Abstract
Introduction Neurosurgical anatomy is traditionally taught via anatomic and operative atlases; however, these resources present the skull base using views that emphasize three-dimensional (3D) relationships rather than operative perspectives, and are frequently written above a typical resident's understanding. Our objective is to describe, step-by-step, a retrosigmoid approach dissection, in a way that is educationally valuable for trainees at numerous levels.
Methods Six sides of three formalin-fixed latex-injected specimens were dissected under microscopic magnification. A retrosigmoid was performed by each of three neurosurgery residents, under supervision by the senior authors (C.L.W.D. and M.J.L.) and a graduated skull base fellow, neurosurgeon, and neuroanatomist (M.P.C.). Dissections were supplemented with representative case applications.
Results The retrosigmoid craniotomy (aka lateral suboccipital approach) affords excellent access to cranial nerve (CN) IV to XII, with corresponding applicability to numerous posterior fossa operations. Key steps include positioning and skin incision, scalp and muscle flaps, burr hole and parasigmoid trough, craniotomy flap elevation, initial durotomy and deep cistern access, completion durotomy, and final exposure.
Conclusion The retrosigmoid craniotomy is a workhorse skull base exposure, particularly for lesions located predominantly in the cerebellopontine angle. Operatively oriented neuroanatomy dissections provide trainees with a critical foundation for learning this fundamental skull base technique. We outline a comprehensive approach for neurosurgery residents to develop their familiarity with the retrosigmoid craniotomy in the cadaver laboratory in a way that simultaneously informs rapid learning in the operating room, and an understanding of its potential for wide clinical application to skull base diseases.
Keywords: retrosigmoid, vestibular schwannoma, acoustic neuroma, meningioma, skull base, education, simulation
Introduction
The retrosigmoid craniotomy (aka lateral suboccipital or retromastoid approach) is a foundational exposure in skull base surgery, and among the most important for skull base trainees to master during their core clinical training. A flexible and efficient exposure, the retrosigmoid is the preferred posterior fossa approach for many skull base operations involving both routine and complex pathologies. This is particularly true if the lesion resides predominantly in the cerebellopontine angle (CPA), or arises from the lateral brainstem or internal auditory canal (IAC) in patients with useful hearing. Additionally, allows ready access to the petroclival junction and the foramen magnum.
In its most common iteration, the retrosigmoid craniotomy is anchored at the transverse-sigmoid junction, from which it extends inferiorly along the sigmoid and posteriorly along the transverse sinus, which constitute the superficial limits of the exposure, along with the adjacent petrous temporal bone and tentorium cerebelli within the intradural compartment. As the bony removal is predominantly suboccipital, the retrosigmoid eliminates the need for potentially time-consuming temporal bone drilling, while still providing a generous working corridor into the posterior fossa.
Although the retrosigmoid approach has been described in several preceding atlases, none has been optimized for the education of junior level residents via a dissection-based neuroanatomic model. Some works have relied on illustrations and diagrams (e.g., Meyer, 1 , Tew et al 2 ). Still others have incorporated neuroanatomic cadaver studies, yet they've employed radical dissections that demonstrate three-dimensional (3D) relationships rather than stepwise operative approaches (e.g., Rhoton 3 ). Frequently, these dissections are also described in language more suitable for a more experience audience that is well versed in skull base techniques (e.g., Wanibuchi et al 4 ), as opposed to a relatively junior trainee. Accordingly, we set out to create a novel educational resource using high-quality, cadaver-based, operatively oriented dissections that would serve two key purposes. First, we sought to provide a step-by-step walkthrough for laboratory dissection of a retrosigmoid craniotomy that parallels the operative experience. Second, we supplemented the core neuroanatomic guide with contextualizing details, example cases, and other insights that we anticipate will assist trainees at any level in not simply learning but in truly understanding the retrosigmoid approach.
Methods
All aspects of this study were approved by our institutional review board and biospecimens committee, as required by standard protocols. Three specimens were formalin-fixed and latex-injected using a six-vessel technique by the study staff, as we have described. 5 Six unilateral exposures were then completed under microscopic magnification, two each by the three dissecting authors with limited neuroanatomical dissection experience at training levels post graduate year (PGY)-3 (LPC), PGY-5 (CSG), and PGY-6 (AP) at time of study. Supervision was provided by the senior authors (C.L.W.D. and M.J.L.) and a graduated clinical skull base fellow neurosurgeon with advanced neuroanatomy experience (MPC). Retrosigmoid approaches were completed once for preparation and once for formal 3D photo documentation by the dissecting authors. Following successful dissection, representative cases were reviewed to emphasize salient principles of approach selection and planning.
Results: Step-by-Step Surgical Approach
Positioning and Incision Planning in the Operating Room
Following anesthesia induction, intubation, and preparation of cranial nerve (CN) monitoring, the patient is positioned lateral decubitus on an axillary roll in place to protect the contralateral brachial plexus. We extensively pad the contralateral hip and utilize a footboard to prevent any pressure palsies or inadvertent changes in positioning intraoperatively. Reverse Trendelenburg's positioning of approximately 10 to 15 degrees is then applied, the skull is placed in three-point pinion fixation. A modest degree of chin flexion and contralateral coronal plane extension are used to orient the superior sagittal sinus parallel to the floor while minimizing compression of the contralateral internal jugular vein (IJV).
With the patient successfully positioned, attention is turned to planning the surgical incision, which is oriented using an imaginary line projected from the inion to the root of the zygoma, approximating the location of the transverse sinus ( Fig. 1A ). The mastoid tip and digastric groove are used to approximate the locations of the sigmoid sinus and transverse-sigmoid junction, and a postauricular semilunar incision is drawn centered on the external auditory meatus (EAM), approximately 5-cm posterior to the digastric notch (6-cm posterior to the posterior aspect of the helix).
Fig. 1.
