Abstract
BACKGROUND AND PURPOSE: Evaluation of the spinal cord is important in the diagnosis and follow-up of patients with multiple sclerosis. Our purpose was to investigate diffusion tensor imaging (DTI) changes in different regions of normal-appearing spinal cord (NASC) in relapsing-remitting multiple sclerosis (RRMS).
METHODS: Axial DTI of the cervical spinal cord was performed in 24 patients with RRMS and 24 age- and sex-matched control subjects. Fractional anisotropy (FA) and mean diffusivity (MD) were calculated in separate regions of interest (ROIs) in the anterior, lateral, and posterior spinal cord, bilaterally, and the central spinal cord, at the C2-C3 level. Patients and control subjects were compared with respect to FA and MD with the use of an exact Mann-Whitney test. Logistic regression and receiver operating characteristic (ROC) curve analysis assessed the utility of each measure for the diagnosis of RRMS.
RESULTS: DTI metrics in areas of NASC in MS were significantly different in patients compared with control subjects; FA was lower in the lateral (mean ± SD of 0.56 ± 0.10 versus 0.69 ± 0.09 in control subjects, P < .0001), posterior (0.52 ± 0.11 versus 0.63 ± 0.10, P < .0001), and central (0.53 ± 0.10 versus 0.58 ± 0.10, P = .049) NASC ROIs. Assessing DTI metrics in the diagnosis of MS, a sensitivity of 87.0% (95% confidence interval [CI], 66.4 to 97.1) and a specificity of 91.7% (95% CI, 73.0 to 98.7) were demonstrated.
CONCLUSION: The NASC in RRMS demonstrates DTI changes. This may prove useful in detecting occult spinal cord pathology, predicting clinical course, and monitoring disease progression and therapeutic effect in MS.
Evaluation of the spinal cord has become increasingly important in both the diagnosis and follow-up of multiple sclerosis (MS), in that changes in the spinal cord function of patients with MS have been shown to correlate with clinical disability.1,2 The mechanism by which MS affects the normal-appearing spinal cord (NASC) is unknown and may relate to Wallerian degeneration, a primary ischemic/vasculitic process, or early local demyelination. Diffusion tensor imaging (DTI) shows promise as a technique for the evaluation of the cervical spinal cord3 and may be able to detect changes in NASC that precede detectable spinal cord signal intensity change and volume loss in patients with MS. Changes in patients with MS have recently been detected in the cervical spinal cord with the use of sagittal DTI technique and evaluating large regions of interest (ROIs) with the use of histogram analysis.4,5 However, it is generally accepted that lesions in primary demyelination have a predilection for the posterior columns of the spinal cord6; therefore, analysis of DTI metrics in different regions of the spinal cord may demonstrate spatial differences. In this study, we used axial DTI to evaluate focal regions of the NASC corresponding to the known location of various white matter tracts, comparing DTI metrics of patients with relapsing-remitting MS (RRMS) to those of age- and sex-matched normal volunteers. Our primary hypothesis was that changes in DTI metrics are present in the NASC in the setting of RRMS. Our secondary hypothesis was that spatial information obtained with axial DTI will improve the sensitivity and specificity of DTI in the detection of MS compared with the mean of metrics or the interrogation of large ROIs, thereby illustrating the importance of investigating different spinal cord tracts.
Methods
Subjects.
We studied 24 patients with RRMS referred to our tertiary care hospital as part of ongoing research. There was no clinical suspicion of an acute MS attack in any of the patients at the time of imaging. The mean patient age was 46.4 years (range, 29.6–68.8 years; median, 48.1 years). There were 18 female and 6 male patients. We also studied 24 age- and sex-matched healthy volunteers using the same protocol, as described below. The mean age of the healthy volunteers was 46.4 years (range, 28.1–67.3 years; median, 45.9 years, P = .8). Approval for this study was obtained from the Institutional Board of Research Associates.
MR Imaging.
