quarter of 170 established BMI SNPs studied and 29 out of 36 fine-mapped BMI loci replicated in these analyses [90]. Further, the investigators found novel loci at LYPLAL1, COBLL1, IRS1, SLC39A8, TFAP2B, and STK33/TRIM66, expanding our understanding of the heterogeneity in genetic architecture of obesity across diverse populations.
Polygenic Risk Scores in Obesity
More recently, aggregating these common variants in polygenic risk scores (PRS) has demonstrated a stronger combined effect of many variants [91–95]. The most recent robust PRS, created from 2.1 million genetic variants identified from a GWAS of BMI [56], found a strong correlation with BMI (0.292, p < 0.001) and a 13-kg gradient in average weight comparing the top and bottom deciles of PRS.. The effect of the PRS was comparable to that of rare monogenic obesity-associated variants; individuals with the top 1.6% of PRS had a mean BMI 4.1 kg/m2 higher than the rest of population, equivalent to the BMI increase seen in individuals with rare MC4R mutations [57••]. A high PRS (i.e., the top decile of the 306,134 participants studied) was associated with a 25-fold gradient risk of severe obesity, including earlier in life with a mean higher weight of 3.5 kg by 8 years of age and 12.3 kg (both p < 0.0001) by 18 years of age as compared with those in the bottom decile. However, polygenic risk of obesity is not deterministic, as greater than 17% of these adults with a high PRS remained normal weight or underweight [57••]. In addition to being a risk factor for obesity, Khera et al. also found that a high BMI PRS is associated with increased risk of cardiometabolic disease and mortality, with a 23% increased risk of ischemic stroke, 27% increased risk of coronary artery disease, 33% increased risk of heart failure, 35% increased risk of hypertension, 40% increased risk of venous thromboembolism, and 70% increased risk of T2DM [57••]. Taken together, these findings suggest that genetic variants, and in particular the combination of many common variants such as through a BMI PRS, do not only increase susceptibility to obesity, but also may help identify patients most at risk for severe obesity and cardiometabolic complications to best target intervention strategies.
Genes, Obesity, and Lifestyle
BMI-associated genetic variants have been shown to be associated with food preferences, thereby potentially modulating the impact of diet on obesity and cardiometabolic outcomes [96–98], although the data are inconsistent. Concordantly, in a pooled study of 30,904 individuals, an interaction of a PRS of 97 BMI-associated SNPs and diet quality found an attenuated effect of PRS on obesity risk in individuals who reported eating a healthier diet [99]. Conversely, in a randomized trial of 609 overweight or obese individuals comparing low-fat vs. low-carbohydrate diets for weight loss, patterns of alleles in three SNPs in genes relevant to fat and carbohydrate metabolism (FABP2, PPARG, ADRB2) showed no significant interaction with diet on amount of weight loss at 12 months [100]. Genetic susceptibility also interacts with other lifestyle factors in association with obesity and related metabolic consequences. For example, genetic susceptibility to obesity may be “unmasked” by increasingly obesogenic environmental factors, such as ease of access to inexpensive calorie-dense foods and reduced need for physical activity [101]. Although initial studies showed inconsistent results, a meta-analysis of FTO variants found a modest interaction between genotype and physical activity on obesity risk [102]. Multiple additional studies have described the interaction of unhealthy diet and sedentary behaviors in individuals with polygenic predisposition to obesity (32–69 variants) showing that the combination of lifestyle factors and genetic risk is interactive on obesity risk [103–106]. Although the detailed biology of potential pleiotropy warrants further study, the impact of genetic variants associated with obesity on cardiometabolic phenotypes appears to be driven through altered food intake and energy homeostasis.
Epigenetics and Obesity
Epigenetic changes are modifications to DNA that are heritable and can affect gene activity and expression without changing the underlying DNA sequence, for example, DNA methylation and histone modifications. Epigenetic modifications can be induced by environmental factors and can contribute to disease risk across the life span, including in utero modifications that can transmit future disease risk. The study of epigenetic modifications, which have both genetic and environmental effects, provides additional insight into obesity and cardiometabolic disease risk. The most well-studied epigenetic modification, DNA methylation, has been investigated through genome-wide, genetic variant and candidate gene approaches in obesity and obesity-associated traits. Genome-wide methylation analyses of whole blood and adipose tissue show associations between methylation patterns of hypoxia-induced factor 3A (HIF3A) and other genes involved in adipogenesis, insulin, and glucose metabolism with higher BMI [107]. Candidate gene approaches to methylation identified pathways in eating behavior and lipid metabolism associated with obesity and related traits. FTO may provide key epigenetic regulation as it has been identified as a N6-methyladenosine (m6A) demethylase. An FTO SNP associated with obesity and increased FTO expression shows reduced m6A ghrelin methylation and increased ghrelin expression in blood cells, providing a mechanism for increased food intake and preference for energy-dense food [73]. The m6A activity of FTO provides a biologic link between obesity and cancer risk, as FTO provides transcriptional regulation impacting tumorigenesis [69].
Obesity Metabolomics: Novel Molecular Pathways and Metabolic Biomarkers
Germline genetic variants provide a static view of risk of obesity and related cardiometabolic disease. Environmental influences that change throughout the lifetime can dynamically alter biologic pathways that may contribute to obesity and cardiometabolic disease risk. Novel omic technologies have yielded the opportunity to examine how biomarkers change over time as chronic diseases develop and may provide more proximal insight into dysregulated disease biology. These technologic advances have allowed for the high-throughput measurement of hundreds to thousands of metabolites in small amounts of biospecimen samples enabling biomarker discovery work. Molecular profiling of circulating metabolomic measurements integrate environmental and genetic factors, and thus can provide insight into obesity and risk of cardiometabolic complications, allowing simultaneous identification of biology and biomarkers.
Branched-Chain Amino Acid Catabolic Pathway in Obesity and T2DM
Early studies applied high-throughput metabolomics to studies of obesity in an unbiased fashion to identify potential novel biological pathways and biomarkers related to this disease over a decade ago. In a study of 74 obese and 67 lean individuals from the STEDMAN study, Newgard et al. used targeted tandem flow injection mass spectrometry to measure 53 metabolites in plasma and identified a cluster of branched-chain amino acid (BCAA) and related mitochondrial catabolic byproducts that discriminated obese from lean individuals. Further, this cluster of BCAA and related metabolites was associated with homeostatic model assessment (HOMA, a marker of insulin resistance) in both obese and lean individuals [108]. To determine whether BCAAs are markers of the obesity and insulin resistance process or are potentially involved in the causative pathway, Zucker obese rats were fed standard chow vs. high fat chow vs. chow supplemented with BCAAs. After 13 weeks, despite lesser weight gain due to lesser food intake, the BCAA-supplemented rats were equally insulin resistant to those fed high fat chow, suggesting that BCAAs are not merely markers but appear to be involved in induction of insulin resistance in the setting of obesity. Supportive of this, in other studies in both mice and rodents, BCAA restriction has been shown to improve insulin sensitivity and metabolic health [109, 110]. Potential mechanisms of BCAA interference with insulin signaling were identified: insulin resistance was accompanied by phosphorylation of mTOR and was reversed by treatment with rapamycin, an mTOR inhibitor [108]. More recently, Vogelzangs et al. used nuclear magnetic-resonance spectroscopy to measure 17 serum metabolites in 634 overweight or obese adults without T2DM and found BCAA levels associated with both hepatic and muscle insulin resistance [111•].
