Abstract
Coronavirus pandemic has emerged as an extraordinary healthcare crisis in modern times. The SARS-CoV-2 novel coronavirus has high transmission rate, is more aggressive and virulent in comparison to previously known coronaviruses. It primarily attacks the respiratory system by inducing cytokine storm that causes systemic inflammation and pulmonary fibrosis. Decorin is a pluripotent molecule belonging to a leucine rich proteoglycan group that exerts critical role in extracellular matrix (ECM) assembly and regulates cell growth, adhesion, proliferation, inflammation, and fibrogenesis. Interestingly, decorin has potent anti-inflammatory, cytokine inhibitory, and anti-fibrillogenesis effects which make it a potential drug candidate against the COVID-19 related complications especially in the context of lung fibrosis. Herein, we postulate that owing to its distinctive pharmacological actions and immunomodulatory effect, decorin can be a promising preclinical therapeutic agent for the therapy of COVID-19.
Keywords: Decorin, COVID-19, Lung injury, Fibrosis, Immunomodulation
Introduction
The SARS-CoV-2 (severe acute respiratory syndrome corona virus 2) infection has swiftly advanced from its origin in China to the remaining world [1]. Coronaviruses have an enveloped architecture and a genome of size ranging from 26 to 31.7 kb. The shape of coronaviruses is characterized by crown like projections of spike protein on its outer surface. They can attain either a spherical or pleomorphic overall shape with a diameter of 80 to 120 nm. The clinical manifestations of SARS CoV-2 are very distinct, varying from asymptomatic stage to severe respiratory syndrome and dysfunctioning of multiple organs. The most common clinical symptoms include cough, fatigue, headache, breathlessness, sore throat, myalgia, and fever [2], [3], [4]. In some of the patients, after the end of one week, infection can progress into dyspnea, respiratory failure, pneumonia, and death. These modifications in severe stages are linked with cytokine storm which is characterized by excessive increase in inflammatory cytokines such as interleukin (IL)-7, IL-10, IL-2, tumor necrosis factor alpha (TNF-α), granulocyte-colony stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP1), and macrophage inflammatory protein 1 alpha (MIP1A). The present outbreak of SARS-CoV-2 has challenged the healthcare, public, and economic infrastructure of the whole world [5], [6]. Currently, there is no effective therapeutic regimen for this disease. Therefore, it is the need of the hour to identify new candidate therapeutics for combating the COVID-19.
Decorin is an extracellular matrix (ECM) protein which is a part of the small leucine-rich proteoglycan family which was cloned in 1986 and found to be an important structural protein of the ECM. It is extensively present in the body and plays diverse roles in epithelial cells and the stroma. It has been reported that the growth hormone can significantly enhance the concentration of circulating decorin in a gender dimorphic manner i.e., the increased effect is more in men as compared to women [7]. Additionally, a marked increase in the concentration of decorin has been observed upon exposure to high glucose at both transcriptional and translational levels in human mesangial cells [8]. Pearson et al., also observed the enhanced concentration of decorin by retinoic acid at protein and mRNA level in bovine articular chondrocytes. Retinoic acid dose dependently increases the concentration of decorin mRNA. However, the possible mechanism behind this accumulating mRNA is under investigation [9]. Principally, decorin was identified as a useful collagen-binding partner and to regulate key biomechanical factors of tissue architecture in muscle, skin, cornea, lungs, and tendon [10]. The involvement of decorin is not restricted to matrix structural proteins and it also affects a diverse variety of biological functions like cell growth, adhesion, migration, proliferation, and differentiation. Additionally, it modulates the process of inflammation and fibrillogenesis [11]. In numerous pre-clinical studies, decorin has been reported to play a protective role against inflammation, cancer, and fibrosis [12]. It has been reported that decorin is an effective moiety for reducing transforming growth factor beta (TGF-β) bioavailability as it neutralizes and represses TGF-β activity by formation of complexes with TGF-β leading to reduction in fibrotic scar. Decorin effectively impedes the TGF-β RI/II activation followed by signaling via Smad2, Smad3, and the extracellular-signal regulated kinase (Erk) proteins and also diminishes the inflammatory cytokine signaling by modulating the mitogen-activated protein kinases (MAPK) and nuclear factor kappa light chain enhancer of activated B cells (NFκB) activity. Moreover, decorin binds to the endogenous ligands of toll like receptor 2/4 (TLR2/4) in the tumor stroma in order to repress the inflammation and tumor growth [12], [13], [14], [15], [16]. In conclusion, we propose that decorin could be a potent therapeutic agent against COVID-19 mediated pulmonary fibrosis and systemic inflammation.
