Abstract
Summary: We describe a previously unreported case of cranial arterial dolichoectasia associated with spontaneous dissection of the petrous (C2) segment of the internal carotid artery (ICA) with 2 patent lumena. Dolichoectasia of the cranial arteries and different types of double lumen of ICA are discussed. A review of previously reported cases is included.
Cranial arterial dolichoectasia (CADE) is angiopathy that is characterized by dilation and elongation of cranial arteries. The prevalence of dolichoectasia has been estimated to be 0.05%–0.06%.1,2 The main etiologic factors of dolichoectasia are atherosclerosis, defects or destruction of the internal elastic, and hypertension.2–5 Dolichoectasia preferentially involves the intracranial vertebral and basilar arteries. Involvement of carotid arteries and branches of the carotid arteries is relatively rare.6 Dolichoectasia is frequently associated with ischemia and rarely with intracranial hemorrhage.3 Intracranial hemorrhages may be caused by the rupture of associated aneurysm, rupture of perforating blood vessels originating from ectatic arteries, or dissection of an ectatic artery.2,7,8 Dissections of the cranial arteries are sometimes misinterpreted as duplications or fenestrations of these arteries. We present a case of vertebral, basilar, and bilateral carotid dolichoectasia associated with an unusual type of dissection of the petrous segment of the internal carotid artery (ICA) revealed by conventional angiography.
Case Report
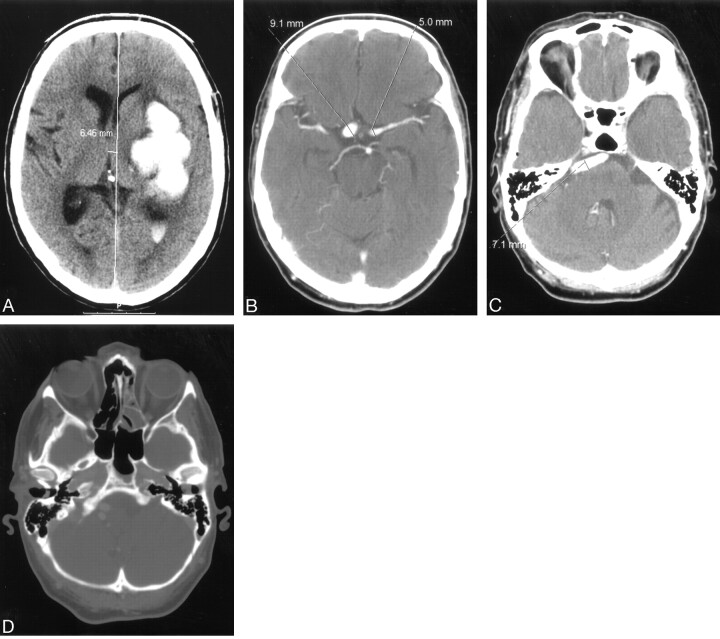
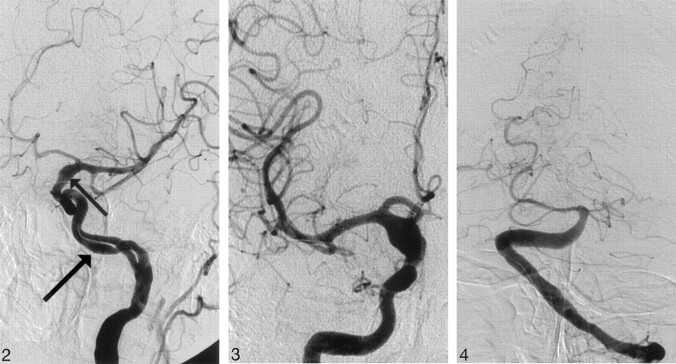
A 58-year-old male smoker, with arterial hypertension, was admitted to the hospital because of a sudden onset of right-sided weakness and pronounced dysphasia. The blood pressure on admission measured 150/100 mmHg. Emergency CT showed intracerebral hematoma localized in the left thalamus, the posterior limb of the internal capsule, and the left lenticular nucleus, as well as the extension of the hematoma into the left lateral ventricle (Fig 1A). The same study showed fusiform dilation of the supraclinoid segments of both ICAs. The finding was more pronounced on the right side. The supraclinoid segment of the right ICA measured 9 mm and the supraclinoid segment of the left ICA measured 5 mm, whereas the diameter of the basilar artery measured 7 mm (Fig 1B, -C). The bone carotid channels appeared to be normal (Fig 1D). The patient was referred for angiographic evaluation. The aortic arch was slightly elongated and dilated. The branches originating from the aortic arch were moderately tortuous and had normal lumen. The left ICA was tortuous and had irregular lumen with 2 fusiform dilations localized, respectively, distally to the bifurcation of the common carotid artery and proximally to the inlet of carotid canal. From this point the left internal carotid artery had 2 lumena that coursed parallel through the carotid canal (Fig 2). Both limbs had smooth contours but slightly irregular, partly fusiform dilated lumen. These limbs were reconnected at the level of the C3 segment. The supraclinoid segment of the ICA was fusiform dilated. The right ICA was dilated distally to the bifurcation of the common carotid artery and distally to the origin of the ophthalmic artery (Fig 3). This fusiform widening involved supraclinoid segment up to the bifurcation of the ICA. Anterior and middle cerebral arteries had normal lumena and branching on both sides. The basilar artery was tortuous and moderately dilated (Fig 4). The branches of vertebral and basilar arteries displayed slightly reduced lumen but normal pattern of branching.