MR imaging of the cervical spine was performed at 1.5T. Localizing sagittal and coronal T1-weighted imaging was obtained followed by turbo spin-echo sagittal T1- and T2-weighted imaging, as well as axial turbo spin-echo T2-weighted and spin-echo T1-weighted imaging. Axial DTI of the upper cervical spinal cord was then performed using pulsed gradient, spin-echo, echo-planar imaging (repetition time [TR]/echo time [TE], 2000/74; matrix, 128 × 128; field of view, 140 × 140 mm; 10 contiguous 4-mm sections; b = 1000 s/mm2; acquisition time, 2 minutes, 20 seconds). Diffusion weighting was applied along 6 noncollinear axes, (±1, 1, 0), (±1, 0, 1), and (1, ±1, 0). A seventh null image without diffusion weighting (b = 0) was also obtained for each section. The pixel size was 1.1 × 1.1 mm2. The DTI imaging plane was parallel to the conventional axial images, perpendicular to the long axis of the spinal cord.
Image Processing.
Quantitative analysis of DTI data were performed using Leonardo VD10B software on a Syngo VX49B imaging software platform (Siemens Medical Solutions, Erlangen, Germany). DTI maps were generated by the DTI task card developed at the Magnetic Resonance Center of Massachusetts General Hospital (Boston, Mass). Mean diffusivity (MD) and fractional isotropy (FA) were calculated for each voxel from the eigenvalues λn as follows:
 |
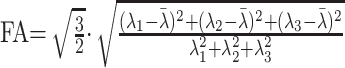 |
Gray-scale maps of these DTI metrics were generated from which the FA and MD can be measured in specific ROIs. For each patient, FA and MD were measured in ROIs placed in NASC at the anterior, lateral, and posterior regions of the spinal cord at the C2–C3 vertebral level, with separate bilateral ROIs at each of these positions (Fig 1). To approximate the known locations of the spinothalamic tracts anteriorly and posterior columns posteriorly, and to allow for consistent placement in patients with varying cord morphology, the anterior and posterior ROIs were placed in a paramidline location. A small distance was maintained between the ROIs and the edge of the cord to minimize volume averaging with the adjacent CSF. The lateral ROIs were placed to approximate the location of the corticospinal tracts. A seventh, larger ROI was also placed centrally within the cord, covering the expected location of both gray and white matter.
Fig 1.

Diagram of the cervical spinal cord in cross-section (A) showing regions of interest (ROIs) placed for the measurement of fractional anisotropy (FA) and mean diffusivity in the anterior, lateral, and posterior spinal cord, bilaterally.
B, Color FA map obtained in control patient at the C2-C3 level.
C, The corresponding black and white FA map is shown with ROIs placed, including a central region of interest. Values have been scaled by a factor of 1000.
The FA metrics in each ROI, respectively, are henceforth referred to as FAant,left, FAant,right, FApost,left, FApost,right, FAlat,left, FAlat,right, and FAcentral. The mean FA, FAmean, was calculated by using these 6 metrics, approximating the overall FA of spinal cord white matter tracts. MD metrics are referred to in a similar fashion, with the mean MD, MDmean, calculated as well. Metrics in the larger central ROI are referred to as FAcentral and MDcentral, respectively.
Statistical Analysis.
The data for each subject consisted of 1 FA and 1 MD measure within each of 7 spinal cord ROIs at the C2-C3 level (central, left and right anterior, left and right posterior, left and right lateral), each measure computed as the average overall voxels within the respective region of interest, as well as the average FA and the average MD overall ROIs except the central. Patients and control subjects were compared with respect to the FA and MD measures in each location with the use of an exact Mann-Whitney test. Logistic regression and receiver operating characteristic (ROC) curve analysis was used to assess the utility of the regional and average FA and MD measures for the diagnosis of RRMS after accounting for patient age and sex. To assess and compare the relative utility of FA versus MD and the regional versus the average measures, stepwise variable selection was used in the context of the logistic regression analysis to identify a subset of significant and independent predictors of disease status (RRMS versus control) from among: case 1, all available FA measures; case 2, all FA measures except the mean (to assess whether the regional assessments are sufficient); case 3, all available MD measures; case 4, all regional MD measures; case 5, all available FA and MD measures together. The diagnostic prediction models identified by the 5 cases were pairwise compared in terms of area under the ROC curve (AUC) by using a jackknife procedure. All statistical computations were performed by using SAS version 9.0 (SAS Institute, Cary, NC) and results were declared statistically significant at the 2-sided 5% significance level.
