Abstract
BACKGROUND AND PURPOSE: Diffusion tensor imaging (DTI) allows direct visualization and volumetric analysis of the corticospinal tract (CST). The purpose of this study was to determine whether color maps and fiber tracking derived from DTI data are valuable in detecting and quantifying CST degeneration in patients with amyotrophic lateral sclerosis (ALS).
METHODS: Sixteen patients with ALS with clinical signs of upper motor neuron (UMN) involvement and 17 healthy subjects were studied with the use of DTI. Disease severity was determined by means of the ALS Functional Rating Scale-Revised (ALSFRS-R) and an UMN involvement score. DTI was acquired with a 12-direction, single-shot, spin-echo echo-planar sequence. The CST from the lower pons to the corona radiata at the level of the corpus callosum on 4 contiguous coronal sections was manually segmented by using color maps generated from the DTI data. The left and right CST volumes were measured separately and normalized to the total intracranial volume. Normalized CST volumes were compared between patients with ALS and healthy subjects.
RESULTS: The CST volumes of patients with ALS were significantly reduced (P < .01, unpaired t test) compared with healthy subjects, in both affected and nonaffected hemispheres. No significant correlation was found between CST volumes and any of the clinical parameters, including disease duration, ALSFRS-R, or UMN involvement score.
CONCLUSION: This study shows that volumetric analysis by using DTI-based color maps is valuable in detecting and monitoring structural degeneration of the CST. This will lead to objective and quantitative assessment of axonal degeneration in ALS.
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease affecting both upper and lower motor neurons. The diagnosis of ALS is currently based on clinical features, electromyography (EMG), and exclusion of other diseases with similar symptoms. Lower motor neuron (LMN) dysfunction can be confirmed by EMG and muscle biopsy, whereas upper motor neuron (UMN) involvement is more difficult to detect, particularly in the early phase.1,2 Because damage to the UMN in the cerebral cortex leads to corticospinal tract (CST) degeneration along the course of the tract,2 a sensitive and reliable measure of CST degeneration may be helpful in diagnosing ALS at an earlier stage and in monitoring the course of the disease.
Diffusion tensor imaging (DTI) provides quantitative information about the magnitude and directionality of water diffusion in 3D space. Diffusion is anisotropic in white matter tracts, because axonal membranes and myelin sheaths present barriers to the motion of water molecules in directions that are not parallel to their orientation.3 The direction of maximum diffusivity is consistent with white matter tract orientation. The architecture of the tract can be mapped in color, where the brightness of the color is modulated by the fractional anisotropy (FA).4-6 The CST, therefore, can be readily identified in color on every cross-section along its course, making it possible to perform segmentation and volumetric analysis of CST.
The CST can also be depicted by using diffusion tensor tractography, a postprocessing technique that allows tracking of major white matter tracts.5,7-9 Fiber tracking is generally performed by using a line propagation technique based on continuous number fields called fiber assignment by continuous tracking (FACT)10,11 and a multiple region of interest (ROI) approach.5,7,10
The purpose of this study was to determine whether color maps and fiber tracking derived from diffusion tensor image data are valuable in detecting and quantifying CST degeneration in patients with ALS.
Methods
Patients
Sixteen patients (13 men and 3 women; age range, 44–72 years; mean age, 58.3 ± 7.8 years) with ALS and 17 age-matched healthy subjects (10 men and 7 women; age range, 41–63 years; mean age, 50.1 ± 8.2 years) were included in this study. All of the patients with ALS had clinical evidence of UMN involvement and were classified, according to the El Escorial criteria,12 as definite ALS (9 patients), probable ALS (5 patients), or possible ALS (2 patients). The study was approved by the institutional review board, and all subjects gave their written informed consent.
Clinical Assessment of Patients with ALS
All patients underwent physical examination before MR scanning. Disease severity was estimated by using the ALS Functional Rating Scale-Revised (ALSFRS-R) and an UMN involvement score. The ALSFRS-R was a validated measure of motor disability,13 with a maximum score of 48 and lower scores indicating greater impairment. The UMN involvement score reflected the number of segments with UMN findings on physical examination. UMN findings were defined as the presence of pathologic reflexes, hyperactive reflexes (or a missing abdominal reflex), preserved reflexes in a weak or wasted limb, or increased tone/spasticity.14 Six segments were considered (bulbar, right and left cervical, thoracic, right and left lumbosacral). The UMN score was the number of segments with UMN signs and ranged from 0 to 6. Disease duration was calculated in months from the date of symptom onset to the date of the scan. Clinical characteristics of these patients are provided in Table 1.