Fig 1.
A, nonenhanced computerized tomography showing left-sided ganglionic bleeding with penetration of the blood into the left lateral ventricle.
B and C, contrast-enhanced computerized tomography (CECT) showing supraclionoid segments of both internal carotid arteries and tortuous and dilated basilar artery.
D, CECT, bone window shows symmetrical and normally shaped carotid channels within hyperaerated petrous bones.
Fig 2.
Angiogram of the left internal carotid artery, left anterior oblique projection showing double lumen of C2 segment of the internal carotid artery. Note the smooth contours of slightly dilated vascular channels (arrow) and fusiform dilation of the supraclinoid segment of the internal carotid artery (small arrow).
Fig 3.
Angiogram of the right internal carotid artery, anteroposterior (AP) projection, shows symmetric fusiform dilation of the supraclinoid segment of the right internal carotid artery.
Fig 4.
Angiogram of the basilar artery, AP projection, shows moderately dilated distal segment of the left vertebral artery as well as dilated and extremely tortuous basilar artery.
The patient was treated conservatively and referred to rehabilitation. Symptoms related to the bleeding have partly regressed, and 3 months after discharge the patient is doing well.
Discussion
CADE is an angiopathy that is characterized by dilation, elongation, and tortuosity of the brain arteries, most frequently distal vertebral arteries, the basilar artery, or distal ICAs.2,4 Because the dilation seems to be the most important feature, the condition is now referred to as dilatative arteriopathy.6 Dolichoectasia that involves both the vertebrobasilar and carotid systems is rare entity and is seen in only 0.5% of patients suffering a first cerebral infarction caused by this angiopathy.9 Despite the observation that approximately 1 of 8 imaged brains has some increase in the length and diameter of intracranial arteries, the prevalence of dolichoectasia is relative low and varies between 0.05% and 0.06% depending on the source.1,2,6 According to Greenfield’s Pathology, a basilar artery lumen >4.5 mm is considered to be ectatic.10 Some authors, however, emphasize that there are no generally accepted quantitative criteria for dilatative arteriopathy.6,11,12 Extremely dilated and elongated intracranial arteries are referred to as fusiform or giant fusiform aneurysms. According to Drake and Peerless, large S-shaped aneurysms of the vertebrobasilar system in a patient with hypertensive arteriopathy have been known as the classic form of the giant fusiform aneurysm.13 In our case, the process was bilateral and asymmetrical, involving extracranial and intracranial segments of both ICAs and basilar artery. The lumena of all these arteries were >4.5 mm but did not fit the criteria for fusiform or giant fusiform aneurysm.