Determinants of elevations in circulating BCAA levels in obesity are diverse. Decreased rates of BCAA oxidation in adipose tissue secondary to suppression of catabolic enzymes likely contribute to circulating levels [112]. Genetics appears to also influence circulating BCAA levels: a genome-wide meta-analysis of 16,596 individuals found the strongest association near the protein phosphatase 1K (PPM1K) gene with higher circulating levels of BCAAs and risk of T2DM; this gene encodes an activator of mitochondrial branched-chain alpha-ketoacid dehydrogenase [113]. The microbiome also appears to play a role in BCAA pathways in obesity and in BCAA levels. Ridura et al. performed an elegant study of fecal transplantation from human twins discordant for obesity into gnotobiotic mice [114]. Fecal communities clustered based on twin obesity phenotype and transplantation with the obese twin’s feces was sufficient to induce obesity in the mice, with coordinated upregulation of BCAA pathways in the mice as they developed obesity. A 2016 study of 277 lean and obese individuals without T2DM found BCAAs to correlate with both insulin resistance and gut microbiota, enriched with species for biosynthetic potential of BCAAs and with fewer genes related to inward amino acid transport, further showing the interrelated metabolomic and microbiome pathways underlying obesity and cardiometabolic risk [115]. These and other studies display the multifactorial reasons and complex feedback loops that determine elevated circulating BCAA levels in obesity which are determined partially by genetics; dietary sources including meat, fish, dairy products, and eggs; and the microbiome [116, 117].
Recently, the liver has emerged as an important organ for BCAA catabolism in obesity. Insulin signaling inhibits branched-chain α-keto acid dehydrogenase (BCKDH, the first irreversible step in BCAA catabolism); fructose feeding in rats, as seen in obesity, inhibits hepatic BCKDH via phosphorylation of its inhibitor kinase BDK leading to hepatic lipogenesis, suppression of fatty acid oxidation, and increased fat storage in the lipid [118]. In Zucker obese rats, a BDK inhibitor molecule relieved the inhibition of BCKDH, lowering circulating branched-chain keto acid (BCKA) and BCAA levels, improving glucose tolerance and insulin resistance and decreasing hepatic fat storage. Overexpression of the phosphatase PPM1K increased BCKDH activity and had similar effects on BCAA levels and hepatic lipid metabolism. In the setting of insulin resistance, impaired hepatic BCAA metabolism is also linked to a compensatory upregulation of skeletal muscle BCAA oxidation, altering mitochondrial substrate utilization and decreasing acylglycine efflux [110]. Heart failure is an important cardiometabolic consequence of both obesity and T2DM and BCAA restriction in obese rats shifts myocardial fuel metabolism in favor of fatty acids over glucose metabolism and reduces myocardial triglyceride stores independent of BCKDH [119].
In addition to highlighting a novel biological pathway underlying obesity and insulin resistance, BCAAs have also been shown to serve as biomarkers in obesity and obesity-related diseases [120], including risk of incident T2DM, even independent of BMI [121, 122]. In a study of metabolomic profiling in 2422 individuals from the Framingham Offspring Study, Wang et al. found that BCAA and related metabolites were the metabolites most associated with measures of insulin resistance, and further, these metabolites predicted risk of incident DM up to 12 years in the future in individuals free of insulin resistance or T2DM at baseline [121]. Circulating BCAAs have also been identified as a biomarker for discrimination of metabolic wellness independent of BMI. In a study of 1872 individuals classified as metabolically well or unwell based on impaired fasting glucose, hypertension, high triglycerides, low HDL, or impaired insulin resistance, BCAA levels were higher in metabolically unwell overweight individuals compared with metabolically well obese individuals, suggesting these metabolites may be more granular markers of metabolic health than traditional clinical lab values [123]. Relatedly, BCAAs and related metabolites have been found to be similarly upregulated between overweight-obese individuals and normal weight-obese individuals (defined as BMI < 25 and body fat > 30% for women and > 25% for men) compared to lean individuals [124]. Finally, circulating BCAA levels have been shown to discriminate patients with coronary artery disease incremental to clinical risk factors [125, 126]. A summary of BCAA studies relevant to obesity, as well as the subsequent metabolic pathways discussed, is provided in Table 2.
Table 2.
Overview of metabolomic studies of obesity
| Study population and sample size | Platform | Key Significant Metabolites | Other Outcomes |
|---|---|---|---|
| Obesity/BMI | |||
| STEDMAN [104] - 74 Obese - 67 Lean |
Targeted MS/MS |
|
Insulin resistance |
| European Diogenes Study [107] - 634 Overweight/Obese |
NMR |
|
Hepatic insulin resistance and Skeletal muscle insulin sensitivity |
| MURDOCK [119] - 610 Overweight - 852 Obese - 410 Lean |
MS/MS |
|
Metabolic health |
| - 43 Normal Weight Obesity [120] (BMI <25 and body fat > 30% for women and > 25% for men) - 110 Overweight/Obesity - 26 Lean |
LC ESI-MS |
|
Body Composition Subtypes |
| SAFHS [123] - 1431 Adults |
LC ESI-MS/MS |
|
Anthropometric and biochemical measurements |
| Western Australian Pregnancy Cohort [124] - 1176 Women |
MS |
|
Waist Circumference Insulin resistance |
| - 34 Metabolically Healthy Obese [125] - 38 Metabolically Unhealthy Obese |
LC/MS & GC/MS |
|
Metabolic Health |
| - 107 Metabolically Healthy Obese [126] - 100 Metabolically Unhealthy Obese - 78 Normal Weight Metabolically Healthy |
LC-MS/MS |
|
Metabolic Health Cardiometabolic Biomarkers |
| - 10 Obese without T2DM [127] - 9 Obese T2DM - 11 Lean |
LC/MS |
|
|
| Boston Puerto Rican Health Study [128] - 781 Adults |
LC/MS |
|
Sugar-sweetened beverage (SSB) consumption and obesity risk |
| - 14 Obese without T2DM [129] - 10 T2DM - 12 Lean |
MS/MS |
|
Glycemic control Acylcarnitines levels following insulin infusion |
| - 6 Obese [130] - 6 Lean Before and after 5-day high-fat diet |
MS/MS |
|
Plasma and Skeletal Muscle metabolomics |
| Six independent cohorts [131] - 739 Adults |
Targeted MS |
|
Age |
| - 35 Men [132] - 47 Women |
NMR |
|
Metabolic health Activity Energy Expenditure Sedentary Time and Activity Reporting Questionnaire |
| TwinsUK Registry [133] - 1,969 Twins |
UPLC-MS/MS |
|
Cardiovascular events |
| - 85 Obese [134] - 42 Non-Obese |
LC ESI-MS/MS |
|
Interleukin-6 C-reactive protein |
| Gestational Obesity | |||
| HAPO Study [135••] - 1600 Pregnant Women |
Targeted MS/MS |
|
Insulin resistance |
| HAPO Study [136] - 1412 Pregnant Women |
Targeted MS/MS and Non-targeted GC-MS |
|
GWAS Insulin resistance |
| Pediatric Obesity | |||
| - 80 Obese Children [137] - 40 Normal Weight Children |
LC-MS/MS |
|
Pubertal stage |
| - 2191 Healthy Participants (age 3 months to 18 years) [138] | LC-MS/MS |
|
Pubertal stage |
| - 524 Adolescents (age ~ 13 years) [139] | Non-targeted LC-MS |
|
Metabolic health |
| Systematic Review [140] - 10 Studies - 2,673 Participants |
Varied |
|
Insulin resistance |
| Behavioral Weight Loss Intervention | |||
| Weight Loss Maintenance Study [141] - 500 Participants with ≥ 4kg Weight Loss - 22 Participant Independent Validation Cohort |
Targeted MS/MS |
|
Change in insulin resistance independent of amount of weight lost |
| Surgical Weight Loss Intervention | |||
| - 16 Gastric Bypass [142] - 17 Dietary Intervention Matched 10 kg weight loss |
MS/MS |
|
Insulin resistance |
| - 10 Gastric Bypass [143] - 10 Laparoscopic adjustable gastric banding ~20% weight loss |
MS/MS |
|
Insulin resistance |