Hypothesis
Decorin plays role in the regulation of inflammation and fibrosis. It is known that decorin showed potent anti-inflammatory and anti-fibrotic properties by modulating MAPKinase activity and inhibiting the excess synthesis of inflammatory cytokines. Moreover, it strengthens the immune system and improves the oxidative stress mediated conditions. Based on these preclinical evidence, we hypothesized that administering appropriate formulation of decorin in the form of aerosol or nanoparticles may potentially abrogate the COVID-19 related complications.
Justification of hypothesis
Decorin can abrogate lung fibrosis mediated by COVID-19
Imbalance in the process of generation and degradation of ECM proteins results in the development of fibrosis in any organ [17]. Decorin is a dermatan sulfate proteoglycan which is secreted by human peritoneal mesothelial cells (HPMC), and acts as an important biological tool for cell growth and ECM assembly along with various pathological conditions such as cancer and fibrotic disorders [18]. Several salient chemotactic factors such as TNF-α and TGF-β1 have been found to be culpable for stimulating and modulating these fibrotic responses and may also play a climacteric role in controlling the expression of decorin [18]. Additionally, it is implicated in reversing cardiac remodeling by promptly impeding the collagen synthesis and fibrotic effects incited by TGF-β. Decorin wields its defensive upshot against fibrosis by regulating the collagen fibrillogenesis along with hampering the biological activity of TGF-β1 and the maturation process of collagen fibrils [19].
It is known to antagonize multiple types of receptor tyrosine kinases, such as the insulin-like growth factor receptor I (IGF-IR) and epidermal growth factor receptor (EGFR) [20]. Decorin protein sequesters all isoforms of TGF-β1 and confines it in the ECM before its interaction with the cell surface. In addition, it also binds and neutralizes another TGF-β molecule that can instigate fibrosis inside the body tissues [21]. It transacts with a vast array of signaling molecules involved in cancer progression. Moreover, decorin prompts the obstruction of platelet derived growth factor (PDGF) over the target cell surface that leads to the attenuation of cancer cell migration. One of the proteins from ECM, periostin is expressed by a large number of cancerous cells, gets coupled with decorin and neutralizes it [22]. Jiang et al., showed the effect of decorin and the mechanism of pro-fibrotic cellular response in HPMC. They showed that the deposition of fibronectin is repressed by decorin when HPMC were unveiled to peritoneal dialysis fluid. This effect was carried out by the downregulation of TGF-β1 and enhanced phosphorylation of glycogen synthase kinase 3 beta (GSK-3β) [23].
