Abstract
Objective
To evaluate the efficacy of colchicine therapy in pediatric patients with PFAPA syndrome who present with an incomplete response to the standard treatment or with frequent episodes (an interval of less than 14 days between two disease flares).
Methods
A multicenter cohort study of children diagnosed with PFAPA syndrome and treated with colchicine was performed in three separate hospitals located in Spain. The patients clinical and laboratory data were reviewed by accessing their medical records. Response to colchicine was evaluated after 12 months of treatment for frequency, duration, and intensity of PFAPA episodes.
Results
A total of 13 children were included in our study, 43% of whom were boys. Median age of the colchicine therapy initiation was 6 years (interquartile range (IQR)=3–9.5). Following a 12-month period of colchicine therapy (median dosage of 0.02 mg/kg/day; IQR=0.02–0.03), a significant decrease in the median number of flares (median 8; IQR=7–14 vs 3; IQR=2–4; p=0.005) and the duration of disease episodes (median 4 days; IQR=3.25–5.125 vs 1 day; IQR=1–2; p=0.003) was observed. Furthermore, the highest degree of fever during disease flares was reduced from median 40ºC (IQR=39.5–40) to 38.5ºC (IQR=37.7–38.9) (p=0.002).
Conclusion
Colchicine therapy decreased the frequency and intensity of PFAPA. The use of colchicine could be an effective treatment in pediatric patients with PFAPA syndrome who present with frequent or severe relapses.
Keywords: Colchicine, children, periodic fever, autoinflammatory diseases
Introduction
Periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome is considered to be the most common periodic fever syndrome during childhood. It is characterized by repeated episodes of fever associated with distinctive clinical features, including aphthous stomatitis, pharyngitis, and/or cervical adenitis (1). The regular timing of fever flares (predictable recurrence between 2 and 8 weeks) is a cardinal feature of this disease (2). In most patients, PFAPA syndrome begins before 5 years of age. Additionally, episodes become less severe and less frequent with time, although some patients continue to have flares in adulthood (2, 3).
The pathogenesis of PFAPA syndrome remains unclear. Polygenic susceptibility, dysregulation of the innate immune system, and the tonsillar microbiome may all play a role in the development of PFAPA syndrome (2, 4–6).
A low-dose corticosteroid therapy (1–2 mg/kg oral dosage) is the main treatment for children with PFAPA syndrome. Corticosteroids rapidly relieve symptoms in PFAPA episodes but cannot prevent subsequent disease episodes over time. Moreover, the corticosteroid therapy may result in shorter disease-free periods between flares. Alternate therapies such as tonsillectomy have proven to be successful in pediatric patients with PFAPA syndrome refractory to corticosteroid treatment (2, 7). In addition, colchicine prophylaxis has shown efficacy in patients with an increase in episode frequency, although further evidence is required (8).
Colchicine is an alkaloid with anti-inflammatory properties that inhibit the expression and the release of an enzyme called nonpancreatic phospholipase A2 (sPLA2). This enzyme has inhibitory effects on microtubule assembly, cell adhesion, and inflammasome activation (9). Although colchicine is not routinely used in the management of PFAPA patients, it has been proposed that colchicine prophylaxis is an effective treatment in reducing the number of PFAPA episodes in patients with frequent disease flares (10).
This study aims to evaluate the efficacy of colchicine in reducing the number, intensity, and duration of flares in pediatric patients with PFAPA syndrome presenting with more than one relapse per month and/or an incomplete response to conventional corticosteroid therapy. Secondary objectives include to describe the clinical and genetic profile of this group of patients.
Methods
Study design
A multicenter retrospective cohort study of children diagnosed with PFAPA syndrome and treated with colchicine was conducted between January 2015 and February 2020. The three participating hospitals were La Paz University Hospital (Madrid), Gregorio Marañón University Hospital (Madrid), and the University Hospital of Virgen del Rocío (Seville). Ethics committee approval was received for this study from the Ethics Committee of the La Paz University Hospital (Approval Date: May 11, 2020; Approval Number: PI-4243). Written informed consent was obtained from the parents of the patients who participated in this study.