Except where specified by (−), higher metabolites levels are associated with increased BMI or other outcomes noted in the table. In studies where examined, metabolic health is defined as a combination of one or more abnormal measures of waist circumference, blood glucose, hypertension, dyslipidemia, and insulin resistance
Abbreviations: MS, mass spectrometry; MS/MS, tandem mass spectrometry; LC, liquid chromatography; GC, gas chromatography; LC ESI-MS, liquid chromatography-electrospray ionization-mass spectrometry; UPLC-MS/MS, ultra-high-performance liquid chromatography-tandem mass spectrometry; NMR, nuclear-magnetic-resonance; BCAA, branched-chain amino acid; AA, amino acids; NEFA, non-esterified fatty acids; SCACs, short-chain acylcarnitines; MCACs, medium-chain acylcarnitines; LCACs, long-chain acylcarnitines
Lipid-Related Metabolic Pathways in Obesity Identified Through Metabolomic Profiling
Evolution of mass spectrometry and other methods for comprehensive metabolomic profiling has enabled discovery of a broader set of metabolic pathways underlying obesity and related phenotypes, including many lipid-related pathways. For example, lipid profiling of 1076 individuals revealed higher ceramide levels (β = 2.01) and lower lysophospholipids (β = − 1.44 to − 2.04) associated with BMI (all p < 0.001) [127]. A second large study of 1176 women found higher sphingomyelin and diacylphosphatidylcholine levels and lower lysophosphatidylcholines associated with waist circumference and BMI; a subset of the associated lipids also correlated with HOMA [144]. Two smaller studies replicated BCAA findings and similarly identified choline-containing phospholipids to be lower in individuals with metabolic unhealthy obesity as compared with normal weight metabolically healthy or metabolically health obesity [128, 145]. A factor composed of lysophosphatidylcholines was inversely associated with cardiometabolic biomarkers such as hemoglobin A1C (β = − 0.010, p = 0.028, 95% CI [− 0.018 to − 0.001]) and CRP (β = − 0.22, p < 0.001, 95% CI [− 0.334 to − 0.110]) in metabolically healthy obese, and with HOMA (β = − 0.118, p = 0.021, 95% CI [− 0.217 to − 0.018]) in metabolically unhealthy obese; and a diacyl-phosphatidylcholine factor was directly correlated with cardiometabolic markers in metabolically unhealthy obese individuals [145]. In mice, 12 weeks of high-fat feeding resulted in reduction of lysophosphatidylcholines with the greatest changes occurring in the first week [146]. Mechanistically, treatment of isolated adipocytes and mice with lysophosphatidylcholines improves glucose uptake in a dose-dependent manner [147]. A recent study investigating the metabolic mechanisms linking sugar-sweetened beverage intake and obesity used plasma metabolomic profiling of 781 individuals and found phosphatidylcholine and lysophospholipid pathways to be enriched with amount of beverage intake and BMI [148]. Lending further support, 8 of 10 genes in these pathways interacted with sugar-sweetened beverage intake on BMI. Based on data from isolated perfused rat livers [149] and obese beagles [150], decreased activity of lecithin:cholesterol acyltransferase (LCAT) may contribute to altered lysophosphatidylcholine levels. Further, genetic deficiencies or polymorphisms in phosphatidylethanolamine N-methyltransferase (PEMT) may contribute to altered lysophosphatidylcholine levels as data from both mice and humans show risk of NAFLD in PEMT KO or polymorphisms [129, 151, 152].
Fatty Acid–Related Pathways in Obesity Identified Through Metabolomic Profiling
Plasma long-chain acylcarnitines, byproducts of mitochondrial fatty acid oxidation, are elevated in individuals with obesity and T2DM compared to lean individuals without metabolic syndrome and are associated with insulin resistance [130]. A study of 12 lean, 14 obese without T2DM, and 10 participants with T2DM found elevated levels of long-chain acylcarnitines (C14:1, C16, C18, C18:1) in subjects with obesity and T2DM compared to lean controls [153]. Insulin infusion reduced plasma levels of long-chain acylcarnitines in all groups but patients with T2DM had a blunted response. In rodents, high-fat feeding and obese, insulin-resistant Zucker rats have accumulation of long-chain acylcarnitines in skeletal muscle, representing incompletely oxidized lipid species and reporting on impaired mitochondrial fatty acid β-oxidation [154]. This substrate accumulation leads to mitochondrial stress and ultimately impaired responsiveness to insulin [131]. A study of plasma and skeletal muscle metabolomics from 6 obese and 6 lean individuals before and after high-fat diet found increased medium-chain acylcarnitines (C6, C8, C10:2, C10:1, C20 and C12:1) in skeletal muscle of obese subjects after high-fat diet, but decreased in lean subjects, potentially providing further evidence to the mechanisms of altered skeletal muscle metabolism in obesity [132]. Obesity and cardiometabolic disease represent pro-inflammatory states and though the detailed causal mechanisms and tissue-specific sources remain to be further elucidated, it has been shown that medium- and long-chain acylcarnitines activate pro-inflammatory signaling pathways [133, 155].
Other Major Metabolic Pathways in Obesity
Beyond BCAAs, lipids, and fatty acid pathways, other biologic markers of metabolism have been identified in obesity. Metabolomic profiling of 739 subjects found in addition to the association of clusters of lipids and amino acid metabolites with BMI, glycine had an inverse association with BMI and has been found to be directly related to insulin sensitivity [156]. Similarly, a small study identified lower serine and glycine concentrations in patients with metabolic syndrome risk factors and greater adiposity that increased with activity energy expenditure [157]. A large study of 1969 unrelated individuals identified 49 significant metabolites associated with BMI across multiple time points that validated in an independent cohort [135••]. In addition to amino acids and lipids, the investigators found nucleotides related to purine metabolism (percent variation in BMI explained by metabolites 7.3–16.4%) and peptides (4.6 to 6.9%) associated with obesity, with urate being the most significant metabolite overall (p = 1.2E−40). Assessment of CVD outcomes found lower event rates in participants with healthier metabolomic profiles compared to normal/overweight BMI with obese metabolic profile and obese individuals (2.6 events per hundred individuals, 3.4 events and 4.4 events, respectively, p = 0.003).
Metabolic Pathways and Biomarkers of Obesity in Pregnancy and in Childhood and Adolescence
In addition to genetic predictors of metabolic signatures and obesity, pregnancy represents an important time point in the lifespan, as obesity is associated with adverse pregnancy outcomes (pre-eclampsia [OR 3.15, CI 2.96–3.35], gestational hypertension [OR 2.91, CI 2.76–3.07], and gestational diabetes [OR 3.56, CI 3.05–4.21]) and may predict future risk of obesity and cardiometabolic disease for both the mother and newborn [158–160]. For example, a large targeted metabolomics study of 1600 pregnant women from four different ancestry groups identified BCAA, their carnitine esters, aromatic amino acids, triglycerides, non-esterified fatty acids, and medium- and long-chain acylcarnitines to be associated with maternal BMI, glucose levels, and insulin sensitivity [136]. This study also replicated the negative correlation of glycine with both maternal BMI and insulin resistance. Maternal BMI is also associated with fetal metabolites and after correction for maternal BMI and blood glucose, maternal levels of branched-chain and other amino acids, acylcarnitines, lipids, and fatty acids have been identified to correlate with fetal growth, adiposity, and hyperinsulinemia [137–139]. A recent study performed integrated GWAS and metabolomic analyses of insulin resistance in 1412 pregnant women and identified variants in the glucokinase regulatory protein gene that associated with palmitoleic acid levels [140].
