Abstract
SUMMARY: We present our initial experience with the use of a modified 3-point Dixon technique to obtain reliable fast spin-echo T1- and T2-weighted fat-suppressed images in the soft-tissue neck. The method has less sensitivity to magnetic field inhomogeneity than frequency-selective radiofrequency fat saturation and provides uniform fat suppression even near tissue-tissue and air-tissue interfaces. Clinical advantages and limitations of the method are discussed and several examples are shown.
MR imaging of the extracranial head and neck requires effective fat signal intensity suppression. Conventional chemical shift selection suppression (CHESS) is strongly dependent on main magnetic field (B0) homogeneity. B0 inhomogeneity in head and neck imaging is substantial due to tissue-tissue and air-tissue interfaces and difficult to completely remove by shimming. Residual inhomogeneity may result in inadequate fat suppression or suppression of water signal intensity. The modified 3-point Dixon (3PD) technique has evolved into a robust and clinically practical tool for fat signal intensity suppression.
Description of the Technique
The original 2-point Dixon method1 was developed for fat and water image separation by utilizing differences in chemical shifts but was sensitive to field inhomogeneity. Modified Dixon techniques include a third acquisition that results in a robust sequence that is able to reliably provide for fat and water separation, even in the presence of magnetic field inhomogeneity.2,3 There has been increasing interest in using 3PD methods in clinical practice because uniform and reliable fat suppression can be of substantial clinical value.4,5 The 3PD method may be implemented in a variety of pulse sequences. Most of our head and neck protocols include fast spin-echo (FSE) T1- or T2-weighted images. Thus, in this work, we utilize the FSE-IDEAL technique (Iterative Decomposition of water and fat with Echo Asymmetry and Least squares estimation), a prerelease General Electric 3PD-FSE pulse sequence that can be used for both T1 and T2-weighted imaging.6
In general, 3PD imaging consists of a repetitive, phase-shifted acquisition (pulse sequence) and specialized reconstruction software. For each echo train acquired by a conventional FSE sequence, the modified 3PD sequence repeats the acquisition 3 times, with differing echo shifts for each repetition. For FSE-IDEAL, echo shifts of −π/6, π/2, and 7π/6 are chosen, an asymmetric sampling scheme that optimizes the signal intensity–to-noise ratio (SNR).7 The echo shift is obtained by increasing the interecho spacing of the readout train.8,9
Specialized reconstruction software is required to analyze the phase information from the 3 acquisitions to calculate separate fat and water images. Postprocessing of the acquired data is performed at the scanner console. The reconstruction software uses an iterative phase correction algorithm.6,9,10 In this way, the phase offset due to inhomogeneity can be calculated and eliminated, allowing the additional information from phase shifting to be used to generate separate fat and water images. For multichannel coils, the data from each coil element are analyzed separately and then combined to form the final image.
Our institution has institutional review board approval for investigational pulse sequences to be used for clinical patients. Additional institutional review board approval was obtained to retrospectively evaluate the utility of the method. Imaging was performed by using a 1.5T Signa scanner with EXCITE 11.0 software (GE Medical Systems, Milwaukee, Wis) and 4- and 8-channel neurovascular phased-array coils. Prerelease GE FSE-IDEAL pulse sequence and reconstruction software were used. The acquisition parameters were chosen to approximate the parameters of a conventional fat-saturation sequence frequently used in clinical practice. Typical parameters for our T1-weighted images were TR 600, TE(eff) 11.5, 256 × 192 matrix, and an echo train length of 4. Typical parameters for our T2-weighted images were TR 2553, TE(eff) 97.3, 256 × 192 matrix, and an echo train length of 11. The largest variation from the equivalent fat saturated FSE sequence is the number of excitations (NEX). Any 3PD method has essentially a multiple NEX acquisition intrinsic to the pulse sequence design (3 excitations are required for each phase encode) which all contribute to SNR. As we routinely chose 2–3 NEX for our CHESS-FSE thin-section head and neck imaging protocols to maintain adequate SNR, scan times are not greatly different. For acquisition of sections through the lower neck, we used a 2 NEX FSE-IDEAL for additional SNR improvement. Images were available for on-line review typically several minutes after the scan completed.
Discussion
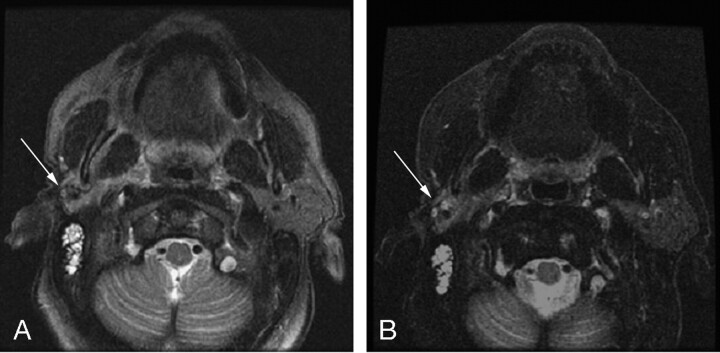
We have found the 3PD method of fat suppression to be a useful tool in our clinical practice. With CHESS methods for fat suppression, we occasionally have situations in which, despite careful shimming, fat signal intensity is inadequately suppressed. Figure 1 shows a clinical example of benefits of FSE-IDEAL over a CHESS-FSE sequence. This patient was referred for follow-up imaging after undergoing a right parotidectomy. The clinical question was whether palpable fullness was due to residual parotid tissue or recurrent tumor. Initial images obtained with CHESS-FSE (Fig 1A) showed inconsistent fat suppression despite careful shimming, and it is difficult to separate parotid tissue from poorly saturated fat. FSE-IDEAL images (Fig 1B) provided better fat suppression and more confident identification of residual parotid tissue.