Dolichoectasia is commonly seen in patients with advanced cerebral atherosclerosis and arterial hypertension.2–5 Besides atherosclerosis and hypertension, there are several factors and conditions that have been implicated in the pathogenesis of dolichoectasia, including a defect or destruction ofthe internal elastic lamina, Ehlers-Danlos syndrome,14 Marfan syndrome,15 and tuberous sclerosis.16 The significance of thinning and disruption of internal elastic lamina in the process of elongation and dilation of intracranial arteries has been emphasized by several authors.4,6,10,13,17 The results of recently published studies indicate that the relationship between these pathologic changes and atherosclerosis in the pathogenesis of dolichoectasia remains unclear. The same studies clearly showed that dilatative arteriopathy was associated with increasing age, male sex, arterial hypertension, and history of myocardial infarction.11,12 In our case, the patient is a smoker and has been treated for hypertension since his 30s. The pathologic background of the dolichoectasia in this patient cannot be determined on the basis of available image material. The shape of the arterial lumen with dilation, elongation, and smooth irregularities of the contours strongly suggest atherosclerotic changes of the wall. The possible presence of thinning and discontinuity of internal elastic lamina, as well as atrophic or fibrotic tunica media that could have contributed to the dilation and elongation of arteries, however, cannot be excluded.
There are several clinical manifestations of the dolichoectasia: compression on cranial nerves or brain stem, obstructive hydrocephalus, and ischemia.2,5,18 Ischemic lesions may possibly be caused by emboli derived from thrombi or fragments of plaques in the walls of the enlarged arterial segment. The occurrence of spontaneous intracranial hemorrhages is less common than that of ischemia in patients with dolichoectasia.10 Intracranial hemorrhages may be caused by the dissection of ectatic artery, rupture of associated aneurysm, or rupture of stretched perforating arteries exposed to chronic hypertension.2,7,8,19 The shape, size, and localization of the hematoma revealed in our case fitted the criteria for hypertensive bleeding caused by rupture of perforating arteries.20,21 Angiography of the left ICA showed ordinary lumen of both anterior and middle cerebral arteries as well as a normal pattern of branching of these arteries. We did not notice changes in course or lumen of the perforating arteries that could have indicated pathologic process related to the dolichoectasia. Of course, possible microscopic defects of the walls of these arteries sometimes associated with dolichoectasia cannot be excluded.
The most remarkable finding in our case was the double lumen of C2 segment of the left ICA. In general, a double lumen of one artery may represent duplication or fenestration of one artery or dissection of its wall with 2 patent lumena. The anatomic variant consisting of 2 arteries running parallel instead of one is referred to as duplication. Fenestration is a duplication of a shorter segment of an artery. Duplications and fenestrations are frequently seen in the vertebrobasilar system but very rarely in the carotid system. There have been only 7 reported cases of duplication or fenestration of the ICA prior to 2004: 3 cases of fenestration of the supraclinoid segment, a single case of multichannel, short-segment duplication of the petrous segment of ICA, and 3 cases of duplication of the C1 segment of ICA.22–28 The main features of all of these cases, except the multichannel fenestration of the C2 segment, are parallel but clearly separate courses, smooth contours, and almost equal diameters of lumena. Gailloud et al reported 6 cases of “pseudofenestration” of ICA.29 The common feature of all these cases was relatively short involvement of the cervical ICA with pronounced asymmetry of the limbs of fenestration. Additional common features are irregularity of the contours and segmental narrowing and enlargement of the lumen. The authors pointed out that such changes are not typical in fenestration of arteries in other locations. On the other hand, described features are commonly associated with arterial dissection. Gailloud et al pointed out that review of the 4 previously reported cases of cervical ICA fenestration revealed similar findings. In all of these cases, asymmetry of the limbs, pronounced irregularity of the contours, segmental narrowing, and enlargement of the lumen of the ICA were described as additional findings to the fenestration. Gailloud et al ended their article with the conclusion that all described cases represent segmental arterial dissection with a double lumen sign rather than an anatomic variant.