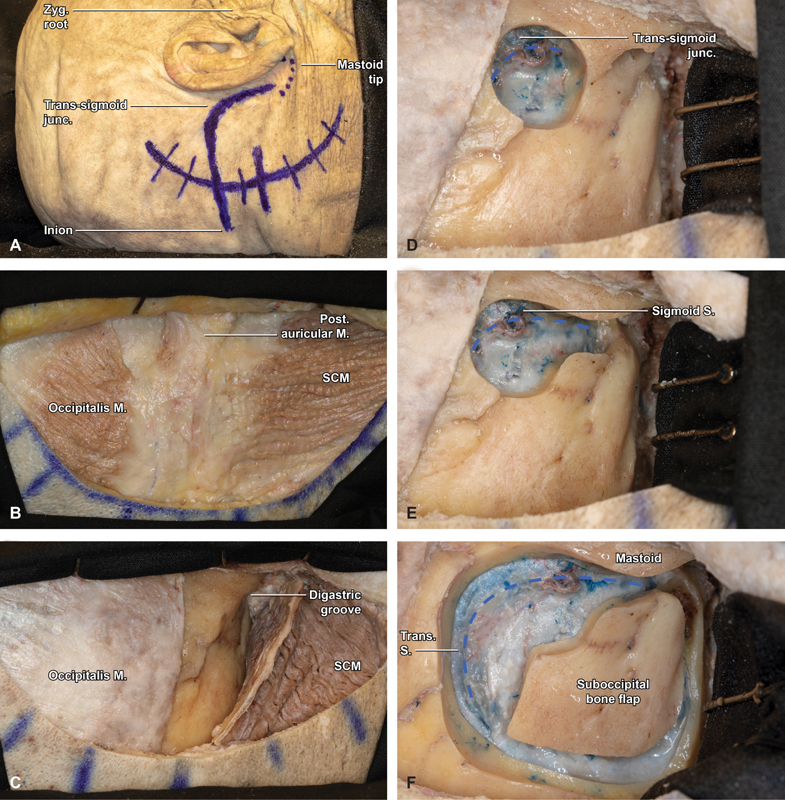
Step-by-step retrosigmoid craniotomy in an anatomical specimen (right side). ( A ) Marked skin incision approximately 5-cm posterior to the digastric notch (6-cm posterior to the helix). The transverse sinus is approximated by connecting an imaginary line from the inion to the zygomatic root, while the sigmoid follows the digastric groove. ( B ) With the postauricular scalp flap reflected anterior, the underlying musculature is visualized, including the occipitalis, posterior auricular, and sternocleidomastoid (SCM) muscles. ( C ) Three cuts are made in SCM roughly paralleling the digastric groove, SCM insertion, and medial margin of the skin incision. ( D ) With the SCM flap protected, reflected inferiorly, and secured with 2 fish hooks, a large burr hole is fashioned overlying the transverse sigmoid junction (blue hash). ( E ) Bone removal is carried from the burr hole inferiorly, tracing the course of the sigmoid sinus to the level of the mastoid tip (blue hash). ( F ) After carefully and extensively stripping the dura from the inner table of the occipital bone, a small, rectangular bone flap is turned with the spiral bit and footplate attachment, with the superior and lateral bony exposure revealing the margins of the transverse and sigmoid sinuses (blue hash). Junc, junction; M, muscle; Post, posterior; S, sinus; SCM, sternocleidomastoid; Trans, transverse; Zyg, zygomatic.
Scalp and Muscle Flaps
The skin is incised sharply and monopolar electocautery is used to dissect through the superficial soft tissue to the subfascial supramuscular plane. The scalp with the Galea, just above the musculature, is then elevated laterally along the full length of the incision until the mastoid is reached. Care must be taken to not inadvertently perforate the scalp that thins as the mastoid is approached. Similarly, the dissection cannot breach the skin of the external auditory meatus (EAM) which lies just beyond the anterior limit of the exposure ( Fig. 1B ). With the scalp flap elevated, the occipital belly of the occipitalis muscle is visualized superiorly, sternocleidomastoid and splenius capitis inferiorly, and posterior auricular muscle laterally. Monopolar cautery is again used to fashion a roughly rectangular, inferiorly pedicled, muscular flap down to the bone. The superior cut is made at the inferior margin of superior nuchal line, to provide a sturdy cuff for closure. The lateral cut traces the mastoid ridge from superior to inferior and stopping just proximal to the mastoid tip. Finally, the posterior cut follows the curvilinear margin of the scalp incision for approximately 3 to 4 cm, medial to the trapezius, but incising the splenius capitis and semispinalis ( Fig. 1C ). Care should be taken to identify and protect the occipital artery and greater occipital nerve that are typically found along the medial margin of the muscle flap. The occipital artery is covered by a deeper layer formed by the splenius capitis muscle and a more superficial layer formed, from lateral-to-medial, by the sternocleidomastoid and trapezius. The occipital artery pierces the fascia between the trapezius and sternocleidomastoid, near the superior nuchal line and ascends in the superficial fascia of the scalp, where it is accompanied by the greater occipital nerve. The lesser occipital nerve crosses superficial to the splenius capitis, being more lateral is more often to be encountered as the flaps are raised. The scalp and muscle flaps are each covered with antibiotic-soaked sponges and retracted using fish hooks, to minimize interference with intraoperative ergonomics.
Burr Holes, Sigmoid Trough, and Craniotomy Completion
The retrosigmoid craniotomy is initiated by fashioning a burr hole at the inferomedial angle of the transverse-sigmoid junction. The borders of both the transverse and medial sigmoid sinuses should be definitively appreciated prior to dissecting the posterior fossa dura from the inner calvarium of the skull and performing the overlying bony cuts ( Fig. 1D ). Although the asterion is widely reported to be a useful landmark in identifying the junction, in our experience and numerous preceding works—including those of Professor Rhoton—there is too much variability for it to constitute a reliable landmark. 6 7 8 We recommend using the inion-to-zygomatic-root technique detailed above to approximate the transverse sinus, and the digastric groove to approximate the medial limit of the sigmoid. This typically results in burr hole placement immediately superior to the superior aspect of the digastric groove where the mastoid bone begins to flatten.
With the junction safely identified, the high-speed drill is used again to extend a trough from superior to inferior, tracing the medial edge of the sigmoid sinus to the level of the mastoid tip ( Fig. 1E ). If the dura and sinus are adherent to the bone, we recommend consideration for egg-shelling the bone with the drill, followed by intermittent gentle dural dissection and Kerrison's 3 and 4 mm rongeurs to eliminate the final layer of bone overlying the sigmoid sinus.
A roughly rectangular craniotomy is then fashioned using the spiral bit and footplate attachment, with approximate final dimensions of 3-cm anteroposterior × 4-cm superoinferior ( Fig. 1F ). Additional dural dissection may be required to safely elevate the bone flap, while preserving the integrity of the underlying dura, so as to facilitate a secure closure and minimize postoperative cerebrospinal fluid (CSF) leaks. Once removed, the flap is placed in antibiotic-containing normal saline on the back table. It may be necessary to extend the margins of the craniotomy if the sinuses aren't fully exposed. This is best conducted using diamond drill bits and Kerrison's rongeurs of varying sizes, taking care to carefully release the venous sinus dura before attempting to remove the last bony fragments. The diamond drill bits also provide a useful tool to drill around and expose large emissary veins that can be later coagulated with bipolar and divided, as well as to limit venous ooze, from the medullary margins of the craniotomy. When the bone removal has been performed, all exposed edges and mastoid air cells are extensively waxed, the Budde Halo retractor (Integra Life Sciences, Plainsboro, New Jersey) is fixed to the Mayfield head holder (Integra Life Sciences, Plainsboro, New Jersey) in a semicircular configuration fitted with a single ⅜-inch blade, and the operating microscope is brought into the surgical field.