Inclusion criteria
This study included patients with PFAPA syndrome who were previously treated with colchicine at any of the hospitals mentioned above during the study period. The diagnosis of PFAPA syndrome was established according to the Thomas criteria (regularly recurring fevers with the early age of onset (<5 years of age); symptoms in the absence of upper respiratory tract infections with at least one of the following: aphthous stomatitis, cervical lymphadenitis, or pharyngitis; exclusion of cyclic neutropenia; completely asymptomatic intervals between episodes and normal growth and development) (11). Monogenic autoinflammatory disease was excluded according to the PRINTO (Pediatric Rheumatology International Trials Organization) clinical classification criteria and the diagnostic score for hereditary recurrent fevers (12–14).
Indications for colchicine therapy included the following: patients with more than one relapse per month and/or an incomplete response to conventional corticosteroid therapy (1–2 mg/kg single dose). A response to colchicine therapy was evaluated following a 12-month treatment period for the frequency, duration, and intensity of PFAPA episodes.
Exclusion criteria
Patients who did not meet all of the above inclusion criteria were excluded.
Data collection
Each patient’s clinical and laboratory data were retrospectively reviewed by accessing their medical records. The following items were examined: age at the disease onset, age at the colchicine initiation, family history, characteristics of the febrile relapses (highest degree of fever during disease flares, frequency and duration of fever episodes per month), clinical features accompanying fever (oral aphthosis, pharyngitis, cervical adenitis, abdominal pain, vomiting, diarrhea, thoracic pain, arthralgia, arthritis, myalgia, skin rash, and headache), the presence or absence of neutropenia, any increase of inflammatory markers (erythrocyte sedimentation rate and/or C-reactive protein) during asymptomatic and symptomatic intervals, and the presence or absence of clinical manifestations during asymptomatic intervals. In addition, we reviewed genetic testing for autoinflammatory diseases including molecular analysis of MEFV, MVK, TNFRSF1A, and NLRP3 genes, respectively, responsible for the following periodic fever syndromes: familial Mediterranean fever, mevalonate kinase deficiency, tumor necrosis factor receptor-associated periodic syndrome, and cryopyrin-associated periodic syndrome.
Statistical analysis
Statistical data analysis was performed using IBM Statistical Package for Social Sciences software for Windows, version 25.0 (IBM SPSS Corp.; Armonk, NY, USA). Results were expressed as median and interquartile range for quantitative variables as well as frequency counts and percentages for categorical variables. The nonparametric Wilcoxon test was used to compare quantitative data before and after the treatment with colchicine (number of flares per year, duration of flares, and highest degree of fever). The differences between data before and after colchicine therapy were considered significant when the test showed a p<0.05.
Results
Clinical characteristics of the cohort
A total of 105 patients with PFAPA syndrome were followed-up by three different hospitals in Spain between January 2015 and February 2020. A total of 13 children with PFAPA syndrome underwent colchicine therapy and met the inclusion criteria (46% boys, 54% girls). Each patient’s characteristics are summarized in Tables 1 and 2. The median age of the disease onset was 4 years (interquartile range (IQR)=1–7). All patients had at least one of the three cardinal clinical signs: pharyngitis (11 of 13, 84%), cervical adenitis (9 of 13, 69%) and aphthous stomatitis (8 of 13. 61%). Additional symptoms were also reported during PFAPA episodes, especially abdominal pain (5 of 13, 38%) and arthralgia (2 of 13, 15%). None of the patients had clinical manifestations and positive laboratory inflammatory markers during fever-free intervals.
Table 1.
Demographic and clinical features of patients with PFAPA syndrome.
| Demographic features n (%) | |
|---|---|
| Age at onset (median years; IQR) | 4; 1–7 |
| Age at colchicine initiation (median years; IQR) | 6; 3–9.5 |
| Sex (M/F) | 6/7 |
| Clinical features | |
| Aphthous stomatitis | 8 (61) |
| Pharyngitis | 11 (84) |
| Cervical adenitis | 9 (69) |
| Abdominal pain | 5 (38) |
| Diarrhea | 0 (0) |
| Thoracic pain | 0 (0) |
| Headaches | 0 (0) |
| Arthritis/skin rash | 0 (0) |
| Arthralgia | 2 (15) |
| Myalgia | 0 (0) |
| Symptomatic intervals between episodes | 0 (0) |
| Neutropenia | 0 (0) |
| Increased inflammatory markers during attacks | 13 (100) |
| Normal inflammatory markers during fever-free Intervals | 13 (100) |
IQR: interquartile range; M: male; F: female.
Table 2.
