Abstract
Summary: Primary amebic meningoencephalitis and granulomatous amebic meningoencephalitis are central nervous system infections caused by free-living amebae. We describe the neuroimaging findings in 5 such cases on CT and MR imaging. A spectrum of findings was seen in the form of multifocal parenchymal lesions, pseudotumoral lesions, meningeal exudates, hemorrhagic infarcts, and necrosis in the brain. Familiarity with the imaging findings is important for the diagnosis and management of this nearly universally fatal disease.
Imaging features of amebic meningoencephalitis are nonspecific and have only rarely been described in previous literature.1,2 We describe the imaging of 5 proved cases of amebic meningoencephalitis and try to familiarize both the unsuspecting radiologist and the clinician with the diverse spectrum of neuroimaging findings. To the best of our knowledge, this is the largest single short series describing the CT and MR imaging features of this entity.
Subjects and Methods
The imaging findings on initial CT and MR imaging (1.5T unit) of the brain in 5 proved cases of amebic meningoencephalitis were retrospectively analyzed. Both non contrast–enhanced and contrast-enhanced MR imaging were performed. The diagnosis was confirmed at autopsy and histopathologic examination in 4 cases and CSF culture in 1 case. The first 4 cases were of granulomatous amebic meningoencephalitis (GAE) and the fifth case was of primary amebic meningoencephalitis (PAM).
Case 1
A 14-year-old presented to the neurosurgery department after she collapsed from an episode of tonic-clonic seizures. She had experienced a similar history of seizures with loss of consciousness 3 months before the present episode. There was no history of opportunistic infections and no other significant medical or travel history. On presentation, she was afebrile and had a mild right hemiparesis with neck rigidity. CSF examination revealed a normal glucose level (63 mg/dL), normal white blood cell count, but an elevated protein count (52 mg/dL). CT examination of the brain revealed an ill-defined hypoattenuated lesion in the left temporoparietal area. MR imaging revealed multifocal involvement of the brain. (Fig 1A-C). The largest lesion in the left parietal lobe resembled a mass lesion and showed a linear pattern of enhancement (Fig 1D). A diagnosis of central nervous system (CNS) lymphoma was considered and a brain biopsy was performed.
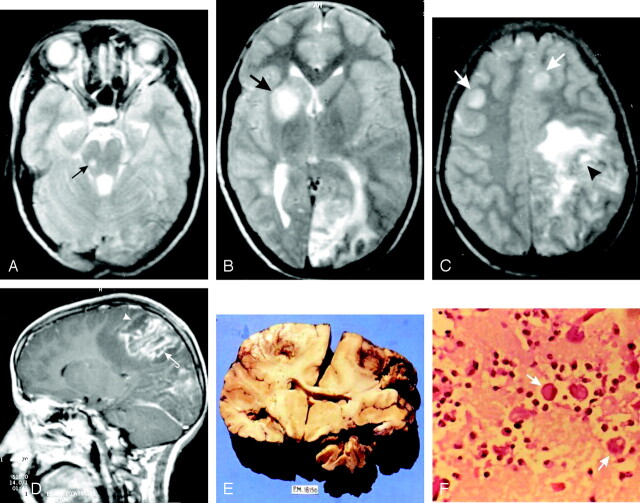
Fig 1.
A 14-year-old girl with a history of seizures and loss of consciousness (GAE).
A–C, Axial T2-weighted images show multiple discrete focal hyperintense lesions at the corticomedullary junctions, basal ganglia, and midbrain (arrows). A masslike lesion is seen in the left parietal lobe with perilesional edema (arrowhead, C).
D, Postcontrast sagittal T1-weighted image shows a masslike lesion in the left parietal lobe (arrowhead), with a linear pattern of enhancement (curved arrow). The other focal lesions show no postcontrast enhancement.
Coronal cut section of the brain (E) shows multiple bilateral hemorrhagic necrotic lesions largely confined to the gray-white junctions. High-power photomicrograph (F) shows several large rounded amebic trophozoites (arrows), surrounded by inflammatory cells against a background of necrosis (H&E, original magnification ×40).