Table 1:
Clinical characteristics of patients with amyotrophic lateral sclerosis (ALS)
| Patient No./ Age (y)/Sex | Duration (mo) | ALSFRS-R | UMN Score | Affected Side | ELESC |
|---|---|---|---|---|---|
| 1/49/M | 16 | 39 | 6 | L | Definite |
| 2/44/M | 5 | 25 | 3 | B | Definite |
| 3/68/M | 52 | 27 | 2 | L | Probable |
| 4/54/F | 6 | 44 | 3 | L | Definite |
| 5/54/M | 8 | 34 | 3 | B | Probable |
| 6/63/M | 33 | 48 | 5 | L | Definite |
| 7/64/M | 11 | 40 | 3 | R | Probable |
| 8/72/M | 20 | 42 | 5 | L | Definite |
| 9/58/M | 10 | 40 | 5 | R | Definite |
| 10/57/M | 4 | 46 | 6 | R | Definite |
| 11/63/F | 5 | 47 | 4 | B | Probable |
| 12/54/M | 30 | 40 | 5 | L | Definite |
| 13/39/F | 50 | 46 | 5 | L | Possible |
| 14/56/M | 13 | 45 | 3 | B | Possible |
| 15/49/F | 4 | 47 | 5 | L | Probable |
| 16/69/M | 60 | 36 | 5 | L | Definite |
Note:—ALSFRS-R indicates ALS Functional Rating Scale–Revised13; UMN, upper motor neuron; ELESC, El Escorial12; L, left; R, right; B, bilateral.
Data Acquisition
MR studies were performed on a Siemens Magneton Trio 3T whole body scanner (Siemens, Erlangen, Germany) with the use of a product transmit-receive head coil. All patients and healthy subjects were positioned in the magnet along their anterior commissure (AC) to posterior commissure (PC) line. Their alignment was adjusted and verified by using quick scout imaging before data acquisition. Routine MR pulse sequences included T1-weighted, 3D, magnetization prepared rapid acquisition gradient echo (MPRAGE) (repetition time [TR]/echo time [TE]/flip angle, 1620/3.9/15), fast fluid-attenuated inversion recovery (FLAIR) (TR/effective TE [TEeff]/inversion time [TI], 9190/97/2500) and T2-weighted (TR/TEeff, 4000/85) images. DTI was acquired with a 12-direction, single-shot, spin-echo echo-planar sequence. Imaging parameters were as follows: TR, 6500 ms; TE, 99 ms; field of view (FOV), 22 × 22 cm2; section thickness, 3 mm, matrix, 128 × 128; b values 0 and 1000 s/mm2, and 40 sections covering the whole brain.
Image Processing
The data were transferred to a Leonardo workstation (Siemens). DTI-based color maps and fiber tracking was generated by using the “DTI-Task-Card” (version 1.69; MGH, Boston, Mass). Color maps were created by mapping the major eigenvector x, y, and z components into red, green, and blue colors that were weighted by FA. Red tracts were oriented in the right/left (transverse) direction; green, in the anterior/posterior direction; and blue, in the superior/inferior direction. The axially acquired images were reformatted into the coronal orientation to facilitate anatomic interpretation (Figs 1 and 2). Axial and coronal color maps were visually evaluated for CST abnormality. The CST was also reconstructed by using FACT method.10 Tracking was initiated from a “seed” ROI in both retrograde and antegrade directions defined by the major eigenvector in the ROI. The propagation was terminated when it reached a voxel with FA < 0.15 or when the angle between 2 consecutive steps was greater than 41°. The seed region of interest was placed at the precentral gyrus and a second in the anterior half of the lower pons on the ipsilateral side. Both ROIs were drawn on b0 image and color map at a single axial section, respectively (as shown in Fig 3). Fiber tracts that passed through both ROIs were designated as the CST. These fiber tracking images were used to map the location of the CST on the axial and coronal color maps.
