Abstract
During SARS-CoV-2 pandemic, we adopted a personalized delayed protocol for ocrelizumab infusions in Relapsing Remitting Multiple Sclerosis (RRMS) patients according to the national recommendations. Out of the 83 RRMS patients whose infusion was scheduled between March and December 2020, 56 patients experienced a delay in treatment based on MS severity and SARS-CoV2 infection risk profile. In most cases, the immunophenotype was performed monthly to guide re-infusions. Specifically, B CD19 + cells repopulation rate was monitored. Mean infusion delay was 103,1 [SD 40,6] days, and none of the patients presented relapses or active disease at MRI at the end of the observation period. Treatment naïve status and the interval between immunophenotyping and the last ocrelizumab infusion were predictors of earlier B CD19 + cells repopulation. Two patients contracted SARS-CoV2 with complete recovery. Definitive data about Sars-Cov2 vaccine efficacy in patients treated with ocrelizumab are still lacking. Our findings suggest that a personalized treatment with a delayed infusion schedule does not compromise ocrelizumab short-term efficacy and may help to lengthen the therapeutic window for an effective response to SARS-CoV2 vaccine.
Keywords: Multiple sclerosis, Treatment, Ocrelizumab, COVID-19, Efficacy, Safety
1. Introduction
SARS-CoV2 pandemic led neurologists to modify the therapeutic management of patients with multiple sclerosis (MS), especially with regard to immunodepleting treatments. During the first wave of Sars-Cov2 pandemic, the impact of ocrelizumab on the risk and severity of infection was limited to single case reports [1,2].
International [3] and local recommendations [4] suggested to stop treatment or to adopt an extended dose regimen according to patients' clinical status and SARS-CoV2 infection risk profile.
The aim of this study was to investigate the effect of delayed ocrelizumab infusions on clinical, radiological and immunological outcomes in a cohort of patients with relapsing-remitting MS.
2. Methods
In this retrospective study, we identified 83 RRMS patients whose treatment with Ocrelizumab were scheduled between 1st March 2020 and 1st December 2020.
For 56 RRMS patients included in this cohort, we decided to delay ocrelizumab infusion. Delay in treating with ocrelizumab was personalized for each patient, considering MS severity and the risk of developing severe COVID-19 related complications. With regard to MS severity, we identified “aggressive MS” patients [5], as patients showing MRI activity and at least one relapse in the year before ocrelizumab start associated with accelerated accrual of disability (EDSS≥ 4.0). Of the remaining patients, those who did not present clinical activity after ocrelizumab start were defined “clinically stable”. Age and cardiovascular comorbidities were evaluated for each patient and considered to outline the individual risk profile related to COVID19 infection.
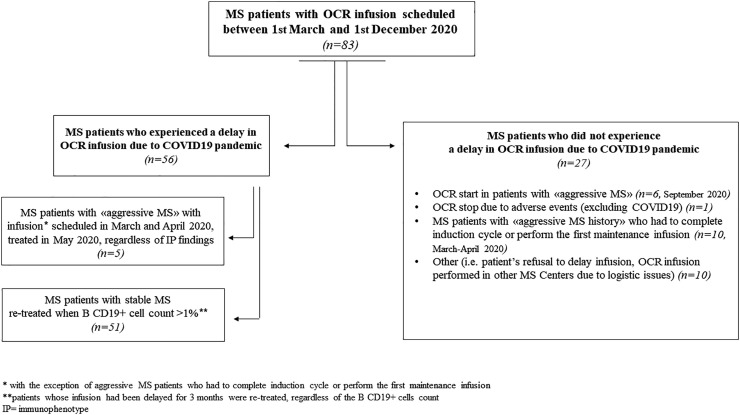
In Fig. 1 , we reported the whole cohort of patients involved in the study, including patients who did not experienced any delay in ocrelizumab treatment in the abovementioned timeframe and the reasons of this therapeutic choice.
Fig. 1.
Composition of the whole cohort of patients involved in the study.
More specifically:
- from March to April 2020 (first pandemic wave), ocrelizumab was administered only to patients with “aggressive MS” who had to complete induction cycle or to perform the first maintenance infusion.
- at the end of April 2020 (when pandemic wave was slowly decreasing), treatment administration was re-introduced. In this phase, the majority of patients performed immunophenotype (IP) monthly and were managed as follows:
-
•
RRMS patients with “aggressive MS” whose maintenance infusions had been delayed were re-infused as soon as possible regardless of IP findings.