Review of the medical records did not reveal any trauma that could have caused dissection of the ICA in our patient. Moreover, the localization and extent of the double lumen is not typical for dissection caused by trauma. Typical traumatic dissection of the ICA is located immediately bellow the skull base, at the entrance of the carotid canal. Only 4 cases of isolated atraumatic dissections of the petrous segment of the ICA have been published to date.30–32 All of these cases were symptomatic with radiologically confirmed disturbances of the blood flow in the dissected segment of the ICA. The absence of symptoms in our case suggests a very slowly progressing dissection that has not produced significant flow disturbances. One can assume that the combination of hypertension, weakness of the arterial wall, and the shearing forces of the blood flow at the entrance of carotid channel could have promoted a dissection. One can also hypothesize that distribution of flow forces in the limited space of the relatively narrow carotid channel could have contributed to such an unusual form of 2 patent arterial channels.
In conclusion, we have presented a case of a relatively rare form of CADE associated with a previously unreported form of dissection of the petrous segment of the ICA. The knowledge of such rare entities may have importance in diagnosis and treatment of vascular lesions of the brain.
Acknowledgments
We thank roentgenology technologists Kerstin Englund, Hans Olov Karlsson, and Carina Olofsson for the technical assistance and Jonathan Jones, MD, for editing of the text.
References
- 1.Courville CB. Arteriosclerotic aneurysms of the circle of Willis: some notes of their morphology and pathogenesis. Bull Los Angeles Neurol Soc 1962;27:1–13 [PubMed] [Google Scholar]
- 2.Yu YL, Moseley IF, Pullicino P, et al. The clinical picture of ectasia of the intracranial arteries. J Neurol Neurosurg Psychiatry 1982;45:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ince B, Petty GW, Brown RD, et al. Dolichoectasia of the intracranial arteries in patients with first ischemic stroke: a population-based study. Neurology 1998;50:1694–97 [DOI] [PubMed] [Google Scholar]
- 4.Sacks JG, Lindenburg R. Dolicho-ectatic intracranial arteries: symptomatology and pathogenesis of arterial elongation and distension. Johns Hopkins Med J 1969;125:95–106 [PubMed] [Google Scholar]
- 5.Moseley IF, Holland IM. Ectasia of the basilar artery, the breadth of the clinical spectrum, and the diagnostic value of computed tomography. Neuroradiology 1979;18:83–91 [DOI] [PubMed] [Google Scholar]
- 6.Caplan L. Dilatative arteriopathy (dolichoectasia): what is known and not known. Ann Neurol 2005;57:469–71 [DOI] [PubMed] [Google Scholar]
- 7.Amano T, Inamura T, Matsukado K, et al. Ruptured saccular aneurysm of a dolichoectatic internal carotid artery in a patient with agenesis of the contralateral internal carotid artery. Neurol Med Chir (Tokyo) 2004;44:20–23 [DOI] [PubMed] [Google Scholar]
- 8.Passero S, Calchetti B, Bartalini S. Intracranial bleeding in patients with vertebrobasilar dolichoectasia. Stroke 2005;36:1421–25 [DOI] [PubMed] [Google Scholar]
- 9.Romi F, Krakenes J, Thomassen L, et al. Dolichoectasia of the intracranial arteries and stroke. Tidsskr Nor Laegeforen 1999;119:3004–3005 [PubMed] [Google Scholar]
- 10.Kalimo H, Kaste M, Haltia M. Dolichoectasia and fusiform aneurysms. In: Graham DI, Lantos PL, eds. Greenfield’s neuropathology. 7th ed. London: Arnold Publishing;2002. :281–355
- 11.Pico F, Labreuche J, Cohen A, et al. Intracranial arterial dolichoectasia is associated with enlarged descending thoracic aorta. Neurology 2004;63:2016–21 [DOI] [PubMed] [Google Scholar]
