Abstract
Summary: Palatal tremor (PT), also known as palatal myoclonus, is defined by short rhythmic contractions of the palatal musculature. Functional MR imaging (fMRI) revealed prominent bilateral neuronal activation in the putamen associated with essential palatal tremor (EPT) in a 41-year-old man. This implies a central role of the putamen in EPT, most likely as a consequence of diminished inhibition in an afferent pathway. Because fMRI primarily detects activations, dysfunctional areas remain obscure. The present functional study complements previous pathologic studies, which associated PT with lesions to dentate nucleus, red nucleus, and the inferior olive (Guillain-Mollaret triangle).
Palatal tremor (PT), formerly also called palatal myoclonus, is defined by short, mostly rhythmic contractions of the palatal musculature and may occasionally be stimulus-sensitive. PT can be associated with synchronous movements of adjacent structures, including the pharynx, larynx, face, and diaphragm.1,2 PT has been subdivided into an essential form (EPT),3 where no origin can be found and MR imaging is usually normal,4 and symptomatic forms (SPT).5 In SPT, stroke (46%) is the most common cause, followed by trauma (11%) and demyelinating lesions (10%).6,7 Early pathologic studies of PT2,8 outlined an important role of lesions affecting the dentatorubral-olivary pathway, or Guillain-Mollaret triangle.9 This circular pathway connects the red nucleus to the inferior olivary nucleus and the contralateral dentate nucleus of the cerebellum via central tegmental tract (red nucleus–inferior olive), inferior cerebellar peduncle (inferior olive–dentate nucleus), and superior cerebellar peduncle (dentate nucleus–red nucleus).9,10 Although it is an anatomic triangle, SPT is associated with lesions in the rubodentate and rubo-olivary fibers, but not in the olivodentate fibers.10 Further, hypertrophic degeneration of the inferior olive was reported in SPT.7,11 Common radiologic changes in SPT are an increase in T2 or proton attenuation MR imaging signal intensity and hypertrophy within the olivary nucleus.10 There are generally no structural lesions in EPT, and, consequently, the MR imaging is usually normal.10
The pathomechanism generating the rhythmic contractions per se in PT remains unexplained. In the present study, we used functional MR imaging (fMRI) to identify neuronal activations associated with PT in a patient with stimulus-sensitive EPT.
Case Report
A 41-year-old man was admitted to our service because of progressive contractions of the palatal and neck musculature. He reported being in a minor motor vehicle crash without loss of consciousness 2 years prior to admission. A pulsatile tinnitus of the left ear evolved several months later and progressed into a rhythmic ear click. Approximately 2 months before admission, contractions in the palatal musculature occurred. The further spread of the contractions with involvement of the larynx lead to a first neurologic consultation. Physical examination showed intermittent bilateral short rhythmic contractions of the palatal and inframandibular musculature, compatible with segmental myoclonus. The segmental myocloni occurred in clusters of 5 rhythmic contractions, followed by a variable silent period. The contractions could be triggered by external sensory stimuli (eg, by touching his left arm). Head movement to the left transiently suppressed the myoclonus. The myoclonus was associated with ear clicks in the left ear and bilateral tinnitus. Otoscopy revealed a high-frequency myoclonus of the M. levator tympani that was not synchronous with breathing. The remainder of the general, psychiatric, neurologic, and otorhinolarygologic examinations—as well as MR imaging scans of the skull, brain, and brain stem and EEG recordings—were normal. Time-resolved MR projection angiography showed no signs of dural AV fistula. The PT did not respond to any medication, including diazepam and gabapentin in the further course of the disease, which led to the diagnosis of an EPT. The patient took no medication before admission and gave written informed consent before inclusion into the study.
Functional Imaging
Blood oxygenation level–dependent (BOLD) fMRI was performed to take advantage of the stimulus sensitivity of the EPT, by using a whole-body 1.5T MR scanner (Sonata; Siemens, Erlangen, Germany). PT occurred spontaneously or could be evoked by tactile stimulation of the patient’s left arm by a physician inside the scanning room. During the functional scanning, 22 clusters of PT occurred, lasting on average 23.4 seconds (minimum, 5 seconds; maximum, 60 seconds). Functional T2*-weighted images were obtained based on echo-planar single-shot pulse sequence (EPI)—matrix size, 64 × 64; field of view (FOV), 220 mm × 220 mm; 25 sections; 4-mm section thickness; 1-mm gap covering the whole brain; flip angle, 90°; repetition time (TR), 2.5 seconds; echo time (TE), 40 milliseconds; 400 measurements lasting 16 minutes 40 seconds. After functional scanning, a high-resolution 1-mm isovoxel T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) (matrix 256 × 256, 176 sections) was acquired. To confirm the findings of the first imaging session, a second functional scanning was performed 3 days later. Appropriate frequency and duration of PT occurred only during the first half of the measurement. Therefore, only the first 200 scans were analyzed, which included 19 clusters of PT lasting on average 9.9 seconds (minimum, 2.5 seconds; maximum, 30 seconds).