Treatment response, colchicine side effects, genetic testing, and family history of patients with PFAPA syndrome.
| Patients | Flares before colchicine (N) | Duration flares before colchicine (days) | Highest degree of fever before colchicine (ºC) | Flares after colchicine (N) | Duration flares after colchicine (days) | Highest degree of fever after colchicine (ºC) | Side effects of colchicine | Genetic testing | Genetic testing results | Family history |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 3.75 | 39.5 | 2 | 1 | 38.5 | No | Yes | No mutation | No |
| 2 | 7 | 4 | 40 | 2 | 1.25 | 39 | No | Yes | MEFV p. I640M | No |
| 3 | 6 | 5.25 | 39 | 2 | 1 | 38.5 | No | No | Not available | No |
| 4 | 7 | 6 | 39.8 | 3 | 1.5 | 38.9 | No | Yes | MEFV p.I591T | No |
| 5 | 12 | 3.75 | 39.3 | 12 | 3.25 | 39 | Abdominal pain | Yes | No mutation | Mother with PFAPA-like |
| 6 | 8 | 4.25 | 39.8 | 3 | 1 | 38.8 | No | Yes | No mutation | No |
| 7 | 4 | 2.25 | 40.5 | 4 | 2.5 | 39 | Vomiting | Yes | No mutation | Both parents with PFAPA-like |
| 8 | 8 | 3.5 | 40 | 4 | 1.5 | 38.6 | Diarrhea | Yes | MEFV p.R202Q | No |
| 9 | 15 | 3 | 39.5 | 4 | 1 | 37.5 | No | Yes | TNFR61A pR121Q | Both parents with PFAPA-like |
| 10 | 9 | 10 | 40 | 3 | 3 | 38 | No | No | Not available | No |
| 11 | 14 | 5 | 40 | 2 | 1 | 37.5 | No | Yes | No mutation | Mother with PFAPA-like |
| 12 | 14 | 3 | 40 | 11 | 1 | 38 | No | No | Not available | No |
| 13 | 14 | 4 | 40 | 4 | 1 | 37.5 | No | No | Not available | No |
N: number; ºC: degrees celsius; PFAPA: periodic fever, aphthous stomatitis, pharyngitis, and adenitis.
Effectiveness of colchicine treatment
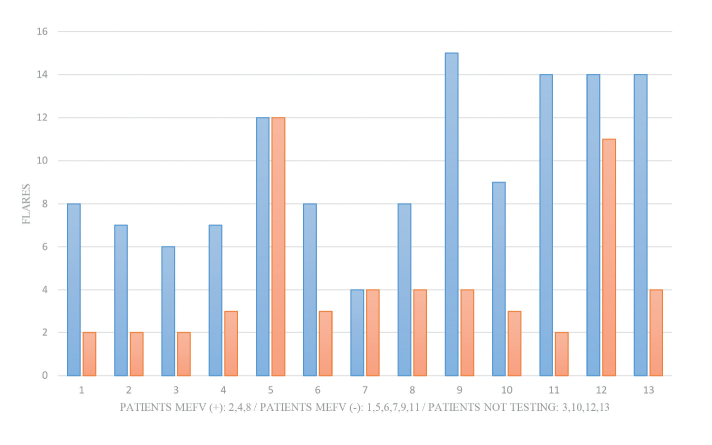
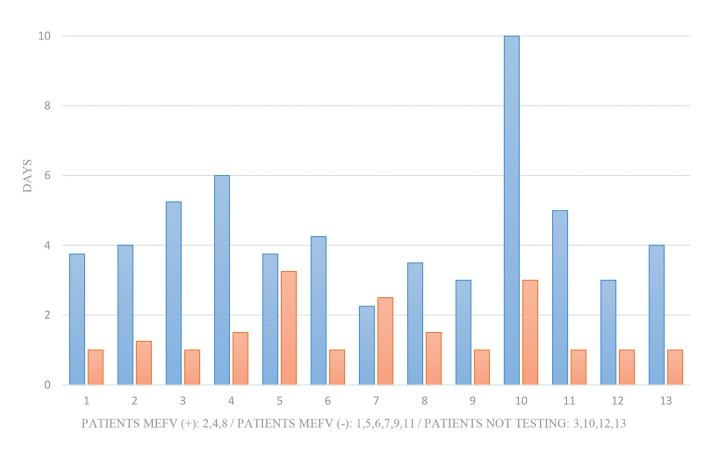
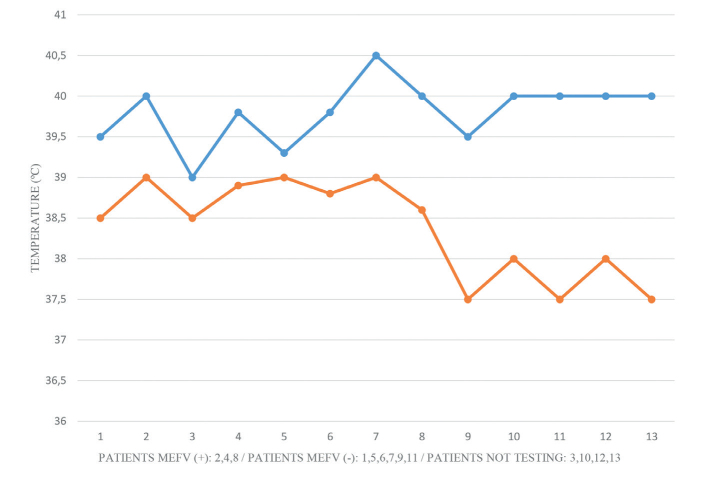
Prior to colchicine therapy, the median number of PFAPA flares was 8 (IQR=7–14) and the median duration of disease episodes was 4 days (IQR=3.25–5.125). The median age of colchicine therapy initiation was 6 years (IQR=3–9.5) and the median colchicine dose was 0.02 mg/kg/day (IQR=0.02–0.03). Twelve months after colchicine therapy, a significant decrease in the number of flares (p=0.005) and duration (p=0.003) of the episodes was observed (median: 3 episodes; IQR=2–4 and 1 day; IQR=1–2, respectively) (Figures 1 and 2). Furthermore, the highest degree of fever recorded during flares was reduced from median 40ºC (IQR=39.5–40) to 38.5ºC (IQR=37.7–38.9; p=0.002) (Figure 3).
Figure 1.
Number of flares per months before (blue) and after (red) colchicine treatment. A significant decrease in the median number of flares (8; IQR=7–14 vs 3; IQR=2–4; p=0.005) was observed [MEFV (+): cases with heterozygous MEFV gene mutations; MEFV (−): cases without heterozygous, IQR: interquartile range].
Figure 2.
Duration of flares before (blue) and after (red) colchicine treatment. A significant decrease in median duration of flares (4 days; IQR=3.25–5.125 vs 1 day; IQR=1–2; p=0.003) was observed. [MEFV (+): cases with heterozygous MEFV gene mutations; MEFV (−): cases without heterozygous, IQR: interquartile range].
Figure 3.
Highest degree of fever in flares before (blue) and after (red) colchicine treatment. Highest degree of fever in flares was reduced from median 40ºC (IQR=39.5–40) to median 38.5ºC (IQR=37.7–38.9; p=0.002). [MEFV (+): cases with heterozygous MEFV gene mutations; MEFV (−): cases without heterozygous, IQR: interquartile range].
Colchicine-related side effects were reported in three patients (23%) and consisted of mild gastrointestinal symptoms (abdominal pain, diarrhea, or vomiting). There were no cases of discontinuation of colchicine treatment due to adverse effects during the study.
Family history and genetic testing for autoinflammatory diseases
A positive family history of recurrent fever with pharyngitis was observed in five cases (38%). Nine patients (69%) had undergone genetic testing for autoinflammatory diseases, and three of these nine patients were found to be MEFV (Familial Mediterranean fever gene) heterozygotes (one I640M pathogenic mutation, one uncertain significance I591T mutation and one nonpathogenic R202Q mutation). In addition, a heterozygous mutation in TNFR61A was found in one patient, who additionally had a mutation in R92Q of uncertain significance. The PRINTO clinical classification criteria for monogenic autoinflammatory disease were not met by the study participants and all presented with a low-risk diagnostic score (<1.32).
Discussion
To the best of our knowledge, this is the first multicenter study in Spain to describe the effect of colchicine therapy in pediatric PFAPA patients. The results obtained from our study indicate a notable decrease in the frequency of relapses and in the intensity of the disease. We observed an average decrease of five flares after 1 year of colchicine therapy in our study population. In addition, an improvement in the intensity of the episodes was observed, reflected by a decrease in both maximum body temperature and duration of disease episodes (median reduction of 3 days).
Previous studies have reported colchicine therapy to be an effective treatment for PFAPA with overall good clinical profile (15). However, there is little evidence available regarding its use in pediatric patients. A clinical trial by Butbul et al. (16) is the only randomized controlled trial of colchicine versus placebo. The study included 18 children diagnosed with PFAPA syndrome. Patients who received colchicine therapy showed a significant decrease in PFAPA flares (nearly to 4 episodes per month) following 3 months of treatment.
Interestingly, we observed improvement of symptoms in three of our patients (23%) with heterozygous MEFV mutations following treatment with colchicine (patients 2, 4, and 8). Several studies have reported the presence of MEFV heterozygous variants in patients with PFAPA syndrome (17–19) and further observed that MEFV mutations potentially influence treatment outcomes of PFAPA syndrome (20–23). In this way, clinical improvements in PFAPA children with MEFV mutations following colchicine therapy have been reported. In the study performed by Pehlivan et al. (20), PFAPA patients with the coexistence of MEFV gene mutation responded better to colchicine treatment compared with patients without MEFV variants (response rate: 66% vs 33%, respectively; p=0.03). In contrast, a poorer response to surgical treatment (tonsillectomy) were found in children with the coexistence of MEFV mutation (response rate: 52% vs 85%, respectively; p=0.002). Gunes et al. (21) reported a retrospective cohort study of 356 children on a 12-month colchicine prophylaxis in Turkey and observed a decrease in the number of flares in about 85% of patients. Moreover, MEFV pathogenic variants (96%) showed a more effective response represented by a decrease in PFAPA episode frequency compared with children without mutation (80%). In the same way, Dusser et al. (22) reported a retrospective study on 20 Turkish pediatric patients with PFAPA syndrome who underwent colchicine prophylaxis and found better outcomes in children with heterozygous MEFV mutations.
Five of our patients (38%) had a positive family history of recurrent fever, pharyngitis, or tonsillectomy supporting the hypothesis of inheritance for PFAPA syndrome (24). Although the heritability of PFAPA is unclear, several familial cases have been reported in the literature. In a case-controlled study, Manthiram et al. (25) compared the family history of patients with PFAPA to healthy controls and found that 23% of patients had at least one family member with symptoms consistent with PFAPA. Furthermore, a higher risk of pharyngitis and/or aphthous stomatitis was found in the first-degree family members of patients with PFAPA compared with the relatives of healthy controls. A Genome-wide analysis and whole-exome sequencing of familial cases performed to date have not revealed variants in a single gene that could relate to the genetic basis of PFAPA syndrome. Di Gioia et al. (26) examined 68 individuals from 14 families with PFAPA syndrome by whole-genome and exome sequencing. No common disease gene associated with PFAPA was found; however, the pedigree analysis of PFAPA syndrome showed an autosomal dominant inheritance pattern. Similarly, we have not found genetic mutations in five of our patients who have a positive family history of recurrent fever and pharyngitis.
The observed mild gastrointestinal side effects of colchicine therapy did not result in treatment discontinuation and were improved by decreasing the drug dosage. The combination of colchicine and dicycloverine, the later an antispasmodic and anticholinergic agent, reduced the gastrointestinal side effects in our patients. Therefore, dicycloverine might be a good option to treat such side effects.
This study has several limitations. First, the retrospective nature and second, the small sample size and the absence of a control group, clearly limit to draw firm conclusions about the efficacy of colchicine therapy. Additionally, flares in PFAPA syndrome usually decrease in frequency and intensity over time; their improvements could be mistakenly associated with colchicine therapy.
Despite the limitations of our study, in our series treatment with colchicine decreased the frequency and intensity of PFAPA flares in children with very frequent or partially controlled flares with corticosteroid therapy, especially in those patients carrying MEFV gene mutations. We consider that colchicine could be a good option for persistent or “severe” forms of PFAPA, including patients with more than one relapse per month and/or with insufficient control of flares. Genetic testing for autoinflammatory diseases could help to select patients for this treatment. A double-blinded, controlled clinical trial comparing intermittent corticosteroid therapy with placebo versus intermittent corticosteroid therapy with colchicine during PFAPA disease flares would allow a better understanding of the therapeutic and prophylactic potential of colchicine.
Main Points.
This is the first multicenter study in Spain describing the effect of colchicine in pediatric PFAPA patients.
The results obtained from our study indicate a decrease in the frequency of relapses as well as their intensity.
Colchicine therapy could be a good option in pediatric patients with PFAPA syndrome presenting with more than one relapse per month and/or with insufficient control of flares with conventional corticosteroid therapy.
Acknowledgements
The authors gratefully acknowledge Kinga Amália Sándor-Bajusz, MD for her contribution to this project.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of the La Paz University Hospital (Approval Date: May 11, 2020; Approval Number: PI-4243).
Informed Consent: Written informed consent was obtained from the parents of the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.Q.O., C.U., R.A.; Design - C.Q.O., C.U., R.A.; Supervision - E.S.R., L.F., M.C.; Materials - C.Q.O., M.C., C.U., R.A.; Data Collection and/or Processing - C.Q.O., C.U., A.R., C.C., R.A.; Analysis and/or Interpretation - C.Q.O., P.O., O.N., S.M., C.U., A.R., C.C., R.A.; Literature Search – L.F., P.O., O.N., S.M., A.R., C.C.; Writing Manuscript - C.Q.O., E.S.R., L.F., M.C., C.U., A.R., C.C., R.A.; Critical Review - E.S.R., L.F., M.C., P.O., O.N., S.M.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Rigante D. Autoinflammatory syndromes behind the scenes of recurrent fevers in children. Med Sci Monit. 2009;15:RA179–RA187. [PubMed] [Google Scholar]
- 2.Adrovic A, Sahin S, Barut K, Kasapcopur O. Familial Mediterranean fever and periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome: Shared features and main differences. Rheumatol Int. 2019;39:29–36. doi: 10.1007/s00296-018-4105-2. [DOI] [PubMed] [Google Scholar]
- 3.Cantarini L, Vitale A, Bartolomei B, Galeazzi M, Rigante D. Diagnosis of PFAPA syndrome applied to a cohort of 17 adults with unexplained recurrent fevers. Clin Exp Rheumatol. 2012;30:269–71. [PubMed] [Google Scholar]
- 4.Gentileschi S, Vitale A, Frediani B, Galeazzi M, Rigante D, Cantarini L. Challenges and new horizons in the periodic fever, aphthous stomatitis, pharingitis and adenitis (PFAPA) syndrome. Expert Opin Orphan Drugs. 2017;5:165–71. doi: 10.1080/21678707.2017.1279049. [DOI] [Google Scholar]
- 5.Esposito S, Bianchini S, Fattizzo M, Baggi E, Marchisio P, Rigante D. The enigma of periodic fever, aphthous stomatitis, pharyngitis and adenitis syndrome. Pediatr Infect Dis J. 2014;33:650–2. doi: 10.1097/INF.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 6.Kolly L, Busso N, von Scheven-Gete A, Bagnoud N, Moix I, Holzinger D, et al. Periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome is linked to dysregulated monocyte IL-1β production. J Allergy Clin Immunol. 2013;131:1635–43. doi: 10.1016/j.jaci.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 7.Cattalini M, Soliani M, Rigante D, Lopalco G, Iannone F, Galeazzi M, et al. Basic characteristics of adults with periodic fever, aphthous stomatitis, pharyngitis, and adenopathy syndrome in comparison with the typical pediatric expression of disease. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/570418. 570418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaggiano C, Rigante D, Sota J, Grosso S, Cantarini L. Treatment options for periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome in children and adults: A narrative review. Clin Rheumatol. 2019;38:11–7. doi: 10.1007/s10067-018-4361-2. [DOI] [PubMed] [Google Scholar]
- 9.Pruzanski W, Kennedy BP, van den Bosch H, Stefanski E, Vadas P. Microtubule depolymerization selectively down-regulates the synthesis of proinflammatory secretory nonpancreatic phospholipase A2. Lab Invest. 1997;76:171–8. [PubMed] [Google Scholar]
- 10.Tasher D, Stein M, Dalal I, Somekh E. Colchicine prophylaxis for frequent periodic fever, aphthous stomatitis, pharyngitis and adenitis episodes. Acta Paediatr. 2008;97:1090–2. doi: 10.1111/j.1651-2227.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas KT, Feder HM, Jr, Lawton AR, Edwards KM. Periodic fever syndrome in children. J Pediatr. 1999;135:15–21. doi: 10.1016/S0022-3476(99)70321-5. [DOI] [PubMed] [Google Scholar]