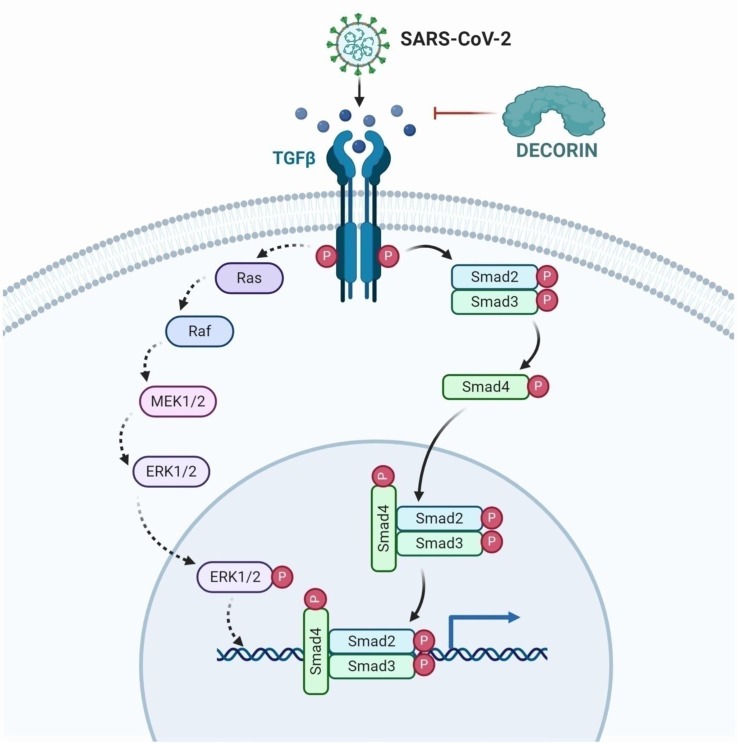
Decorin is also known as “the guardian from the matrix” due to its copious ECM protein binding partners along with regulation of cell growth and differentiation. Neill et al., stated that the binding of decorin molecule with TLR-2 and TLR-4 leads to escalated generation of the pro-inflammatory protein programmed cell death 4 (PDCD4) and hence, simultaneously fostering decrement in the synthesis of anti-inflammatory cytokines (IL-10) [24]. This induces the synthesis of various other pro-inflammatory modulators leading to the suppression of tumors and fibrotic growth [25]. Based on these evidence, targeting decorin could significantly reverse the lung fibrosis associated with COVID-19 (Fig. 1 ).
Fig. 1.
TGF-β triggers the downstream activation of important signaling events of rat sarcoma (RAS) which are involved in the cooperation with the TGF-β/Smad family including the RAS-activated factor (RAF)/mitogen activated protein kinase (MAPK), extracellular regulated kinase (ERK) kinase (MEK)/ERK pathway (RAS-RAF-MEK-ERK signaling). Moreover, TGF-β phosphorylation activates the Smad2, Smad3, and Smad4 signaling cascade involved in the fibrotic process. SARS-CoV-2 stimulates the excess activation of TGF-β which in turn triggers the process of fibrosis. Decorin can effectively inhibit TGF-β, thereby sequestering the whole signaling pathway resulting in the reduction of fibrotic scar. The figure was created with BioRender.com.
Decorin against inflammation and reactive oxygen species
In COVID-19 patients, disparity between the generation of reactive oxygen species (ROS) and their obliteration from the tissues causes oxidative stress inside the body resulting into chronic inflammation [26], [27]. ROS are cardinal in maintaining the cellular homeostasis, receptor activation, and signal transduction when produced in a bound quantity [28]. In COVID-19 patients, an increase in oxidative stress can stimulate various transcription factors that can lead to differential expression of some genes which participate in different inflammatory pathways [29]. Additionally, the expression of vascular endothelium growth factor (VEGF) is enhanced which is considered as the fundamental growth factor that helps in the transportation of various inflammatory cells and macromolecules from the systemic circulation into the tissues [30]. Further, an upsurge in the generation of ROS by polymorphonuclear neutrophils (PMN) can catalyze tissue damage and endothelial dysfunction at the site of inflammation [31], [32]. This gives rise to the opening of inter-endothelial junctions and aids in the migration of various inflammatory cells which assists in the clearance of foreign particles and pathogens from the tissue but also leads to tissue injury. Here, the small leucine rich proteoglycan (SLRP), decorin plays a vital role in the regulation of these cellular pathways involved in the process of inflammation [33].
Decorin helps in the perpetuation of the structural cohesion of ECM and regulates cellular processes by modifying downstream signaling pathways. It interacts with toll like receptors (TLR2/4) present on macrophage surface with exorbitant analogy that simultaneously results into the activation of MAPK along with stimulation of NFκB signaling pathway. As a result, there is an increase in the secretion of various inflammatory mediators like TNF-α, IL-10, and IL-12p70 [34], [35].
