Abstract
SUMMARY: Four patients with encephalitis/encephalopathy and parenchymal lesions accompanying reversible splenial lesions were retrospectively evaluated. In 3 patients, reversible lesions with transiently reduced diffusion were seen in the splenium and symmetrically in the peripheral frontoparietal white matter, clinical signs and symptoms were mild, and recovery was complete. These and previous observations suggest a less severe course and outcome for patients with reversible lesions isolated to the splenium or to the splenium and peripheral frontoparietal white matter.
An MR imaging finding of a reversible lesion in the central portion of the splenium of the corpus callosum (SCC) without any accompanying lesions has been reported in patients with epilepsy receiving antiepileptic drugs.1,2 Fifteen patients with clinically mild encephalitis/encephalopathy with a reversible isolated SCC lesion (MERS) were recently reported, 7 of whom had no history of seizures or administration of antiepileptic drugs.3 All the patients with MERS had mild clinical courses and recovered completely without any sequelae. Because the SCC lesion in the 2 conditions revealed transiently homogenous reduced apparent diffusion coefficient (ADC) values, we speculated that these 2 conditions might share the same unknown pathogenesis.
The previous report of MERS excluded those patients with parenchymal lesions in addition to the reversible SCC lesion.3 The purpose of this study was to evaluate clinical and radiologic findings in 4 patients having additional lesions to clarify the clinical and radiologic characteristics of these other abnormalities.
Patients and Methods
Patients with possible encephalitis/encephalopathy and a reversible SCC lesion on MR imaging were collected retrospectively by sending out a questionnaire to the members of the annual Zao Conference on Pediatric Neurology, the Japanese Society of Pediatric Neurology, and the Japanese Society of Neuroradiology. The MR imaging scans and charts of these patients were reviewed. A reversible SCC lesion was defined as a lesion involving the central portion of SCC that disappeared on the follow-up study. The diagnosis of encephalitis has been defined as acute onset of brain dysfunction in association with inflammatory changes of CSF. When there was no evidence of inflammatory change, we made the diagnosis of encephalopathy. Patients with abnormal signal intensity in regions other than the SCC were separated from those with isolated SCC lesions based on examination of their MR imaging studies.
Results
We identified 4 patients with encephalitis or encephalopathy having parenchymal lesions in addition to the reversible SCC lesion among the 29 patients whose clinical records and MR imaging examinations were referred for this study. The clinical and radiologic findings of the 4 patients are summarized in Tables 1 and 2.
Table 1:
Clinical data
| Patient No./Age (y)/Sex | Pathogen | Initial Symptom | Premedication | CNS Manifestation (onset day) | CNS Diagnosis | Therapy | Prognosis | CSF | EEG |
|---|---|---|---|---|---|---|---|---|---|
| 1/6/M | Unknown | Fever, vomiting | None | Headache (d 1), delirium (d 4) | Encephalopathy | None | CR (day 8) | Normal | Normal |
| 2/8/M | Unknown | Fever | None | Drowsiness (1) | Encephalitis | ACV | CR (day 10) | CC, 19 | Rolandic discharge |
| 3/4/F | EBV | Fever | None | Seizure, drowsiness (3) | Encephalopathy | ACV | CR (day 8) | Normal | Slow wave |
| 4/5/F | Unknown | Fever | None | Seizure, drowsiness (1) | Encephalopathy | PSL | MR (IQ29) | Normal | Normal |
Note:— CNS indicates central nervous system; CSF, cerebrospinal fluid; EEG, electroencephalograph; EBV, Epstein-Barr virus; ACV, aciclovir; PSL, prednisolone; CR, complete recovery; MR, mental retardation; CC, cell count (per mm3).
Table 2:
MR imaging data
| Patient No. | Scan Date* | Callosal Lesion | Shape | T2WI | T1WI | GD | DWI | ADC | Other Lesions | T2WI | T1WI | GD | DWI | ADC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Day 7 (3) | SCC | Extended | H | I | — | H | L | sym P WM | H | I | — | H | L |
| Day 14 | None | None | ||||||||||||
| 2 | Day 2 (2) | None | sym F, P WM | I | I | NE | H | L | ||||||
| Day 5 | SCC | Extended | s1 H | I | NE | H | L | sym F, P WM | s1 H | I | NE | H | L | |
| Day 12 | None | None | ||||||||||||
| 3 | Day 4 (2) | SCC | Extended | sl H | I | NE | H | L | sym F, P WM | s1 H | I | NE | H | L |
| Day 10 | None | None | ||||||||||||
| 4 | Day 1 (1) | None | None | |||||||||||
| Day 2 | SCC | Ovale | s1 H | I | — | H | L | None | ||||||
| Day 7 | None | F GM, WM | I | I | NE | H | L | |||||||
| Day 70 | None | F GM† | s1 H | I | NE | I | I |
Note:— GD indicates gadolinium enhancement; DWI, diffusion-weighted imaging; ADC, apparent difusion coefficient; SCC, splenum of corpus callosum; H, high signal; I, iso signal; L, low signal; NE, not examined; sym, symmetric; P, parietal; F, frontal; WM, white matter; GM, gray matter.
Days after central nervous system manifestation are in parentheses following the scan date.
Frontal atrophy.