Results
DTI metrics were calculated at the C2-C3 spinal level within a total of 168 ROIs in 24 patients with MS as well as 168 ROIs in 24 age- and sex-matched healthy volunteers. Table 1 shows the mean FA and MD in the various regions of the spinal cord. FA was significantly lower in the lateral, posterior, and central spinal cord ROIs in the patients with MS compared with control subjects. MD was higher in all regions of the ROIs in the patients with MS, failing to meet statistical significance except in the right lateral and left posterior ROIs (Table 1). Evaluation of conventional imaging revealed no T2 hyperintense lesions in the region of DTI interrogation in any of the patients.
Table 1:
Average fractional anisotropy and mean diffusivity in regions of interest in the anterior, lateral, posterior, and central spinal cord at the C2–C3 level, in 24 patients with multiple sclerosis and 24 age- and sex-matched normal volunteers
| Anterior |
Lateral |
Posterior |
Central | ||||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||
| Fractional anisotropy | |||||||
| Cases (n = 24) | 0.50 ± 0.12 | 0.50 ± 0.12 | 0.56 ± 0.12 | 0.55 ± 0.10 | 0.52 ± 0.11 | 0.52 ± 0.12 | 0.53 ± 0.10 |
| Controls (n = 24) | 0.51 ± 0.09 | 0.49 ± 0.10 | 0.69 ± 0.11 | 0.69 ± 0.10 | 0.63 ± 0.11 | 0.64 ± 0.11 | 0.58 ± 0.10 |
| P values | .63 | .67 | <.0001 | <.0001 | .001 | .001 | .05 |
| Mean diffusivity (×10−3 mm−2s−1) | |||||||
| Cases | 0.92 ± 0.21 | 0.99 ± 0.30 | 0.91 ± 0.29 | 0.92 ± 0.26 | 0.92 ± 0.22 | 0.92 ± 0.24 | 0.89 ± 0.18 |
| Controls | 0.86 ± 0.17 | 0.86 ± 0.17 | 0.79 ± 0.16 | 0.75 ± 0.25 | 0.80 ± 0.11 | 0.81 ± 0.12 | 0.81 ± 0.14 |
| P values | .33 | .06 | .06 | .02 | .03 | .06 | .077 |
Logistic regression analysis in the 5 cases (see Statistical Analysis, above) returned a total of 3 distinct prediction models: cases 1 and 5 resulted in a model based on FAmean, FAant,right, and FAcentral (model 1); case 2 resulted in a model based on FAant,right and FAlat,left (model 2); and cases 3 and 4 resulted in a model based on MDlat,right (model 3). Results are summarized for each of these 3 prediction/diagnostic models in Table 2. The most accurate model, based on FAmean, FAant,right, and FAcentral (model 1), demonstrates a sensitivity of 87.0% (95% confidence interval [CI], 66.4 to 97.1) and a specificity of 91.7% (95% CI, 73.0 to 98.7).
Table 2:
Three models for the prediction of multiple sclerosis
| Model | Effect | df | Wald Chi-Square | P Value |
|---|---|---|---|---|
| 1 | Sex* | 1 | 2.5362 | .1113 |
| Age* | 1 | 0.8098 | .3682 | |
| FAmean | 1 | 8.5093 | .0035 | |
| FAant, right | 1 | 7.3482 | .0067 | |
| FAcentral | 1 | 4.5314 | .0333 | |
| 2 | Sex* | 1 | 0.8292 | .3625 |
| Age* | 1 | 0.0219 | .8823 | |
| FAant, right | 1 | 4.8448 | .0277 | |
| FAlat, left | 1 | 13.6320 | .0002 | |
| 3 | Sex* | 1 | 0.0031 | .9555 |
| Age* | 1 | 0.0008 | .9776 | |
| MDlat, right | 1 | 5.1438 | .0233 |
Note:—FA indicates fractional anisotropy; MD, mean diffusivity; subscripts ant and lat, anterior and lateral; df, degrees of freedom.
Forced into model.
Pairwise comparison of model 1 with a model that used only FAmean showed a significantly higher AUC for the former (Fig 2A, AUC = 0.928 and 0.748, respectively, P = .012). Comparison of model 1 with a model that used only FAcentral also showed a significantly higher AUC for model 1 (Fig 2B, AUC = 0.928 and 0.654, respectively, P = .001). Model 1, which uses only FA measures, also showed a significantly higher AUC than model 3, which uses only MDlat,right (AUC = 0.928 and 0.879, respectively, P = .001). Model 2, which also uses spatial FA measures, showed a significantly higher AUC than model 3 (AUC = 0.879 and 0.669, respectively, P = .008).