Moreover, it intercepts translational subjugation of PDCD4 which ultimately makes the cytokine profile more proinflammatory. Neill et al., stated that decorin null mice with delayed type hypersensitivity showed the role of decorin in activating an enhanced pro-inflammatory environment. Furthermore, lower quantities of decorin associates with low TNF-α levels whereas more leukocyte adhesion molecules show increment in adherence of leukocytes to the endothelium [36], [37]. This engenders inflammatory environs in the tissue microdomain which indicates the efficacy of decorin in inflammation. Therefore, therapeutic intervention by targeting decorin in COVID-19 patients might be a plausible approach.
Decorin as an immunomodulator
Patients having immuno-compromised conditions or other diseases are more vulnerable to COVID-19 complications, specifically the elder people [38]. So, boosting the immune system by pharmacological agents might be of significance in this case. ECM is a heterogeneous and convoluted network of structural and functional molecules surrounding the cells [39]. Decorin is present in the ECM that has the competence to bind with various cells [12]. It is highly expressed in adult heart valves and possesses immunomodulatory potential. Decorin is used in immunotherapeutics that attunes inflammation by restraining VEGF, TGF-β, and EGFR [40]. Hill et al., demonstrated the immunomodulatory effects of decorin for the treatment of glioblastoma multiforme. In this experiment, intracerebral injections of decorin were employed to investigate the microglial response and local immune responses. Rat models were administered with these injections for a week after which cerebrospinal fluid (CSF) samples were collected to ascertain the presence of any change in inflammatory markers or the presence of decorin in samples. It was observed that there were no symptoms of any inflammatory reaction or any cavity generation in the brain. It had also subdued the activity of microglial response when direct injections were given into the brain. As a result, a safe toxicological and pharmacokinetic profile of decorin was demonstrated because of the deprivation of any dose limiting toxicity while its immunomodulatory effects were maintained in the model [41].
Decorin has a structure encompassing central domain of leucine rich repeats (LRR) along with cysteine rich domains at both ends. It acts as damage associated molecular pattern (DAMP) after releasing from the ECM followed by secretion from activated macrophages and other immune cells [42]. Their association with different pathogen recognition receptors harmonizes various signaling cascades that trigger an immune response and initialize pathogen mediated inflammation [43]. Hinderer et al., demonstrated human immune responses actuated with the help of generated human recombinant decorin. Numerous immune cells were analyzed by in vitro test settings for the study of cytokine secretion, and other immune cell features. Relative number of migrated cells were evaluated with the help of spontaneous migration value in the absence of decorin molecules. It was observed that a significant dose dependent migration of immune cells such as monocytes and PMN cells had transpired which upregulated CD206 receptor in the presence of decorin. Conclusively, it has been observed that ECM decorin not only inhibits proliferation but also protects macrophages from the induction of apoptosis inside a tissue [44], [45]. In conclusion, employing decorin in COVID-19 cases may be a plausible approach.