-
•
“Clinically stable” RRMS patients who received ocrelizumab re-infusion when the B CD19+ cell population reached the cut-off of 1% of total lymphocyte count. [6,7]
Then, we decided to use a conservative approach and patients whose infusion would have been delayed for more than 3 months, were re-treated, regardless of the B CD19+ cells count.
When possible, 3 T brain MRI (Prisma, Siemens) was planned before ocrelizumab re-infusion.
Data about relapses, Expanded Disability Status Scale (EDSS) progression and MRI activity before the ocrelizumab infusion were acquired. Adverse events (AEs) were also recorded. Analyses were performed using SPSS 22.0 (IBM; X). Distribution of data were analyzed using Kolmogorov Smirnov test. Demographic differences between groups (evidence of B CD19+ cells repopulation +/− at 3 months of delay) were assessed using Chi-Square test, independent samples t-test, Mann-Whitney test as appropriate. Correlations between demographic and clinical variables and the evidence of B CD19+ cells repopulation was explored using binary logistic regression analyses adjusted for age, sex and BMI. All p values were 2-sided and considered statistically significant when p ≤ 0.05.
All patients involved in the study signed the informed consent. The study was approved by the Local Ethic Committee.
3. Results
Demographic and clinical features of the 83 RRMS patients whose treatment with ocrelizumab was scheduled between March and December 2020 are reported in Table 1 and Fig. 1.
Table 1.
MS = multiple sclerosis, RR = Relapsing Remitting, BMI = Body Mass Index, DMT = disease modifying treatment, ARR = Annualized Relapse Rate, EDSS = Expanded Disability Status Scale, CMT = Charcot Marie Tooth.
| Delayed treatment | Scheduled treatment | |
|---|---|---|
| Number of patients (%) | 56 (67.4) | 27 (32.6) |
| Age, mean (SD) | 38.1 (10.2) | 42.2 (9.8) |
| Female, number (%) | 38 (67.9%) | 16 (59.1) |
| BMI, mean (SD) | 24.3 (3.8) | 24.6 (3.8) |
| Disease duration at ocrelizumab start, mean (SD) years | 10.5 (9.7) | 9.1 (8.7) |
| Previous DMTs, mean (SD) number | 1.9 (1.6) | 1,7 (1.6) |
Last DMT before ocrelizumab start, number (%)
|
17 (30.4) 7 (12.5) 7 (12.5) 5 (8.9) 3 (5.4) 2 (3.6) 0 (0) 2 (3.6) |
7 (25.9) 3 (11,1) 0 (0) 2 (7,4) 2 (7,4) 3 (11,1) 2 (7,4) 0 (0) |
| Treatment naïve patients, number (%) | 13 (23.2) | 8 |
| ARR 1y before ocrelizumab start, mean (SD) | 0.7 (0.7) | 0.9 (0.8) |
| Patients with MRI activity 1y before ocrelizumab start, number (%) | 43 (76.8) | 23 (85.1) |
| EDSS at ocrelizumab start, median (IQR) | 2.5 (2–4) | 2.8 (2–4) |
| Patients with radiological activity during ocrelizumab treatment before the delay due to Sars-Cov2 pandemic | 17 (30.9) | 9 (33.3) |
| Patients with clinical activity after ocrelizumab start before the delay | 2 (3.6) | n.a. |
| Ocrelizumab infusions performed at the delay, mean (SD) number | 3 (0.8) | n.a. |
| Total delay, mean (SD), days | 103.1 (40.6) | n.a. |
Delay for each ocrelizumab infusion timepoint, mean (SD), for number (%) of patients (pts)
|
0 (0) for 0 (0) pts. 102.8 (42.6) for 17 (30.3) pts. 92.5 (32.4) for 26 (46.4) pts. 124.6 (46.7) for 13 (23.2) pts. |
n.a. |
| Reason for exceeding scheduled delay, number of patients | previous bacterial pneumonia, 1 concomitant CMT, 1 COVID infection, 2 contact with COVID+ subject, 5 patient decision, 2 |
n.a. |
| Ocrelizumab treatment follow-up duration, mean (SD), years | 1.5 (0.4) | 1.9 (0.6) |
Fifty-six RRMS patients (67.4%) experienced a delay in ocrelizumab infusion, while for 27 RRMS patients (32.6%) the treatment was regularly performed.
With regard to patients who had a delay in ocrelizumab treatment, 5 (8.9%) were patients who fulfilled the criteria of “aggressive MS”; these patients were re-treated as soon as possible (May 2020), regardless of IP findings. The remaining 51 RRM patients (91%) were “clinically stable” and received ocrelizumab re-infusion when the B CD19+ cell population reached the cut-off of 1% of total lymphocyte count.