Fig 2.
Receiver operating characteristic (ROC) curve for diagnostic model using fractional anisotropy (FA)mean, FAant,right, and FAcentral (model 1, solid line in 2A and 2B, area under ROC curve (AUC), 0.928; 95% confidence interval [CI], 0.813 to 0.982), showing a significantly higher AUC compared with a model using only FAmean (2A, thick dotted line, difference between AUCs = 0.180, P = .012) as well as a model using only FAcentral (2B, thick dotted line, difference between AUCs = 0.274, P = .001). In other words, the best performing test is one in which multiple ROIs provide spatial information.
None of the FA and MD measures exhibited a significant association with age (P > .15). This was true for the data as a whole (data from both patients and control subjects included in one analysis) as well as when the data from patients and control subjects were analyzed separately. Scatterplots of the diffusion tensor metrics versus age in the control group are shown in Fig 3.
Fig 3.
Diffusion tensor imaging (DTI) metrics of healthy patients within ROIs placed in the anterior, lateral, and posterior spinal cord (bilaterally, with 2 data points per location per patient) at the C2-C3 level. Fractional anisotropy (FA) versus age (A) and mean diffusivity (MD) versus age (B). None of the FA or MD measures exhibited a significant association with patient age (P > .15).
Discussion
The current recommended radiologic diagnostic criteria for MS involve assessing the number and location of brain and spinal cord lesions, as well as presence of enhancing lesions, on MR imaging.7 However, in clinical practice, patients often present with clinically suspected MS, with conventional MR findings that fail to meet criteria for diagnosis. Furthermore, MS is a disease that affects both the brain and spinal cord, including areas of gray matter and white matter that appear normal on conventional MR imaging.8–10 In recent years, changes in both normal-appearing gray matter (NAGM) and normal-appearing white matter (NAWM) in the brain have been demonstrated with the use of advanced MR techniques such as dynamic perfusion imaging, MR spectroscopy, and magnetization transfer, as well as DTI.11–18 These findings may prove clinically useful in the radiologic diagnosis of MS in patients with equivocal findings at conventional MR imaging. In this cross-sectional case-control study, we have shown the ability of axial DTI to detect occult changes in the cervical spinal cord. In future investigations, DTI (in combination with other MR imaging techniques such as spectroscopy and perfusion MR imaging) may provide insight into whether the pathophysiology of MS within the spinal cord is related to Wallerian degeneration or a primary ischemic phenomenon.
In the diagnosis of RRMS by using DTI metrics from the NASC, we have found that models using spatial DTI metrics are significantly more accurate than models using the mean of metrics or central cord interrogation alone; we also found that models that used FA were superior to those that used MD. These findings serve to illustrate the importance of spatial information in DTI evaluation of the spinal cord.