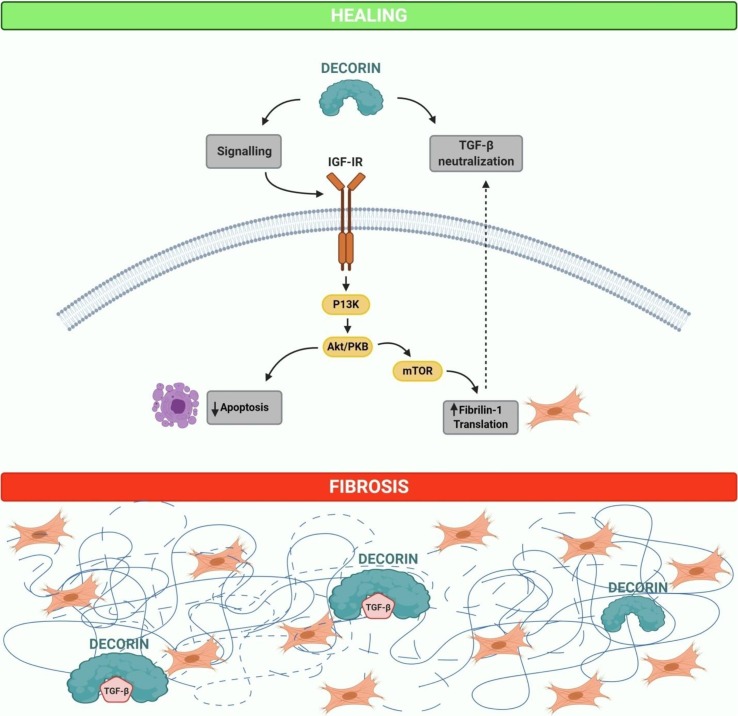
Decorin has a high affinity for IGF-IR and even nanomolar concentrations of decorin can downregulate the IGF-IR (Fig. 2 ) [46]. Addition of decorin leads to IGF-IR phosphorylation and activation, which is accompanied by receptor down-regulation [47]. Schaefer et al., also showed that in renal fibroblasts decorin binds to and induces phosphorylation of IGF-IR. Silencing of the IGF-I receptor tyrosine kinase and its downstream signaling molecule phosphoinositide-3 kinase inhibits fibrillin-1 synthesis which is mediated by decorin [48]. Zhang et al., demonstrated that decorin directly prevents the translation of fibrillin-1 by binding to IGF-IR and results in negative regulation of the pathway. The study concluded that decorin can be a potential anti-fibrotic molecule that displays pivotal effects in sequestering fibrogenesis through a number of distinctive pathways. Interestingly, Zhu et al., reported that decorin antagonizes the EGFR activity at the surface of tumor cells through the downregulation of tyrosine kinases. Decorin binds directly to EGFR receptor, promotes dimerization which leads to caveolin-mediated internalization and finally degradation in the lysosomes [49]. Furthermore, decorin also decreases the activity of the ErbB2 and ErbB4 receptors via degradation [25], [50]. In addition, decorin negatively regulates the hepatocyte growth factor receptor Met, vascular endothelial growth factor receptor 2 (VEGFR2) and platelet-derived growth factor receptor (PDGFR) [51], [52], [53]. Hence, decorin might be an efficacious therapeutic agent against COVID-19 associated pulmonary fibrosis (Fig. 2).
Fig. 2.
Role of soluble form of decorin in comparison with matrix-bound decorin in healing and fibrosis. Soluble decorin works as a signaling molecule of the insulin like growth factor I receptor (IGF-IR), hence, shielding epithelial cells against apoptosis or inducing the production of fibrillin-1 in fibroblasts. Furthermore, decorin is capable of neutralizing the activity of TGF-β directly or indirectly through the modulation of fibrillin-1. In the event of fibrosis, most of the decorin is bound to cellular matrix components, mainly to collagen type I, as a part of fibrotic scar and consequently is incapable to act as a signaling molecule. However, matrix-bound decorin is still capable to sequester TGF-β in the matrix, thus, withdrawing the cytokine from its cell membrane receptors. The figure was created with BioRender.com.
Possible formulations and targeting of decorin for COVID-19 treatment therapy
Dosage form is an important feature of any drug for effective pharmacotherapy. So, it is critical to develop a rational formulation to achieve superior therapeutic benefit. In this context, nanotechnology has opened novel avenues for the researchers and pharmaceutical sector [54]. Principally, COVID-19 attacks the lungs and renders them incompetent to function, so local and site specific targeted drug delivery to the lungs ought to be integrated as a restorative approach for desired pharmacological activity [55], [56], [57]. However, peptide delivery is associated with several challenges or limitations. As peptides/proteins are amphiphilic in nature and undergo aggregation and unfolding leading to protein instability [58]. Further, they are prone to enzymatic degradation and can be degraded before reaching the lungs [59]. To overcome these challenges, use of excipients in the formulation such as polyethylene glycol (PEG) could help in enhancing protein resistance and prevent proteolysis by proteases present in the lung mucosa. Additionally, the use of pH stabilizers, surfactants, encapsulation, aerosol or a nanoformulation can help in target specific delivery [60], [61].