Dural Opening
The dura is first opened sharply at the inferior edge, using a single, linear, 15-mm cut, placed just medial to the edge of the sigmoid sinus and 5-mm superior to the inferior bony margin ( Fig. 2A , B ). With an assistant elevating the inferior edge and a ½ inch × 3 inch pattie used to protect the cerebellum from retraction injury, the inferior CPA cistern is accessed via sharply opening the arachnoid ( Fig. 2C ). This is most safely performed by opening the arachnoid just posterior to the ascending CN XI. Care must be taken to not put too much stretch on intradural bridging veins which are predisposed to avulsion and may bleed copiously directly over CNs IX, X, and XI. Once CSF is released, the posterior fossa becomes pulsatile and relaxed, protecting the patient and providing more optimal conditions for the remainder of the opening and approach.
Fig. 2.
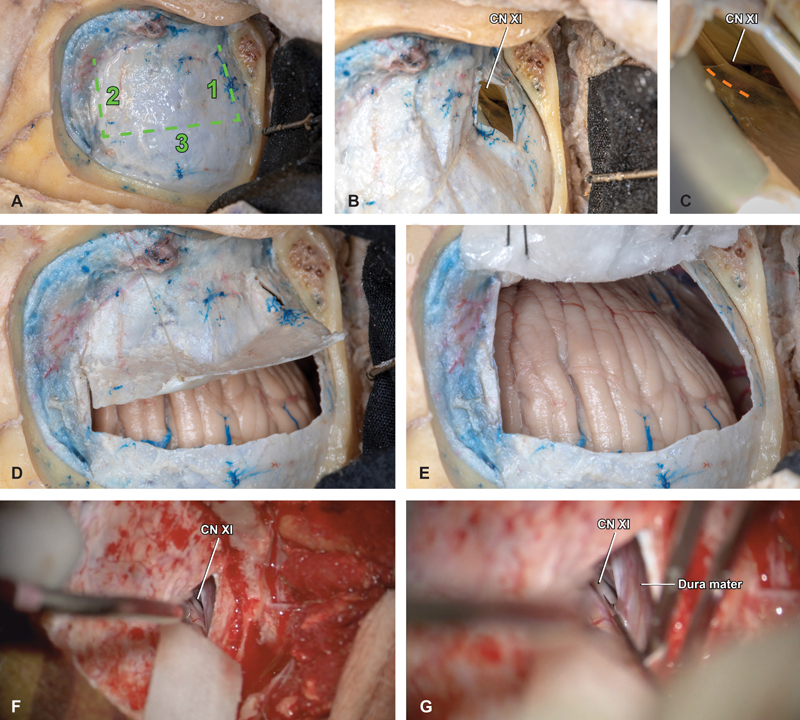
Dural opening and cisterna magna access. ( A ) With the bone flap removed, a trap-door durotomy is planned in three cuts, with the base abutting the sigmoid sinus and the superior cut placed approximately 1 to 2 mm inferior to the inferior margin of the transverse sinus. ( B ) The inferior cut is made first, 5 mm above the inferior bony margin at the posterior border of the descending sigmoid sinus. This small initial dural opening facilitates early foramen magnum access and CSF drainage, to achieve posterior fossa relaxation. Within the deep, inferior aspect of the intradural space, the spinal component of cranial nerve XI is visualized ascending through the foramen magnum. ( C ) Safe opening of the arachnoid is best performed sharply, just posterior to the ascending nerve (orange hash). ( D ) The medial and superior cuts are performed in a stepwise fashion and the dural flap is elevated carefully, ( E ) covered with an antibiotic-soaked nonadherent surgical strip pattie, and secured in place using 3–0 silk sutures. ( F ) Representative intraoperative photographs demonstrate the technique of using a ½” x 3” pattie and dynamic retraction to expose XI and ( G ) incise the overlying arachnoid. CN, cranial nerve.
Attention is then turned to completing the superior and medial dural opening, which is completed sharply with a 15-blade scalpel and connected using a small scissor. This results in a 2.5 cm × 3.5 cm rectangular aperture, based on the sigmoid sinus with the superior margin leaving a 1 to 2 mm cuff of dura just below the distal transverse sinus ( Fig. 2D ). The dural flap is protected with nonadherent surgical strip pattie and secured in place using two 3–0 silk sutures, placed with a through-and-through technique at the base of the flap immediately adjacent to the sigmoid. Sutures are secured to the scalp flap and tied in place, and the covering surgical strip pattie should be moistened periodically throughout the case to optimize dural integrity and a successful closure ( Fig. 2E ).
Posterior Fossa Dissection and Final Retrosigmoid Exposure
With the dural flap opened and secured, attention is turned to the cerebellar hemisphere that is coated with oxidized cellulose polymer (Surgicel, Ethicon, Somerville, New Jersey) to distribute compressive forces and protect the cerebellar cortex. Successive pattie placement is combined with gentle, dynamic retraction to slowly displace the cerebellum medially. Sharp arachnoid dissection is then used to safely expose the posterior fossa neurovascular structures and protect them from unintentional traction injury. Dissection is typically performed inferior to superior that allows for early identification, isolation, and stimulation of XI, providing a critical positive control for the intraoperative neuromonitoring.
Subsequent structures identified in essentially all posterior fossa operations include the lower CNs, the posterior inferior and anterior inferior cerebellar arteries, the VII and VIII CN complex and the trigeminal (V) nerve ( Fig. 3A ). In operations involving the facial nerve such as vestibular schwannoma (VS) resection, we attempt an “infrafloccular” dissection at the first safe opportunity. This allows for early identification of the facial nerve root exit zone at the brainstem by gently elevating the cerebellar flocculus and visualizing VII inferomedial to the vestibulocochlear nerve, immediately superior to IX ( Fig. 3B ). Other dissection techniques will similarly be tailored to the case in question. Of note, a well-executed retrosigmoid craniotomy provides excellent access from CNs III and IV at its superior limit ( Fig. 3C ), to the contents of the foramen magnum including XII, as well as to the ipsilateral vertebral artery, and posterior inferior cerebellar artery ( Fig. 3D ).
Fig. 3.