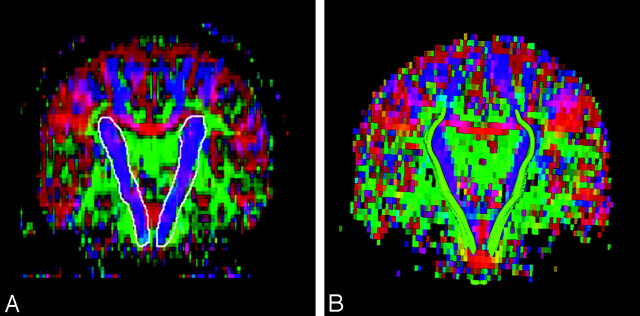
Fig 1.
Diffusion tensor imaging-based color map of a healthy subject. Colors indicate directions as follows: red, left-right; green, anteroposterior; blue, superior-inferior. The white line delineates manually segmented corticospinal tract (CST) (A). Reconstructed CSTs (green) are overlaid on color maps (B).
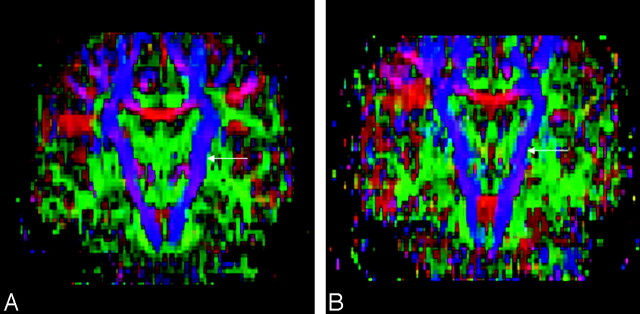
Fig 2.
Diffusion tensor imaging-based color maps of a healthy subject (A) and a patient with amyotrophic lateral sclerosis (ALS) (B). The left corticospinal tract (arrows) appears thinner in the patient with ALS (B).
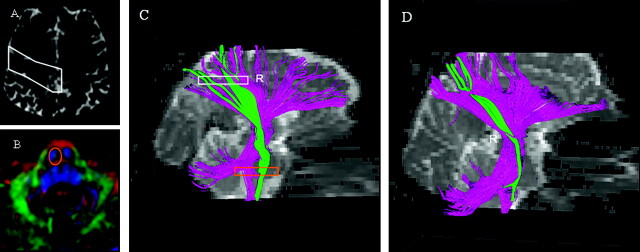
Fig 3.
Region of interest (ROI) placement on the reconstruction of corticospinal tract (CST) at the level of precentral gyrus (A, white) and lower pons (B, orange). Fiber tracking images of a healthy subject (C) and a patient with amyotrophic lateral sclerosis (ALS) (D). The rectangle in C indicates section location of ROIs. Descending fibers connecting the cortex and brain stem are shown in purple. CSTs are green. The CST fibers are diminished in the patient with ALS (D).
Specifically, progressing through the coronal color maps, the voxels pertaining to the CST were determined by 2 factors: relative location based on fiber tracking images and orientation of diffusion properties of the tissue. The CST consisted of voxels with tensor information that was oriented superior-inferiorly, shown in blue. A neuroradiologist (S.W.) who was blinded to the neurologic diagnosis and clinical findings manually performed segmentation of the CST. The CST on 4 contiguous coronal sections in the region from the lower pons to the corona radiata at the level of the corpus callosum was visually outlined based upon color by using fiber tracking images to map the location (Fig 1). It should be pointed out that these 4 sections constitute the stem portion of CST. The remaining fiber tracts either fan out into sprays where the fibers originate or are indiscernible from voxels belonging to other fiber bundles, and could not be investigated in the present study. This is secondary to the inability of DTI to distinguish among multiple fiber orientations within a single voxel.15
The left and right CST volumes were then obtained separately by calculating and converting the number of voxels contained in the segmented area into cubic millimeters by using code written in Matlab 6.1 (MathWorks, Natick, Mass). Because the volume of brain structures depends directly on the total intracranial volume, which can vary among individual subjects, we normalized the CST volume to the total intracranial volume for each subject. The total intracranial volumes excluding the cerebellum were calculated based on the high-resolution T1-MPRAGE images. A high-dimensional elastic type of spatial normalization transformation16 was used to transfer a standardized stereotaxic T1 image with total intracranial volume manually outlined to each subject space. The intracranial volume was then calculated from the warped template.
