Abstract
Objective
Several long-lasting health complications have been reported in previous coronavirus infections. Therefore, the aim of this study was to review studies that evaluated physical and mental health problems post-COVID-19.
Methods
Articles for inclusion in this scoping review were identified by searching the PubMed, Scopus, Web of Science and Google Scholar databases for items dated from 1 January to 7 November 2020. Observational studies evaluating physical health (musculoskeletal symptoms, functional status) or mental health status with a follow-up period longer than 1 month after discharge or after the onset of symptoms were included.
Results
This scoping review included 34 studies with follow-up periods of up to 3 months post-COVID-19. The most commonly reported physical health problems were fatigue (range 28% to 87%), pain (myalgia 4.5% to 36%), arthralgia (6.0% to 27%), reduced physical capacity (six-minute walking test range 180 to 561 m), and declines in physical role functioning, usual care and daily activities (reduced in 15% to 54% of patients). Common mental health problems were anxiety (range 6.5% to 63%), depression (4% to 31%) and post-traumatic stress disorder (12.1% to 46.9%). Greater fatigue, pain, anxiety and depression were reported in female patients and individuals admitted to intensive care. An overall lower quality of life was seen up to 3 months post-COVID-19.
Conclusions
This review highlights the presence of several physical and mental health problems up to 3 months post-COVID-19. The findings point to the need for comprehensive evaluation and rehabilitation post-COVID-19 to promote quality of life.
Keywords: Coronavirus, Follow up, Health, Post-discharge, Quality of life
Abbreviations: COVID-19, Coronavirus disease 2019; SARS, Severe acute respiratory syndrome; MERS, Middle East respiratory syndrome; HRQOL, Health-related quality of life; PTSD, Post-traumatic stress disorder; ICF, International Classification of Functioning, Disability and Health; SD, Standard deviation; IQR, Interquartile range; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; EQ-5D, European Quality of Life 5-Dimensions instrument; EQ-5D-5L, European Quality of Life 5-Dimensions, 5-levels instrument; SF-36, Short Form Health Survey-36; SGRQ, St. George's Respiratory Questionnaire; 6MWT, Six-minute walk test; STS, Sit to stand test; ICU, Intensive care unit; FIM, Functional independence measure; PCFS, Post-COVID-19 Functional Status Scale; FVC, Forced vital capacity; CBT, Cognitive Behavioral Therapy
1. Background
Coronavirus disease 2019 (COVID-19) has spread throughout the world, leading to a global pandemic [1]. The World Health Organization (WHO) consequently declared COVID-19 to be a public health emergency of international concern [2]. A rapidly growing body of literature is available on the clinical presentations and treatments of the acute phase of COVID-19. Common characteristics of the acute stage of the disease are fever, musculoskeletal symptoms (fatigue, myalgia, and joint pain), dry cough, dyspnea, gastrointestinal symptoms, and anosmia with or without ageusia [3].
Importantly, after viral infection different types of damage occur in multiple body organs, especially the brain [4]. In addition, peripheral and central inflammatory responses (neuroinflammation) may be triggered by the infection, and can lead to long-lasting musculoskeletal problems, cognitive impairment, and psychological distress [[5], [6], [7], [8]]. A review of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) reported several long-lasting clinical complications that affect different aspects of health, including chronic fatigue, reduced physical capacity, muscles weakness, increased depression, anxiety, post-traumatic stress disorder (PTSD), and sleep problems [9]. An overall decline in quality of life has been observed as long as 1 year after major coronavirus outbreaks [9]. According to the WHO International Classification of Functioning, Disability and Health (ICF) framework, rehabilitation programs should be planned in order to improve individual functioning at three levels: (1) body function and structure, (2) activity and participation, and (3) environmental factors and personal factors to improve quality of life [10]. Current recommendations advise implementation of the ICF model in the fields of evaluation, diagnosis, and interventions. This makes it important to document post-COVID-19 physical and mental health consequences that affect quality of life, as a way to improve rehabilitation services and help healthcare organizations to plan efficient rehabilitation programs. Therefore, the aim of this study was to review studies on post-COVID-19 physical and mental health complications.
2. Methods
2.1. Study design
The methodological framework proposed by Arksey and O'Malley was used to conduct this scoping review [11]. The following five steps were used: a) identifying a clear research objective and search strategies, b) identifying relevant research articles, c) selecting research articles, d) extracting and charting data, and e) summarizing, analyzing, discussing, and reporting the results.
2.2. Identifying the review questions
The main review question was as follows: “What are the physical and mental health complications in adult patients post-COVID-19 infection?”
2.3. Literature search strategies
Studies for this scoping review were identified by searching the PubMed, Scopus, Web of Science and Google Scholar databases for items dated from 1 January 2020 to 7 November 2020. The search terms were (“COVID-19” OR “2019 novel coronavirus” OR “SARS-CoV-2” OR “2019-nCoV” OR “Coronavirus 2019”) AND (“Follow up” OR “Discharge” OR “Post discharge” OR “Long-term effect” OR “Post-covid”). The PubMed syntax is shown in Appendix A.
2.4. Eligibility criteria
The following inclusion criteria were used: Original articles (observational studies such as cohort, cross-sectional and case series) that were peer reviewed or preprinted, that assessed physical health status (musculoskeletal symptoms, physical and/or functional performance) with functional tests or by questionnaire, that assessed mental health status, that evaluated patients after a follow-up period of at least 1 month after the onset of COVID-19 or after hospital discharge, and that were published in English. Studies were excluded if they were randomized clinical trials, review articles, guidelines and recommendations, protocol studies, or case reports; if they examined only clinical factors such as laboratory tests, cardiovascular, pulmonary, endocrine, renal, neurological disorders, or specific disease groups with coronavirus infection; if they included non-adult patients; and if they were published in a language other than English.
2.5. Identification and selection of studies
All studies involving adults with a confirmed diagnosis of COVID-19 were included in this review. After searching the databases, a total of 7398 articles were found. After 3802 duplicate articles were removed, the titles and abstracts of all remaining articles were screened by two independent reviewers (M.T., S.A.A.) in two steps. Subsequently, the same two reviewers independently screened the full texts of studies to verify that they met the eligibility criteria. In cases of disagreement, a third researcher was consulted (S·S.) to reach a final decision.
2.6. Data extraction from included studies
The data from all included studies were extracted by two independent reviewers (M.T., S.A.A.), and are presented in Table 1, Table 2, Table 3 . The data extracted for each article were author, study design, country, sample size, age, follow-up period, hospitalization period, outcomes and their measurements tools, prevalence of outcomes and mean (standard deviation [SD]) or median (interquartile range [IQR]) score of each outcome.
Table 1.
Quality of life outcomes in COVID-19 survivors.