With rising rates of childhood obesity, it is important to understand metabolic pathways and predictors of obesity early in life, to better understand earlier biological underpinnings and potential lifespan “setpoints,” and to target earlier therapeutic interventions. Interestingly, metabolomic profiling in children and adolescents suggests some similar but also some potentially different dysregulated metabolic pathways. A study of 80 obese children and 40 normal weight children between the ages of 6 and 15 found increased levels of two long-chain acylcarnitines (C12:1 and C16:1) and decreased levels of the amino acids glutamine, methionine, and proline and of 9 phosphatidylcholines in obese children compared to controls, suggesting changes in oxidative stress, β-oxidation, and sphingomyelin metabolism in pediatric obesity [161]. No correlations in metabolite concentrations were seen with pubertal stage. Concordant with studies in adults, acylcarnitine levels were higher in obese children and phosphatidylcholine and lysophosphatidylcholines were lower, but in contrast to adult metabolomic findings in obesity, this study found no significant differences in BCAA levels. Conversely, a study of 2191 children between the ages of 3 months and 18 years did find associations between elevated levels of BCAAs, aromatic AAs and C3 carnitines (an intermediate of BCAA metabolism), and BMI, and found distinct metabolite patterns when comparing pubertal stages, with sex-dependent differences with males having higher metabolite levels in later pubertal stages [162]. The investigators also found negative associations between citrulline and glycine and BMI, but only in females. Similarly, a study of 524 overweight or obese adolescents found higher levels of BCCAs and related metabolites, as well as long-chain acylcarnitines, diacylglycerols, and steroid hormones in overweight/obese adolescents with high metabolic risk compared to non-overweight/obese with low metabolic risk [163]. A systematic review of metabolomic markers of insulin resistance in childhood obesity identified BCAAs, aromatic amino acids, and acylcarnitines to be most frequently associated with insulin resistance [164]. BCAAs and tyrosine were associated with future metabolic risk in cohorts with long-term follow-up; however, overall small scale and heterogeneity of study design limits these results. Larger metabolomic studies of children and adolescents that consider pubertal stage and include racially diverse cohorts are needed to better understand the metabolomics of obesity and cardiometabolic risk across the life span.
Integrated Genomics and Metabolomics for Greater Understanding of Obesity Biology
The combination of genomic and metabolomic research can enhance our understanding of the biology of obesity. For example, metabolomic profiling of six individuals with homozygous loss-of-function mutations in LEP before and after leptin replacement showed decreases in BCAAs and phospholipids. These changes were not seen after caloric restriction, suggesting that leptin administration leads to changes in substrate utilization but that the effect of leptin on these metabolites is independent of caloric intake [165]. These patients also had increases in fatty acids and acylcarnitines, and overall a metabolomic score of 37 metabolites was similar to that of individuals with obesity in the absence of monogenic syndromes, suggesting the utility of metabolomic scores regardless of genetic risk of obesity. Beyond GWAS of BCAA discussed above, GWAS of metabolomic traits and even metabolome-wide genome-wide association studies have emerged as methods to understand the biologic impact on disease phenotypes. These studies allow for integrated analysis of genetic, metabolite, and environmental influences on disease phenotypes and genetic variants can in part explain metabolomic variance [166, 167]. For example, a study of 1809 individuals with targeted metabolomics identified that the genetic variant is located in or near genes encoding the enzymes associated with metabolic traits, including those related to lipid and fatty acid metabolism [141]. Interestingly, a metabolic score of 49 BMI-associated metabolites was not associated with a PRS for obesity, suggesting distinct pathways for these molecular predictors of obesity risk [135••]. Taking a broader approach to systems biology, integrating genetics and metabolomics, or genetics and gene expression, investigators have identified biologic pathways of adipogenesis and fatty acid metabolism associated with weight [168] and new genes associated with obesity [169, 170].
Personalized Approaches to Obesity Using Genomics and Metabolomics: One Size Does Not Fit All
There are many potential interventions for obesity, including behavioral, dietary, exercise, pharmacologic, and surgical therapies; however, there is marked heterogeneity among individuals in weight loss response to interventions and improvement in cardiometabolic risk factors like insulin resistance [171, 172]. Despite attempts to characterize predictors of response to weight loss intervention, only early weight loss response in the first few weeks of intervention and adherence to the weight loss program have been consistently identified predictors [173]. Many studies have documented this heterogeneity of response of metabolic risk factors in the context of weight loss [174]. This suggests that improvement in cardiometabolic health is not directly related to amount of weight lost and that additional genomic and metabolomic measures may be important in predicting heterogeneity.
As proof of this concept, a study evaluating a PRS comprised of 25 BMI-associated SNPs showed greater weight loss after dietary counseling in individuals with low PRS than in individuals with higher PRS, with the PRS explaining 2.4% of weight change variance at 1 year [175]. Conversely, a study of a 15 SNP PRS was not associated with differential response to lifestyle interventions in children and adolescents with obesity [176]. Metabolites have also been shown to predict response to weight loss interventions. For example, baseline levels of BCAAs and related metabolites are responsive and predictive of improvements in insulin resistance in behavioral weight loss [171]. However, acylcarnitines levels, although elevated in obesity, have not been found to respond to weight loss and do not correlate with improvement in insulin resistance [177]. This suggests that certain metabolite levels could be used to identify individuals whose metabolic health would benefit most from specific types of weight loss intervention.
Surgical weight loss interventions also result in heterogeneous improvements in cardiometabolic risk factors. Three years after bariatric surgery in obese individuals, gastric bypass resulted in resolution of dyslipidemia in 62% of participants compared to only 27% of patients treated with laparoscopic gastric banding [178]. Similar heterogeneity was seen in this population and others for improvement in T2DM and hypertension after surgery [78, 79, 179–183]. As surgical interventions are costly and pose both short-term surgical complications and longer-term complications, the obesity guidelines indicate the need to characterize patients “most likely to benefit from and least likely to suffer adverse consequences of bariatric surgical procedures” [184]. Studies of genetic predictors of weight loss after gastric banding or bypass have identified polymorphisms in UCP2 to be predictive of lesser weight and fat-free mass loss [142, 185]. A GWAS of 693 individuals found a variant at 15q26.1 to be associated with greater weight loss after gastric bypass; higher expression of ST8SIA2, a gene near this locus, in omental fat was associated with greater weight loss after gastric bypass surgery [143]. Patients undergoing gastric bypass have been observed to have greater improvement in metabolic health measures compared to dietary interventions, even after accounting for the amount of weight lost [75, 76]. Concomitantly, BCAAs and related metabolites have been shown to decrease to a greater extent after gastric banding or bypass than after dietary weight loss in weight loss–matched individuals and correlate with levels of insulin resistance [80, 81]. As such, there is already a robust literature supporting the use of genetic and metabolic biomarkers for identifying those individuals who may benefit the most from a given weight loss intervention with regard to its weight loss and metabolic health benefits. However, while these discovery studies are interesting and in parallel highlight important biology, biomarker-guided implementation studies to determine efficacy of such a personalized approach to weight loss interventions are necessary. As well, studies that incorporate genomic and metabolomic measures may aid in prediction of response to weight loss pharmacotherapy to limit exposure to risks and costs of therapies for those who do not respond with weight loss.
Future Directions: Multifaceted Approaches to Tackle the Obesity Epidemic
While metabolomics lends itself well scientifically to understanding the biology and biomarkers of obesity given its metabolic basis, the future landscape of obesity omics research will include a more comprehensive perspective incorporating epigenomics, proteomics, and the exposome and other environmental factors for a deep understanding of biology and a more precise prediction of obesity-related outcomes. The integration of these layers in parallel with advances in bioinformatics and computational biology is creating opportunities for clinical translation to improve patient outcomes, and to create a more personalized approach to obesity management and prevention of cardiometabolic disease. Stratifying individuals based on more personalized needs will hopefully allow for allocation of health care resources to individuals with the greatest need and to tailor interventions to a given patient. However, while discovery studies are vital, implementation research to show efficacy and utility of these personalized approaches is also necessary. Clinical implementation of these strategies will necessitate development of decision support tools, digital health device integration, and scaling for population-level strategies, with dynamic return of data to patients and communities stand to improve current outcomes for obesity and cardiometabolic disease.
Importantly, tackling the obesity epidemic will have to include partnerships between health systems, public health re-searchers, and local and federal governments to create policies and interventions that support the health of communities throughout their lifespan. This will require addressing socioeconomic and health disparities that impact access to healthy foods and the creation of opportunities for community education and engagement. These endeavors require a multidisciplinary team approach incorporating primary care physicians, endocrinologists, cardiologists, surgeons and dietitians, patients, and communities.