Of the 56 RRMS patients who experienced a delay, no patients showed clinical relapses or confirmed disability progression during the delay period.
Thirty-three (58.9%) patients performed 3 T brain MRI before the delayed ocrelizumab re-infusion. No patients showed gadolinium enhancing lesions; 2 patients presented 1 new T2 lesion.
Two patients were infected by SARS-CoV2. One patient performed Sars-Cov2 swab in the context of the contact tracing program without developing any symptom. The second one developed interstitial pneumonia requiring hospitalization; treatment with remdesivir was performed and the patient completely recovered without sequelae. Both patients showed no CD19+ cells at the time of COVID19 infection.
No other AEs were reported.
IP within 3 months of delay was available for 53/56 (94.6%) RRMS patients. Thirty-five (66%) RRMS patients presented B CD19+ cells repopulation within 3 months of delay. The mean age, sex, BMI, disease duration and previous DMTs number were similar between patients with and without evidence of B CD19+ cell repopulation.
The model of binary logistic analysis, adjusted for age, sex and BMI (Nagelkerke R2 = 0.419, p = 0.004), evidenced that the time interval between IP and last ocrelizumab infusion date [OR = 1.03 (1–1.1) p = 0.011] and to being treatment naïve [OR = 6.7 (1.2–36.8) p = 0.028] were significant predictors of B CD19+ cells repopulation within 3 months of delay, while a trend was found for the number of ocrelizumab infusions at the delay [0.39 (0.1–1.1) p = 0.083]. Neither the cumulative number nor the specific type of previous treatments were predictors of the B CD19 + cells repopulation rate.
4. Discussion
SARS-CoV2 pandemic has led neurologists to rethink therapeutic strategies in MS, especially regarding immunodepleting treatments, as ocrelizumab. Concerns about the pejorative impact of anti-CD20 treatments on COVID-19 infection [8] and the possibility to adopt an extended dosing interval to de-risk the chance of severe COVID19 infection in patients treated with rituximab have been recently published [9].
In this scenario, in addition to rapid infusion protocols implementation [10], our MS centre adopted a personalized infusion schedule based on MS severity and the risk of developing severe COVID-19 related complications. In particular, we decided to delay ocrelizumab treatment in 56 of the 83 RRMS patients whose infusion was scheduled between March and December 2020. With the exception of “aggressive MS” patients who were re-treated immediately after the first pandemic wave regardless of IP findings, for the remaining patients (51/56, 91%) the evidence of B CD19+ cells repopulation guided the re-infusions time schedule. No patients showed relapses nor disability progression during the delay, in line with a recent study performed in a smaller cohort [11]. Furthermore, none of the patients who performed brain MRI before the delayed re-infusions showed active lesions. Two patients presented a new T2 lesion. Nevertheless, it was not possible to rule out if they had developed due to the delay, because the previous MRIs (used for comparison) had been performed 1 year and 9 months before the lockdown, respectively.
In our cohort, a 7-fold increase of the probability of B CD19+ cells repopulation within 3 months of delay was observed in treatment naïve patients. Moreover, the time interval between IP and last ocrelizumab infusion date was a significant predictor [6] of B CD19+ cells repopulation within 3 months of delay while a trend was found for the number of ocrelizumab infusion at the delay.
Moreover, the efficacy of a tailored infusion regimen has been previously demonstrated in patients treated with rituximab [12] and ocrelizumab [13]. Guided by the findings obtained in patients treated with rituximab for MS [6] and neuromyelitis optica spectrum disorder [14], we used the threshold of 1% of B CD19+ cells to guide re-infusions. Although a long-lasting delay [12,13] have demonstrated to provide a good disease control, we chose to adopt a more conservative approach, not exceeding 3 months of delay from the last ocrelizumab infusion. The evidence of an earlier B cell repopulation in some patients and the lack of definitive data about the impact of delaying ocrelizumab on disability progression guided our therapeutic choice.
In line with previous findings [6,12,13], our results should prompt the design of prospective studies based on B cells repopulation. Furthermore, the possible interfering activity of anti-CD20 treatments on the response to vaccines is a well-known issue in clinical practice [15] although definitive data about Sars-Cov2 vaccine efficacy in patients treated with ocrelizumab are still lacking. In the meanwhile, a safe and personalized ocrelizumab treatment with a delayed infusion schedule may help to lengthen the therapeutic window and increase the possibility to obtain an effective humoral response to SARS-CoV2 vaccine.