| Author | Study design | Country | Sample size (N) Male/Female |
Age (years) Mean (SD) |
Follow-up period month/or daysM ean (SD) |
Hospitalization period (days) Mean (SD) |
Measurement tools | Results (Mean, SD), Prevalence of outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qi et al. [12] | Retrospective cohort Study | China | 80 48/32 |
64.88 (16.98) | 1 and 3 months after hospital discharge | 15 (4.5) | SGRQ | SGRQ | Mean (SD) | |||
| At 1 month | 51.74 (9.56) | |||||||||||
| At 3 months | 46.36 (9.52) * | |||||||||||
| Daher et al. [15] | Case series | Germany | 33 22/11 |
64 (3) | 56 (48–71) days after hospital discharge | 15 (1.8) | EQ-5D-5L, SRGQ |
Median [IQR] EQ-5D-5L at follow-up Mobility (walking) 2 [1–3] Self-care 1 [1–1] Usual activities 2 [1–3] Pain/Discomfort 2 [1–3] Anxiety/Depression 2 [1–2] EQ VAS 63 [53–80] Slight to moderate problems in all domains of EQ-5D-5L Median [IQR] SRGQ Symptoms score 34 [9–57] Activity score 54 [19–78] Impacts score 12 [2−33] Total score 26 [7–42] Mainly reduced activity domain score in SRGQ |
||||
| Arnold et al. [16] | Prospective cohort study | England | 110 68/42 |
59.64 (20.28) | 88.94 (12.77) days after symptom onset |
5 (4.5) | SF-36 | Severity of COVID-19 | ||||
| Mild (n=27) |
Moderate (n=65) |
Severe (n=18) |
||||||||||
| Mean (SD) | ||||||||||||
| MCS | 45 (11) | 46 (11) | 40 (17) | |||||||||
| PCS | 41 (12) | 41 (12) | 36 (7) | |||||||||
| Health-related quality of life scores were lower in patients with more severe COVID-19. | ||||||||||||
| Arab-Zozani et al. [17] | Cross-sectional study | Iran | 409 247/162 |
58.4 (18.21) | 21.6 (14.8) days after hospital discharge | 8 (7) | EQ-5D-5L | EQ-5D-5L 0.6125 (SD 0.006) | ||||
| No problem | Slight | Moderate | Severe | Unable | ||||||||
| Mobility | ||||||||||||
| 53.34% | 24% | 12% | 12% | 0% | ||||||||
| Self-care | ||||||||||||
| 87.75% | 3% | 0% | 0% | 0% | ||||||||
| Usual activity | ||||||||||||
| 58.97% | 41% | 8% | 2% | 1% | ||||||||
| Pain/Discomfort | ||||||||||||
| 57.97% | 11% | 22% | 6% | 0% | ||||||||
| Anxiety/Depression | ||||||||||||
| 41.26% | 21% | 20% | 10% | 4% | ||||||||
| Santus et al. [18] | Cohort study | Italy | 20 17/3 |
55 (15) | 15 days after hospital discharge | 17.7 (11.5) | SGRQ | At follow-up Mean (SD) Total score 16.9 (13.2) Symptoms 16.7 (12.9) Activity limitation 28.3 (23.3) Social and emotional impact 10.6 (10.7) |
||||
| Garrigues et al. [19] | Cohort study | France | 120 75/45 |
63.2 (15.7) | 110.9 (11.1) days after admission | 11.2 (13.4) | Health related quality of life (EQ-5D-5L) |
EQ-5D index 0.86 (0.20) EQ-VAS% 70.3 (21.5) |
||||
| Carfi et al. [20] | Retrospective cohort study | Italy | 143 90/53 |
56.5 (14.6) | 60.3 (13.6) days after symptom onset | 13.5 (9.7) | EQ-5D VAS | Worse QoL: 63/143 (44.1%) | ||||
| Halpin et al. [21] | Cross-sectional study | England | 48 (10.3) days after hospital discharge | ICU patients: 12.71 (4.65) Ward patients: 8.26 (7.57) |
EQ-5D-5L | Worse EQ-5D ICU patients 22/32 (68.8%) Ward patients 30/68 (45.6%) Percent of worse individuals |
||||||
| ICU (n=32) | Ward (n=68) | |||||||||||
| Mobility | 16 (50%) | 21 (30.9%) | ||||||||||
| Self-care | 4 (12.5%) | 12 (17.6%) | ||||||||||
| Usual activities | 19 (29.4%) | 25 (36.8%) | ||||||||||
| Pain/Discomfort | 9 (28.1%) | 10 (14.7%) | ||||||||||
| Anxiety/Depression | 12 (37.5%) | 11 (16.2%) | ||||||||||
| Goertz et al. [22] | Retrospective cohort study | Netherlands, Belgium | 2113 310/1803 |
46.64 (11.12) | 79 (17) days after symptom onset |
N/A | Self-reported health status (Good, Moderate, Poor) | During follow-up ↑Poor health status 28.6% ↑Moderate health status 64.2% ↓Good health status 7.2% |
||||
| Cody et al. [28] |
Case series study | England, Wales, Northern Ireland | 45 37/8 |
55 (11.2) | 6–8 weeks after hospital discharge | 24.54 (18.37) | Quality of life outcomes: (EQ-5D) |
Moderate to severe problems with each domain (n = 31): Mobility 14 (45.2%) Self-care 9 (29.0%) Usual activities 19 (61.3%) Anxiety/Depression 11 (35.5%) Pain/Discomfort 16 (51.6%) |
||||
| Chen et al. [29] | Cross-sectional study | China | 361 186/175 |
47.22 (13.03) |
1 month after hospital discharge | 19.13 (7.60) | SF-36 | Low PCS (15.5%) Low MCS (48.5%) COVID-19 patients vs. Chinese population norm ↓ Physical function ↓Social functioning † ↓ Physical role scores † ↑ Bodily pain † ↑ Vitality † ↑ Mental health † ↑ General health † |
||||
| Mendez et al. [33] | Prospective cohort study | Spain | 179 105/74 |
57.7 (13.45) | 2 months after hospital discharge | 13.05 (6.72) | SF-12 | Poor QoL (40%) | ||||
| Mean (SD) | ||||||||||||
| PCS | 42.5 (11.2) | |||||||||||
| MCS | 45.5 (11.5) | |||||||||||
| Temperoni et al. [34] | Retrospective cohort study | Italy | 104 56/48 |
41.1 (7.4) | 1 month after hospital discharge | N/A | SF-36 | (N = 64/104) Mean (SD) PCS 49.87 (24.25) MCS 55.54 (23.22) Physical functioning 74.3 (25.48) Social functioning 45.12 (29.52) Physical role 30.47 (42.13) Emotional role 46.87 (45.50) Bodily pain 54.34 (30.39) Vitality 48.44 (23.20) Mental health 59.06 (20.35) General health 63.06 (17.91) The lowest scores (<50) were observed in physical role, vitality, social functioning and emotional role domains. No significant differences between outpatient (n = 49) and inpatient (n = 15) groups |
||||
| De Lorenzo et al. [36] | Retrospective and prospective cohort study |
Italy | 185 123/62 |
57.35 (14.19) | 24.05 (6.72) days after hospital discharge |
10.2 (6.72) | WHOQOL-BREF | Median [IQR] Total QoL score 99 [90–107.2] Physical health/Level of independence 16 [IQR 13.7–17.1] Psychological 14.7 [13.3–16.7] Social relations 16 [14.7–17.3] Environment 15 [13.5–17] Patients discharged from hospital, compared to hospitalized patients, had lower quality of life, especially in the psychological domain (P = 0.0084). |
||||
ICU: intensive care unit, EQ-5D: EuroQol 5-dimensional, EQ-5D-5L: EuroQol 5-dimensional 5-levels, WHOQOL-BREF: World Health Organization Quality of Life, SF-12: Short Form Health Survey 12-item, PCS: Physical component score, MCS: Mental component score, HRQoL: health-related quality of life, SF-36: Short Form Health Survey 36-item, VAS: visual analog scale, SGRQ: St. George's Respiratory Questionnaire, N/A: Not available, * Significantly different from the previous value. † Significantly different between patients vs. population norm.
Table 2.
Physical health outcomes in COVID-19 survivors.
| Author | Study design | Country | Sample size (N) Male/Female |
Age (years) Mean (SD) |
Follow-up period month/or days Mean (SD) |
Hospitalization period (days) Mean (SD) |
Outcomes (Measurement tools) | Results (Mean, SD), Prevalence of outcomes n/N (%) |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Qi et al. [12] | Retrospective cohort study | China | 80 48/32 |
64.88 (16.98) | 30 and 90 days after hospital discharge | 15 (4.5) | Independent functional status (FIM) ADL (Barthel index) Muscle strength (MRC score) Functional capacity (6MWT) |
Mean (SD) | 1 month | 3 months |
| Barthel index | ↑82.25 (10.55) | 86.26 (7.70)* | ||||||||
| FIM | ↑100.28 (13.30) | 103.82 (10.71) | ||||||||
| MRC | ↑49.95 (7.36)* | 50.67 (5.64) | ||||||||
| ICU-AW | ↓26 (34.21%) | 14 (18.42%) | ||||||||
| 6MWT | ↑280.08 (37.82)* | 357.57 (56.98)* | ||||||||
| Sonnweber et al. [13] | Prospective cohort study |
Austria | 109 65/44 |
58 (14) | 60 (12) days after symptom onset |