Fig 1.
T2-weighted axial images obtained by using (A) CHESS-FSE sequence with a scan time of 2:32 minutes and (B) FSE-IDEAL sequence with a scan time of 2:33 minutes. The patient had a history of right parotidectomy for poorly differentiated parotid carcinoma. Despite careful shimming, fat signal intensity in the region of the operative bed (arrow) in the CHESS-FSE sequence is not clearly suppressed. The FSE-IDEAL image has uniform fat suppression throughout the field, allowing for better visualization of residual parotid parenchyma (arrow).
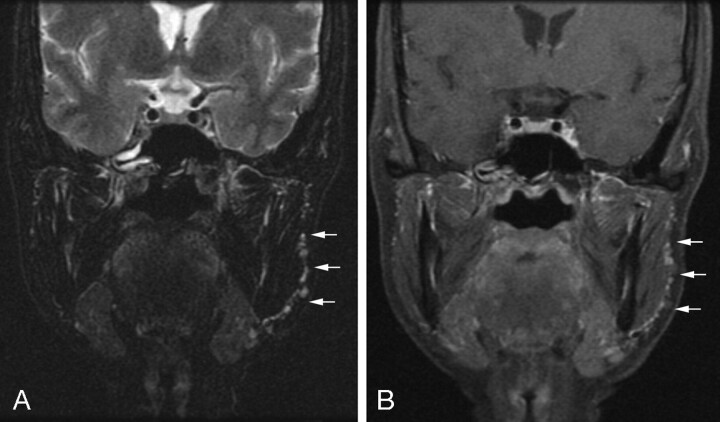
In general, the method can provide uniform fat suppression consistently throughout the field of view. Figure 2 shows coronal T2 and postgadolinium T1-weighted images obtained by using the FSE-IDEAL in a patient who had previously undergone left parotidectomy for pleomorphic adenoma. Uniform fat suppression is seen throughout the field of view. Tiny foci of increased T2 signal intensity and enhancement are easily identified overlying the left masseter, consistent with residual spilled pleomorphic adenoma. In many situations, the availability of a 3PD sequence as an additional (or primary) method for uniform fat suppression made clinical decisions substantially easier by effectively suppressing confounding fat signal intensity.
Fig 2.
Coronal T2-weighted (A) and postgadolinium T1-weighted (B) FSE-IDEAL water images. Numerous small tumor droplets (arrows) from a spilled pleomorphic adenoma are seen overlying the left masseter.
Two primary limitations of 3PD imaging include the additional time required for acquisition/reconstruction and the need for specialized reconstruction software. The method does require 3 acquisitions for each phase encode, which imposes some limitations of coverage and resolution to maintain reasonable scan times. Most head and neck protocols at our institution, however, use multiple NEX acquisitions to guarantee an adequate SNR. The 3 acquisitions required with the Dixon technique do contribute to increasing SNR, and the overall image signal intensity is theoretically equivalent to a 3-NEX acquisition6,7 Scan times remain feasible for use under clinical time restraints. We think that the value of the uniform fat suppression obtained by using the method justifies modestly increased scan times.
Reconstruction times have also greatly improved and are no longer clinically limiting. Earlier uses of Dixon methods were restrained by the computational power required for performing the phase corrections or the need for off-line reconstruction. This is becoming less of a factor with newer implementations. Reconstructions are set up and performed at the scanner console and can be performed while running subsequent sequences. With the current implementation of the reconstruction software, reconstructions are typically completed by the end of our next sequence (several minutes). Reconstruction time can be anticipated to decrease with further improvements in computational power and efficiency of reconstruction.