The biopsy revealed necrotizing vasculitis with perivascular lymphocytic infiltrate and plasma cells suggestive of infection with rare organisms. Subsequently, the child developed signs of increased intracranial tension, was unresponsive to stimuli, and died 12 days after admission. A partial autopsy and brain examination revealed that a large part of the left cerebral hemisphere on the convexity was necrotic and soft. Findings of microscopy revealed acute and chronic nongranulomatous vasculitis, thrombosis, fibrinoid necrosis, and a large number of single and groups of round large organisms, 15–25 μm, with vacuolated cytoplasm and single nuclei. In addition, axon retraction balls and reactive astrocytes were seen in the involved brain. The meninges were chronically inflamed and showed acute necrosis and a few organisms (Fig 1E, -F) consistent with a diagnosis of GAE.
Case 2
A 10-year-old boy presented with a history of bifrontal headaches for 2 and a half months, with vomiting, but with no history of any febrile illness. On examination, no cranial nerve involvement was found. There was mild neck rigidity and bilateral papilledema. Blood biochemistry values were within normal limits. Previous CT and MR imaging showed a hypointense masslike lesion on T1-weighted images with isointense signal intensity on T2-weighted images (Fig 2A, -B). Postcontrast images showed a poorly enhancing lesion. A chronic infective etiology was considered, and a CSF examination revealed an elevated protein count (64 mg/dL). A CSF smear was negative for fungal infection, but culture revealed Acanthamoeba organisms. The patient was treated with mannitol, dexamethasone, ketoconazole, and rifampicin. He showed symptomatic improvement and was discharged. Follow-up MR imaging after a gap of approximately 2 months, however, showed persistence of the masslike lesion (Fig 2C).
Fig 2.
A 10-year-old boy with headache and vomiting for 2 and a half months (GAE).
A, Contrast-enhanced CT shows an ill-defined pseudotumoral lesion in the left frontotemporal lobe (arrows), with a patchy linear type of enhancement.
B, T2-weighted MR image shows that the mass lesion is isointense to gray matter (arrows). No perilesional edema or any significant mass effect is seen. There were no other focal lesions.
C, Two-month follow-up contrast-enhanced MR image reveals persistence of the mass lesion, with no significant contrast enhancement.
Case 3
A 19-year-old woman presented with a history of headache for 6 months, vomiting for 1 month, and unsteadiness of gait for 15 days. Her history was not significant. Fundoscopy revealed bilateral gross papilledema with positive neck stiffness. CSF analysis revealed the following values: proteins, 60 mg/dL; glucose, 91 mg/dL; and an adenosine deaminase level, <1 U/L. CT of the brain revealed multiple focal oval lesions (Fig 3A, -B) with basilar exudates. Multiple T2 hyperintense oval lesions with perilesional edema and intense postcontrast enhancement were seen on MR imaging (Fig 3C, -D). The patient was started on empiric antitubercular treatment, and antiedema measures were instituted. A biopsy was planned; however, the patient had a recurrent seizure and sustained cardiorespiratory arrest from which she could not be revived. At autopsy, there were multiple discrete hemorrhagic areas in the brain, with a large cystic necrotic area in the left parietal lobe, corresponding with the imaging findings (Fig 3E). Microscopy revealed acute vasculitis, thrombosis, fibrinoid necrosis, and a large number of single and groups of round large organisms consistent with amebic trophozoites on an inflammatory background (Fig 3F).
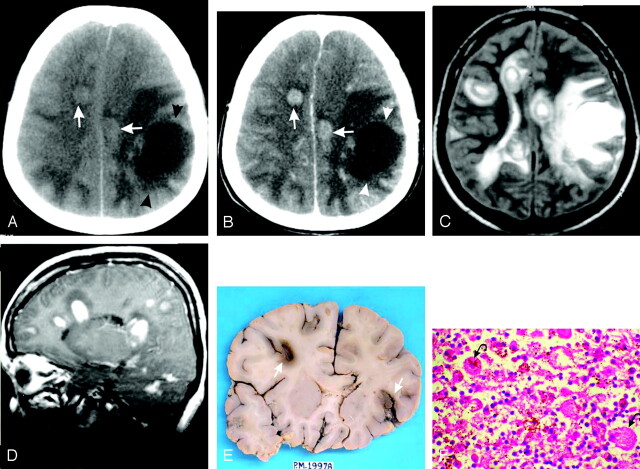
Fig 3.