To present this rationale, we propose that aerosol based mouth spray dosage form may be a viable approach as it is patient compliant and provides quick relief. Aerosol based delivery of decorin specifically to the lungs could provide better effect against SARS-CoV-2. Decorin based formulation may also be administered by i.v. route which can achieve 100% bioavailability. Moreover, decorin can be used as an adjuvant treatment in combination with other drugs, which can serve as a synergistic approach in COVID-19 therapy.
Implications of the hypothesis
We present pre-clinical evidence for COVID-19 therapy by employing decorin. This hypothesis is based on the rationale that decorin may halt the progression of cytokine storm, and modulate the epithelial-to-mesenchymal transition (EMT) signaling. Pre-clinical studies revealed that decorin may improve the pulmonary functions during COVID-19 related complications including lung fibrosis. Thus, we envision that our hypothesis may stimulate future research in the area of COVID-19 and the potential benefit of using decorin.
CRediT authorship contribution statement
Prince Allawadhi: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing. Vishakha Singh: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing. Isha Khurana: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing. Pushkar Singh Rawat: Methodology, Writing - review & editing. Akshata Patangrao Renushe: Methodology, Writing - review & editing. Amit Khurana: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing. Umashanker Navik: Methodology, Writing - review & editing. Sachin Allwadhi: Methodology, Writing - review & editing. Satish Kumar Karlapudi: Methodology, Writing - review & editing. Anil Kumar Banothu: Methodology, Writing - review & editing. Kala Kumar Bharani: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Perlman S. Another Decade, Another Coronavirus. N Engl J Med. 2020;382:760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.-J., Ni Z.-Y., Hu Y., et al. Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2019;382(2020):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G., Fan Y., Lai Y., et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh V., Allawadhi P., Khurana A., Banothu A.K., Bharani K.K. Critical neurological features of COVID-19: Role of imaging methods and biosensors for effective diagnosis. Sensors International. 2021 doi: 10.1016/j.sintl.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allawadhi P., Khurana A., Allwadhi S., Joshi K., Packirisamy G., Bharani K.K. Nanoceria as a possible agent for the management of COVID-19. Nano Today. 2020;35 doi: 10.1016/j.nantod.2020.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allawadhi P., Khurana A., Allwadhi S., et al. Potential of electric stimulation for the management of COVID-19. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahl N., Stone G., McLean M., Ho K.K., Birzniece V. Decorin, a growth hormone-regulated protein in humans. Eur J Endocrinol. 2018;178:145–152. doi: 10.1530/EJE-17-0844. [DOI] [PubMed] [Google Scholar]
- 8.Wahab N.A., Parker S., Sraer J.-D., Mason R.M. The decorin high glucose response element and mechanism of its activation in human mesangial cells. J Am Soc Nephrol. 2000;11:1607–1619. doi: 10.1681/ASN.V1191607. [DOI] [PubMed] [Google Scholar]
- 9.Pearson D., Sasse J. Differential regulation of biglycan and decorin by retinoic acid in bovine chondrocytes. J Biol Chem. 1992;267:25364–25370. [PubMed] [Google Scholar]
- 10.Chen S., Young M.F., Chakravarti S., Birk D.E. Interclass small leucine-rich repeat proteoglycan interactions regulate collagen fibrillogenesis and corneal stromal assembly. Matrix biology : journal of the International Society for Matrix Biology. 2014;35:103–111. doi: 10.1016/j.matbio.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson P.S., Huang T.F., Kazam E., Iozzo R.V., Birk D.E., Soslowsky L.J. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W., Ge Y., Cheng Q., Zhang Q., Fang L., Zheng J. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget. 2018;9:5480–5491. doi: 10.18632/oncotarget.23869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y., Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988;336:244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- 15.Border W.A., Noble N.A., Yamamoto T., et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi Y., Mann D.M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 17.Khurana Amit, Sayed Nilofer, Allawadhi Prince, Weiskirchen Ralf. It’s all about the spaces between cells: role of extracellular matrix in liver fibrosis. Annals of Translational Medicine. 