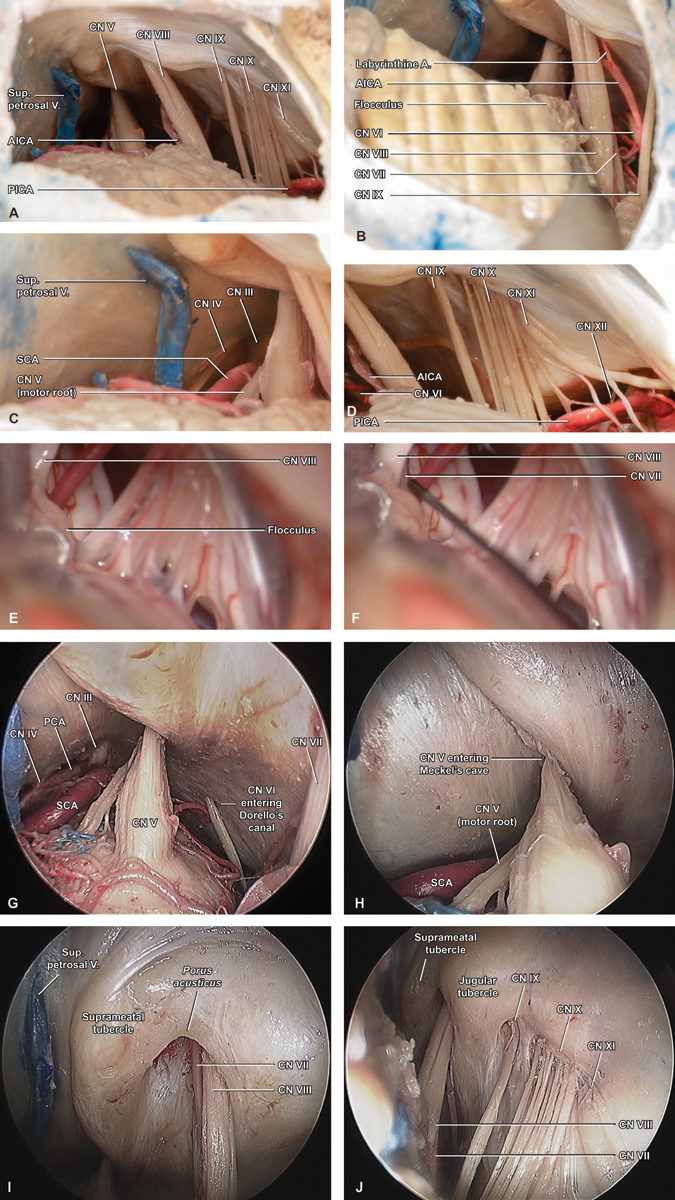
Intradural exposure. ( A ) An initial overview of the final intradural exposure demonstrates how the retrosigmoid craniotomy allows for ready identification of cranial nerves V, VIII, IX, X, and XI, as well as the anterior and posterior inferior cerebellar arteries (AICA/PICA), and superior petrosal vein. ( B ) Gentle elevation of the cerebellar flocculus reveals cranial nerve VII at the brainstem root entry zone via the “infrafloccular maneuver,” while high-magnification views at the ( C ) superior and ( D ) inferior limits of the exposure highlight cranial nerves III and IV adjacent to the deep medial margin of the tentorium, and cranial nerve XII within the foramen magnum, where its rootlets are interdigitated with branches of PICA and the ipsilateral vertebral artery. ( E ) Intraoperative photographs prior to and ( F ) during an infrafloccular maneuver highlight how this technique allows for early identification and stimulation of VII. ( G ) Endoscopic images captured at 0-degree provide additional, close-up views of the upper retrosigmoid exposure, where cranial nerves III–VI are encountered, including VI entering Dorello's canal and ( H ) V entering Meckel's cave. ( I ) Adjacent endoscopic images captured inferiorly at 45-degree highlight the anatomic details surrounding the IAC and jugular foramen, including the relationship between cranial nerves VII and VIII at the porus acusticus , ( J ) as well as cranial nerves IX and X-XI at the jugular foramen. The root exit zone of VII is also appreciated, via this relative infra-floccular perspective. A, artery; AICA, anterior inferior cerebellar artery; CN, cranial nerve; PICA, posterior inferior cerebellar artery; Sup., superior; V, vein.
Although infrequently utilized during open posterior fossa operations, an endoscopic view allows dramatic close-up visualization of both the 3D relationships between the CN root entry zones, CPA trajectories, and exit points via their respective skull base foramina ( Fig. 3G ). These include CN VI entering Dorello's canal and ( Fig. 3H ), CN V entering Meckel's cave ( Fig. 3I ), CNs VII and VIII at the porus acusticus ( Fig. 3J ), and CNs IX and X to XI at the pars nervosa and pars venosa of the jugular foramen, respectively.
Internal Auditory Canal Exposure
A common addition to the retrosigmoid craniotomy is opening the IAC, a particularly important maneuver in VS operations where it is critical to achieving a gross total resection (GTR). Commonly employed options for bone removal include an ultrasonic aspirator fitted with a cutting attachment, which is our preferred technique or sequentially smaller diamond drill bits, typically beginning with 3.5-mm diameter. A semicircular region of the medial petrous temporal bone, immediately overlying the VII and VIII CN complex as it traverses the CPA is exposed and subsequently removed ( Fig. 4A ). When hearing preservation is a goal of surgery the lateral extent of the dissection is limited primarily by the endolymphatic duct and posterior semicircular canal. Depending on the length of the IAC and tumor extent bone is removed along the axis of the nerves, to a width of approximately 3 mm on either side to allow for subsequent trough formation ( Fig. 4B ). Once these boundaries have been established, the bone is carefully thinned until the IAC dura is exposed ( Fig. 4C ). Attention is then focused on the regions immediately superior and inferior to the IAC, where longitudinal troughs are extended until at least 180 degrees of circumferential bone removal has been extended from the porus to near the fundus ( Fig. 4C , D ), allowing maximal access to the full lengths of the intracanalicular nerve segments without breaching the posterior semicircular canal or vestibule ( Fig. 4E ).
Fig. 4.
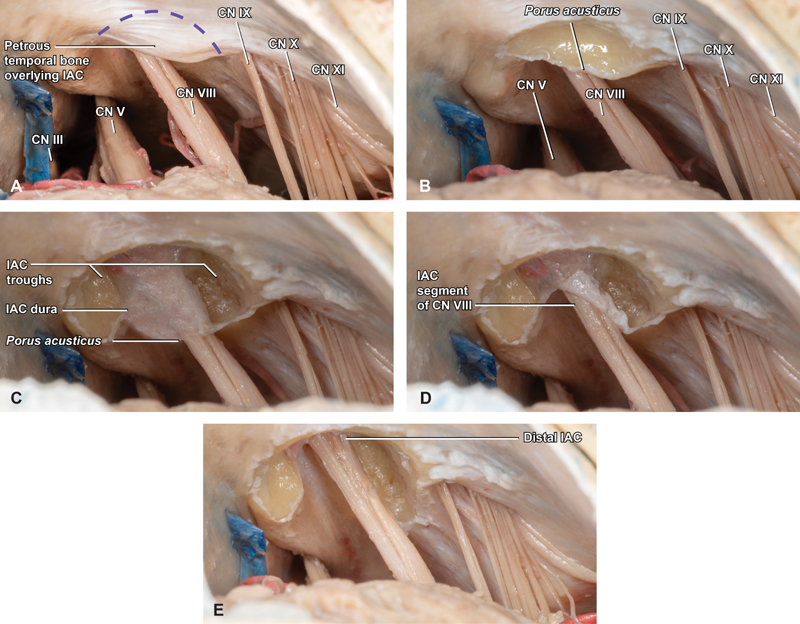
Internal auditory canal (IAC) drilling. ( A ) Intradural exposure of the IAC is initiated by removing a semilunar region of the medial petrous temporal bone (purple hash) overlying the porus acusticus ( B ), to a lateral depth based on IAC length, tumor extent and location of posterior semicircular canal. ( C ) With these boundaries established, saucerization of the region overlying the porus carefully proceeds until the IAC dura is identified overlying cranial nerve VIII. ( D ) Taking care to protect the IAC dura and contents, two troughs are extended at the superior and inferior limits of the region of bony removal and carried longitudinally from the porus to the fundus, and ( E ) deepened until at least 180 degrees of circumferential IAC exposure is completed. CN, cranial nerve; PCA, posterior cerebral artery; SCA, superior cerebellar artery; Sup., superior; V, vein.