DTI measures free diffusion of water molecules, with directional information obtained by applying diffusion gradients along 6 or more noncollinear directions. In that the free diffusion of water molecules is hindered by factors including cell membranes and myelin, DTI has shown theoretic promise in detecting and evaluating the integrity of white matter tracts; in animal models, DTI metrics in the various spinal cord tracts have been shown to correlate with specific histologic measures including axon counts, extracellular and myelin volume, and axon diameter and spacing.3
Bot et al19 have investigated the spinal cords of patients with MS postmortem using high-field MR imaging with histopathologic correlation. At histopathology, they demonstrated significant axonal loss, increase in axonal diameter, and decreased myelin attenuation in NASC of patients with MS. A strong correlation between demyelination and quantitative MR imaging parameters (T1 and T2 relaxation times and magnetization transfer measurements) was demonstrated; however, these parameters were insensitive to changes in axonal attenuation. The researchers also suggested that the decrease in spinal cord area seen in MS may relate to demyelination in addition to axonal attenuation, potentially limiting the utility of this quantitative technique in the assessment of axonal damage. Given these postmortem findings as well as the histopathologic correlation in animal models, it is plausible that the measurement of DTI metrics may prove superior to other quantitative techniques in the quantification of axonal pathology. In addition, DTI may prove superior to measurement of spinal cord area in evaluating axonal damage, though the latter technique of atrophic measure has been shown to correlate significantly with patient disability.1
In formulating quantitative diagnostic criteria for MS by using DTI metrics, DTI interrogation of the NASC may be more useful than interrogation of regions of signal intensity abnormality. Changes in DTI metrics have been demonstrated within focal spinal cord plaques.20 However, these findings are nonspecific; similar changes in DTI metrics have also been demonstrated in regions of signal intensity abnormality in patients with myelomalacia secondary to chronic spondylotic spinal cord compression.21,22 Furthermore, changes seen in the regions of spinal cord plaques, which probably reflect only the local effects of MS, may not relate to patient morbidity as well as changes seen in NASC, which are likely reflect both the local effects of MS and indirect effects related to brain lesions. In a large cross-sectional postmortem study, Evangelou et al23 suggested axonal degeneration or an alternative atrophic process as the primary contributor to spinal cord atrophy in MS, rather than local lesion load; the researchers also suggested that spinal cord atrophy in MS is more prominent in the cervical and upper thoracic spinal cord as opposed to the lower spinal cord. Bergers et al24 similarly demonstrated extensive axonal loss in postmortem analysis of the spinal cords of patients with MS, largely independent of local lesion load seen at T2-weighted MR imaging.24
Clinical diffusion-weighted imaging and DTI of the cervical spinal cord remains technically difficult. The small size of the spinal cord necessitates the employment of small voxel sizes for spatial resolution at the expense of signal intensity–to-noise ratio. Images may be degraded because of macroscopic motion related to CSF pulsation, patient breathing, or gross patient motion. Local field inhomogeneity also contributes to image degradation. Echo-planar DTI can be performed in a reasonable amount of time for clinical use, and single-shot technique may limit the adverse effect of in-plane bulk motion to some extent. In the future, new techniques using parallel imaging,25 as well as pulse-triggering or cardiac gating, should further minimize image degradation.
In the analysis of DTI maps, care must be taken to avoid including CSF in the ROIs, which would produce a misleading change in DTI metrics (decreased FA and increased MD). To guard against this effect in this study, we correlated the DTI maps with conventional images when placing ROIs. The calculated SDs of the DTI metrics within the individual ROIs remained relatively low, implying a homogenous sample and rendering CSF partial volume-averaging effects less likely to be present. Given the known normal morphometry of the cervical spinal cord in cross section as well as the resolution of our technique, it is likely that some gray matter was included in the anterior spinal cord ROIs (Fig 1A). This may explain the relatively lower FA seen in the anterior spinal cord in both the patients with MS and in the control group, as well as explain the decreased ability of metrics measured in this region to discriminate disease from control groups. The ability to resolve the anterior and anterolateral spinal cord tracts from both gray matter and CSF may improve as higher-resolution techniques at higher field strength become available.
Conclusion
FA was significantly lower in the NASC of patients with MS in the lateral, posterior, and central cord compared with control subjects. The measurement of DTI metrics in the cervical spinal cord may prove useful in aiding the diagnosis of MS, correlating with clinical disability, as well as monitoring disease progression and therapeutic effect.
Footnotes
This work was presented at the annual meeting of the American Society of Neuroradiology, May 2005, Toronto, Canada.