Conclusion
The past two decades have ushered in an era that has enabled application of high-throughput genomic and metabolomic technologies to identify the biology and biomarkers of obesity. These multi-omic measures have created a framework of important discoveries that can be used for precision medicine approaches to tackle obesity throughout the lifespan. Implementation studies are necessary to determine effectiveness and clinical utility of these approaches. Regardless, a multifaceted, interdisciplinary approach is essential to tackle the obesity epidemic and thereby mitigate the downstream adverse health consequences.
Funding
J. Regan is supported by a grant from the National Heart, Lung and Blood Institute (1R38HL143612).
Conflict of Interest
J. Regan declares no conflict of interest. S. Shah holds an unlicensed patent (10317414) on a related research finding, and receives research support through sponsored research agreements through Verily Life Sciences Inc. and Lilly Inc.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hales CM, Carrol MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360. [PubMed] [Google Scholar]
- 2.WHO. Global status report on noncommunicable diseases 20142014 Contract No.: 978 92 4 156485 4.
- 3.Hales CM, Carrol MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288. [PubMed] [Google Scholar]
- 4.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282(16):1530–8. 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 5.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187–93. 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694–703. 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–105. 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 8.Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–53. 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev. 2016;17(1):56–67. 10.1111/obr.12316. [DOI] [PubMed] [Google Scholar]
- 11.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113 (6) : 898–918. 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar S, Lipworth L, Kabagambe EK, Bian A, Stewart TG, Blot WJ, et al. A description of risk factors for non-alcoholic fatty liver disease in the southern community cohort study: a nested case-control study. Front Nutr. 2020;7:71. 10.3389/fnut.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czernichow S, Kengne AP, Huxley RR, Batty GD, de Galan B, Grobbee D, et al. Comparison of waist-to-hip ratio and other obesity indices as predictors of cardiovascular disease risk in people with type-2 diabetes: a prospective cohort study from ADVANCE. Eur J Cardiovasc Prev Rehabil. 2011;18(2):312–9. 10.1097/HJR.0b013e32833c1aa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prospective Studies C, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016;374(25):2430–40. 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- 17.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596. 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 18.Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(9):928–35. 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis JP, Loria CM, Lewis CE, Powell-Wiley TM, Wei GS, Carr JJ, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310(3):280–8. 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finer N Medical consequences of obesity. Medicine. 2015;43(2): 88–93. 10.1016/j.mpmed.2014.11.003. [DOI] [Google Scholar]
- 21.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–13. 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 22.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 23.Aune D, Sen A, Schlesinger S, Norat T, Janszky I, Romundstad P, et al. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(3): 181–92. 10.1007/s10654-017-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi Y, Yan S, Lu Y, Liang Y, Li C. Venous thromboembolism has the same risk factors as atherosclerosis: a PRISMA-compliant systemic review and meta-analysis. Medicine (Baltimore). 2016;95(32):e4495. 10.1097/MD.0000000000004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wattanakit K, Lutsey PL, Bell EJ, Gornik H, Cushman M, Heckbert SR, et al. Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. A time-dependent analysis. Thromb Haemost. 2012;108(3):508–15. 10.1160/TH11-10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotta LA, Wittemans LBL, Zuber V, Stewart ID, Sharp SJ, Luan J, et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA. 2018;320(24):2553–63. 10.1001/jama.2018.19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson SC, Back M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2020;41(2):221–6. 10.1093/eurheartj/ehz388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey SG. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: mendelian randomisation study. BMJ. 2020;369:m1203. 10.1136/bmj.m1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Censin JC, Peters SAE, Bovijn J, Ferreira T, Pulit SL, Magi R, et al. Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet. 2019;15(10):e1008405. 10.1371/journal.pgen.1008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167(7):642–8. 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 31.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168(15):1617–24. 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 32.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emdin CA, Khera AV, Natarajan P, Klarin D, Zekavat SM, Hsiao AJ, et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA. 2017;317(6):626–34. 10.1001/jama.2016.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur Y, de Souza RJ, Gibson WT, Meyre D. A systematic review of genetic syndromes with obesity. Obes Rev. 2017;18(6):603–34. 10.1111/obr.12531. [DOI] [PubMed] [Google Scholar]
- 35.Heon E, Kim G, Qin S, Garrison JE, Tavares E, Vincent A, et al. Mutations in C8ORF37 cause Bardet Biedl syndrome (BBS21). Hum Mol Genet. 2016;25(11):2283–94. 10.1093/hmg/ddw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novas R, Cardenas-Rodriguez M, Irigoin F, Badano JL. Bardet-Biedl syndrome: is it only cilia dysfunction? FEBS Lett. 2015;589(22):3479–91. 10.1016/j.febslet.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer E, Stoetzel C, Scheidecker S, Geoffroy V, Prasad MK, Redin C, et al. Identification of a novel mutation confirms the implication of IFT172 (BBS20) in Bardet-Biedl syndrome. J Hum Genet. 2016;61(5):447–50. 10.1038/jhg.2015.162. [DOI] [PubMed] [Google Scholar]
- 38.Cheon CK. Genetics of Prader-Willi syndrome and Prader-Will-like syndrome. Ann Pediatr Endocrinol Metab. 2016;21(3):126–35. 10.6065/apem.2016.21.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choquet H, Meyre D. Genomic insights into early-onset obesity. Genome Med. 2010;2(6):36. 10.1186/gm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barsh GS, Farooqi IS, O’Rahilly S. Genetics of body-weight regulation. Nature. 2000;404(6778):644–51. 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- 41.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–95. 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 42.Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4(10):569–77. 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- 43.Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87(2):398–404. 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- 44.Silventoinen K, Magnusson PK, Tynelius P, Kaprio J, Rasmussen F. Heritability of body size and muscle strength in young adulthood: a study of one million Swedish men. Genet Epidemiol. 2008;32(4):341–9. 10.1002/gepi.20308. [DOI] [PubMed] [Google Scholar]
- 45.Hager J, Dina C, Francke S, Dubois S, Houari M, Vatin V, et al. A genome-wide scan for human obesity genes reveals a major susceptibility locus on chromosome 10. Nat Genet. 1998;20(3):304–8. [DOI] [PubMed] [Google Scholar]
- 46.Price RA, Li WD, Bernstein A, Crystal A, Golding EM, Weisberg SJ, et al. A locus affecting obesity in human chromosome region 10p12. Diabetologia. 2001;44(3):363–6. 10.1007/s001250051627. [DOI] [PubMed] [Google Scholar]
- 47.Saar K, Geller F, Ruschendorf F, Reis A, Friedel S, Schauble N, et al. Genome scan for childhood and adolescent obesity in German families. Pediatrics. 2003;111(2):321–7. 10.1542/peds.111.2.321. [DOI] [PubMed] [Google Scholar]
- 48.Boutin P, Dina C, Vasseur F, Dubois S, Corset L, Seron K, et al. GAD2 on chromosome 10p12 is a candidate gene for human obesity. PLoS Biol. 2003;1(3):361–71. ARTN e68. 10.1371/journal.pbio.0000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swarbrick MM, Waldenmaier B, Pennacchio LA, Lind DL, Cavazos MM, Geller F, et al. Lack of support for the association between GAD2 polymorphisms and severe human obesity. PLoS Biol. 2005;3(9):e315. 10.1371/journal.pbio.0030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groves CJ, Zeggini E, Walker M, Hitman GA, Levy JC, O’Rahilly S, et al. Significant linkage of BMI to chromosome 10p in the U.K. population and evaluation of GAD2 as a positional candidate. Diabetes. 2006;55(6):1884–9. 10.2337/db05-1674. [DOI] [PubMed] [Google Scholar]
- 51.Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39(6):724–6. 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 52.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94. 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115. 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40(6):768–75. 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41(2):157–9. 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 56.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.••.Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177(3):587–96.e9. 10.1016/j.cell.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used 2.1 million common genetic variants associated with obesity to quantify a PRS that associated with obesity risk across the life span, with a similar effect size to rare, monogenic obesity variants and is a strong risk factor for adverse obesity-associated outcomes including coronary disease, heart failure, and mortality.