Results: Representative Case Review
Case One: Vestibular Schwannoma
A 19-year-old boy presented with a 4-year history of slowly progressive left-sided hearing loss, tinnitus, and new skin lesions, ultimately prompting magnetic resonance imaging (MRI) of the brain. Neurofibromatosis II was diagnosed on the basis of multiple intracranial schwannomas and meningiomas, including a small, intracanalicular right-sided VS, and a large, 3.5 cm, enhancing, left-sided VS with prominent hypervascularity and causing marked brainstem compression ( Fig. 5A ). Resection was recommended, and the patient was taken to surgery for a left-sided retrosigmoid craniotomy.
Fig. 5.
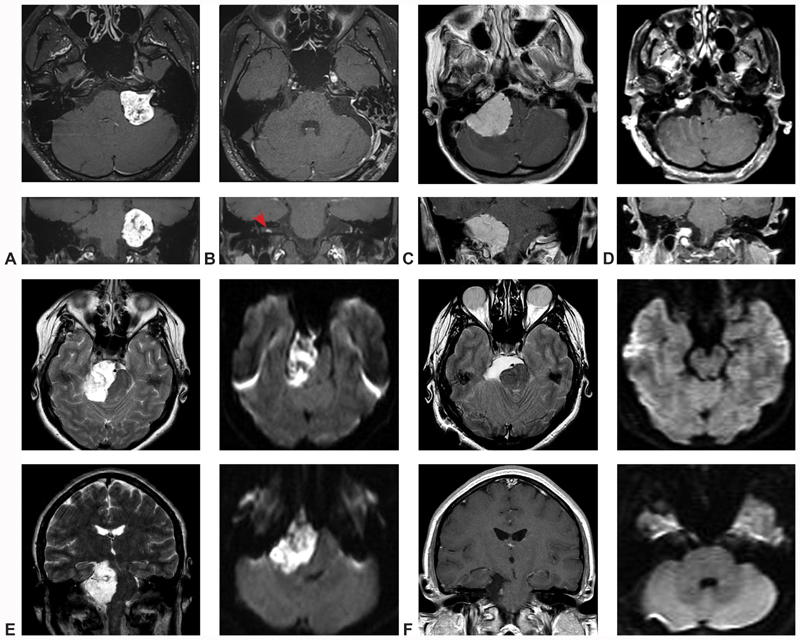
Illustrative cases. ( A – case one ) Preoperative T1-weighted MRI slices in the axial and coronal planes demonstrate a large, heterogeneous, vividly enhancing CPA mass with IAC extension, most consistent with vestibular schwannoma. ( B – case one ) Comparable postoperative T1-weighted MRI slices in the axial and coronal planes confirm gross total resection of the lesion, as well as a small, contralateral, intracanalicular mass, diagnostic of NF-II (red arrow). ( C – case two ) Preoperative T1-weighted MRI slices in the axial and coronal planes identify a very large, homogenously enhancing, dural-based CPA mass extending from the tentorium to the foramen magnum and causing severe compression and displacement of the brainstem, consistent with a jugular foramen meningioma. ( D – case two ) Following resection, a small, anticipated tumor residuum was noted at the jugular foramen, with aggressive subtotal resection of all other tumor. ( E – case three ) Preoperative T2-weighted axial and coronal MRI identified a very large hyper-intense CPA mass causing dramatic midbrain compression and mass effect. Diffusion-weighted sequences demonstrated marked restricted diffusion, confirming the diagnosis of epidermoid cyst. ( F – case three ) Postoperative T2-weighted axial, T1-weighted coronal, and diffusion-weighted axial and coronal sequences confirmed gross total resection of the lesion, with interval improvement in midline shift of the midbrain and pons. CN, Cranial nerve; CPA, cerebellopontine angle; IAC, Internal auditory canal; MRI, magnetic resonance imaging; NF, neurofibromatosis.
Intraoperatively, a large, yellow-appearing tumor was identified filling the right CPA. After stimulation of the presenting surface of the tumor revealed no firing from the facial nerve, the tumor was debulked extensively, until the inferior pole could be dissected free from the lower CNs and rolled superiorly. This revealed the vestibulocochlear nerve and, via superior mobilization of the flocculus, the facial nerve exiting the brainstem. The tumor was sharply dissected off the brainstem, taking care to preserve several large adjacent arteries including a loop of the anterior inferior cerebellar artery. With the brainstem decompressed and the CPA component removed, drilling of the IAC proceeded to its maximal lateral extent, followed by opening of the IAC dura and careful resection of the intracanalicular tumor. Following gross-total resection of the mass, precautions were taking to minimize risk of CSF leak. These included thorough application of bone wax and fibrin sealant to all IAC air cells, followed by closure of the dura, additional waxing of extracranial air cells, replacement of the craniotomy flap, and approximation of the musculature, and scalp in layers.
Postoperatively, the patient awoke with intact facial nerve function, and expected ipsilateral hearing loss. Follow-up MRI demonstrated complete resection of the left VS, with no evidence of recurrence at his 3-year postoperative visit ( Fig. 5B ).
Case Two: Meningioma
A 70-year-old woman presented with a 3-year history of worsening vertigo, imbalance, headaches, and mild right-sided hearing loss. MRI identified a large, 3.9 cm, homogenously enhancing mass centered on the right jugular tubercle, with minimal IAC extension ( Fig. 5C ). Given the size, edema, and disabling symptoms, surgical resection was recommended, via retrosigmoid craniotomy.