2021;9(8):728. doi: 10.21037/atm-20-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaka Y., Brees D.K., Ikegaya K., et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 19.Kolb M., Margetts P.J., Sime P.J., Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-β-mediated fibrotic responses in the lung. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2001;280:L1327–L1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 20.Kehlet S.N., Bager C., Willumsen N., et al. Cathepsin-S degraded decorin are elevated in fibrotic lung disorders–development and biological validation of a new serum biomarker. BMC pulmonary medicine. 2017;17:1–10. doi: 10.1186/s12890-017-0455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolb M., Margetts P.J., Galt T., et al. Transient Transgene Expression of Decorin in the Lung Reduces the Fibrotic Response to Bleomycin. Am J Respir Crit Care Med. 2001;163:770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- 22.Giri S.N., Hyde D.M., Braun R.K., Gaarde W., Harper J.R., Pierschbacher M.D. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997;54:1205–1216. doi: 10.1016/s0006-2952(97)00343-2. [DOI] [PubMed] [Google Scholar]
- 23.Jiang N., Zhang Q., Chau M.K.M., et al. Anti-fibrotic effect of decorin in peritoneal dialysis and PD-associated peritonitis. EBioMedicine. 2020;52 doi: 10.1016/j.ebiom.2020.102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neill T., Schaefer L., Iozzo R.V. Decorin as a multivalent therapeutic agent against cancer. Adv Drug Deliv Rev. 2016;97:174–185. doi: 10.1016/j.addr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bi X.L., Yang W. Biological functions of decorin in cancer. Chinese journal of cancer. 2013;32:266–269. doi: 10.5732/cjc.012.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khurana A., Anchi P., Allawadhi P., et al. Superoxide dismutase mimetic nanoceria restrains cerulein induced acute pancreatitis. Nanomedicine (London, England) 2019;14:1805–1825. doi: 10.2217/nnm-2018-0318. [DOI] [PubMed] [Google Scholar]
- 27.Khurana A., Anchi P., Allawadhi P., et al. Yttrium oxide nanoparticles reduce the severity of acute pancreatitis caused by cerulein hyperstimulation, Nanomedicine: Nanotechnology. Biology and Medicine. 2019;18:54–65. doi: 10.1016/j.nano.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Advances in Biological Regulation. 2020;77 doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ntyonga-Pono M.P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? The Pan African medical journal. 2020;35:12. doi: 10.11604/pamj.2020.35.2.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarma J.V., Ward P.A. In: Encyclopedia of Medical Immunology: Autoimmune Diseases. Mackay I.R., Rose N.R., Diamond B., Davidson A., editors. Springer; New York, New York, NY: 2014. Neutrophils in Endothelial Damage; pp. 777–784. [Google Scholar]
- 32.Allawadhi P., Khurana A., Sayed N., Kumari P., Godugu C. Isoproterenol-induced cardiac ischemia and fibrosis: Plant-based approaches for intervention. Phytother Res. 2018;32:1908–1932. doi: 10.1002/ptr.6152. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer L., Tredup C., Gubbiotti M.A., Iozzo R.V. Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. Febs j. 2017;284:10–26. doi: 10.1111/febs.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neill T., Schaefer L., Iozzo R.V. Decorin: a guardian from the matrix. The American journal of pathology. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi J.-H., Sun S.-C. Tumor Necrosis Factor Receptor-Associated Factor Regulation of Nuclear Factor κB and Mitogen-Activated Protein Kinase Pathways. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidler D.G., Mohamed N.A., Bocian C., et al. The role for decorin in delayed-type hypersensitivity. J Immunol. 2011;187:6108–6119. doi: 10.4049/jimmunol.1100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neill T., Schaefer L., Iozzo R.V. Oncosuppressive functions of decorin. Molecular & cellular oncology. 2015;2 doi: 10.4161/23723556.2014.975645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vessey Judith A., Betz Cecily L. Everything Old is New again: COVID-19 and Public Health. J Pediatr Nurs. 2020;52:A7–A8. doi: 10.1016/j.pedn.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alkhouli N., Mansfield J., Green E., et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. American Journal of Physiology-Endocrinology and Metabolism. 2013;305:E1427–E1435. doi: 10.1152/ajpendo.00111.2013. [DOI] [PubMed] [Google Scholar]