- 58.Muller MJ, Geisler C, Blundell J, Dulloo A, Schutz Y, Krawczak M, et al. The case of GWAS of obesity: does body weight control play by the rules? Int J Obes. 2018;42(8):1395–405. 10.1038/s41366-018-0081-6. [DOI] [PubMed] [Google Scholar]
- 59.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–U53. 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akiyama M, Okada Y, Kanai M, Takahashi A, Momozawa Y, Ikeda M, et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat Genet. 2017;49(10):1458–67. 10.1038/ng.3951. [DOI] [PubMed] [Google Scholar]
- 61.Gill R, Cheung YH, Shen Y, Lanzano P, Mirza NM, Ten S, et al. Whole-exome sequencing identifies novel LEPR mutations in individuals with severe early onset obesity. Obesity (Silver Spring). 2014;22(2):576–84. 10.1002/oby.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhatt A, Purani C, Bhargava P, Patel K, Agarbattiwala T, Puvar A, et al. Whole exome sequencing reveals novel LEPR frameshift mutation in severely obese children from Western India. Mol Genet Genomic Med. 2019;7(7):e00692. 10.1002/mgg3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paz-Filho G, Boguszewski MC, Mastronardi CA, Patel HR, Johar AS, Chuah A, et al. Whole exome sequencing of extreme morbid obesity patients: translational implications for obesity and related disorders. Genes (Basel). 2014;5(3):709–25. 10.3390/genes5030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiao H, Kulyte A, Naslund E, Thorell A, Gerdhem P, Kere J, et al. Whole-exome sequencing suggests LAMB3 as a susceptibility gene for morbid obesity. Diabetes. 2016;65(10):2980–9. 10.2337/db16-0522. [DOI] [PubMed] [Google Scholar]
- 65.•.Marenne G, Hendricks AE, Perdikari A, Bounds R, Payne F, Keogh JM, et al. Exome sequencing identifies genes and gene sets contributing to severe childhood obesity, linking PHIP variants to repressed POMC transcription. Cell Metab. 2020;31(6):1107–19.e12. 10.1016/j.cmet.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified novel genes associated with severe childhood obesity using WES, including PHIP which the investigators found regulates POMC expression in the leptin-melanocortin pathway.
- 66.Tachmazidou I, Suveges D, Min JL, Ritchie GRS, Steinberg J, Walter K, et al. Whole-genome sequencing coupled to imputation discovers genetic signals for anthropometric traits. Am J Hum Genet. 2017;100(6):865–84. 10.1016/j.ajhg.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Bruning JC, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458(7240):894–8. 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 68.Lee YS. Genetics of nonsyndromic obesity. Curr Opin Pediatr. 2013;25(6):666–73. 10.1097/MOP.0b013e3283658fba. [DOI] [PubMed] [Google Scholar]
- 69.Deng X, Su R, Stanford S, Chen J. Critical enzymatic functions of FTO in obesity and cancer. Front Endocrinol (Lausanne). 2018;9: 396. 10.3389/fendo.2018.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, et al. FTO obesity variant circuitry and adipocyte browning in humans. N Engl J Med. 2015;373(10):895–907. 10.1056/NEJMoa1502214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stratigopoulos G, Martin Carli JF, O’Day DR, Wang L, Leduc CA, Lanzano P, et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 2014;19(5):767–79. 10.1016/j.cmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berulava T, Horsthemke B. The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet. 2010;18(9):1054–6. 10.1038/ejhg.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karra E, O’Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest. 2013;123(8):3539–51. 10.1172/JCI44403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villalobos-Comparan M, Teresa Flores-Dorantes M, Teresa Villarreal-Molina M, Rodriguez-Cruz M, Garcia-Ulloa AC, Robles L, et al. The FTO gene is associated with adulthood obesity in the Mexican population. Obesity (Silver Spring). 2008;16(10):2296–301. 10.1038/oby.2008.367. [DOI] [PubMed] [Google Scholar]
- 75.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 76.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–85. 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amigo L, Mendoza H, Castro J, Quinones V, Miquel JF, Zanlungo S. Relevance of Niemann-Pick type C1 protein expression in controlling plasma cholesterol and biliary lipid secretion in mice. Hepatology. 2002;36(4 Pt 1):819–28. 10.1053/jhep.2002.35617. [DOI] [PubMed] [Google Scholar]
- 78.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26): 2683–93. 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 79.Adams ST, Salhab M, Hussain ZI, Miller GV, Leveson SH. Obesity-related hypertension and its remission following gastric bypass surgery - a review of the mechanisms and predictive factors. Blood Press. 2013;22(3):131–7. 10.3109/08037051.2012.749570. [DOI] [PubMed] [Google Scholar]
- 80.Laferrere B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3(80):80re2. 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, et al. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes. 2013;62(8):2757–61. 10.2337/db13-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pulkkinen L, Uitto J. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol. 1999;18(1):29–42. 10.1016/s0945-053x(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 83.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101(16):6062–7. 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492): 371–5. 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikonen E Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9(2):125–38. 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 86.Vance JE. Lipid imbalance in the neurological disorder, Niemann-Pick C disease. FEBS Lett. 2006;580(23):5518–24. 10.1016/j.febslet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Serria MS, Ikeda H, Omoteyama K, Hirokawa J, Nishi S, Sakai M. Regulation and differential expression of the c-maf gene in differentiating cultured cells. Biochem Biophys Res Commun. 2003;310(2):318–26. 10.1016/j.bbrc.2003.08.144. [DOI] [PubMed] [Google Scholar]
- 88.Tsuchiya M, Taniguchi S, Yasuda K, Nitta K, Maeda A, Shigemoto M, et al. Potential roles of large mafs in cell lineages and developing pancreas. Pancreas. 2006;32(4):408–16. 10.1097/01.mpa.0000220867.64787.99. [DOI] [PubMed] [Google Scholar]
- 89.Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15(3):269–72. 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 90.Fernandez-Rhodes L, Gong J, Haessler J, Franceschini N, Graff M, Nishimura KK, et al. Trans-ethnic fine-mapping of genetic loci for body mass index in the diverse ancestral populations of the Population Architecture using Genomics and Epidemiology (PAGE) Study reveals evidence for multiple signals at established loci. Hum Genet. 2017;136(6):771–800. 10.1007/s00439-017-1787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Golan D, Lander ES, Rosset S. Measuring missing heritability: inferring the contribution of common variants. Proc Natl Acad Sci U S A. 2014;111(49):E5272–81. 10.1073/pnas.1419064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90(1):7–24. 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang J, Manolio TA, Pasquale LR, Boerwinkle E, Caporaso N, Cunningham JM, et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat Genet. 2011;43(6): 519–25. 10.1038/ng.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang J, Bakshi A, Zhu Z, Hemani G, Vinkhuyzen AA, Lee SH, et al. Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat Genet. 2015;47(10):1114–20. 10.1038/ng.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Z, Bakshi A, Vinkhuyzen AA, Hemani G, Lee SH, Nolte IM, et al. Dominance genetic variation contributes little to the missing heritability for human complex traits. Am J Hum Genet. 2015;96(3):377–85. 10.1016/j.ajhg.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferguson JF, Allayee H, Gerszten RE, Ideraabdullah F, Kris-Etherton PM, Ordovas JM, et al. Nutrigenomics, the microbiome, and gene-environment interactions: new directions in cardiovascular disease research, prevention, and treatment: a scientific statement from the American Heart Association. Circ Cardiovasc Genet. 2016;9(3):291–313. 10.1161/HCG.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pirastu N, Kooyman M, Traglia M, Robino A, Willems SM, Pistis G, et al. A genome-wide association study in isolated populations reveals new genes associated to common food likings. Rev Endocr Metab Disord. 2016;17(2):209–19. 10.1007/s11154-016-9354-3. [DOI] [PubMed] [Google Scholar]