Following exposure of the CPA, a large, firm tumor was encountered, extending from the tentorium to the foramen magnum. In spite of the marked inferior extension of the tumor, the foramen magnum could be readily accessed to draw-off CSF and produce early relaxation of the posterior fossa contents. After stimulating the tumor, the ultrasonic aspirator was used to conduct an extensive internal debulking, with interval interruptions to advance the circumferential dissection and mobilization of the tumor poles. Cranial nerves III to V superiorly and XII to VII inferiorly were identified. In spite of the tumor's apparent IAC extension on preoperative imaging, it was readily removed from the porus without the need for and intracranial drilling of the petrous temporal bone. A small nodule of meningioma was left at the jugular foramen, to preserve vagus nerve function, but all other visible tumor was resected, and the wound was closed in the usual fashion.
Postoperatively, the patient developed mild vocal cord paresis, with no other evidence of neurologic injury. Follow-up MRI confirmed an aggressive subtotal resection, with anticipated residuum overlying the jugular foramen ( Fig. 5D ). Future follow-up will include serial MR studies at regular intervals, with a plan to treat the tumor remnant using stereotactic radiosurgery if significant growth is observed.
Case Three: Epidermoid cyst
A 25-year-old woman presented with tinnitus, headaches, disequilibrium, and mild right-sided hearing loss, all progressive over 3 months. MRI identified a very large T1-hypointense T2-hyperintense, diffusion-restricted, nonenhancing mass, which extended from the upper midbrain to the foramen magnum, with significant brainstem displacement and partial encasement of the basilar artery ( Fig. 5E ). Given the sizeable, symptomatic lesion, the tumor was recommended for resection, and a right retrosigmoid craniotomy was planned.
Intraoperatively, an extensive epidermoid cyst was encountered filling the CPA, foramen magnum, and ipsilateral prepontine and peduncular cisterns. Initially working above the IAC, the superior cyst pole was debulked and its capsule sequentially resected, exposing the upper pontine brainstem and basilar artery with its perforators medially. The lesion was then sharply dissected off the trigeminal, facial, and vestibulocochlear nerves. An interneural window was then used to decompress the ventral brainstem, identify the abducens nerve, and dissect it free of tumor from its origin to Dorello's canal. The lower CNs were subsequently dissected free from the capsule, and the resection was carried down to the distal pole at the foramen magnum. Still further cyst was removed superiorly, revealing the oculomotor and trochlear nerves, the superior cerebellar artery, and ultimately the pituitary infundibulum. Each neuroanatomic structure was carefully isolated and protected, and debulking proceeded until a gross-total resection had been achieved. Given the dramatic deformation of the posterior fossa contents created by the epidermoid, CNs III to XII were readily visualized and anatomically intact at the conclusion of the resection.
The patient awoke from surgery at her neurologic baseline, and subsequently recovered normal hearing in her ipsilateral ear. Postoperative MRI confirmed gross total resection of the epidermoid cyst ( Fig. 5E ). An episode of transient partial ipsilateral facial palsy occurred several weeks after surgery and was treated with oral steroids, with complete resolution of the deficit.
Discussion
Key Features of the Retrosigmoid Craniotomy
As described in detail throughout this guide, the retrosigmoid approach provides a wide, safe, rapidly accessible corridor that is a reliable option for the vast majority of posterior fossa operations. This is particularly the case for those lesions with a large CPA component, predominant involvement of the lateral brainstem or cerebellar hemisphere or a goal of hearing preservation. There are numerous variations of the skin incisions that have been described for a retrosigmoid exposure in the literature, including linear incisions over the craniotomy flap. The senior authors have found that the incision presented in this work allows for a wider craniotomy posteromedially, and due to the anatomical angle of the IAC, optimal IAC exposure. Even with very large tumors, our team does not utilize lumbar drain intraoperatively or postoperatively. Careful exposure and opening of the arachnoid membrane of the cisterna magna just behind CN XI, as detailed in the surgical approach, releases CSF early in the operation and allows optimal relaxation of the cerebellum before further opening of the dura mater. This allows to perform surgery with dynamic retraction, and fixed cerebellar retraction is only used as cerebellar protection at the time of the IAC drilling.
Anatomical Comparison to the Presigmoid Approaches
Surgical decision making in CPA surgery is typically oriented toward identifying the relative advantages and disadvantages of a retrosigmoid versus presigmoid approach. The latter category encompasses the commonly employed posterior petrosectomy (aka presigmoid retrolabyrinthine approach) and translabyrinthine approaches, as well as their uncommon variants, the transcrusal, transotic, and transcochlear approaches. For most routine operations in patients with functional hearing, the retrosigmoid is considered by many neurosurgeons to be the most familiar—and therefore safest—means of accessing the posterior fossa. Additionally, as there is essentially no need for extensive temporal bone drilling prior to the critical portion of the procedure, the retrosigmoid is a very efficient means of accessing the CPA.
Collectively, these features of the retrosigmoid approach have rendered it the neurosurgical workhorse for posterior fossa surgery. However, some circumstances may warrant consideration of a presigmoid transtemporal craniotomy. Notwithstanding, for lesions arising medial to the trigeminal nerve, or that extend past the midline, the working distance and light exposure may become major challenges during a retrosigmoid exposure. Other disadvantages of the retrosigmoid approach that have been variably posited in literature include a slightly longer recovery time, increased risk of protracted headache in the postoperative period, and increased risk of hydrocephalus or chemical meningitis from intradural temporal bone drilling. 9 10 11 12 However, most of these findings have been derived from observational studies, and the clinically meaningful difference between the approaches appears to be trivial, particularly from the patient quality-of-life perspective, as we have previously shown. 13 14
The presigmoid family of approaches provides a more lateral angulation relative to the retrosigmoid approach which in several more anteriorly seated cases may helpfully decrease the need for retraction of the cerebellum and brainstem. 15 Other potential advantages of a presigmoid approach include a shorter working distance, particularly for lesions of the petrous apex, clivus, or prepontine cistern, and a larger, shallower aperture that maximizes light influx to the intradural operative site.
Hearing preservation is theoretically possible in the less-aggressive presigmoid variants. Although the nature of the pathology typically limits the capacity for hearing preservation in almost all VS operations, for non-VS pathologies, the transtemporal approaches have increased risk of hearing loss, relative to the retrosigmoid craniotomy. 16 17 18 19 Additionally, the presigmoid craniotomies share the relative disadvantages of requiring a more prolonged bony dissection, making them potentially less efficient than the retrosigmoid. Also, a large fat graft for closure is usually felt necessary to try and prevent postoperative CSF leak which adds some small risk of donor site morbidity. The anterior inferior limit of the presigmoid approach is the jugular bulb, thus it may not be an optimal exposure to access the inferior aspect of the CPA closer to the lower CNs, or to access lesions that extend inferiorly toward the foramen magnum. Anatomical variations, such as high jugular bulbs, which are close to the labyrinth, reduce significantly the working space in this area and the anatomy of the patient should be carefully studied before the operation to choose the optimal surgical approach ( Fig. 6 ). The presigmoid approaches combined with supratentorial exposure and tentorial division provide optimal working spaces for certain pathologies. 9 Although the risk of complications increases markedly with the more aggressive transotic and transcochlear variants, in the hands of an experienced neurootologist, the relative risk to the facial nerve function of a posterior petrosectomy or translabyrinthine operation is equivocal, as compared with a retrosigmoid craniotomy with intradural opening of the IAC. 20 21 22 23 24 25
Fig. 6.