- 40.Gubbiotti M.A., Vallet S.D., Ricard-Blum S., Iozzo R.V. Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biology : Journal of the International Society for Matrix Biology. 2016;55:7–21. doi: 10.1016/j.matbio.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill L.J., Moakes R.J.A., Vareechon C., et al. Sustained release of decorin to the surface of the eye enables scarless corneal regeneration. NPJ Regen Med. 2018;3:23. doi: 10.1038/s41536-018-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreth K., Iozzo R.V., Schaefer L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell cycle (Georgetown, Tex.) 2012;11:2084–2091. doi: 10.4161/cc.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mogensen Trine H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinderer S., Sudrow K., Schneider M., et al. Surface functionalization of electrospun scaffolds using recombinant human decorin attracts circulating endothelial progenitor cells. Sci Rep. 2018;8:110. doi: 10.1038/s41598-017-18382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinderer S., Schesny M., Bayrak A., et al. Engineering of fibrillar decorin matrices for a tissue-engineered trachea. Biomaterials. 2012;33:5259–5266. doi: 10.1016/j.biomaterials.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 46.Schönherr E., Sunderkötter C., Iozzo R.V., Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 47.Iozzo R.V., Buraschi S., Genua M., et al. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem. 2011;286:34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer L., Tsalastra W., Babelova A., et al. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and Mammalian target of rapamycin. The American journal of pathology. 2007;170:301–315. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J.X., Goldoni S., Bix G., et al. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 50.Reed C.C., Waterhouse A., Kirby S., et al. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 51.Goldoni S., Humphries A., Nyström A., et al. Decorin is a novel antagonistic ligand of the Met receptor. The Journal of cell biology. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan G.A., Girish G.V., Lala N., Di Guglielmo G.M., Lala P.K. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast, Molecular endocrinology (Baltimore. Md.) 2011;25:1431–1443. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baghy K., Horváth Z., Regős E., et al. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. The FEBS journal. 2013;280:2150–2164. doi: 10.1111/febs.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khurana A., Tekula S., Saifi M.A., Venkatesh P., Godugu C. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 55.Mansour H.M., Hickey A.J. Raman characterization and chemical imaging of biocolloidal self-assemblies, drug delivery systems, and pulmonary inhalation aerosols: A review. AAPS PharmSciTech. 2007;8:140. doi: 10.1208/pt0803064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farokhzad O.C., Langer R. Impact of Nanotechnology on Drug Delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 57.Khurana I., Allawadhi P., Khurana A., et al. Can bilirubin nanomedicine become a hope for the management of COVID-19? Med Hypotheses. 2021;149 doi: 10.1016/j.mehy.2021.110534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otvos L., Jr, Wade J.D. Current challenges in peptide-based drug discovery. Front Chem. 2014;2:62. doi: 10.3389/fchem.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruge C.A., Kirch J., Lehr C.-M. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers—therapeutic possibilities and technological challenges. The lancet Respiratory medicine. 2013;1:402–413. doi: 10.1016/S2213-2600(13)70072-9. [DOI] [PubMed] [Google Scholar]
- 60.Ho D.-K., Nichols B.L., Edgar K.J., Murgia X., Loretz B., Lehr C.-M. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur J Pharm Biopharm. 2019;144:110–124. doi: 10.1016/j.ejpb.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Khurana A., Allawadhi P., Khurana I., et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021 doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]