Funding
This study did not receive any funding support.
Availability of data and material
Not applicable
Code availability
Not applicable.
Ethical approval
This study was approved by local ethical committee
Consent for publication
All patients signed a written informed consent concerning the publication of the data included in this paper.
Declaration of Competing Interest
Dr. F. Tazza has nothing to disclose.
Dr. C. Lapucci has nothing to disclose.
Dr. M. Cellerino has nothing to disclose.
Dr. G. Boffa has nothing to disclose.
Dr. G. Novi has nothing to disclose.
Ms. I. Poire has nothing to disclose.
Dr. E. Mancuso has nothing to disclose.
Dr. N. Bruschi has nothing to disclose.
Dr. E. Sbragia has nothing to disclose.
Dr. A. Laroni received grants from Italian MS Society, Italian Ministry of Healthy, Italian Ministry for University; fees for consultation from Biogen, Novartis, Merck, Roche.
Dr. E Capello received honoraria for consultation from Merck, Novartis and Almirall.
Dr. M. Inglese received grants NIH, NMSS, FISM; received fees for consultation from Roche, Genzyme, Merck, Biogen and Novartis.
References
- 1.Novi G., Mikulska M., Briano F., et al. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord. 2020 Jul;42:102120. doi: 10.1016/j.msard.2020.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laroni A., Schiavetti I., Sormani M.P., et al. COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult. Scler. 2020 Nov;18 doi: 10.1177/1352458520971817. 1352458520971817. [DOI] [PubMed] [Google Scholar]
- 3.Amor S., Baker D., Khoury S.J., et al. SARS-CoV-2 and multiple sclerosis: not all immune depleting DMTs are equal or bad. Ann. Neurol. 2020 Jun;87(6):794–797. doi: 10.1002/ana.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coles A., Lim M., Giovannoni G., et al. ABN guidance on the use of disease-modifying therapies in multiple sclerosis in response to the threat of a coronavirus epidemic. https://cdn.ymaws.com/www.theabn.org/resource/collection/65C334C7-30FA-45DB-93AA-74B3A3A20293/02.04.20_ABN_Guidance_on_DMTsforMS_and COVID19VERSION4April2nd.pdf
- 5.Rush C.A., MacLean H.J., Freedman M.S. Aggressive multiple sclerosis: proposed definition and treatment algorithm. Nat. Rev. Neurol. 2015 Jul;11(7):379–389. doi: 10.1038/nrneurol.2015.85. (Epub 2015 Jun 2. PMID: 26032396) [DOI] [PubMed] [Google Scholar]
- 6.Zecca C., Bovis F., Novi G., et al. Treatment of multiple sclerosis with rituximab: a multicentric Italian-Swiss experience. Mult. Scler. 2020 Oct;26(12):1519–1531. doi: 10.1177/1352458519872889. (Epub 2019 Oct 1. PMID: 31573386) [DOI] [PubMed] [Google Scholar]
- 7.Ellrichmann G., Bolz J., Peschke M., et al. Peripheral CD19+ B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J. Neurol. 2019;266(1):57–67. doi: 10.1007/s00415-018-9092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sormani M.P., De Rossi N., Schiavetti I., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. (Epub 2021 Feb 9. PMID: 33480077; PMCID: PMC80134409)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maarouf A., Rico A., Boutiere C., et al. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol Neuroimmunol Neuroinflamm. 2020 Jun 25;7(5):e825. doi: 10.1212/NXI.0000000000000825. (PMID: 32587103; PMCID: PMC7357416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rath L., Bui M.V., Ellis J., et al. Fast and safe: Optimising multiple sclerosis infusions during COVID-19 pandemic. Mult Scler Relat Disord. 2020 Dec 1;47:102642. doi: 10.1016/j.msard.2020.102642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barun B., Gabelić T., Adamec I., et al. Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness. Mult Scler Relat Disord. 2020 Dec 21;48:102704. doi: 10.1016/j.msard.2020.102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser S., Waubant E., Arnold D.L., et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008 Feb 14;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 13.Baker D., Pryce G., James L.K., et al. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord. 2020 Sep;44:102279. doi: 10.1016/j.msard.2020.102279. 32645640 Epub 2020 Jun 8. [DOI] [PubMed] [Google Scholar]
- 14.Kim S., Huh S., Lee S., et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013 Sep 1;70(9):1110–1117. doi: 10.1001/jamaneurol.2013.3071. [DOI] [PubMed] [Google Scholar]
- 15.Baker D., Roberts A.K., Pryce G., et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020 Nov;202(2):149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable