The introduction of biological disease-modifying antirheumatic drugs (bDMARDs) in the last two decades has deeply changed the management of inflammatory arthritides. In the last 5 years, as the originator bDMARDs patents expired, less-expensive biosimilars were introduced in the market (1). The EMA and FDA defined the biosimilar as a biological agent that contains a similar version of the active substance of an already approved original biological agent (reference product). To date infliximab, etanercept, adalimumab, and rituximab biosimilars have been approved in rheumatology. The growing evidence concealing the use of biosimilar drugs in rheumatological diseases has been recently analyzed in an international consensus (2). Nevertheless, implementation of biosimilars into real world practice is still a matter of controversy among rheumatologists (3).
To evaluate the real world impact of biosimilar use in rheumatologic diseases, we retrospectively analyzed the baseline characteristics and the 18-month retention rate in a cohort of patients who received at least a course of bDMARDs in our Rheumatology Unit from January 2000 to December 2019. Patients switching from originator to biosimilar were excluded from the analysis. We stratified the study population according to biosimilar use. Descriptive data are presented by medians (interquartile range [IQR]) for continuous data or as numbers (percentages) for categorical data. Drug survival distribution curves were computed by the Kaplan-Meier method and compared by a stratified log-rank test. A Cox proportional hazards regression analysis stratified by indication, drug, age, disease duration, sex, treatment line, biosimilar use, and prescription year was performed. Statistical analyses were performed using Medcalc statistical software, version 18.2.1 (MedCalc Software bvba, Ostend, Belgium). P values ≤0.05 were considered statistically significant.
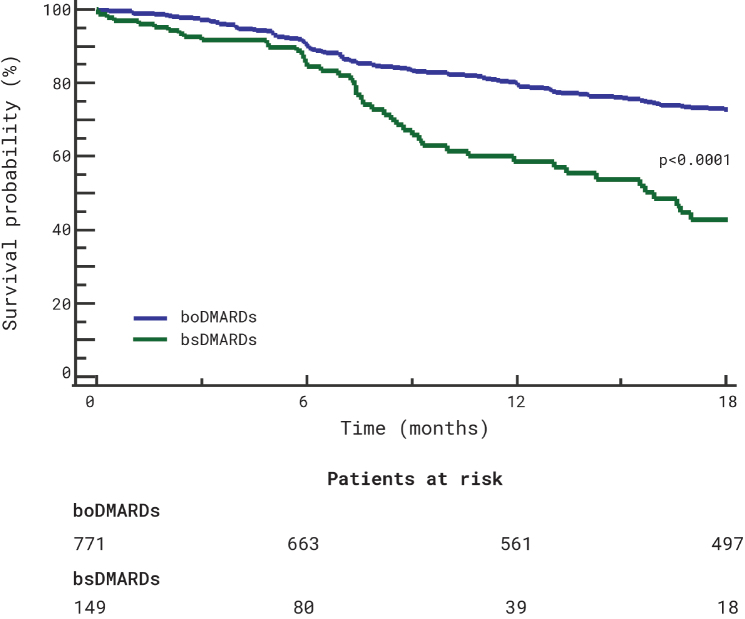
The analysis included 920 patients, 580 (63%) female, median age (IQR) 54 (44–64) years, median disease duration (IQR) 71 (24–167.5) months, affected by rheumatoid arthritis (477, 51.8%), psoriatic arthritis (239, 26%), and axial spondyloarthritis (204, 22.2%). The patients were treated with TNFi (infliximab, n=160 [17.4%]; etanercept, n=334 [36.3%]; adalimumab, n=394 [42.8%]) or rituximab (n=32 [3.5%]). Hundred and forty nine (16.2%) patients were treated with a biosimilar drug (infliximab, n=49 [32.9%]; etanercept, n=64 [43%]; adalimumab, n=34 [22.8%]; and rituximab, n=2 [1.3%]). The overall 18-month retention rate was 70.1%. The estimated proportions of patients maintaining originators and biosimilars were 79.3 and 58.5%, respectively, after 12 months, and 72.8 and 42.8% after 18 months (Figure 1). Originators showed a higher survival on treatment (Hazard ratio [HR] 0.43, 95% confidence intervals [CI] 0.28 to 0.67, p<0.0001). However, the Cox proportional hazard regression analysis stratified by indication, drug, age, disease duration, sex, treatment line, biosimilar use, and prescription year, highlighted that the predictors significantly associated with an overall higher risk of treatment discontinuation were the year of prescription (HR 1.12, 95% CI 1.09 to 1.54; p<0.0001) and female sex (HR 1.42, 95% CI 1.09 to 1.85). No other significant associations were found.
Figure 1.
18-month drug survival of originator bDMARDs (boDMARDs) and biosimilar bDMARDs (bsDMARDs) treatment.
In conclusion, our preliminary real-life clinical data reported a 70.1% 18-month retention rate of bDMARDs among all rheumatologic indications. Originators showed an overall higher retention rate (72.8% vs 42.8%). However, similarly to previous observations (4, 5), the only predictors of treatment discontinuation were the year of treatment prescription and female sex. Although limited by its retrospective design and by its relatively short follow-up, our study confirms that biosimilar integration into clinical practice is effective and safe.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.A., A.B.; Design - A.A., A.B.; Data Collection and/ or Processing - A.A., A.B., S.G., F.L., E.D.D., D.S., F.M., M.R., G.L.; Analysis and/or Interpretation - A.A., A.B., S.G., F.L., E.D.D., D.S., F.M., M.R., G.L.; Writing Manuscript - A.A., A.B.; Critical Review - A.A., A.B., S.G., F.L., E.D.D., D.S., F.M., M.R., G.L.
Conflict of Interest: A.B. served as a speaker for Sanofi-Genzyme, UCB, AbbVie and Amgen, outside the submitted work. The other authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Smolen JS, Goncalves J, Quinn M, Benedetti F, Yongkwon JY. Era of biosimilars in rheumatology: Reshaping the healthcare environment. RMD Open. 2019;5:e000900. doi: 10.1136/rmdopen-2019-000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kay J, Schoels MM, Dörner T, Emery P, Kvien TK, Smolen JS, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis. 2018;77:165–74. doi: 10.1136/annrheumdis-2017-211937. [DOI] [PubMed] [Google Scholar]
- 3.Mysler E, Pineda C, Horiuchi T, Singh E, Mahgoub E, Coindreau J, et al. Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol Int. 2016;36:613–25. doi: 10.1007/s00296-016-3444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neovius M, Arkema EV, Olsson H, Eriksson JK, Kristensen LE, Simard JF, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 2015;74:354–60. doi: 10.1136/annrheumdis-2013-204128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok CC, Chan KY, Lee KL, Tam LS, Lee KW Hong Kong Society of Rheumatology. Factors associated with withdrawal of the anti-TNFα biologics in the treatment of rheumatic diseases: Data from the Hong Kong Biologics Registry. Int J Rheum Dis. 2014;17(Suppl 3):1–8. doi: 10.1111/1756-185X.12264. [DOI] [PubMed] [Google Scholar]