A 19-year-old woman with holocranial headaches for 6 months, vomiting for 1 month, and an unsteady gait for 15 days (GAE).
Non contrast–enhanced CT (A) and contrast-enhanced CT (B) scans reveal multiple hyperattenuated oval lesions on the non contrast–enhanced CT scan (arrows), suggestive of hemorrhage. These lesions show intense enhancement on contrast-enhanced CT (arrows). A large nonenhancing cystic lesion is seen in the left parietal lobe (arrowheads).
Axial T2-weighted MR image (C) shows multiple hyperintense oval lesions with perilesional edema and the cystic lesion in the left parietal lobe. Intense postcontrast enhancement of the lesions is seen on the sagittal contrast-enhanced MR image (D).
Coronal cut section of the brain (E) shows multiple hemorrhagic lesions (arrows). Photomicrograph (F) shows the characteristic rounded amebic trophozoites (curved arrows) on an inflammatory background (H&E, original magnification ×40).
Case 4
A 38-year-old man presented in our neurosurgical emergency department with deteriorating vision and persistent vomiting for a week. There was history of headache and occasional vomiting for about 2 months. On examination, the patient was in altered sensorium with papilledema and visual field defects. A contrast-enhanced CT (Fig 4A) and MR imaging (Fig 4B–D) of the brain were performed. The patient underwent surgery with a presumed diagnosis of a mass lesion. Subsequently, his condition deteriorated, and he died on the seventh postoperative day. At autopsy, the pathologic findings were consistent with GAE.
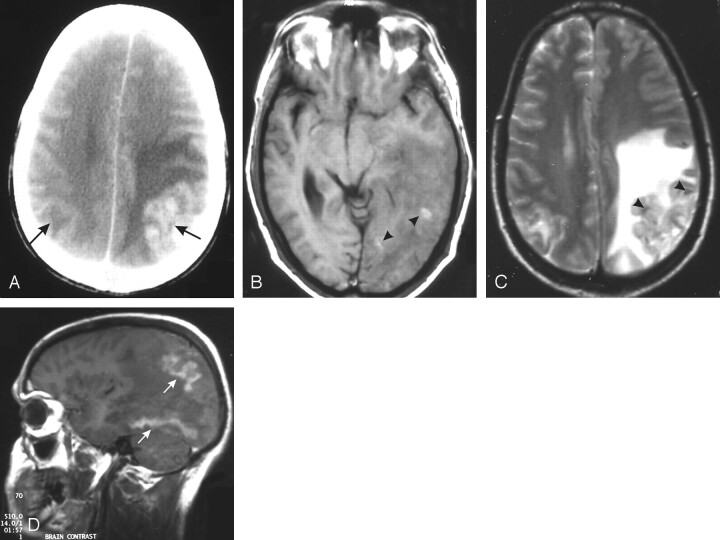
Fig 4.
A 38-year-old man with deteriorating vision and persistent vomiting for a week (GAE).
A, Contrast-enhanced CT scan of the brain shows an enhancing cortical-based lesion in the left parietal lobe with extensive perilesional edema. Another smaller hypoattenuated lesion is seen on the opposite side (arrows).
B, T1-weighted MR image shows hemorrhagic foci (arrowheads) in the left parietooccipital lesion with ill-defined gray-white matter distinction.
C, T2-weighted MR image shows heterogeneous signal intensity in the swollen cortex (arrowheads) with extensive white matter edema. The right-sided lesion also shows mild edema and cortical signal-intensity changes.
D, Contrast-enhanced T1-weighted sagittal image shows gyriform enhancement of the affected cortex with nonenhancing edematous white matter. Similar enhancement is also seen in the undersurface of the occipital lobe (arrows).