References
- 1.Lin X, Tench CR, Turner B, et al. Spinal cord atrophy and disability in multiple sclerosis over four years: application of a reproducible automated technique in monitoring disease progression in a cohort of the interferon {beta}-1a (Rebif) treatment trial. J Neurol Neurosurg Psychiatry 2003;74:1090–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losseff N, Webb S, O’Riordan J, et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996;119:701–08 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz ED, Cooper ET, Chin C-L, et al. Ex vivo evaluation of ADC values within spinal cord white matter tracts. AJNR Am J Neuroradiol 2005;26:390–97 [PMC free article] [PubMed] [Google Scholar]
- 4.Valsasina P, Rocca MA, Agosta F, et al. Mean diffusivity and fractional anisotropy histogram analysis of the cervical cord in MS patients. NeuroImage 2005;26:822–28 [DOI] [PubMed] [Google Scholar]
- 5.Agosta F, Benedetti B, Rocca MA, et al. Quantification of cervical cord pathology in primary progressive MS using diffusion tensor MRI. Neurology 2005;64:631–35 [DOI] [PubMed] [Google Scholar]
- 6.Thielen KR, Miller GM. Multiple sclerosis of the spinal cord: magnetic resonance appearance. J Comput Assist Tomogr 1996;20:434–38 [DOI] [PubMed] [Google Scholar]
- 7.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–27 [DOI] [PubMed] [Google Scholar]
- 8.Miller DH, Barkhof F, Frank JA, et al. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 2002;125:1676–95 [DOI] [PubMed] [Google Scholar]
- 9.De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 2003;60:1157–62 [DOI] [PubMed] [Google Scholar]
- 10.Sailer M, Fischl B, Salat D, et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain 2003;126:1734–44 [DOI] [PubMed] [Google Scholar]
- 11.Law M, Saindane AM, Ge Y, et al. Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology 2004;231:645–52 [DOI] [PubMed] [Google Scholar]
- 12.Davie CA, Barker GJ, Thompson AJ, et al. 1H magnetic resonance spectroscopy of chronic cerebral white matter lesions and normal appearing white matter in multiple sclerosis. J Neurol Neurosurg Psychiatry 1997;63:736–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husted CA, Goodin DS, Hugg JW, et al. Biochemical alterations in multiple sclerosis lesions and normal-appearing white matter detected by in vivo 31P and 1H spectroscopic imaging. Ann Neurol 1994;36:157–65 [DOI] [PubMed] [Google Scholar]
- 14.Filippi M, Rocca MA, Minicucci L, et al. Magnetization transfer imaging of patients with definite MS and negative conventional MRI. Neurology 1999;52:845–48 [DOI] [PubMed] [Google Scholar]
- 15.Ge Y, Law M, Johnson G, et al. Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 2004;20:1–7 [DOI] [PubMed] [Google Scholar]
- 16.Wilson M, Tench CR, Morgan PS, et al. Pyramidal tract mapping by diffusion tensor magnetic resonance imaging in multiple sclerosis: improving correlations with disability. J Neurol Neurosurg Psychiatry 2003;74:203–07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loevner LA, Grossman RI, Cohen JA, et al. Microscopic disease in normal-appearing white matter on conventional MR images in patients with multiple sclerosis: assessment with magnetization-transfer measurements. Radiology 1995;196:511–15 [DOI] [PubMed] [Google Scholar]
- 18.Inglese M, Ge Y, Filippi M, et al. Indirect evidence for early widespread gray matter involvement in relapsing-remitting multiple sclerosis. NeuroImage 2004;21:1825–29 [DOI] [PubMed] [Google Scholar]
- 19.Bot JCJ, Blezer ELA, Kamphorst W, et al. The spinal cord in multiple sclerosis: relationship of high-spatial-resolution quantitative MR imaging findings to histopathologic results. Radiology 2004;233:531–40 [DOI] [PubMed] [Google Scholar]
- 20.Clark CA, Werring DJ, Miller DH. Diffusion imaging of the spinal cord in vivo: estimation of the principal diffusivities and application to multiple sclerosis. Magn Reson Med 2000;43:133–38 [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya K, Katase S, Fujikawa A, et al. Diffusion-weighted MRI of the cervical spinal cord using a single-shot fast spin-echo technique: findings in normal subjects and in myelomalacia. Neuroradiology 2003;45:90–94 [DOI] [PubMed] [Google Scholar]
- 22.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging 2005;22:38–43 [DOI] [PubMed] [Google Scholar]
- 23.Evangelou N, DeLuca GC, Owens T, et al. Pathological study of spinal cord atrophy in multiple sclerosis suggests limited role of local lesions. Brain 2005;128:29–34 [DOI] [PubMed] [Google Scholar]
- 24.Bergers E, Bot JCJ, De Groot CJA, et al. Axonal damage in the spinal cord of MS patients occurs largely independent of T2 MRI lesions. Neurology 2002;59:1766–71 [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya K, Fujikawa A, Suzuki Y. Diffusion tractography of the cervical spinal cord by using parallel imaging. AJNR Am J Neuroradiol 2005;26:398–400 [PMC free article] [PubMed] [Google Scholar]