Anatomic and functional images were analyzed by using BrainVoyager QX (Brain Innovation, Maastricht, the Netherlands). Preprocessing included 3D motion correction, section-time correction, Gaussian spatial filtering (full width half maximum 4 mm), and high-pass temporal filtering of 3 cycles in time course (removal of low-frequency drifts). The functional images were coregistered to anatomic MPRAGE images. Normalization was not performed. The PT time courses were convolved with a hemodynamic reference function to create basis regressors. All reported activations are based on a fixed-effects general linear model (GLM) of the first run with statistical threshold of P < .01 (corrected Bonferroni; corresponding to t >5.3) and extent threshold of 500 mm3. The first run was re-evaluated with additional motion-correction predictors. No motion-related activation was found in the putamen. The results of the second run are not shown.
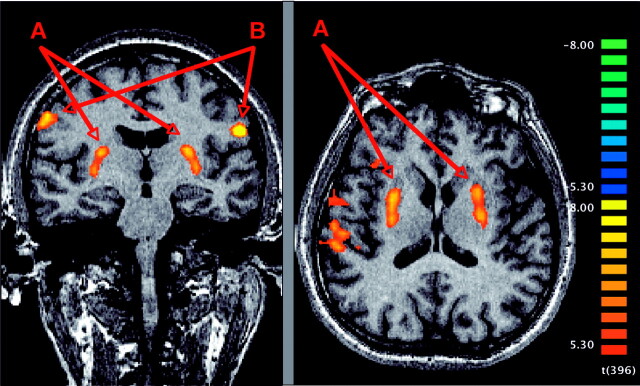
Peak activation associated with PT was identified in basal ganglia bilaterally and closely resembled the anatomic boundaries of the putamen. Additional activations were present in the precentral gyrus bilaterally and right superior temporal and angular gyrus (Fig 1). The activation in the putamen bilaterally could be reproduced in a second functional scan at day 3. The level of significance was, however, lower because of different alternation of myoclonus clusters and silent periods (not illustrated). No focal activation could be observed in prefrontal motor cortex, the brain stem, or the cerebellum.
Fig 1.
Neuronal activations associated with the initiation and maintenance of EPT in a 41-year-old patient. Peak activation assessed by fMRI is present in the putamen bilaterally (A). Additional activation is found in precentral gyrus bilaterally (B) and right superior temporal and angular gyrus (not shown). No focal activation could be observed in prefrontal motor cortex, the brain stem, or the cerebellum. The analysis is based on a fixed-effects GLM with statistical threshold of P < .01 (corrected Bonferroni) and spatial threshold of 500 mm3. Radiologic convention: right hemisphere is depicted on the left-hand side.
Discussion
fMRI was used to identify neuronal activations associated with PT in a 41-year-old patient with stimulus-sensitive EPT. PT was associated with peak neuronal activation in the putamen bilaterally. No focal activation was detected in structures of the Guillain-Mollaret triangle, which were previously associated with PT.2,8 Initially, the present investigation appears to contradict these previous investigations; however, these previous anatomic/pathologic studies identified damaged or dysfunctional brain areas. We reason that a dysfunctional brain area per se unlikely evokes overshooting muscular activations in PT. It seems more plausible that a dysfunctional area modulates another area—for example, as a consequence of diminished inhibition. Because fMRI preferentially detects activations, the dysfunctional structures remain obscure. On the basis of the previous anatomic/pathologic studies, the damage might reside in the Guillain-Mollaret triangle. Consistent with these considerations, an inhibitory effect of the Guillain-Mollaret triangle on the basal ganglia has been described in the literature.12,13 The present fMRI study therefore does not contradict, but complements, previous anatomic/pathologic studies and implies that disinhibition of the putamen bilaterally is an essential component of EPT. Of interest, activation of the putamen was observed during eyelid spasms in patients with benign essential blepharospasm,14 which points to an as yet not precisely understood involvement of the putamen in hyperkinesia.