Case 5
A 40-year-old man presented with fever, headache, and vomiting for a week and altered sensorium for 3 days. The headache was holocranial in nature with associated nonprojectile vomiting. His history was not significant. On examination, the patient was febrile with positive neck rigidity. There was no papilledema. The CSF sample showed the following values: proteins, 95 mg/dL; glucose, 27 mg/dL, with random blood glucose of 91 mg/dL. CT of the brain revealed exudates in the perimesencephalic cistern, with right basal ganglia infarction (Fig 5A, -B). The possibility of tubercular or fungal meningitis versus a vasculitis was considered. The patient was started on empiric antitubercular treatment and ceftriaxone; however, he developed refractory hypotension and died. An autopsy revealed a softened brain with multiple infarcts in the basal ganglia, deep cerebellar white matter, and the corticomedullary junctions of both parietooccipital lobes. There was also meningeal thickening and gross exudates, especially in the basilar cisterns. On microscopy, the exudates were positive for the characteristic amebic trophozoites.
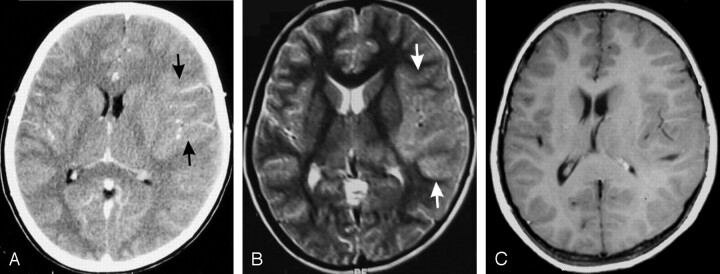
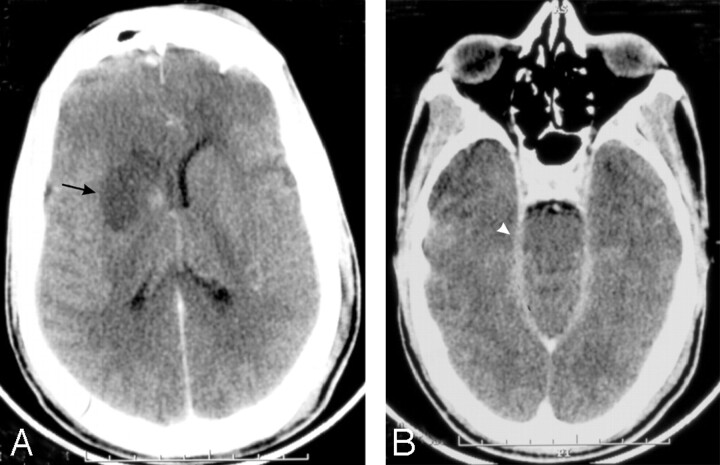
Fig 5.
A 40-year-old man who presented with fever and headache for a week and altered sensorium for 3 days (PAM).
Contrast-enhanced CT brain scan shows right basal ganglia infarction (arrow, A) and enhancing exudates in the perimesencephalic cistern (arrowhead, B).
Discussion
Rare CNS infections in humans caused by free-living amebae of the genera Naegleria or Acanthamoeba were first described in 1965 by Fowler and Carter 3 and in 1966 by Butt.4 Naegleria and Acanthamoeba organisms are responsible for causing primary PAM and GAE, respectively,2 having distinctive epidemiology, pattern of presentation, clinical course, pathology, and imaging findings.