- 12.Pico F, Labreuche J, Touboul PJ, et al. Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology 2003;61:1736–42 [DOI] [PubMed] [Google Scholar]
- 13.Drake CG, Peerless JS. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg 1997;87:141–62 [DOI] [PubMed] [Google Scholar]
- 14.Schoolman A, Kepes JJ. Bilateral spontaneous carotid-cavernous fistulae in Ehlers-Danlos syndrome: case report. J Neurosurg 1967;26:82. [DOI] [PubMed] [Google Scholar]
- 15.Finney LH, Roberts TS, Anderson RE. Giant intracranial aneurysm associated with Marfan’s syndrome: case report. J Neurosurg 1976;45:342–47 [DOI] [PubMed] [Google Scholar]
- 16.Blumenkopf B, Huggins MJ. Tuberous sclerosis and multiple intracranial aneurysms: case report. Neurosurgery 1985;17:797–800 [DOI] [PubMed] [Google Scholar]
- 17.Mizutani T, Yoshimasa M, Kojima H, et al. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 1999;45:253–60 [DOI] [PubMed] [Google Scholar]
- 18.Passero S, Filosomi G. Posterior circulation infarcts in patients with vertebrobasilar dolichoectasia. Stroke 1998;29:653–59 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein SJ, Tibbs PA. Recurrent subarachnoid hemorrhage complicating cerebral arterial ectasia: case report. J Neurosurg 1982;55:139–42 [DOI] [PubMed] [Google Scholar]
- 20.Dickinson CJ. Why are strokes related to hypertension? Classic studies and hypothesis revisited. J Hypertension 2001;19:1515–21 [DOI] [PubMed] [Google Scholar]
- 21.Qureshi A, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2005;344:140–46 [DOI] [PubMed] [Google Scholar]
- 22.Banach MJ, Flamm ES. Supraclinoid carotid artery fenestration with an associated aneurysm. J Neurosurg 1993;79:438–41 [DOI] [PubMed] [Google Scholar]
- 23.Yock DH. Fenestration of the supraclionid internal carotid artery with rupture of associated aneurysm. AJNR Am J Neuroradiol 1984;5:634–36 [PMC free article] [PubMed] [Google Scholar]
- 24.Findlay JM, Chui M, Muller PJ. Fenestration of the supraclinoid internal carotid artery. Can J Neurol Sci 1987;14:159–61 [DOI] [PubMed] [Google Scholar]
- 25.Mangla S, Teitelbaum GP. Multichannel fenestration of the petrous segment of the internal carotid artery. AJNR Am J Neuroradiol 99;20:590–92 [PMC free article] [PubMed] [Google Scholar]
- 26.Chess AM, Barsotti JB, Chang JK, et al. Duplication of the extracranial internal carotid artery. AJNR Am J Neuroradiol 1995;16:1545–47 [PMC free article] [PubMed] [Google Scholar]
- 27.Killien FC, Wyler AR, Cromwell LD. Duplication of the internal carotid artery. Neuroradiology 1980;19:101–02 [DOI] [PubMed] [Google Scholar]
- 28.Koenigsberg RA, Zito JL, Patel M, et al. Fenestration of the internal carotid artery: a rare mass of the hypotympanum associated with persistence of the stapedial artery. AJNR Am J Neuroradiol 1995;16:908–10 [PMC free article] [PubMed] [Google Scholar]
- 29.Gailloud P, Carpenter J, Heck DV, et al. Pseudofenestration of the cervical internal carotid artery: a pathologic process that stimulates an anatomical variant. AJNR Am J Neuroradiol 2004;25:421–24 [PMC free article] [PubMed] [Google Scholar]
- 30.Saeed RM, Hinton AE, Ramsed RT. Spontaneous dissection of the intrapetrous internal carotid artery. J Laryng Otol 1990;104:491–93 [DOI] [PubMed] [Google Scholar]
- 31.Bilbao R, Amoros S, Murubic J. Horner syndrome as an isolated manifestation of an intrapetrous internal carotid artery dissection. Am J Ophthalmol 1997;123:562–64 [DOI] [PubMed] [Google Scholar]
- 32.Robertson DI, Stuckey SL. Isolated intrapetrous carotid canal atraumatic internal carotid artery dissection: MRI and digital subtraction angiography findings. Austral Radiol 2003;47:462–64 [DOI] [PubMed] [Google Scholar]