N/A | Performance evaluation (6MWT) |
Lower 6MWT in individuals with persisting hyper-ferritinemia (n = 12) (200 m) compared to patients without (n = 11) (400 m) Lower 6MWT in individuals with pathological CT findings (180 m) compared to without (426 m) (P = 0.011) |
||
| Huang et al. [14] | Retrospective cohort study | China | 57 26/31 |
46.72 (13.78) | 30 days after hospital discharge | 20.89 (7.22) | Respiratory muscle strength (PImax), (PEmax) Functional capacity (6MWT) |
Mean (SD) 6MWT 561.97 (45.29) Severe patients had significantly lower 6MWT vs. non-severe patients. |
||
| Daher et al. [15] | Case series | German | 33 22/11 |
64 (3) | 58.49 (17.82) days after hospital discharge | 15 (1.8) | Functional capacity (6MWT) Fatigue (Borg Scale) Tiredness, myalgia |
During follow-up ↓Fatigue 15/33 (45%) ↓Tiredness 15/33 (45%) ↓Myalgia 5/33 (15%) Median [IQR] 6MWT 380 m [80–470 m] |
||
| Arnold et al. [16] | Prospective cohort study | England | 110 68/42 |
59.64 (20.28) | 88.94 (12.77) days after symptom onset | 5 (4.5) | Fatigue, myalgia, arthralgia, sit to stand (face-to-face outpatient follow-up) | (%) ↓Fatigue (39%) ↓Myalgia (23%) * ↓Arthralgia (6%) * |
||
| Garrigues et al. [19] | Cohort study | France | 120 (24 in ICU group, 96 in Ward group) 75/45 |
63.2 (15.7) | 110.9 (11.1) days after hospital admission | 11.2 (13.4) | Fatigue, myalgia, professional and physical activities (short phone questionnaire) |
Persistent fatigue 66/120 (55%) Myalgia 19/120 (15.8%) Returned to work/Worked before hospitalization 38/56 (67.9%) Resumed sports/Practiced sports regularly before hospitalization 28/39 (71.8%) |
||
| Carfi et al. [20] | Retrospective cohort study | Italy | 143 90/53 |
56.5 (14.6) | 60.3 (13.6) days after symptom onset | 13.5 (9.7) | Fatigue, joint pain, myalgia (COVID-19 standardized questionnaire) |
During follow-up ↓Fatigue (53.1%) ↓Joint pain (27.3%) ↓Myalgia (6%) |
||
| Halpin et al. [21] | Cross-sectional study | England | 100 (19/13 in ICU group, 35/33 in Ward group) |
ICU patients: 58.85 (38.8) Ward patients: 60.61 (55.29) |
48 (10.3) days after hospital discharge | ICU patients: 12.71 (4.65) Ward patients: 8.26 (7.57) |
Fatigue (COVID-19 rehabilitation telephone screening tool, Likert scale) |
Fatigue 72% of ICU patients, 60.3% of Ward patients Did not return to work 60% of ICU patients 15% of Ward patients |
||
| Goertz et al. [22] | Retrospective cohort study | Netherlands, Belgium | 2113 310/1803 |
46.64 (11.12) | 79 (17) days after symptom Onset |
N/A | Fatigue, muscle pain, pain between shoulder blades, joint pain, chest tightness (questionnaires with yes/no items) | During follow-up ↓Fatigue 87% ↓Muscle pain 36% ↓Joint pain 22% ↓Pain between shoulder blades 33% ↓Chest tightness 44% |
||
| Mandal et al. [23] | Cross-sectional study | London, United Kingdom | 384 238/146 |
59.9 (16.1) |
53.29 (8.9) days after hospital discharge |
7.1 (5.02) | Fatigue (graded as absent or present on an 11-point scale) | Fatigue 69%. Patients graded their overall health recovery as 90% [IQR 75–100%] compared to 100% best health. |
||
| Townsend et al. [24] | Cross-sectional study | Ireland | 128 59/69 |
49.5 (15) | 73.75 (18.74) days after hospital discharge |
Non-severe fatigue group (n = 61): 11.61 (9.87) Severe fatigue group (n = 67): 10.47 (8.33) |
Fatigue (CFQ-11 questionnaire) |
Mean (SD) Fatigue 15.8 (5.9) Physical fatigue 11.38 (4.22) Psychological fatigue 4.72 (1.99) |
||
| Wang et al. [25] | Prospective cohort study | China | 131 59/72 |
49 (19.48) | 120 days post hospital discharge | 15.35 (5.2) | Myalgia, chest tightness, fatigue (customized questionnaire) | Fatigue 0 (0%) Chest tightness 1 (0.76%) Myalgia 0 (0%) |
||
| Kamal et al. [26] |
Cross-sectional study | Egypt | 287 103/184 |
32.3 (8.5) | >20 days from the last negative test | N/A | Fatigue, joint pain (Post-COVID-19 Symptoms Collect questionnaire) |
Fatigue 72.8% Joint pain 31.4% |
||
| Xiong et al. [27] | Prospective cohort study | China | 538 245/293 |
51.64 (15.6) | 98.05 (5.2) days after hospital discharge |
17.4 (10.4) | Fatigue, myalgia, arthralgia (small-scale clinical pretrial assessment) | N (%) Fatigue 152/538 (28.3%) * Myalgia 24 /538 (4.5%) * Arthralgia 41/538 (7.6%) * |
||
| Carvalho-Schneider et al. [30] | Prospective cohort study | France | 150 66/84 |
49 (15) | 32.7 (2.5) and 59.7 (1.7) days after symptom onset |
N/A | Asthenia (WHO performance status classification) Arthralgia, myalgia (standardized case report form completed) |
Day 30 (n=150) | Day 60 (n=130) | |
| Asthenia/myalgia | 54 (36.0%) | 28 (21.5%) | ||||||||
| Arthralgia | 13 (9.8%) | 21 (16.3%) | ||||||||
| Tomasoni et al. [31] | Cross-sectional study | Italy | 105 77/28 |
54.29 (16.53) | 45.64 (3.75) days after virological clearance |
8.35 (3.75) | Asthenia, pain (electronic case report form) |
Asthenia 33/105 (31.4%) Burning pain 11/105 (10.5%) |
||
| Mohamed- Hussein et al. [32] | Cross-sectional study | Egypt | 444 192/252 |
33.09 (12.09) | 35.31 (18.75) days after symptom onset | N/A | Post-COVID-19 Functional Status Scale (PCFS) | Functional limitations (%) Negligible (63.1%) Slight (14.1%) Moderate (2.5%) Severe (0.5%) No functional limitations (20%) 80% of patients who recovered from COVID-19 had some degree of functional restriction. |
||
| Yuan et al. [38] | Retrospective cohort study | China | 96 (27/27 in normal group, 20/22 in self-reported depression group) | Normal group = 45.2 (13.2), Self-reported depression group = 49.6 (13.2) | 14 days after discharge | Normal group = 24.4 (6.7) Self-reported depression group = 24.8 (6.5) |
Chest tightness (online questionnaire) | Patients reported chest tightness. | ||
| Landi et al. [72] | Prospective cohort study | Italy | 131 80/51 |
55.8 (14.8) | 55.8 (10.8) days after symptom onset | Patients with negative test: 12.1 (7.9) Positive test: 10.0 (6.5) |
Fatigue, joint pain | Fatigue 67/131 (51.1%) Joint pain 33/131 (25.1%) |
||
| Du et al. [73] | Retrospective cohort study | China | 126 61/65 |
62.83 (11.24) | 60 days after hospital discharge | 25.64 (11.24) | Fatigue, muscle or joint ache (standardized data collection form) |
Fatigue 49/126 (38.9%) Muscle/joint ache 16/126 (12.7%) |
||
| Ismael et al. [74] | Retrospective cohort study | Brazil | 895 354/541 |
40.79 (0.45) | 56.6 days after treatment | N/A | Fatigue, asthenia, myalgia, joint pain (online assessment, website/phone) | Fatigue 324 (36.94%) Asthenia 179 (20.41%) Myalgia 259 (29.57%) Joint pain 84 (9.58%) |
||
| Weerahandi et al. [75] | Prospective cohort study | New York, USA | 152 95/57 |
59.54 (12.72) | 36.64 (9.72) days after hospital discharge |
19.75 (15.71) | Physical health status (PROMIS Global Health-10 instrument) Survey completed by phone or online |
Mean (SD) ↓Physical health 43.8 (9.3) Indicating worse physical health after COVID-9 compared to baseline |
||
WHO: World Health Organization, ADL: activities of daily living, FIM: functional independence measure, MRC: Medical Research Council, 6MWT: Six-Minute Walk Test, ICU-AW: ICU-acquired weakness, CT: computed tomography, PCFS: Post-COVID-19 Functional Status Scale, PImax: maximum static inspiratory pressures; PEmax: maximum static expiratory pressures, PTSD: post-traumatic stress disorder, CFQ-11: Chalder Fatigue Score, FS-14: Fatigue Scale-14, MBS: Modified Borg Dyspnoea Scale, N/A: Not available, * Significantly different from the previous value.
Table 3.