Statistical Analysis
The data were analyzed with the use of statistical software (SYSTAT for Windows; Systat Software, Point Richmond, Calif). The distribution of the measurements was verified by the normal plot method, provided by SYSTAT, which showed that distributions of individual values of the CST volume could be approximated to a Gaussian distribution. Comparisons of the CST volumes between affected and nonaffected hemispheres in the patient group and between patients and healthy subjects were performed with a paired and nonpaired 2-sided t test, respectively.
To quantify the relationship between CST volume in affected and nonaffected hemispheres and clinical parameters such as ALSFRS-R score, UMN involvement score, and duration of disease, a Spearman rank correlation coefficient was used. In the case of bilateral involvement, one hemisphere was assigned to the affected group and the other to the nonaffected group, depending on which side was more severely clinically affected. P < 0.05 was considered significant.
Results
The CST was easily visualized on axial and coronal color maps in all 33 subjects. In 8 of 16 patients with ALS, thinning of the CST was appreciated upon visual inspection of the color maps (Fig 2). Fiber tracking images of the CST were created for all patients. DTI fiber tracts in 7 patients with ALS were similar to those of healthy subjects; however, the remaining 9 patients had fewer fiber tracts than healthy subjects (Fig 3). The mean volume of the CST in the affected hemisphere was not significantly different from the mean volume in nonaffected hemisphere. However, the average total volume of CST from both hemispheres of patients with ALS was substantially lower than the corresponding value for healthy subjects (P <.01; Table 2). The mean values of CST volume from affected and nonaffected hemispheres were also significantly lower than the mean values from either the left or right side of healthy subjects (Table 2).
Table 2:
Corticospinal tract (CST) volumes in patients with amyotrophic lateral sclerosis (ALS) and healthy subjects
| Measurement | Healthy Control Subjects (n = 17) |
ALS Patients (n = 16) |
||
|---|---|---|---|---|
| Left (10−3 mm3) | Right (10-3 mm3) | Affected (10−3 mm3) | Nonaffected (10−3 mm3) | |
| CST volume (mean ± SD) | 10.19 ± 0.94 | 10.43 ± 0.96 | 9.18 ± 0.70* | 9.33 ± 0.64* |
Statistically different by unpaired t-test (P < .01).
No significant correlation was found between CST volumes and any clinical parameters, including disease duration, ALSFRS-R, or UMN involvement score. The highest value of correlation coefficient was found between CST volume and UMN score, in both the affected (r = −0.23) and nonaffected side (r = −0.31); however, the P value varied between 0.2 and 0.4 and did not reach significance.
Discussion
This study showed that the CST volume in patients with ALS is lower than that in the healthy subjects. This result suggests that CST volumetric analysis, as determined with DTI-based color maps, provides valuable information about UMN involvement.
The CST is the largest descending pathway that connects the motor cortex to the brain stem and spinal cord. CST fibers converge into the corona radiata and continue through the posterior limb of the internal capsule to the cerebral peduncle on their way to the lateral funiculus. The main histologic changes seen in the corticospinal system of patients with ALS include the loss of cortical pyramidal motor neurons and degeneration of CST axons with associated astrocytosis, which can be very variable.17,18 Surviving motor neurons tend to be smaller and are histologically abnormal. Postmortem studies from patients with ALS have revealed uneven involvement with variable patterns of degeneration of the CST.19,20
Conventional MR imaging in ALS is currently used to exclude other pathologies rather than for a definitive diagnosis. Hypointensity in the motor cortex on T2-weighted images has been observed in a number of patients with ALS, probably because of the presence of iron deposition,21 whereas high signal intensity along the CST has been described on T2-weighted, proton-attenuation, and FLAIR pulse sequences. These hyperintense regions are most readily identified at the level of the posterior limb of the internal capsule and may reflect demyelination and degeneration of the CST.22-24 There is a general lack of agreement regarding the sensitivity and specificity of these abnormalities in the detection of UMN involvement, probably because of differences in the sequence parameters used, and the subjectivity in identifying the abnormalities. Generally, abnormal MR findings correlate with average or rapid progression of the disease.22
Changes in tissue structure due to CST degeneration modify the diffusion characteristics of water molecules, which can be detected by DTI. Investigations applying DTI to the evaluation of ALS have generally focused on measurements of mean diffusivity and/or anisotropy at different levels of the CST.25-27 The authors reported decreased FA and elevated ADC values in the posterior limb of the internal capsule. Therefore, all these studies are based on the magnitude of these changes in diffusion properties. DTI-based color maps, created directly from the diffusion tensor data, as used in the current study, allow direct visualization of CST based on its orientation. Therefore, evaluation of the color maps represents a potential method to monitor morphologic changes of the CST in patients with ALS. The thinning of the CST on color maps and reduced fiber tracts observed in some of the patients with ALS is consistent with the UMN pathology.