Additional neuronal activations were present bilaterally in the precentral gyrus, which might be attributed to the motor control of the palatine muscles. In line with involuntary activity, no activity was registered in prefrontal areas. Further, activation was present in the right superior temporal and angular gyrus. This activation may reflect auditory stimulation, which occurs simultaneously with PT in the form of audible ear clicks.
Previous fMRI investigations of PT were limited to the brain stem, including the Guillain-Mollaret triangle, and did not cover the putamen in the investigated volume. Dysfunctional activation was found in the dentate nuclei, the left inferior olivary nucleus and the left red nucleus in a 56-year-old patient with PT.13 This investigation demonstrated dysfunction on the basis of MR images sensitized to changes in cerebral blood oxygenation state. In contrast, the present investigation primarily shows activations. In another functional study, voluntary PT was associated with hyperactivation of the inferior olive, a brain stem region, and the cerebellar dentate nuclei8 in a brother of a patient with EPT who was able to elicit, modulate, and stop rhythmic contractions of the soft palate associated with ear clicks voluntarily. This voluntary control of rhythmic contractions obviously differs from the patient in the present investigation with involuntary EPT.
One limitation of the present study is that it is only a single case description, so further investigations are necessary to confirm the presented findings. A prerequisite for fMRI is several repetitions of activation interleaved with rest periods within a few minutes, which is rarely encountered in PT. Putative motion-related pseudoactivations are unlikely, because of the reproducibility of activations with and without nonexplanatory motion regressors and after 3 days. Also, no significant motion regressor–associated activations were present in the putamen.
Conclusion
The present study revealed prominent bilateral activation of the putamen associated with EPT. These results imply a central role of the putamen in the generation of EPT. Further studies will be needed to confirm a putative disinhibition of the putamen in EPT.
Footnotes
S.G.W. was supported, in part, by a grant from the Swiss National Science Foundation (grant 3200-066634/1).
References
- 1.Deuschl G, Wilms H. Clinical spectrum and physiology of palatal tremor. Mov Disord 2002;17(suppl 2):S63–S66 [DOI] [PubMed] [Google Scholar]
- 2.Tomkinson A, Craven C, Brown MJ. Palatal myoclonus affected by neck position. J Laryngol Otol 1995;109:61–62 [DOI] [PubMed] [Google Scholar]
- 3.Vieregge P, Klein C, Gehrking E, et al. The diagnosis of “essential palatal tremor.” Neurology 1997;49:248–49 [DOI] [PubMed] [Google Scholar]
- 4.Samuel M, Torun N, Tuite PJ, et al. Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain 2004;127:1252–68 [DOI] [PubMed] [Google Scholar]
- 5.Deuschl G, Toro C, Valls-Sole J, et al. Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain 1994;117:775–88 [DOI] [PubMed] [Google Scholar]
- 6.Deuschl G, Mischke G, Schenck E, et al. Symptomatic and essential rhythmic palatal myoclonus. Brain 1990;113:1645–72 [DOI] [PubMed] [Google Scholar]
- 7.Dubinsky RM, Hallett M. Palatal myoclonus and facial involvement in other types of myoclonus. Adv Neurol 1988;49:263–78 [PubMed] [Google Scholar]
- 8.Nitschke MF, Kruger G, Bruhn H, et al. Voluntary palatal tremor is associated with hyperactivation of the inferior olive: a functional magnetic resonance imaging study. Mov Disord 2001;16:1193–95 [DOI] [PubMed] [Google Scholar]
- 9.Guillain G, Mollaret P. Deux cas de myoclonies synchrones et rythmees velo-pharygolaryngo-oculo-diaphragmatiques: le probleme anatomique et physio-pathologique de ce syndrome. Rev Neurol 1931;2:545–66 [Google Scholar]
- 10.Goyal M, Versnick E, Tuite P, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol 2000;21:1073–77 [PMC free article] [PubMed] [Google Scholar]
- 11.Drysdale AJ, Ansell J, Adeley J. Palato-pharyngo-laryngeal myoclonus: an unusual cause of dysphagia and dysarthria. J Laryngol Otol 1993;107(8):746–47 [DOI] [PubMed] [Google Scholar]
- 12.Lapresle J. Rhythmic palatal myoclonus and the dentato-olivary pathway. J Neurol 1979;220:223–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boecker H, Kleinschmidt A, Weindl A, et al. Dysfunctional activation of subcortical nuclei in palatal myoclonus detected by high-resolution MRI. NMR Biomed 1994;7:327–29 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt KE, Linden DE, Goebel R, et al. Striatal activation during blepharospasm revealed by fMRI. Neurology 2003;60:1738–43 [DOI] [PubMed] [Google Scholar]