GAE (Cases 1–4)
GAE is a subacute to chronic infection caused by Acanthamoeba and also Leptomyxida organisms.5 Acanthamoeba species are found in all types of environment. Cases have also been associated with amebic keratitis from contaminated contact lens solutions6 or with hematogenous spread from a primary source of infection, either a pulmonary focus or a skin ulcer.7 GAE is known to occur in patients who are debilitated or immunocompromised by AIDS, chemotherapy, or steroid therapy.5 The clinical course is characterized by a long duration of focal neurologic symptoms unlike the rapid progression and fulminant course of patients with PAM.4 All our 4 patients with GAE had a prolonged duration of focal symptoms (eg, seizures) present for an average of 2–3 months. None of our patients had an underlying immune compromise or any significant history such as a long illness or steroid use. Findings of enzyme-linked immunosorbent assay testing for HIV status performed for 2 patients were negative. All 4 patients were from a low socioeconomic status, and possibly GAE can occur in this subset of individuals who are otherwise immunocompetent. Leptomyxida order species are, however, known to cause GAE in immunocompetent hosts.8
Pathologically, the macroscopic appearance in GAE is one of focal edema of the cerebral hemispheres. Multifocal parenchymal lesions with involvement of posterior cranial fossa structures, the diencephalon, and the thalamus are seen. The presence of trophozoites and cysts, along with a chronic granulomatous reaction containing multinucleated giant cells, is characteristic. Microscopically, there is evidence of leptomeningitis, most prominent adjacent to the parenchymal lesions. There may be severe necrotizing angitis. GAE is diagnosed by identifying Acanthamoeba trophozoites or cysts in CSF/brain biopsy. Cultures of brain tissues or CSF can also reveal Acanthamoeba organisms.2
The pathologic literature addresses in detail the findings of GAE, but, to our knowledge, little is written about the imaging findings. Sporadic case reports describing the CT characteristics as multiple enhancing lesions involving the cerebral cortex and underlying white matter with mild mass effect are available.9 On MR imaging, multifocal lesions showing T2 hyperintensity and a heterogeneous or ringlike pattern of enhancement, with a predilection for the diencephalon, thalamus, brain stem, and posterior fossa structures, are seen.3,5 A case report by Kidney and Kim2 described multiple punctate focal areas of enhancement bilaterally throughout the cerebellar hemispheres as well as a few scattered supratentorial lesions. The random corticomedullary location of most of the lesions as seen in our patients (Figs 1B and 3C) and also in another case report of GAE in an HIV patient10 supports a hematogenous mode of spread. Intralesional hemorrhage was considered an important diagnostic feature by these authors.10 In 2 of our patients, there was evidence of intralesional hemorrhage (Figs 3A and 4B), which was confirmed on the macroscopic examination of the cut surface of the brain specimen. Such hemorrhagic lesions can be explained by the necrotizing angitis described in severe cases of GAE and can be an important clue in the diagnosis. The lesions of GAE are thought to represent focal areas of cerebritis or microabscesses.2 The differential diagnosis includes infarcts from septic emboli, abscesses, toxoplasmosis fungal granuloma, or neoplastic lesions.5,10 Solitary space-occupying lesions have also been described in cases of GAE,2,10 mimicking a low-grade glioma or lymphoma. This type of imaging was demonstrated in our second patient (Fig 2B, -C).
Imaging findings of GAE are, therefore, either in the form of a multifocal pattern with discrete focal lesions in the corticomedullary junction, as a larger solitary masslike lesion,11 or a combination of the 2 findings. The masslike lesion (pseudotumoral pattern) often demonstrates a linear and superficial gyriform pattern of enhancement, which could be a useful indicator of the diagnosis. This pattern possibly represents a combination of enhancement in the overlying inflamed meninges covered with exudates and the actual enhancement of the underlying cortex. The amebae possibly infiltrate along the pial vessels into the brain substance leading to an inflammatory response with border zone encephalitis and microabscess formation. The lesion may even suggest a large vessel infarction, except for the history, inordinate amount of associated edema, and multiplicity of the lesions.
Although no consistently effective treatment exists, surgical removal of granulomatous mass lesions, combined with chemotherapy such as amphotericin B or clotrimazole, provides the best chance of survival as seen in our second patient.
PAM (Case 5)
PAM is caused by Naegleria fowleri, with most patients having an acute onset of symptoms with rapid progression and almost always fatal meningoencephalitis within 48–72 hours. The affected patients are children and young adults with no immunologic compromise. The mode of entry of these thermophilic organisms is through the olfactory tract during contact with contaminated water. In our series, none of the patients revealed such a history, and it is most likely that other means, such as inhalation of dust or soil containing the amebic cysts, was the port of entry.
Pathologic changes in cases of PAM are typified by the extensive damage to the brain parenchyma, ependyma, andmeninges.9,12 Congestion of the meningeal vessels, edematous cortex with herniation of uncus and cerebellum are other features. Microscopically, there is a purulent leptomeningeal exudate with hemorrhage and necrosis throughout the cerebral hemispheres, brain stem, cerebellum, and upper spinal cord.9 Our fifth patient demonstrated these typical findings with extensive purulent leptomeningeal exudates mostly along the perimesencephalic cistern. The exudates contained mainly polymorphonuclear cells but were positive for the characteristic amebae, confirming the diagnosis of PAM.