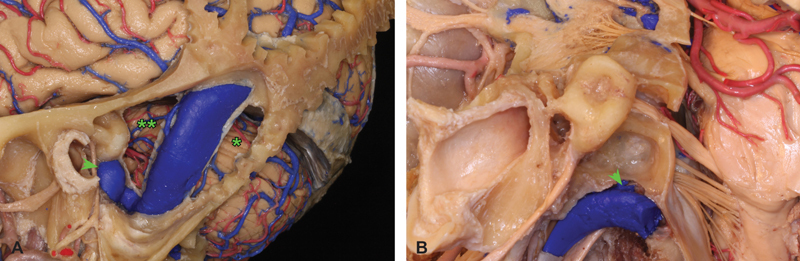
Presigmoid versus retrosigmoid trajectories. ( A ) Extensive dissection of a neuroanatomic specimen centered on the lateral skull base and sigmoid sinus region highlights the relative trajectories afforded by retrosigmoid (*) versus presigmoid (**) approaches to the CPA. Although the dura forming Trautman's triangle between the sinus, bony labyrinth, and superior petrosal sinus has been removed, the very high position of the jugular bulb in this specimen would markedly limit a presigmoid approach in a patient with similar anatomy. ( B ) Comparable dissection of a different neuroanatomic specimen demonstrates how a patient with a low-lying jugular bulb would constitute a more appropriate candidate for presigmoid in addition to retrosigmoid approaches. CPA, cerebellopontine angle.
Clinical Pearls for Selection of a Retrosigmoid Approach
As the cases detailed above highlight, the great strength of the retrosigmoid lies in its versatility and simplicity, resulting in a broad range of indications for both intra- and extra-axial operations. Optimal cases for the retrosigmoid include those in which the bulk of the lesion is located in the CPA, arising from the posterolateral brainstem or cerebellar hemisphere, posterioinferior to the IAC porus. Taken together, these factors build a compelling case for the retrosigmoid as the front-line approach for the majority of posterior fossa tumors, particularly where hearing preservation is a possibility.
Several interesting trends in complex cranial surgery have reinforced the extraordinary versatility of the retrosigmoid craniotomy and its associated central role in the neurosurgical armamentarium. More specifically, although numerous leaders in skull base and cerebrovascular neurosurgery including Majid Samii and Robert Spetzler enthusiastically incorporated the posterior petrosal approach (combined suprainfratentorial exposure) into their practices in the 1980s and 1990s, they have subsequently published follow-up experiences. In these more contemporary perspectives, the authors argue that, for the great majority of lesions, the retrosigmoid is the safer and more efficient approach. 20 23 24 25 26 27 28 29 As we have argued previously, we hold that the posterior petrosectomy continues to serve an important role in carefully selected circumstances, but favor a retrosigmoid for most patients, which has an approximate utilization of 9:1 in our practice, as compared with the posterior petrosectomy. In parallel with the evolution of our own practice, other authors have increasingly demonstrated a role for the endoscope in CPA surgery, either as an adjunct to the microscope, or the primary visualization modality, a trend that may further enhance the utility and dynamism of the retrosigmoid approach across a broad range of clinical contexts. 30 31 32 33 Ultimately, we emphasize the importance of a broad, inclusive, and anatomically oriented skull base education which we anticipate will be well-informed by these dissections and guides that can empower individuals at all stages of training to learn and reproduce the core approach techniques.
Conclusion
The retrosigmoid craniotomy is a foundational approach for complex cranial surgery, and arguably the most versatile and widely used technique for skull base surgery in the posterior fossa. Although the bone work is less sophisticated than in presigmoid transtemporal approaches, a nuanced understanding of the anatomic relationships between cranial landmarks, dural venous sinuses, and intradural structures is among the most important pillars of surgical neuroanatomy for the skull base surgeon. Reliance on a cadaver-based pedagogical framework appears to provide the most reliable and durable 3D understanding of neuroanatomy, and an approach-oriented surgical anatomy curriculum built around resources such as the presented dissections is critical to empower residents and fellows to achieve early operative independence, and develop a true mastery of skull base fundamentals.
Funding Statement
Funding None.
Conflict of Interest None declared.
Co-first authors
References
- 1.Meyer F B. Philadelphia, PA: Churchill Livingstone; 1999. Atlas of Neurosurgery: Basic Approaches to Cranial and Vascular Procedures. [Google Scholar]
- 2.Tew J M, Van Loveren H Keller JT. Philadelphia, PA: Saunders; 1994. Atlas of Operative Microneurosurgery. [Google Scholar]
- 3.Rhoton A L., JrThe cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach Neurosurgery 200047(3, Suppl):S93–S129. [DOI] [PubMed] [Google Scholar]
- 4.Wanibuchi M, Friedman A H, Fukushima T. Stuttgart, Germany: Thieme; 2009. Photo Atlas of Skull Base Dissection: Techniques and Operative Approaches. [Google Scholar]
- 5.Carlstrom L P, Perry A, Graffeo C S.Foundations of advanced neuroanatomy: technical guidelines for specimen preparation, dissection, and 3d-photodocumentation in a surgical anatomy laboratory J Neurol Surg B Skull Base 201980(S 01):S1–S244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day J D, Tschabitscher M. Anatomic position of the asterion. Neurosurgery. 1998;42(01):198–199. doi: 10.1097/00006123-199801000-00045. [DOI] [PubMed] [Google Scholar]
- 7.Ribas G C, Rhoton A L, Jr., Cruz O R, Peace D. Suboccipital burr holes and craniectomies. Neurosurg Focus. 2005;19(02):E1. doi: 10.3171/foc.2005.19.2.2. [DOI] [PubMed] [Google Scholar]
- 8.Uz A, Ugur H C, Tekdemir I. Is the asterion a reliable landmark for the lateral approach to posterior fossa? J Clin Neurosci. 2001;8(02):146–147. doi: 10.1054/jocn.2000.0798. [DOI] [PubMed] [Google Scholar]
- 9.Carlson M L, Tombers N M, Kerezoudis P, Celda M P, Lohse C M, Link M J. Quality of life within the first 6 months of vestibular schwannoma diagnosis with implications for patient counseling. Otol Neurotol. 2018;39(10):e1129–e1136. doi: 10.1097/MAO.0000000000001999. [DOI] [PubMed] [Google Scholar]