Mental health outcomes in COVID-19 survivors.
| Author | Study design | Country | Sample size (N, Male/Female) |
Age (years) Mean (SD) |
Follow-up period month/or days Mean (SD) |
Hospitalization period, days Mean (SD) |
Outcomes (Measurement tools) | Results, Prevalence of outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Daher et al. [15] | Case series | Germany | 33 22/11 |
64 (3) | 58.49 (17.82) days after discharge |
15 (1.8) | Depression (PHQ-9) Anxiety (GAD-7) Cognitive problems |
Median [IQR] Depression 7 [4–11] Anxiety 4 [1–9] Cognitive problems 6/33 (18%) |
||
| Arnold et al. [16] | Prospective cohort study | England | 110 68/42 |
59.64 (20.28) | 88.94 (12.77) days after hospital discharge |
5(4.5) | Mental wellbeing (WEMWBS) Insomnia (NEWS, scored from 1 to 20) |
↑Insomnia (24%) Mental wellbeing in severity of COVID-19 |
||
| Median [IQR] | ||||||||||
| Mild | 52 [44-56] | |||||||||
| Moderate | 53 [42-59] | |||||||||
| Severe | 50 [39-58] | |||||||||
| Mental wellbeing was similar to healthy population norms | ||||||||||
| Garrigues et al. [19] | Cohort study | France | 120 75/45 |
63.2 (15.7) | 110.9 (11.1) days after hospital admission | 11.2 (13.4) | Cognitive and sleep problems (short phone questionnaire) | Attention problem 32/120 (26.7%) Memory loss 41/120 (34.2%) Sleep problems 37/120 (30.8%) |
||
| Halpin et al. [21] | Cross-sectional study | England | 100 (19/13 in ICU group, 35/33 in Ward group) |
ICU patients: 58.85 (38.8) Ward patients: 60.61 (55.29) |
48 (10.3) days after hospital discharge | ICU patients: 12.71 (4.65) Ward patients: 8.26 (7.57) |
PTSD symptoms, cognitive problems (COVID-19 rehabilitation telephone screening tool, Likert scale) |
ICU (n=32) | Ward (n=68) | |
| PTSD | 46.9% | 23.5% | ||||||||
| Worsened anxiety/depression | 12 (37.5%) | 11 (16.2%) | ||||||||
| Thoughts of self‐harm | 1 (3.1%) | 1 (1.5%) | ||||||||
| New or worsened concentration problem | 11 (34.4%) | 11 (16.2%) | ||||||||
| New or worsened short‐term memory problem | 6 (18.8%) | 12 (17.6%) | ||||||||
| Mandal et al. [23] | Cross-sectional study | London, United Kingdom | 384 62/38 | 59.9 (16.1) | 53.29 (8.9) days after hospital discharge | 7.1 (5.02) | Sleep quality (11-point scale) | Sleep quality Median [IQR] Maximum intensity 5 [1–5] Intensity at follow up 3 [0–6] 66.2% reported improvement 29.7% reported no change 4.2% reported deterioration at follow-up. Patients graded their overall health recovery as 90% (IQR 75–100%) compared to 100% best health |
||
| Kamal et al. [26] | Cross-sectional study | Egypt | 287 103/184 |
32.3 (8.5) | More than 20 days after the last negative test | N/A | Anxiety, depression, obsessive-compulsive disorder, dementia (data collection questionnaire) | Anxiety 38% Depression 28.6% Obsessive-Compulsive disorder 4.9% Dementia 28.6% |
||
| Xiong et al. [27] | Prospective cohort study | China | 538 245/293 |
51.64 (15.6) | 98.05 (5.2) days after hospital discharge |
17.4 (10.4) | Psychosocial symptoms, depression, anxiety, dysphoria, feelings of inferiority, sleep problems (small-scale clinical pretrial assessment, telephone follow-up survey) | Psychosocial symptoms 122 (22.7%) † Depression 23 (4.3%) † Anxiety 35 (6.5%) † Dysphoria 9 (1.7%) Feelings of inferiority 3 (0.6%) Sleep problems 95 (17.7%) † Significantly higher than in the comparison group. |
||
| Tomasoni et al. [31] | Cross-sectional study | Italy | 105 77/28 |
54.29 (16.53) | 45.64 (3.75) days after virological clearance |
8.35 (3.75) | Anxiety, depression (HADS) Cognitive problems (MMSE) |
Pathological HADS-A/D 30/100 (30%) [Both anxiety and depression 10/30 (33%), only anxiety 19/30 (63%), only depression 1/30 (4%)] Persistent cognitive problems (n = 25) 17.1% 52.4% showed persistent symptoms. |
||
| Mendez et al. [33] | Prospective cohort study | Spain | 179 105/74 |
57.7 (13.45) | 2 months after hospital discharge | 13.05 (6.72) | Anxiety (GAD-7) Depression (PHQ-2) PTSD (DTS) Neurocognitive function (standardized instruments) By telephone |
39.1% of patients had psychiatric morbidity Anxiety (29.6%) Depression (26.8%) PTSD (25.1%) 58.7% of patients had neurocognitive impairment in at least one function |
||
| Cognitive problems | Moderate | Severe | ||||||||
| Immediate verbal memory | 38% | 11.2% | ||||||||
| Delayed verbal memory | 11.8% | 2.8% | ||||||||
| Working memory | 6.1% | 1.1% | ||||||||
| Semantic verbal fluency | 34.6% | 8.4% | ||||||||
| Mazza et al. [35] | Prospective cohort study | Italy | 402 [admitted patients (n = 300), managed at home (n = 102)] 265/137 |
57.80 (13.33) | 31.29 (15.7) days after hospital discharge | 15.31 (10.32) for admitted patients | Anxiety (STAI-Y) Depression (ZSDS and BDI-13) PTSD (PCL-5, IES-R) Sleep problem (MOS-SS) Obsessive-Compulsive (OCI) |
Anxiety 42% Depression 31% PTSD 28% Obsessive-Compulsive symptoms 20% Sleep problem 40% 55.7% presented at least 1 mental disorder |
||
| De Lorenzo et al. [36] | Retrospective and prospective cohort study |
Italy | 185 123/62 |
57.35 (14.19) | 24.05 (6.72) days after hospital discharge |
10.2 (6.72) | PTSD (IES-R) Anxiety (STAI-Y, Pain (VAS) Insomnia (WHIIRS) Cognitive impairment (MoCA score) |
Cognitive impairment 47/185 (25.4%) Insomnia 51/185 (27.6%) Anxiety 55/185 (29.7%) PTSD 41/185 (22.2%) VAS pain 77.5 [IQR 75–90] Anxiety and PTSD were significantly more frequent in discharged vs. hospitalized patients. |
||
| Wu et al. [37] | Case series | China | 370 203/167 |
50.5 (13.1) | 24.1(7.44) days after hospital discharge |
11.64 (3.72) | Anxiety (GAD-7) Depression (PHQ-9) Sleep problem (single question) |
Anxiety 50 (13.5%) Depression 40 (10.8%) Both anxiety and depression 23 (6.2%) Sleep problem 109 (29.5%) |
||
| Yuan et al. [38] | Retrospective cohort study | China | 96 (27/27 in normal group, 20/22 in self-reported depression group) | Normal group = 45.2 (13.2), Self-reported depression group = 49.6 (13.2) | 14 days after discharge | Normal group = 24.4 (6.7) Self-reported depression group = 24.8 (6.5) |
Zung self-rating depression scale | Depression 42/96 (43%) Significantly increased immune response in individuals with depression . |
||
| Liu et al. [60] | Cross-sectional survey study | China | 675 317/358 |
53.94 (18.57) | 36.75 days after hospital discharge |
27.87 | Anxiety (GAD-7) Depression (PHQ-9) PTSD symptoms (PCL-5) Sleep difficulties (item analysis for PTSD, depression) |
PTSD 84/675 (12.4%) Anxiety 70/675 (10.4%) Depression 128/675 (19%) PCL-5 12 [IQR 4–16] GAD-7 4 [IQR 2–6] PHQ-9 5 [IQR 3–8] Sleep difficulty was the most frequently reported symptom. Perceived discrimination was a central predictor of mental illness. |
||
| Ismael et al. [74] | Retrospective cohort study | Brazil | 895 354/541 |
40.79 (0.45) | 56.6 days after treatment | N/A | Depression (PHQ-9) Anxiety (GAD-7) PTSD (PCL-C) (online assessment, website or phone) |
Depression (n = 235) 26.26% Anxiety (n = 201) 22.46% PTSD (n = 155) 17.32% |
||
| Weerahandi et al. [75] | Prospective cohort study | New York, USA | 152 95/57 |
59.54 (12.72) | 36.64 (9.72) days after hospital discharge |
19.75 (15.71) | Overall and mental health status (PROMIS Global Health-10 instrument) Survey completed by phone or online |
During follow-up: Mean (SD) ↓Mental health 47.3 (9.3) * Indicating worse mental health after COVID-19 compared to baseline |
||
| Chang et al. [76] | Cross-sectional study | Korea | 64 28/36 |
54.7 (16.6) | 75.7 (20.0) days after hospital discharge | 31.2 (18.1) | PTSD (PCL-5) By telephone interview | PTSD 13/64 (20.3%) PCL-5 score in PTSD group vs. Non-PTSD group 46 (SD 11.9) vs. 9.6 (SD 7.6) |
||
| Wu et al. [77] | Case series | China | 14 7/7 |
39 (10) | 44.7 (13.5) days out of clinical services | 12.1 (8.7) | Anxiety/fear (valid questionnaires, website) | Persistent mild to severe anxiety/fear 13/14 (92.9%) | ||
MOS-SS: Medical Outcomes Study Sleep Scale, WHIIRS: Women's Health Initiative Insomnia Rating Scale, MMSE: Mini Mental State Examination, STAI-Y: State-Trait Anxiety Inventory form Y, ZSDS: Zung Self-Rating Depression Scale, BDI-13: 13-item Beck's Depression Inventory, PTSD: posttraumatic stress disorder, PCL-5: PTSD Checklist-5, HADS: Hospital Anxiety and Depression Scale, GAD-7: Generalized Anxiety Disorder Screener, OCI: Obsessive-Compulsive Inventory, PHQ-9: Patient Health Questionnaire-9, PROMIS: Patient-Reported Outcomes Measurement Information System, NEWS: National Early Warning Score, IES-R: Impact of Events Scale-Revised, VAS: visual analog scale, DTS: Davidson Trauma Scale, WEMWBS: Warwick-Edinburgh Mental Well-Being Scale, PTSD-SS: Post-traumatic Stress Disorder Self-rating Scale, GHQ-12: General Health Questionnaire-12, SAS: Zung Self-Rating Anxiety Scale, SDS: Zung Self-Rating Depression Scale, PCL—C: PTSD Checklist-Civilian version, MoCA: Montreal Cognitive Assessment, N/A: Not available, * Significantly different from the previous value.
3. Results
3.1. Search results
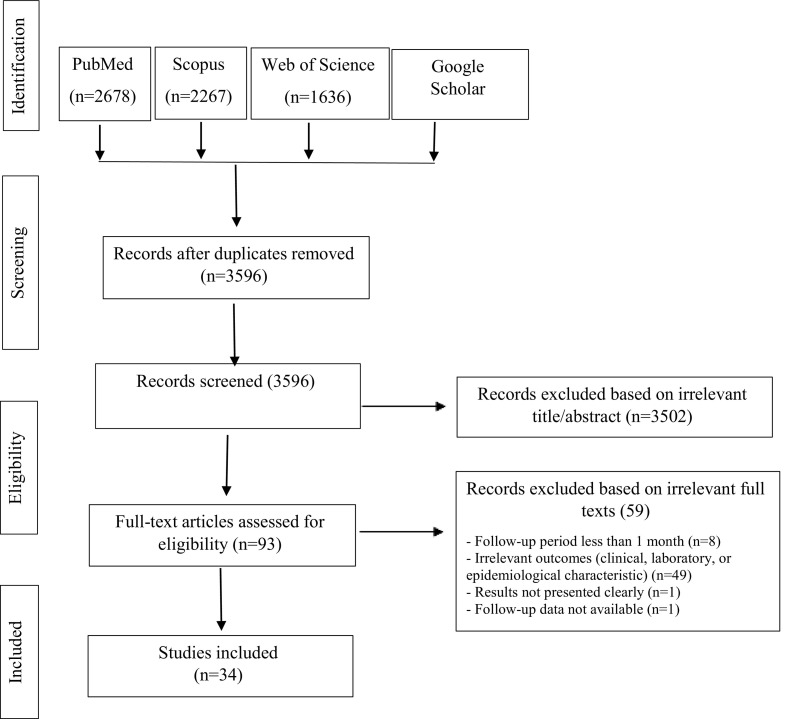
Fig. 1 illustrates a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the process of searching and selecting eligible studies. We found 7398 studies by searching with syntaxes adapted to each database, and removed 3802 as duplicates. Titles and abstracts were screened for residual studies (n = 3596), and 3503 of these were excluded based on irrelevant titles and/or abstracts, which left 93 studies. Full text screening showed that 59 studies did not meet the eligibility criteria, and so were excluded. Finally, 34 studies met our inclusion criteria and were included in this review: 29 studies were peer reviewed and five studies were preprints.
Fig. 1.
PRISMA flowchart for the literature review.
3.2. Characteristics of included studies
The characteristics of all 34 included studies are presented in Table 1, Table 2, Table 3. The total number of participants in all 34 studies was 8932, with sample sizes ranging from a case series of 14 patients to a retrospective cohort study of 2113 patients. Among all patients included, 56.3% were women and 43.7% were men. The majority of studies were from China (n = 10, 29.5%) and Italy (n = 7, 20.6%). The designs of these studies were 20 cohort, 10 cross-sectional, and four case series. Post-infection follow-up times differed among studies, ranging from 1 to 3 months after symptom onset or hospital discharge, and the mean duration of hospitalization was 15.86 days. The overall Health related Quality of Life (HRQOL) score (Table 1) was reported in 13 studies, with six studies using the European Quality of Life 5-Dimensions instrument (EQ-5D) or European Quality of Life 5-Dimensions instrument, 5-levels (EQ-5D-5L), three studies using the Short Form Health Survey (SF-36), and three studies using St. George's Respiratory Questionnaire (SGRQ). Physical health status was evaluated in 23 studies, and was commonly assessed as fatigue/asthenia/tiredness (16 studies), pain including joint pain/arthralgia and myalgia (14 studies), or functional status based on valid questionnaires (6 studies) or exercise capacity tests such as the six-minute walk test (6MWT) or one-minute sit to stand test (STS]) [[12], [13], [14], [15]] (Table 2). Mental health status was assessed in 18 studies, in which the mental health symptoms evaluated most frequently were anxiety (11 studies), depression (10 studies), PTSD (7 studies), sleep problems (8 studies), and cognition problems (8 studies) (Table 3). Psychological symptoms were evaluated with single-item questions or valid instruments (Table 1, Table 3).
3.3. Health status
Overall health status that was evaluated with the HRQOL questionnaire, and was found to be lower post-COVID-19 compared to the normal population [12,16]. Post-COVID-19 patients had slight to moderate declines in HRQOL [[15], [16], [17], [18], [19], [20]]. Intensive care unit (ICU) admission had a significant negative effect on HRQOL scores [[15], [16], [17],21]. One study reported that early physical and pulmonary rehabilitation within 72 h of ICU admission could be effective in improving HRQOL at 1 month and 3 months after hospital discharge [12]. Specific categories of physical and mental health status according to the HRQOL questionnaire are covered in the next two sections, along with the results of other measurements related to physical and mental health.
3.4. Physical health status
3.4.1. Musculoskeletal symptoms
Fatigue was reported in 28% to 87% of individuals after coronavirus infection. This complication was observed in both hospitalized and nonhospitalized patients, and in those admitted to in-patient wards and the ICU [16,19,22,23]. Seven studies assessed the association between fatigue and COVID-19 severity [16,21,[24], [25], [26]]; four found greater fatigue in severely ill individuals [16,21,26], and one study reported greater fatigue or physical decline with longer durations of hospital stay [27]. Five studies found normal laboratory tests at follow-up, and suggested there was no association between fatigue and laboratory parameters such as inflammatory markers, cell turnover (leukocyte, neutrophil or lymphocyte counts, neutrophil-to-lymphocyte ratio, lactate dehydrogenase, C-reactive protein) or pro-inflammatory molecules (IL-6 or soluble CD25), ferritin or D-dimer [15,16,23,24]. Fatigue was reported in a greater proportion of women [21,24,27] and individuals with psychological problems [21,24].
Across all studies, the prevalence of post-COVID-19 myalgia was 4.5–36%, and the prevalence of post-COVID-19 arthralgia was 6.0–27%. Only one study from China reported no pain at follow-up [25]. Pain was evaluated with health-related questionnaires (EQ-5D, EQ-5D-5L and SF-36), and patients reported slight to moderate pain, except in one study that reported moderate to severe pain in 51.6% of individuals admitted to the ICU [28]. Overall, pain was reported by a greater percentage of recovering individuals post-COVID-19 who experienced severe illness in the acute stage and required hospitalization [16,19,26,29,30]. A larger proportion of individuals with anxiety and depression reported musculoskeletal symptoms compared to their counterparts without these diagnoses [15,21,31]. The studies included in this review reported conflicting results regarding the association between comorbidities and the presence of musculoskeletal symptoms post-COVID-19 [[22], [23], [24],26,30], although most reported no association [22,24,30]. Muscle weakness was evaluated in one study that reported ICU-acquired weakness in 18.42% of individuals post-COVID-19 at 3 months' follow-up [12]. Another study found that respiratory muscle strength in post-COVID-19 patients was lower at inspiration (49.1%) and expiration (22.8%) 1 month after hospital discharge [14].
3.5. Physical and functional health status
Four studies evaluated functional capacity with the 6MWT, with results ranging between 180 to 561 m from 1 to 3 months post-COVID-19 [[12], [13], [14], [15]]. Two studies reported no decrease in oxygen saturation during functional capacity tests (6MWT and STS); however, excessive fatigue was found in some individuals [15,16]. One study found very low 6MWT results in individuals with persisting hyperferritinemia [13].
Physical health status was most often evaluated with quality of life questionnaires (EQ-5D, EQ-5D-5L, SF-36 and SGRQ). Only two studies evaluated functional status and level of physical activity with other instruments such as the Barthel index [12], functional independence measure (FIM), or post-COVID-19 functional status scale (PCFS) [32]. Physical activity, mobility, and usual activities were reported to be reduced in 15% to 54% of individuals after their coronavirus infection [12,15,[17], [18], [19],21,28,29,33]. Five studies reported a decline in physical activity especially for usual care and daily activities in individuals admitted to the ICU [12,15,16,21,28]. Three studies reported greater declines in physical role functioning (participation in life despite physical limitations) compared to other components of physical health status [16,29,34].
3.6. Mental health status
The most frequent mental health symptom reported in the studies included in this review was anxiety, with post-COVID-19 prevalences ranging from 6.5% to 63%. The second most frequent psychological symptom was depression, with prevalences ranging between 4% and 31% at follow-up times longer than 1 month post-COVID-19. One third of the patients in Italy and 41.3% of those in Iran had both depression and anxiety after hospital discharge [17,31]. Another common post-COVID-19 mental health problem was PTSD, with prevalences ranging from 12.1% to 46.9%. The severity of COVID-19 was related to the severity and prevalence of mental health symptoms [16], with anxiety and PTSD being significantly more frequent in patients admitted to the ICU compared to wards [19,21,31,36]. Additional mental health symptoms were sleep problems, with prevalences ranging between 17.7% to 30.8% [16,19,27], and cognitive-functional problems, reported in 17.1% to 4.4% of individuals post-COVID-19, especially in ICU survivors [21,31,33]. Neurocognitive parameter including immediate verbal memory and semantic verbal fluency were moderately impaired in 58.7% and severely impaired in 18.4% of post-COVID-19 patients [33]. More than half of the patients presented at least one stress-related symptom (anxiety, depression, and PTSD) [35] or neurocognitive impairment [33].
The associations between mental health symptoms and demographic characteristics were evaluated in most studies. Inconsistent findings were reported for the association between age and psychological symptoms: four studies found an inverse relationship [16,21,31,35,36], whereas three studies reported no association [4,31,33]. Greater psychological impact was observed in females post-COVID-19, with a 2.2- to 2.5-fold higher odds of developing psychiatric morbidity [21,29,33,36]. Comorbidities (arterial hypertension, coronary artery disease, and diabetes mellitus) were not related to a higher incidence of mental health problems [31,36,37]. Previous psychiatric disorders were associated with increased severity of post-COVID-19 mental health symptoms [35,36]. However, even individuals without previously diagnosed mental health morbidity (74%) reported anxiety and depression symptoms post-COVID-19 [21]. One study reported a significant direct relationship between symptoms of depression and baseline immune responses [38], and inverse associations were found elsewhere with lung function parameters (forced vital capacity [FVC]) and post-discharge respiratory symptoms [4,29].
4. Discussion
This review highlights the presence of several physical and mental health problems post-COVID-19 after follow-up periods of up to 3 months. Among the physical health problems, fatigue was the most common musculoskeletal symptom reported post-COVID-19. Long-lasting fatigue has been reported previously after other viral infections [[39], [40], [41], [42]]. Several studies have investigated the associations between fatigue and disease severity, gender, age, comorbidity, and a pre-existing diagnosis of depression and/or anxiety [15,16,26,27,30]. Greater fatigue and pain at follow-up were reported, apparently, by patients who had experienced severe SARS-CoV-2 infection [16,21,26]. However, inconsistent findings were reported in studies that evaluated the association between disease severity and fatigue; differences among studies maybe due to the severity criteria used for COVID-19 and to different admission or discharge protocols [16,21,[24], [25], [26]]. Women tended to experience fatigue to a greater extent compared to men [21,24,27]. This is consistent with the higher rate of chronic fatigue syndrome reported in females, which has been linked to stress-related factors [43].
Physical health declines were found mostly with the physical role functioning, mobility and usual activity [16,29,34], which are related to participation in work and regular daily activities [44]. In addition, reduced physical capacity was evident as lower 6MWT results post-COVID-19 compared to the average value of >650 m in healthy adults [45], with no decrease in oxygen saturation during testing [[12], [13], [14], [15],46]. These findings suggest a role for factors other than pulmonary dysfunction in reduced physical capacity. Previously, declines in physical activity were reported in individuals who recovered from SARS and experienced restrictions in activities of daily living and work participation [47,48]. Physical performance should ideally be assessed in a way that includes both functional capacity measurements (e.g., 6MWT, STS), and muscle strength, to gain better insights that can be used in clinical practice. However, only one study evaluated muscle strength at 3-months' post-COVID-19 follow-up, and reported that 18% of individuals admitted to the ICU had acquired weakness [49]. Muscle weakness and acute sarcopenia have been observed in COVID-19 survivors [8,50]. Among individuals infected with the coronavirus, Paneroni et al. found quadriceps weakness in 86% and biceps brachialis weakness in 73% at hospital discharge [50]. This finding further highlights the importance of evaluating muscle strength at follow-up [51,52], because it is associated with poor functional outcomes and long-term disability [51]. Functional decline and lower physical capacity were evident in both hospitalized (ward and ICU) and non-hospitalized patients at follow-up periods of 1 and 3 months post-COVID-19. Accordingly, physical inactivity as a consequence of quarantine, social distancing and isolation may also be a source of reduced physical capacity and function in persons who are recovering from COVID-19.
Most studies included in the present review reported psychological and neuropsychological issues (anxiety and depression, PTSD, sleep and cognition problems) post-COVID-19, even in individuals with no previously diagnosed mental health problems. This is consistent with the findings of two metaanalyses of survivors of previous coronavirus epidemics [[9], [53]], which found that one third of patients experienced at least one psychological impairment (PTSD, depression, and anxiety) more than 6 months post-discharge [9]. The prevalence of mental health symptoms varies widely among studies, which may be due to differences in the instruments used to measure these outcomes, and to differences among countries in the impact of cultural or spiritual beliefs in efforts to cope with the psychological effect of coronavirus disease [54,55]. Some studies reported an association between anxiety, depression and PTSD with physical symptoms [15,21,24,31]. Wang et al. proposed a chain model to describe the link between physical symptoms and mental health via the need for health information and perceived impact of pandemic mediators. Excessive conflicting health information regarding the physical symptoms of COVID-19 might exaggerate the perceived impact of the pandemic, thus predisposing individuals to a higher risk of anxiety, depression and stress [55].
Sleep problems were another prominent mental health problem post-COVID-19, especially insomnia, which was observed in both acute and chronic stages of the disease [16,19,27,56]. Physical and psychological stress in the acute stage of the infection can trigger physiological mechanisms that stimulate pro-inflammatory cytokine release, which in turn can interfere with metabolic and cardiovascular functions related to sleep [56]. McNally et al. (2015) suggested that sleep problems may impair both emotional and attentional regulation, and cause irritability and concentration problems [57]. Other studies suggested that sleep problems were a central complication perceived among COVID-19 survivors [29,37].
Mental health symptoms were more frequent among females [4,21,29,35]. This may be due to the greater susceptibility of women to stressors, or more likely the higher rate of reporting psychological symptoms [58,59]. Depression, PTSD, and cognitive problems (concentration and short-term memory impairment) were more frequent among ICU survivors than ward patients [21,60]. In addition, post-COVID-19 symptoms of cough, fatigue, and chest distress were risk factors for PTSD [60]. The symptoms of PTSD are a well-recognized component of post-ICU syndrome caused by a variety of factors including fear of dying, invasive treatment, pain, delirium, inability to communicate, weakness, immobility, sensory problems, and sleep deprivation [61]. Stress-related symptoms (anxiety, depression, and PTSD) and delirium were associated with an approximately 4-fold increase in the odds of developing neurocognitive impairment [33], and neurocognitive impairments were related with a 4.5-fold increase in the odds of psychiatric morbidity (anxiety, depression, and PTSD) [33]. Considered together, neuropsychological factors may increase neurocognitive impairments [33,38] and even mortality [62], so it is important to consider psychiatric rehabilitation for post-COVID-19 survivors, and measures to strengthen protective factors such as social support that act as stress-buffering mechanisms.
The post-COVID-19 symptoms studied thus far appear to resemble chronic fatigue syndrome, which has been reported after other viral infection [63]. The diagnosis of this syndrome requires the concurrent presence of at least four symptoms among muscle pain, joint pain, post-exertional fatigue, cognitive problems (memory and concentration), and sleep problems, for more than 6 months [64]. Although the symptoms related to this syndrome are present post-COVID-19, the studies included in the present review were all related to follow-up periods shorter than 6 months.