- 12.Gattorno M, Hofer M, Federici S, Vanoni F, Bovis F, Aksentijevich I, et al. Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis. 2019;78:1025–32. doi: 10.1136/annrheumdis-2019-215048. [DOI] [PubMed] [Google Scholar]
- 13.Federici S, Gattorno M. A practical approach to the diagnosis of autoinflammatory diseases in childhood. Best Pract Res Clin Rheumatol. 2014;28:263–76. doi: 10.1016/j.berh.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Gattorno M, Sormani MP, D’Osualdo A, Pelagatti MA, Caroli F, Federici S, et al. A diagnostic score for molecular analysis of hereditary autoinflammatory syndromes with periodic fever in children. Arthritis Rheum. 2008;58:1823–32. doi: 10.1002/art.23474. [DOI] [PubMed] [Google Scholar]
- 15.Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596–609. doi: 10.1016/j.berh.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Butbul Aviel Y, Tatour S, Gershoni Baruch R, Brik R. Colchicine as a therapeutic option in periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome. Semin Arthritis Rheum. 2016;45:471–4. doi: 10.1016/j.semarthrit.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Dagan E, Gershoni-Baruch R, Khatib I, Mori A, Brik R. MEFV, TNF1rA, CARD15 and NLRP3 mutation analysis in PFAPA. Rheumatol Int. 2010;30:633–6. doi: 10.1007/s00296-009-1037-x. [DOI] [PubMed] [Google Scholar]
- 18.Perko D, Debeljak M, Toplak N, Avcin T. Clinical features and genetic background of the periodic Fever syndrome with aphthous stomatitis, pharyngitis, and adenitis: A single center longitudinal study of 81 patients. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/293417. 293417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniuchi S, Nishikomori R, Iharada A, Tuji S, Heike T, Kaneko K. MEFV variants in patients with PFAPA syndrome in Japan. Open Rheumatol J. 2013;7:22–5. doi: 10.2174/1874312901307010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pehlivan E, Adrovic A, Sahin S, Barut K, Kul Cınar O, Kasapcopur O. PFAPA syndrome in a population with endemic familial Mediterranean fever. J Pediatr. 2018;192:253–5. doi: 10.1016/j.jpeds.2017.08.078. [DOI] [PubMed] [Google Scholar]
- 21.Gunes M, Cekic S, Kilic SS. Is colchicine more effective to prevent periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis episodes in Mediterranean fever gene variants? Pediatr Int. 2017;59:655–60. doi: 10.1111/ped.13265. [DOI] [PubMed] [Google Scholar]
- 22.Dusser P, Hentgen V, Neven B, Koné-Paut I. Is colchicine an effective treatment in periodic fever, aphtous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome? Joint Bone Spine. 2016;83:406–11. doi: 10.1016/j.jbspin.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Haytoglu Z, Gundeslioglu OO. Mediterranean fever gene variants and colchicine therapy in periodic fever, aphthous stomatitis pharyngitis, adenitis syndrome in a Mediterranean region. Expert Rev Clin Immunol. 2019;15:571–5. doi: 10.1080/1744666X.2019.1591275. [Retraction in: Haytoglu Z, Gundeslioglu OO, Expert Rev Clin Immunol, 2020; 16: 229]. [DOI] [PubMed] [Google Scholar]
- 24.Harel L, Hashkes PJ, Lapidus S, Edwards KM, Padeh S, Gattorno M, et al. The first international conference on periodic fever, aphthous stomatitis, pharyngitis, adenitis syndrome. J Pediatr. 2018;193:265–74.e3. doi: 10.1016/j.jpeds.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 25.Manthiram K, Nesbitt E, Morgan T, Edwards KM. Family history in periodic fever, aphthous stomatitis, pharyngitis, adenitis (PFAPA) syndrome. Pediatrics. 2016;138:e20154572. doi: 10.1542/peds.2015-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Gioia SA, Bedoni N, von Scheven-Gête A, Vanoni F, Superti-Furga A, Hofer M, et al. Analysis of the genetic basis of periodic fever with aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome. Sci Rep. 2015;5:10200. doi: 10.1038/srep10200. [DOI] [PMC free article] [PubMed] [Google Scholar]