- 98.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94. 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Ding M, Ellervik C, Huang T, Jensen MK, Curhan GC, Pasquale LR, et al. Diet quality and genetic association with body mass index: results from 3 observational studies. Am J Clin Nutr. 2018;108(6):1291–300. 10.1093/ajcn/nqy203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JPA, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–79. 10.1001/jama.2018.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yanovski SZ, Yanovski JA. Toward precision approaches for the prevention and treatment of obesity. JAMA. 2018;319(3):223–4. 10.1001/jama.2017.20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kilpelainen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8(11):e1001116. 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367(15):1387–96. 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qi Q, Li Y, Chomistek AK, Kang JH, Curhan GC, Pasquale LR, et al. Television watching, leisure time physical activity, and the genetic predisposition in relation to body mass index in women and men. Circulation. 2012;126(15):1821–7. 10.1161/CIRCULATIONAHA.112.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qi Q, Chu AY, Kang JH, Huang J, Rose LM, Jensen MK, et al. Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ. 2014;348:g1610. 10.1136/bmj.g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tyrrell J, Wood AR, Ames RM, Yaghootkar H, Beaumont RN, Jones SE, et al. Gene-obesogenic environment interactions in the UK Biobank study. Int J Epidemiol. 2017;46(2):559–75. 10.1093/ije/dyw337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rohde K, Keller M, la Cour PL, Bluher M, Kovacs P, Bottcher Y. Genetics and epigenetics in obesity. Metabolism. 2019;92:37–50. 10.1016/j.metabol.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 108.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26. 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16(2): 520–30. 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab. 2016;5(7):538–51. 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.•.Vogelzangs N, van der Kallen CJH, van Greevenbroek MMJ, van der Kolk BW, Jocken JWE, Goossens GH, et al. Metabolic profiling of tissue-specific insulin resistance in human obesity: results from the Diogenes study and the Maastricht Study. Int J Obes. 2020;44(6):1376–86. 10.1038/s41366-020-0565-z. [DOI] [PubMed] [Google Scholar]; This study provides recent insight into the metabolic pathways contributing to tissue-specific hepatic and muscle insulin resistance in obesity.
- 112.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293(6): E1552–63. 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med. 2016;13(11):e1002179. 10.1371/journal.pmed.1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–81. 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 116.Shah SH, Svetkey LP, Newgard CB. Branching out for detection of type 2 diabetes. Cell Metab. 2011;13(5):491–2. 10.1016/j.cmet.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 117.White PJ, Newgard CB. Branched-chain amino acids in disease. Science. 2019;363(6427):582–3. 10.1126/science.aav0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman JM, et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 2018;27(6):1281–93.e7. 10.1016/j.cmet.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McGarrah RW, Zhang GF, Christopher BA, Deleye Y, Walejko JM, Page S, et al. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am J Physiol Endocrinol Metab. 2020;318(2): E216–E23. 10.1152/ajpendo.00334.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32(9):1678–83. 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–53. 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palmer ND, Stevens RD, Antinozzi PA, Anderson A, Bergman RN, Wagenknecht LE, et al. Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab. 2015;100(3):E463–8. 10.1210/jc.2014-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Batch BC, Shah SH, Newgard CB, Turer CB, Haynes C, Bain JR, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism. 2013;62(7):961–9. 10.1016/j.metabol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bellissimo MP, Cai Q, Ziegler TR, Liu KH, Tran PH, Vos MB, et al. Plasma high-resolution metabolomics differentiates adults with normal weight obesity from lean individuals. Obesity (Silver Spring). 2019;27(11):1729–37. 10.1002/oby.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3(2):207–14. 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 126.Bhattacharya S, Granger CB, Craig D, Haynes C, Bain J, Stevens RD, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. 2014;232(1):191–6. 10.1016/j.atherosclerosis.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weir JM, Wong G, Barlow CK, Greeve MA, Kowalczyk A, Almasy L, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54(10):2898–908. 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen HH, Tseng YJ, Wang SY, Tsai YS, Chang CS, Kuo TC, et al. The metabolome profiling and pathway analysis in metabolic healthy and abnormal obesity. Int J Obes. 2015;39(8):1241–8. 10.1038/ijo.2015.65. [DOI] [PubMed] [Google Scholar]
- 129.Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3(5):321–31. 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 130.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62(1):1–8. 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15(5):606–14. 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baker PR 2nd, Boyle KE, Koves TR, Ilkayeva OR, Muoio DM, Houmard JA, et al. Metabolomic analysis reveals altered skeletal muscle amino acid and fatty acid handling in obese humans. Obesity (Silver Spring). 2015;23(5):981–8. 10.1002/oby.21046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rutkowsky JM, Knotts TA, Ono-Moore KD, McCoin CS, Huang S, Schneider D, et al. Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab. 2014;306(12):E1378–87. 10.1152/ajpendo.00656.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cussotto S, Delgado I, Anesi A, Dexpert S, Aubert A, Beau C et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front Immunol. 2020;11:557. 10.3389/fimmu.2020.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.••.Cirulli ET, Guo L, Leon Swisher C, Shah N, Huang L, Napier LA, et al. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab. 2019;29(2):488–500.e2. 10.1016/j.cmet.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports novel metabolites associated with BMI and a composite metabolic score of significant metabolites was associated with higher cardiovascular disease outcomes. Interestingly, the metabolic score was not associated with a PRS for obesity, provide insight into potentially distinct obesity risk pathways.