We observed a significant decrease in CST volume in patients with ALS relative to healthy subjects. From a pathologic standpoint, CST degeneration might reasonably cause both FA decrease and CST volume loss. Manual segmentation of CST on coronal color maps was determined by 2 factors: relative location based on fiber tracking images and color. Because FA modulates the brightness of the color, a decrease of FA in patients with ALS may be responsible for a portion of the volume loss observed in these patients. In a recent study, proton attenuation or T2-weighted images were used for the measurements of gray and white matter volumes.28 This study reported a significant reduction of the white matter volume along the CST in ALS. However, inclusion of the entire white matter would not be specific for the CST because such an analysis would include many other fibers as well. In our study, we demonstrated that color maps, generated by DTI, can readily identify the CST and thus provide a more specific assessment of UMN involvement in patients with ALS.
Fiber tracking is a versatile tool for elucidating the trajectory of white matter fiber pathways but less so for their volumetric analysis.29 Fiber tracking is a user-dependent process, the result of which varies significantly depending on the FA, angular threshold, vector step lengths, and seed ROIs. The tracking result is also affected by image resolution and signal intensity–to-noise ratio. The validity of the technique could be assessed by defining a “gold standard” volume for the structure of interest based on anatomic atlases. Threshold parameters, step length, seed ROIs, and other parameters could then be optimized to provide the most faithful reconstruction of the “gold standard” volume.30 Because of the above-mentioned limitation, we believe that a quantitative estimation of the CST volume by using fiber tracking may not be accurate. However, this technique is useful for mapping the location of CST.
We observed no significant correlations between scores of clinical disability and CST volume. This is not surprising, because it is well known that associations between clinical scores and pathologic findings are weak. It has been reported that a significant clinical disability may occur even in the absence of CST involvement; the reverse has also been reported.1,18 In addition, the ALS functional rating scale is not specific to clinical UMN involvement. The UMN score used in the present study, though simple and easily reproducible, may not have been sensitive enough to differentiate between minimal and marked UMN disease.
The CST volume reported in this study might not reflect true volume. An important point to our study is that this noninvasive method, whether a true measurement or just a close approximation, may be useful in patients with ALS. Our study suggests that this measurement is significantly decreased in patients with ALS. The limitation of our study is the relatively small sample size, especially the number of patients with suspected or possible ALS. Future directions should include longitudinal studies in patients with ALS and studying patients with clinical LMN disease who lack apparent UMN disease.
In conclusion, this study highlights the potential of DTI in detecting and monitoring structural degeneration of the CST. This will lead to more objective and quantitative assessment of axonal degeneration in ALS.
Acknowledgments
We acknowledge Christos Davatzikos and Dinggang Shen for their assistance in the measurements of intracranial volume.