Behavioral psychotherapy treatments such as cognitive behavioral therapy (CBT) are recommended for chronic fatigue syndrome [65]. Nevertheless, face-to-face CBT is a time-intensive treatment, and is not applicable during the pandemic because of government restrictions that require social distancing and quarantines. Modern modes of digital communication can, however, enable efficient support in the form of rehabilitation services provided via information and communication technologies, and comprising prevention, assessment, intervention, supervision, education and consultation during the pandemic [[66], [67], [68]]. The advantages of internet consultations and smart phone applications for telehealth (telerehabilitation and telepsychiatry) are that they can minimize viral transmission from face-to-face therapy, reduce travel time and costs, minimize the need to schedule appointments, and improve treatment accessibility [[67], [68], [69]]. Internet CBT (I-CBT) has been reported to be an effective and efficient treatment for psychiatric problems and musculoskeletal symptoms [70,71]. Therefore, telehealth could be considered as a follow-up treatment for post-COVID-19 patients in efforts to prevent long-lasting physical and mental health complications.
4.1. Limitations
According to the WHO description of health, only physical and mental health dimensions were reviewed in this article, therefore other dimensions such as post-COVID-19 social health consequences and its related factors were not considered in this study. This review was restricted to only English language studies. In addition, measurement bias may have influenced the results of studies that used self-reported questionnaires with single-item questions and used telephone contact to collect information at follow-up.
5. Conclusion
This scoping review highlights the persistence, for up to 3 months post-COVID-19, of physical and mental health problems that can reduce quality of life. Early screening and comprehensive rehabilitation planning may be required to effectively prevent and manage post-COVID-19 complications. This approach could reduce economic and clinical health consequences, and prevent long-term disability.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors (S.S., M.T., N.Z., I.E., S.A.A.) participated in the conceptualization of this study. M.T., S.A.A., and S.S participated in the methodology and analysis. S.S., M.T., N.Z., and S.A.A. contributed to the writing. S.S. and M.T. were the main contributors to manuscript writing. All authors (S.S., M.T., N.Z., I.E, and S.A.A.) took part in manuscript revision, and all read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors have no competing interests to report.
Acknowledgements
We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the use of English in the manuscript.
Appendix A
(COVID-19[tiab] OR “2019 novel coronavirus”[tiab] OR SARS-CoV-2[tiab] OR 2019-nCoV[tiab] OR nCoV[tiab] OR “Coronavirus 2019”[tiab]) AND (Follow-Up[tiab] OR “follow up”[tiab] OR Discharge*[tiab] OR postdischarge[tiab] OR post-discharge[tiab] OR “post discharge”[tiab] OR “Longterm Effect*”[tiab] OR “Long-Term Effect*”[tiab] OR “Long Term Effects”[tiab])
References
- 1.Woods J., Hutchinson N.T., Powers S.K., Roberts W.O., Gomez-Cabrera M.C., Radak Z., et al. The COVID-19 pandemic and physical activity. J. Sport Health Sci. 2020 doi: 10.1016/j.smhs.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Team E.E. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. J. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.5.200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cipollaro L., Giordano L., Padulo J., Oliva F., Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J. Orthop. Surg. Res. 2020;15(1):178–184. doi: 10.1186/s13018-020-01702-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. J. Brain. Behav. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. https://doi:10.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., et al. Neurological associations of COVID-19. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wostyn P. COVID-19 and chronic fatigue syndrome: Is the worst yet to come? J. Med. Hypotheses. 146:110469. 10.1016/j.mehy.2020.110469. [DOI] [PMC free article] [PubMed]
- 7.Komaroff A.L., Bateman L. Will COVID-19 lead to myalgic ENcephalomyelitis/chronic fatigue syndrome? J. Front. Med. 2021;7:1132. doi: 10.3389/fmed.2020.606824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Disser N.P., De Micheli A.J., Schonk M.M., Konnaris M.A., Piacentini A.N., Edon D.L., et al. Musculoskeletal consequences of COVID-19. J. Bone Joint Surg. Am. 2020;102:1197–1204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed H., Patel K., Greenwood D.C., Halpin S., Lewthwaite P., Salawu A., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J. Rehabil. Med. 2020;52:1–11. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 10.Steiner W.A., Ryser L., Huber E., Uebelhart D., Aeschlimann A., Stucki G. Use of the ICF model as a clinical problem-solving tool in physical therapy and rehabilitation medicine. J. Phys. Ther. 2002;82:1098–1107. doi: 10.1093/ptj/82.11.1098. [DOI] [PubMed] [Google Scholar]
- 11.Arksey H., O’Malley L. Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 12.Qi D., Yan X., Xiang J., Peng J., Yu Q., Tang X., et al. Effects of early physical and pulmonary rehabilitation for severely and critically ill COVID-19 patients: a retrospective, cohort, and multicenter study. Researchsquare. 2020 doi: 10.21203/rs.3.rs-66798/v1. [DOI] [Google Scholar]
- 13.Sonnweber T., Boehm A., Sahanic S., Pizzini A., Aichner M., Sonnweber B., et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir. Res. 2020;21:1–9. doi: 10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020;21:1–10. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daher A., Balfanz P., Cornelissen C., Müller A., Bergs I., Marx N., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daher A., Balfanz P., Cornelissen C., Müller A., Bergs I., Marx N., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106–112. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arab-Zozani M., Hashemi F., Safari H., Yousefi M., Ameri H. Health-related quality of life and its associated factors in COVID-19 patients. Osong Public Health Res. Perspect. 2020;11:296–302. doi: 10.24171/j.phrp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santus P., Tursi F., Croce G., Di Simone C., Frassanito F., Gaboardi P., et al. Changes in quality of life and dyspnoea after hospitalization in COVID-19 patients discharged at home. Multidiscip. Respir. Med. 2020;15 doi: 10.4081/mrm.2020.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Inf. Secur. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. 1001.2020;324(6):603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J. Med. Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 22.Goërtz Y.M., Van Herck M., Delbressine J.M., Vaes A.W., Meys R., Machado F.V., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6 doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. J. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240784. 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Xu H., Jiang H., Wang L., Lu C., Wei X., et al. Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM: Inter. J. Med. 2020;113:657–665. doi: 10.1093/qjmed/hcaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamal M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2020 doi: 10.1111/ijcp.13746. 2020: e13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Q., Xu M., Li J., Liu Y., Zhang J., Xu Y., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-Centre longitudinal study. Clin. Microbiol. Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cody N.M., Lakey S., McMahon S., Downey M., Duncan M., Hewitt J., et al. Clinical characteristics and post-intensive care outcomes of COVID-19 pneumonia. Researchsquare. 2020 doi: 10.21203/rs.3.rs-58685/v1. [DOI] [Google Scholar]
- 29.Chen K.-Y., Li T., Gong F., Zhang J.-S., Li X.-K. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front. Psychiatry. 2020;11:668. doi: 10.1046/j.1440-1843.2003.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomasoni D., Bai F., Castoldi R., Barbanotti D., Falcinella C., Mulè G., et al. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in Milan, Italy. J. Med. Virol. 2021;93:1175–1179. doi: 10.1002/jmv.26459. 10.1002/jmv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamed-Hussein A., Galal I., Saad M., Zayan H.E., Abdelsayed M., Moustafa M., et al. Post-COVID-19 functional status: relation to age, smoking, hospitalization and comorbidities. medRxiv. 2020 doi: 10.1101/2020.08.26.20182618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendez R., Balanza-Martinez V., Luperdi S.C., Estrada I., Latorre A., Gonzalez-Jimenez P., et al. Short-term neuropsychiatric outcomes and quality of Life in COVID-19 survivors. medRxiv. 2020 doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Temperoni C., Grieco S., Pasquini Z., Canovari B., Polenta A., Gnudi U., et al. Clinical characteristics, management and health related quality of life in young to middle age adults with COVID-19. BMC Infect. Dis. 2021;21:1–10. doi: 10.1186/s12879-021-05841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. 2020;15 doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C., Hu X., Song J., Yang D., Xu J., Cheng K., et al. Mental health status and related influencing factors of COVID-19 survivors in Wuhan, China. Clin. Trans. Med. 2020;10 doi: 10.1002/ctm2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan B., Li W., Liu H., Cai X., Song S., Zhao J., et al. Correlation between immune response and self-reported depression during convalescence from COVID-19. Brain Behav. Immun. 2020;88:39–43. doi: 10.1016/j.bbi.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. J. BMC Neurol. 2011;11:1–7. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S.H., Shin H.-S., Park H.Y., Kim J.L., Lee J.J., Lee H., et al. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in middle east respiratory syndrome survivors. J. Psychiatry Investig. 2019;16:59. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickie I., Davenport T., Wakefield D., Vollmer-Conna U., Cameron B., Vernon S.D., et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jason L.A., Katz B.Z., Shiraishi Y., Mears C.J., Im Y., Taylor R.R. Predictors of post-infectious chronic fatigue syndrome in adolescents. J. Psychol. Health Med. 2014;2:41–51. doi: 10.1080/21642850.2013.869176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.GJOm Ranjith. Vol. 55. 2005. Epidemiology of Chronic Fatigue Syndrome; pp. 13–19. [DOI] [Google Scholar]