- 10.Carlson M L, Tveiten O V, Driscoll C L. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg. 2015;122(04):833–842. doi: 10.3171/2014.11.JNS14594. [DOI] [PubMed] [Google Scholar]
- 11.Carlson M L, Tveiten O V, Driscoll C L. What drives quality of life in patients with sporadic vestibular schwannoma? Laryngoscope. 2015;125(07):1697–1702. doi: 10.1002/lary.25110. [DOI] [PubMed] [Google Scholar]
- 12.Link M J, Lund-Johansen M, Lohse C M. Quality of life in patients with vestibular schwannomas following gross total or less than gross total microsurgical resection: should we be taking the entire tumor out? Neurosurgery. 2018;82(04):541–547. doi: 10.1093/neuros/nyx245. [DOI] [PubMed] [Google Scholar]
- 13.Carlson M L, Tveiten O V, Yost K J, Lohse C M, Lund-Johansen M, Link M J. The minimal clinically important difference in vestibular schwannoma quality-of-life assessment: an important step beyond p <. 05. Otolaryngol Head Neck Surg. 2015;153(02):202–208. doi: 10.1177/0194599815585508. [DOI] [PubMed] [Google Scholar]
- 14.Kerezoudis P, Yost K J, Tombers N M, Celda M P, Carlson M L, Link M J.Defining the minimal clinically important difference for patients with vestibular schwannoma: are all quality-of-life scores significant?Neurosurgery 2018; (e-pub ahead of print) doi: 10.1093/neuros/nyy467 [DOI] [PubMed]
- 15.Graffeo C S, Maria P-C, Perry A, Carlstrom L P, Driscol C LW, Link M J. Anatomical step-by-step dissection of complex skull base approaches for trainees: surgical anatomy of the posterior petrosal approach. J Neurol Surg B Skull Base. 2019;80(04):338–351. doi: 10.1055/s-0038-1675174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross B A, Dunn I F, Du R, Al-Mefty O. Petrosal approaches to brainstem cavernous malformations. Neurosurg Focus. 2012;33(02):E10. doi: 10.3171/2012.6.FOCUS12110. [DOI] [PubMed] [Google Scholar]
- 17.Horgan M A, Delashaw J B, Schwartz M S, Kellogg J X, Spektor S, McMenomey S O. Transcrusal approach to the petroclival region with hearing preservation. Technical note and illustrative cases. J Neurosurg. 2001;94(04):660–666. doi: 10.3171/jns.2001.94.4.0660. [DOI] [PubMed] [Google Scholar]
- 18.Kaylie D M, Horgan M A, Delashaw J B, McMenomey S O.Hearing preservation with the transcrusal approach to the petroclival region Otol Neurotol 20042504594–598., discussion 598 [DOI] [PubMed] [Google Scholar]
- 19.King W A, Black K L, Martin N A, Canalis R F, Becker D P. The petrosal approach with hearing preservation. J Neurosurg. 1993;79(04):508–514. doi: 10.3171/jns.1993.79.4.0508. [DOI] [PubMed] [Google Scholar]
- 20.Bambakidis N C, Kakarla U K, Kim L J.Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review Neurosurgery 2007610502202–209., discussion 209–211 [DOI] [PubMed] [Google Scholar]
- 21.Gross B A, Tavanaiepour D, Du R, Al-Mefty O, Dunn I F. Evolution of the posterior petrosal approach. Neurosurg Focus. 2012;33(02):E7. doi: 10.3171/2012.6.FOCUS12133. [DOI] [PubMed] [Google Scholar]
- 22.Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105(04):527–535. doi: 10.3171/jns.2006.105.4.527. [DOI] [PubMed] [Google Scholar]
- 23.Samii M, Tatagiba M.Experience with 36 surgical cases of petroclival meningiomas Acta Neurochir (Wien) 1992118(1-2):27–32. [DOI] [PubMed] [Google Scholar]
- 24.Samii M, Tatagiba M, Carvalho G A. Resection of large petroclival meningiomas by the simple retrosigmoid route. J Clin Neurosci. 1999;6(01):27–30. doi: 10.1054/jocn.1997.0201. [DOI] [PubMed] [Google Scholar]
- 25.Spetzler R F, Daspit C P, Pappas C T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76(04):588–599. doi: 10.3171/jns.1992.76.4.0588. [DOI] [PubMed] [Google Scholar]
- 26.Daspit C P, Spetzler R F, Pappas C T. Combined approach for lesions involving the cerebellopontine angle and skull base: experience with 20 cases--preliminary report. Otolaryngol Head Neck Surg. 1991;105(06):788–796. doi: 10.1177/019459989110500604. [DOI] [PubMed] [Google Scholar]
- 27.Samii M, Ammirati M.The combined supra-infratentorial pre-sigmoid sinus avenue to the petro-clival region. Surgical technique and clinical applications Acta Neurochir (Wien) 198895(1-2):6–12. [DOI] [PubMed] [Google Scholar]
- 28.Samii M, Ammirati M, Mahran A, Bini W, Sepehrnia A. Surgery of petroclival meningiomas: report of 24 cases. Neurosurgery. 1989;24(01):12–17. doi: 10.1227/00006123-198901000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Spetzler R F, Daspit C P, Pappas C T. Combined approach for lesions involving the cerebellopontine angle and skull base: experience with 30 cases. Skull Base Surg. 1991;1(04):226–234. doi: 10.1055/s-2008-1057102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alicandri-Ciufelli M, Federici G, Anschuetz L. Transcanal surgery for vestibular schwannomas: a pictorial review of radiological findings, surgical anatomy and comparison to the traditional translabyrinthine approach. Eur Arch Otorhinolaryngol. 2017;274(09):3295–3302. doi: 10.1007/s00405-017-4630-8. [DOI] [PubMed] [Google Scholar]
- 31.Marchioni D, Gazzini L, Boaria F, Pinna G, Masotto B, Rubini A. Is endoscopic inspection necessary to detect residual disease in acoustic neuroma surgery? Eur Arch Otorhinolaryngol. 2019;276(08):2155–2163. doi: 10.1007/s00405-019-05442-4. [DOI] [PubMed] [Google Scholar]
- 32.Presutti L, Alicandri-Ciufelli M, Bonali M. Expanded transcanal transpromontorial approach to the internal auditory canal: pilot clinical experience. Laryngoscope. 2017;127(11):2608–2614. doi: 10.1002/lary.26559. [DOI] [PubMed] [Google Scholar]
- 33.Presutti L, Magnaguagno F, Pavesi G. Combined endoscopic-microscopic approach for vestibular schwannoma removal: outcomes in a cohort of 81 patients. Acta Otorhinolaryngol Ital. 2014;34(06):427–433. [PMC free article] [PubMed] [Google Scholar]