References
- 1.Brownell B, Oppenheimer DR, Hughes JT. The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 1970;33:338–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowland LP. Diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 1998;160 Suppl 1:S6–24 [DOI] [PubMed] [Google Scholar]
- 3.Chenevert TL, Brunberg JA, Pipe JG. Anisotropic diffusion in human white matter: demonstration with MR techniques in vivo. Radiology 1990;177:401–05 [DOI] [PubMed] [Google Scholar]
- 4.Albayram S, Melhem ER, Mori S, et al. Holoprosencephaly in children: diffusion tensor MR imaging of white matter tracts of the brainstem–initial experience. Radiology 2002;223:645–51 [DOI] [PubMed] [Google Scholar]
- 5.Melhem ER, Mori S, Mukundan G, et al. Diffusion tensor MR imaging of the brain and white matter tractography. AJR Am J Roentgenol 2002;178:3–16 [DOI] [PubMed] [Google Scholar]
- 6.Pajevic S, Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med 1999;42:526–40 [PubMed] [Google Scholar]
- 7.Mori S, Kaufmann WE, Davatzikos C, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 2002;47:215–23 [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Kaufmann WE, Pearlson GD, et al. In vivo visualization of human neural pathways by magnetic resonance imaging. Ann Neurol 2000;47:412–14 [PubMed] [Google Scholar]
- 9.Mori S, van Zijl PC. Fiber tracking: principles and strategies—a technical review. NMR Biomed 2002;15:468–80 [DOI] [PubMed] [Google Scholar]
- 10.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–69 [DOI] [PubMed] [Google Scholar]
- 11.Xue R, van Zijl PC, Crain BJ, et al. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med 1999;42:1123–27 [DOI] [PubMed] [Google Scholar]
- 12.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 1994;124 Suppl:96–107 [DOI] [PubMed] [Google Scholar]
- 13.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169:13–21 [DOI] [PubMed] [Google Scholar]
- 14.Hong YH, Lee KW, Sung JJ, et al. Diffusion tensor MRI as a diagnostic tool of upper motor neuron involvement in amyotrophic lateral sclerosis. J Neurol Sci 2004;227:73–78 [DOI] [PubMed] [Google Scholar]
- 15.Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology 2004;230:77–87 [DOI] [PubMed] [Google Scholar]
- 16.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging 2002;21:1421–39 [DOI] [PubMed] [Google Scholar]
- 17.Iwanaga K, Hayashi S, Oyake M, et al. Neuropathology of sporadic amyotrophic lateral sclerosis of long duration. J Neurol Sci 1997;146:139–43 [DOI] [PubMed] [Google Scholar]
- 18.Lawyer T, Jr., Netsky MG. Amyotrophic lateral sclerosis. AMA Arch Neurol Psychiatry 1953;69:171–92 [DOI] [PubMed] [Google Scholar]
- 19.Fischer LR, Culver DG, Tennant P, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 2004;185:232–40 [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Yagishita S, Amano N, et al. Amyotrophic lateral sclerosis with numerous axonal spheroids in the corticospinal tract and massive degeneration of the cortex. Acta Neuropathol (Berl) 1997;94:294–99 [DOI] [PubMed] [Google Scholar]
- 21.Oba H, Araki T, Ohtomo K, et al. Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 1993;189:843–46 [DOI] [PubMed] [Google Scholar]
- 22.Cheung G, Gawel MJ, Cooper PW, et al. Amyotrophic lateral sclerosis: correlation of clinical and MR imaging findings. Radiology 1995;194:263–70 [DOI] [PubMed] [Google Scholar]
- 23.Hecht MJ, Fellner F, Fellner C, et al. MRI-FLAIR images of the head show corticospinal tract alterations in ALS patients more frequently than T2-, T1- and proton-density-weighted images. J Neurol Sci 2001;186:37–44 [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Ulug AM, Zimmerman RD, et al. The diagnostic utility of FLAIR imaging in clinically verified amyotrophic lateral sclerosis. J Magn Reson Imaging 2003;17:521–27 [DOI] [PubMed] [Google Scholar]
- 25.Ellis CM, Simmons A, Jones DK, et al. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999;53:1051–58 [DOI] [PubMed] [Google Scholar]
- 26.Sach M, Winkler G, Glauche V, et al. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004;127:340–50 [DOI] [PubMed] [Google Scholar]
- 27.Toosy AT, Werring DJ, Orrell RW, et al. Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2003;1250–57 [DOI] [PMC free article] [PubMed]
- 28.Ellis CM, Suckling J, Amaro E, Jr., et al. Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology 2001;57:1571–78 [DOI] [PubMed] [Google Scholar]
- 29.Lori NF, Akbudak E, Shimony JS, et al. Diffusion tensor fiber tracking of human brain connectivity: aquisition methods, reliability analysis and biological results. NMR Biomed 2002;15:494–515 [DOI] [PubMed] [Google Scholar]
- 30.Clark CA, Barrick TR, Murphy MM, et al. White matter fiber tracking in patients with space-occupying lesions of the brain: a new technique for neurosurgical planning? Neuroimage 2003;20:1601–08 [DOI] [PubMed] [Google Scholar]