- 44.Theofilou P. Quality of life: definition and measurement. Eur. J. Psychol. 2013:9. doi: 10.5964/ejop.v9i1.337. [DOI] [Google Scholar]
- 45.Camarri B., Eastwood P.R., Cecins N.M., Thompson P.J., Jenkins S. Six minute walk distance in healthy subjects aged 55–75 years. J. Respir. Med. 2006;100:658–665. doi: 10.1016/j.rmed.2005.08.003. 10.1016/j.rmed. [DOI] [PubMed] [Google Scholar]
- 46.Townsend L., Dowds J., O’Brien K., Sheill G., Dyer A.H., O’Kelly B., et al. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann. Am. Thorac. Soc. 2021 doi: 10.1513/AnnalsATS.202009-1175OC. https://doi.10.1513/AnnalsATS.202009-1175OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan K., Zheng J., Mok Y., Li Y., LIU Y.N., Chu C., et al. SARS: prognosis, outcome and sequelae. Respirology. 2003;8:S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau H.M.-C., Lee E.W.-C., Wong C.N.-C., Ng G.Y.-F., Jones A.Y.-M., Hui D.S.-C., et al. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch. Phys. Med. Rehabil. 2005;86(6):1134–1140. doi: 10.1016/j.apmr.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Aerde N., Van den Berghe G., Wilmer A., Gosselink R., Hermans G. Intensive care unit acquired muscle weakness in COVID-19 patients. J. Intensive Care Med. 2020;46:2083–2085. doi: 10.1007/s00134-020-06244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paneroni M., Simonelli C., Saleri M., Bertacchini L., Venturelli M., Troosters T., et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am. J. Phys. Med. Rehabil. 2021;100:105–109. doi: 10.1097/PHM0000000000001641. [DOI] [PubMed] [Google Scholar]
- 51.Schweickert W.D., Hall J.J.C. ICU-acquired weakness. Chest. 2007;131:1541–1549. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 52.Jolley S.E., Bunnell A.E., Hough C.L.J.C. ICU-acquired weakness. Chest. 2016;150:1129–1140. doi: 10.1016/j.chest.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tripathy S., Acharya S.P., Singh S., Patra S., Mishra B.R. Kar NJBp. Post traumatic stress symptoms, anxiety, and depression in patients after intensive care unit discharge–a longitudinal cohort study from a LMIC tertiary care Centre. BMC Psychiatry. 2020;20:1–11. doi: 10.1186/s12888-020-02632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C., Chudzicka-Czupała A., Tee M.L., Núñez M.I.L., Tripp C., Fardin M.A., et al. A chain mediation model on COVID-19 symptoms and mental health outcomes in Americans, Asians and Europeans. J. Sci. Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-85943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao F., Tam W., Hu X., Tan W., Jiang L., Jiang X., et al. A quantitative and qualitative study on the neuropsychiatric sequelae of acutely ill COVID-19 inpatients in isolation facilities. J. Transl. Psychiatry. 2020;10(1):1–14. doi: 10.1038/s41398-020-01039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNally R.J., Robinaugh D.J., Wu G.W., Wang L., Deserno M.K., Borsboom D. Mental disorders as causal systems: a network approach to posttraumatic stress disorder. Clin. Psychol. Sci. 2015;3:836–849. doi: 10.1177/2167702614553230. [DOI] [Google Scholar]
- 58.Sandanger I., Nygård J.F., Sørensen T., Moum T. Is women’s mental health more susceptible than men’s to the influence of surrounding stress? Soc. Psychiatry Psychiatr. Epidemiol. 2004;39:177–184. doi: 10.1007/s00127-004-0728-6. [DOI] [PubMed] [Google Scholar]
- 59.Singleton N., Lewis G.J.S.O. 2003. Better Or Worse: A Longitudinal Study of the Mental Health of Adults Living in Private Households in Great Britain: Report Based on Surveys Carried Out by the Office for National Statistics in 2000 and 2001 for the Department of Health and the Scottish Executive Health Department. [Google Scholar]
- 60.Liu D., Baumeister R.F., Veilleux J.C., Chen C., Liu W., Yue Y., et al. Risk factors associated with mental illness in hospital discharged patients infected with COVID-19 in Wuhan, China. J. Psychiatr. Res. 2020;292:113297. doi: 10.1016/j.psychres.2020.113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wade D., Hardy R., Howell D., Mythen M. Identifying clinical and acute psychological risk factors for PTSD after critical care: a systematic review. Minerva Anestesiol. 2013;79:944–963. doi: 10.1177/2167702614553230. [DOI] [PubMed] [Google Scholar]
- 62.Hatch R., Young D., Barber V., Griffiths J., Harrison D.A. Watkinson PJCc. Anxiety, depression and post traumatic stress disorder after critical illness: a UK-wide prospective cohort study. Crit. Care. 2018;22:310. doi: 10.1186/s13054-018-2223-6. 10.1186/s13054-018-2223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hickie I., Davenport T., Wakefield D., Vollmer-Conna U., Cameron B., Vernon S.D., et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575. doi: 10.1007/s11065-005-3588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jason L.A., Corradi K., Torres-Harding S., Taylor R.R., King C. Chronic fatigue syndrome: the need for subtypes. J. Neuropsychol. Rev. 2005;15:29–58. doi: 10.1007/s11065-005-3588-2. [DOI] [PubMed] [Google Scholar]
- 65.Castell B.D., Kazantzis N., Moss-Morris R.E. Cognitive behavioral therapy and graded exercise for chronic fatigue syndrome: a meta-analysis. Clin. Psychol. Sci. Pract. 2011;18(4):311–324. doi: 10.1111/j.1468-2850.2011.01262.x. [DOI] [Google Scholar]
- 66.Zhang M., Ho R.J.T. Moodle: the cost effective solution for internet cognitive behavioral therapy (I-CBT) interventions. Technol. Health Care. 2017;25(1):163–165. doi: 10.3233/THC-161261. [DOI] [PubMed] [Google Scholar]
- 67.Salawu A., Green A., Crooks M.G., Brixey N., Ross D.H., Sivan M. A proposal for multidisciplinary tele-rehabilitation in the assessment and rehabilitation of COVID-19 survivors. Int. J. Environ. Res. Public Health. 2020;17(13):4890. doi: 10.3390/ijerph17134890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho C.S., Chee C.Y., Ho R.C. Mental health strategies to combat the psychological impact of COVID-19 beyond paranoia and panic. J. Ann. Acad. Med. Singapore. 2020;49(1):1–3. doi: 10.47102/annals-acadmedsg.202043. [DOI] [PubMed] [Google Scholar]
- 69.Prvu Bettger J., Resnik L.J. Telerehabilitation in the age of COVID-19: an opportunity for learning health system research. J. Phys. Therapy. 2020;100(11):1913–1916. doi: 10.1093/ptj/pzaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soh H.L., Ho R.C., Ho C.S., Tam W.W. Efficacy of digital cognitive behavioural therapy for insomnia: a meta-analysis of randomised controlled trials. J. Sleep Med. 2020;75:315–325. doi: 10.1016/j.sleep.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 71.Vugts M.A., Joosen M.C., van der Geer J.E., Zedlitz A.M., Vrijhoef H.J. The effectiveness of various computer-based interventions for patients with chronic pain or functional somatic syndromes: a systematic review and meta-analysis. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0196467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Landi F., Carfì A., Benvenuto F., Brandi V., Ciciarello F., Monaco M.R.L., et al. Predictive factors for a new positive nasopharyngeal swab among patients recovered from covid-19. Am. J. Prev. 2021;60:13–19. doi: 10.1016/j.amepre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du H.-W., Chen J.-N., Pan X.-b., Chen X.-L., Fang S.-F., Li X.-Q., et al. Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients. Eur. J. Clin. Microbiol. Infect. Dis. 2020:1–5. doi: 10.1007/s10096-020-04024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ismael F., Bizario J.C., Battagin T., Zaramella B., Leal F.E., Torales J., et al. Post-infection depression, anxiety and PTSD: a retrospective cohort study with mild COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.08.25.20182113. [DOI] [Google Scholar]
- 75.Weerahandi H., Hochman K.A., Simon E., Blaum C., Chodosh J., Duan E., et al. Post-discharge health status and symptoms in patients with severe COVID-19. J. Gen. Intern. Med. 2020:1–8. doi: 10.1101/2020.08.11.20172742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang M.C., Park D. Incidence of post-traumatic stress disorder after coronavirus disease. Healthcare. 2020;8(4):373. doi: 10.3390/healthcare8040373. 2020.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu J., Chen X., Yao S., Liu R. Anxiety persists after recovery from acquired COVID-19 in anaesthesiologists. J. Clin. Anesth. 2020;67:109984. doi: 10.1016/j.jclinane.2020.109984. [DOI] [PMC free article] [PubMed] [Google Scholar]