Imaging features of PAM are nonspecific and, to our knowledge, have rarely been described previously. Findings of CT and MR imaging may be normal early in the disease, with evidence of brain edema and basilar meningeal enhancement subsequently.1,9,11 In the case of PAM reported by Kidney and Kim,2 both CT and MR imaging revealed a pattern of brain edema and hydrocephalus, with rapid progression of the disease. In our fifth patient, there was also evidence of obliteration of the cisterns with enhancing basilar exudates (Fig 5B). In addition, there was infarction of the right basal ganglia (Fig 5A), possibly due to obliteration of the perforating vessels by the extensive exudates. Cerebral infarctions in cases of PAM have been described,12 but to our knowledge, the imaging has never been documented previously. The mainstay of treatment for PAM is amphotericin B, rifampicin, and miconazole. The identification of ameba requires, immunofluorescence, immunohistochemistry, or DNA probes. Direct fluorescent antibody studies confirm the species type. These, however, were not available and were not performed for the patients in our series, and this omission is one of the limitations of this study.
The regular frequency at which our histopathology department has demonstrated an amebic etiology in autopsied brains of patients dying of a suspected atypical CNS infection with an indeterminate radiologic picture has led us to believe that amebic infections of the CNS are much more frequent than actually documented, particularly for patients from a low socioeconomic status in developing countries. Familiarity with some of these imaging findings would be useful for both the clinicians and the radiologists and perhaps would decrease the number of missed cases of amebic CNS infections. An antemortem diagnosis and the institution of aggressive therapy early in course of disease may alter the dismal prognosis of this disease.
References
- 1.Barnett NDP, Kaplan AM, Hopkin RJ, et al. Primary amebic meningoencephalitis with Naegleria fowleri: clinical review. Pediatr Neurol 1996;15:230–34 [DOI] [PubMed] [Google Scholar]
- 2.Kidney DD, Kim SH. CNS infections with free-living amebas: neuroimaging findings. AJR Am J Roentgenol 1998;17:809–12 [DOI] [PubMed] [Google Scholar]
- 3.Fowler M, Carter RF. Acute pyogenic meningitis probably due to Acanthamoeba: a preliminary report. BMJ 1965;2:740–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butt CG. Primary amebic meningoencephalitis. N Engl J Med 1966;271:1425–26 [DOI] [PubMed] [Google Scholar]
- 5.Sell JJ, Rupp FW, Orrison WW Jr. Granulomatous amebic encephalitis caused by Acanthamoeba. Neuroradiology 1997;39:434–36 [DOI] [PubMed] [Google Scholar]
- 6.Stehr-Green JK, Bailey TM, Brandt FH, et al. Acanthamoeba keratitis in soft contact lens wearers: a case-control study. JAMA 1987;258:57–60 [PubMed] [Google Scholar]
- 7.Martinez AJ. Free-living amebas: infection of the central nervous system. Mt Sinai J Med 1993;60:271–78 [PubMed] [Google Scholar]
- 8.Deol I, Robledo L, Meza A, et al. Encephalitis due to a free-living amoeba (Balamuthia mandrillaris): case report with literature review. Surg Neurol 2000;53:611–16 [DOI] [PubMed] [Google Scholar]
- 9.Ma P, Visvesvara GS, Martinez AJ, et al. Naegleria and Acanthamoeba infections: review. Rev Infect Dis 1990;12:490–510 [DOI] [PubMed] [Google Scholar]
- 10.Zagardo MT, Castellani RJ, Zoarski GH, et al. Granulomatous amebic encephalitis caused by leptomyxid amebae in an HIV-infected patient. AJNR Am J Neuroradiol 1997;18:903–08 [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher DJ, Tien RD, Lane K. Neuroimaging findings in rare amebic infections of the central nervous system. AJNR Am J Neuroradiol 1995;16:930–35 [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez AJ, Visvesvara GS. Laboratory diagnosis of pathogenic free-living amoebas: Naegleria, Acanthamoeba and Leptomyxid. Clin Lab Med 1991;11:861–72 [PubMed] [Google Scholar]