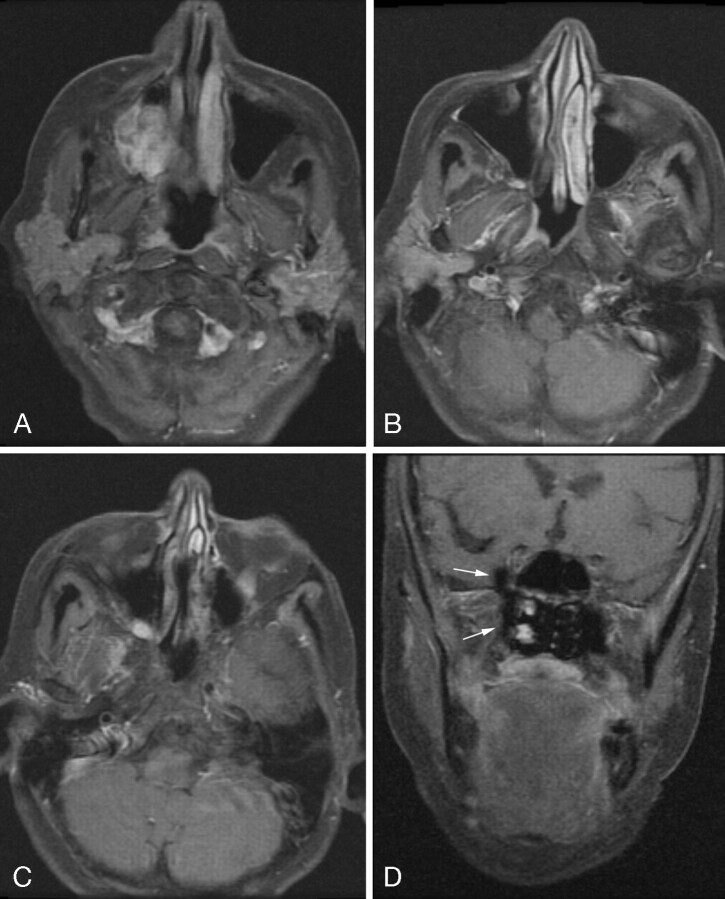
However, because the acquired data are analyzed and fat-water images are generated (rather than the physical fat signal intensity provided by radio frequency selective methods), reconstruction errors may still occur. In our experience, erroneous assignment of fat signal intensity to the water image and vice versa is the primary limitation. In Fig 3, the FSE-IDEAL sequence was used in a patient with a right maxillary sinus polymorphous adenocarcinoma. The coronal postgadolinium FSE T1-weighted 3PD water image (D) is diagnostically limited because of an unfortunate fat-water mismap at the right pterygopalatine fossa. These errors can be exacerbated by slight errors in centering the water peak in prescan. The method still requires per-patient shimming to avoid regions of gross field inhomogeneity, which can otherwise disrupt the reconstruction algorithm. These errors have an artifactual appearance and are usually fairly easily identified. It is expected that with further advances in reconstruction techniques, such as auto frequency centering, these artifacts will be further minimized.
Fig 3.
Axial postgadolinium FSE-IDEAL T1-weighted images (A–C) demonstrate a polymorphous adenocarcinoma of the right maxillary sinus extending through the pterygopalatine fossa to the inferior orbital fissure. Coronal postgadolinium T1-weighted FSE-IDEAL image (D) is diagnostically limited due to unfortunate fat-water mismap at the right pterygopalatine fossa (arrows).
Overall, modified 3PD imaging promises to be an effective and reliable method for fat suppression for imaging the extracranial head and neck. The implementation with a FSE sequence allows for both T1- and T2-weighted imaging with efficient overall scan times. The uniformity of fat suppression appears to be superior to fat saturation in regions of magnetic field inhomogeneity. Scan times are minimally increased compared with a multiple NEX, conventional fat-saturated FSE sequence.
Acknowledgments
We thank Dave Stanley for his assistance with this work.
Footnotes
Presented at the 43rd annual meeting of the American Society of Neuroradiology, Toronto, Ontario, Canada.
References
- 1.Dixon WT. Simple proton spectroscopic imaging. Radiology 1984;153:189–94 [DOI] [PubMed] [Google Scholar]
- 2.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med 1991;18:371–83 [DOI] [PubMed] [Google Scholar]
- 3.Glover GH. Multipoint Dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging 1991;1:521–30 [DOI] [PubMed] [Google Scholar]
- 4.Rybicki FJ, Mulkern RV, Robertson RL, et al. Fast three-point Dixon MR imaging of the retrobulbar space with low-resolution images for phase correction: comparison with fast spin-echo inversion recovery imaging. AJNR Am J Neuroradiol 2001;22:1798–802 [PMC free article] [PubMed] [Google Scholar]
- 5.Ma J, Singh SK, Kumar AJ, et al. T2-weighted spine imaging with a fast three-point Dixon technique: comparison with chemical shift selective fat suppression. J Magn Reson Imaging 2004;20:1025–29 [DOI] [PubMed] [Google Scholar]
- 6.Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med 2005;54:636–44 [DOI] [PubMed] [Google Scholar]
- 7.Pineda AR, Reeder SB, Wen Z, et al. Cramer-Rao bounds for three-point decomposition of water and fat. Magn Reson Med 2005;54:625–44 [DOI] [PubMed] [Google Scholar]
- 8.Hardy PA, Hinks RS, Tkach JA. Separation of fat and water in fast spin-echo MR imaging with the three-point Dixon technique. J Magn Reson Imaging 1995;5:181–85 [DOI] [PubMed] [Google Scholar]
- 9.Reeder SB, Wen Z, Yu H, et al. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med 2004;51:35–45 [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Reeder SB, Shimakawa A, et al. Field map estimation with a region growing scheme for iterative 3-point water-fat decomposition. Magn Reson Med 2005;54:1032–39 [DOI] [PubMed] [Google Scholar]