- 136.Jacob S, Nodzenski M, Reisetter AC, Bain JR, Muehlbauer MJ, Stevens RD, et al. Targeted metabolomics demonstrates distinct and overlapping maternal metabolites associated with BMI, glucose, and insulin sensitivity during pregnancy across four ancestry groups. Diabetes Care. 2017;40(7):911–9. 10.2337/dc16-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kadakia R, Nodzenski M, Talbot O, Kuang A, Bain JR, Muehlbauer MJ, et al. Maternal metabolites during pregnancy are associated with newborn outcomes and hyperinsulinaemia across ancestries. Diabetologia. 2019;62(3):473–84. 10.1007/s00125-018-4781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sandler V, Reisetter AC, Bain JR, Muehlbauer MJ, Nodzenski M, Stevens RD, et al. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia. 2017;60(3):518–30. 10.1007/s00125-016-4182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lowe WL Jr, Bain JR, Nodzenski M, Reisetter AC, Muehlbauer MJ, Stevens RD, et al. Maternal BMI and glycemia impact the fetal metabolome. Diabetes Care. 2017;40(7):902–10. 10.2337/dc16-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu Y, Kuang A, Talbot O, Bain JR, Muehlbauer MJ, Hayes MG, et al. Metabolomic and genetic associations with insulin resistance in pregnancy. Diabetologia. 2020. 10.1007/s00125-020-05198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Illig T, Gieger C, Zhai G, Romisch-Margl W, Wang-Sattler R, Prehn C, et al. A genome-wide perspective of genetic variation in human metabolism. Nat Genet. 2010;42(2):137–41. 10.1038/ng.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen HH, Lee WJ, Wang W, Huang MT, Lee YC, Pan WH. Ala55Val polymorphism on UCP2 gene predicts greater weight loss in morbidly obese patients undergoing gastric banding. Obes Surg. 2007;17(7):926–33. 10.1007/s11695-007-9171-6. [DOI] [PubMed] [Google Scholar]
- 143.Hatoum IJ, Greenawalt DM, Cotsapas C, Daly MJ, Reitman ML, Kaplan LM. Weight loss after gastric bypass is associated with a variant at 15q26.1. Am J Hum Genet. 2013;92(5):827–34. 10.1016/j.ajhg.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rauschert S, Uhl O, Koletzko B, Kirchberg F, Mori TA, Huang RC, et al. Lipidomics reveals associations of phospholipids with obesity and insulin resistance in young adults. J Clin Endocrinol Metab. 2016;101(3):871–9. 10.1210/jc.2015-3525. [DOI] [PubMed] [Google Scholar]
- 145.Bagheri M, Farzadfar F, Qi L, Yekaninejad MS, Chamari M, Zeleznik OA, et al. Obesity-related metabolomic profiles and discrimination of metabolically unhealthy obesity. J Proteome Res. 2018;17(4):1452–62. 10.1021/acs.jproteome.7b00802. [DOI] [PubMed] [Google Scholar]
- 146.Barber MN, Risis S, Yang C, Meikle PJ, Staples M, Febbraio MA, et al. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One. 2012;7(7):e41456. 10.1371/journal.pone.0041456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yea K, Kim J, Yoon JH, Kwon T, Kim JH, Lee BD, et al. Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J Biol Chem. 2009;284(49):33833–40. 10.1074/jbc.M109.024869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou B, Ichikawa R, Parnell LD, Noel SE, Zhang X, Bhupathiraju SN, et al. Metabolomic links between sugar-sweetened beverage intake and obesity. J Obes. 2020;2020:7154738. 10.1155/2020/7154738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sekas G, Patton GM, Lincoln EC, Robins SJ. Origin of plasma lysophosphatidylcholine: evidence for direct hepatic secretion in the rat. J Lab Clin Med. 1985;105(2):190–4. [PubMed] [Google Scholar]
- 150.Angell R, Mitsuhashi Y, Bigley K, Bauer JE. Plasma LCAT activity and lipid subfraction composition in obese beagles undergoing weight loss. Lipids. 2009;44(5):415–24. 10.1007/s11745-009-3290-x. [DOI] [PubMed] [Google Scholar]
- 151.Martinez-Una M, Varela-Rey M, Cano A, Fernandez-Ares L, Beraza N, Aurrekoetxea I, et al. Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology. 2013;58(4):1296–305. 10.1002/hep.26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005;19(10):1266–71. 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring). 2010;18(9):1695–700. 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 155.Sampey BP, Freemerman AJ, Zhang J, Kuan PF, Galanko JA, O’Connell TM, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS One. 2012;7(6):e38812. 10.1371/journal.pone.0038812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kraus WE, Pieper CF, Huffman KM, Thompson DK, Kraus VB, Morey MC, et al. Association of plasma small-molecule intermediate metabolites with age and body mass index across six diverse study populations. J Gerontol A Biol Sci Med Sci. 2016;71(11):1507–13. 10.1093/gerona/glw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Palmnas MSA, Kopciuk KA, Shaykhutdinov RA, Robson PJ, Mignault D, Rabasa-Lhoret R, et al. Serum metabolomics of activity energy expenditure and its relation to metabolic syndrome and obesity. Sci Rep. 2018;8(1):3308. 10.1038/s41598-018-21585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Poorolajal J, Jenabi E. The association between body mass index and preeclampsia: a meta-analysis. J Matern Fetal Neonatal Med. 2016;29(22):3670–6. 10.3109/14767058.2016.1140738. [DOI] [PubMed] [Google Scholar]
- 159.Shin D, Song WO. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Fetal Neonatal Med. 2015;28(14):1679–86. 10.3109/14767058.2014.964675. [DOI] [PubMed] [Google Scholar]
- 160.Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8):2070–6. 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 161.Wahl S, Yu Z, Kleber M, Singmann P, Holzapfel C, He Y, et al. Childhood obesity is associated with changes in the serum metabolite profile. Obes Facts. 2012;5(5):660–70. 10.1159/000343204. [DOI] [PubMed] [Google Scholar]
- 162.Hirschel J, Vogel M, Baber R, Garten A, Beuchel C, Dietz Y, et al. Relation of whole blood amino acid and acylcarnitine metabolome to age, sex, BMI, puberty, and metabolic markers in children and adolescents. Metabolites. 2020;10(4). 10.3390/metabo10040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perng W, Rifas-Shiman SL, Sordillo J, Hivert MF, Oken E. Metabolomic profiles of overweight/obesity phenotypes during adolescence: a cross-sectional study in project viva. Obesity (Silver Spring). 2020;28(2):379–87. 10.1002/oby.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao X, Gang X, Liu Y, Sun C, Han Q, Wang G. Using metabolomic profiles as biomarkers for insulin resistance in childhood obesity: a systematic review. J Diabetes Res. 2016;2016: 8160545. 10.1155/2016/8160545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lawler K, Huang-Doran I, Sonoyama T, Collet TH, Keogh JM, Henning E, et al. Leptin-mediated changes in the human metabolome. J Clin Endocrinol Metab. 2020;105(8). 10.1210/clinem/dgaa251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Adamski J Genome-wide association studies with metabolomics. Genome Med. 2012;4(4):34. 10.1186/gm333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dharuri H, Demirkan A, van Klinken JB, Mook-Kanamori DO, van Duijn CM, t Hoen PA, et al. Genetics of the human metabolome, what is next? Biochim Biophys Acta. 2014;1842(10):1923–31. 10.1016/j.bbadis.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 168.Ghazalpour A, Doss S, Zhang B, Wang S, Plaisier C, Castellanos R, et al. Integrating genetic and network analysis to characterize genes related to mouse weight. PLoS Genet. 2006;2(8):e130. 10.1371/journal.pgen.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.English SB, Butte AJ. Evaluation and integration of 49 genome-wide experiments and the prediction of previously unknown obesity-related genes. Bioinformatics. 2007;23(21):2910–7. 10.1093/bioinformatics/btm483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen Y, Zhu J, Lum PY, Yang X, Pinto S, MacNeil DJ, et al. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452(7186):429–35. 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55(2):321–30. 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stubbs J, Whybrow S, Teixeira P, Blundell J, Lawton C, Westenhoefer J, et al. Problems in identifying predictors and correlates of weight loss and maintenance: implications for weight control therapies based on behaviour change. Obes Rev. 2011;12(9):688–708. 10.1111/j.1467-789X.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 173.Unick JL, Neiberg RH, Hogan PE, Cheskin LJ, Dutton GR, Jeffery R, et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring). 2015;23(7):1353–6. 10.1002/oby.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 175.Lamiquiz-Moneo I, Mateo-Gallego R, Bea AM, Dehesa-Garcia B, Perez-Calahorra S, Marco-Benedi V, et al. Genetic predictors of weight loss in overweight and obese subjects. Sci Rep. 2019;9(1): 10770. 10.1038/s41598-019-47283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hollensted M, Fogh M, Schnurr TM, Kloppenborg JT, Have CT, Ruest Haarmark Nielsen T, et al. Genetic susceptibility for childhood BMI has no impact on weight loss following lifestyle intervention in Danish children. Obesity (Silver Spring). 2018;26(12): 1915–22. 10.1002/oby.22308. [DOI] [PubMed] [Google Scholar]
- 177.Schooneman MG, Napolitano A, Houten SM, Ambler GK, Murgatroyd PR, Miller SR, et al. Assessment of plasma acylcarnitines before and after weight loss in obese subjects. Arch Biochem Biophys. 2016;606:73–80. 10.1016/j.abb.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 178.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–25. 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7(1):102–8. 10.1016/S1091-255X(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 180.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17): 1567–76. 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000;10(3):233–9. 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- 182.Higa K, Ho T, Tercero F, Yunus T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;7(4):516–25. 10.1016/j.soard.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 183.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122–31. 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 185.Nicoletti CF, de Oliveira AP, Brochado MJ, Pinhel MA, de Oliveira BA, Marchini JS, et al. The Ala55Val and −866G>A polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition. 2017;33:326–30. 10.1016/j.nut.2016.07.020. [DOI] [PubMed] [Google Scholar]