All patients had developed normally until the onset of neurologic symptoms and were subsequently diagnosed with encephalitis/encephalopathy. Epstein-Barr virus was identified as the causative agent in patient 3. Two patients (patients 3 and 4) experienced seizures, but none had received antiepileptic drugs at the time of MR studies. Patients 1, 2, and 3 recovered completely within 10 days, whereas patient 4 had residual sequelae.
The imaging features of the SCC lesion were identical to those reported in MERS; the lesion was homogenously slightly hyperintense on T2-weighted images, isointense to slightly hypointense on T1-weighted images, showed homogenously reduced diffusion (hyperintensity on diffusion-weighted images and low ADC values), and completely resolved on follow-up studies.3 The lesion was ovoid and in the center of the SCC in patient 4, but it extended irregularly into the lateral portion of SCC in the other 3 patients.
The additional parenchymal lesions were symmetric and located in the peripheral parietal or frontoparietal white matter in 3 of the 4 patients (patients 1–3). In patient 2, the white matter lesions seemed to be connected through the lesions in the central and lateral aspects of the SCC (Fig 1). The signal intensity (T2 prolongation with markedly reduced ADC) and reversibility of the white matter lesions were identical to those of the SCC lesion. In patient 4, parenchymal lesions were seen in other locations—ie, MR imaging showed diffuse frontal cortical and white matter lesions with transiently reduced diffusion, followed by frontal cortical T2 prolongation and atrophy (Fig 2).
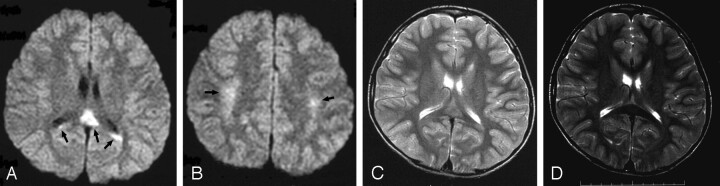
Fig 1.
Diffusion-weighted and T2-weighted images of patient 2 on day 5 showed high-signal-intensity lesions in the central portion of the splenium of the corpus callosum (SCC) and symmetric frontoparietal peripheral white matter (A–C, arrows). Follow-up study on day 12 showed no lesions on any sequences (T2-weighted image, D).
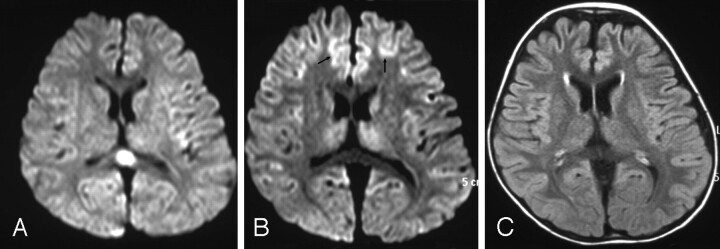
Fig 2.
Diffusion-weighted image (DWI) of patient 4 on day 2 showed an oval shaped splenial lesion (A). DWI on day 7 showed no splenial lesion, but frontal cortical lesions with reduced diffusion (B, arrows), followed by cortical T2 prolongation and atrophy on day 70 (fluid-attenuated inversion recovery, C).
Discussion
The most important finding in this study is the observation that symmetric lesions in the parietal or frontoparietal peripheral white matter had the same signal intensity characteristics and the same reversibility as the SCC lesion seen in MERS.3 Three patients (patients 1–3) who had lesions on only these locations had mild clinical courses and recovered completely within 10 days, features identical to those of MERS.3 Two very similar patients with clinically mild encephalopathy due to influenza type B and Salmonella enteritis have been reported to have MR imaging findings of identical reversible lesions with transiently reduced ADC in both the SCC and bilateral frontoparietal peripheral white matter.4,5 These observations suggest a mild course and good outcome for patients with reversible lesions in the splenium and peripheral frontoparietal white matter, similar to what has been found in patients with MERS. There has been no report of reversible MR imaging lesions (other than those in the SCC) in patients with epilepsy or taking antiepileptic drugs. It is interesting to note that, in this regard, most of the SCC lesions in patients with epilepsy were ovoid or round in shape.1–3 In contrast, the SCC abnormalities in patients with additional hemispheric lesions extended into the lateral portions of splenium (patients 1–3); this finding suggests that the callosal lesions and those in the surrounding white matter might be connected, as was suggested in the MR imaging of patient 2.
In any patient with encephalitis/encephalopathy and lesions in the white matter, acute disseminated encephalomyelitis (ADEM) should be considered in the differential diagnosis.6 ADEM is a monophasic postinfectious inflammatory disorder and presents with seizures, focal neurologic signs, and alteration of consciousness days to weeks after the onset of presumed viral infections. Corticosteroids are accepted as useful treatment for ADEM, and recovery occurs within weeks.6 In contrast, 5 of the reported patients with reversible lesions in SCC and symmetric peripheral white matter (patients 1–3 in this report and the 2 previously reported patients) developed neurologic symptoms quickly after the onset of illness (days 1–4), and recovered completely within 10 days.4, 5 Corticosteroids were not administered to 4 of the 5 patients. MR imaging in ADEM usually shows multiple foci of T1 and T2 prolongation typically bilaterally but asymmetrically in the subcortical white matter.7 Although the corpus callosum may be involved in ADEM, these lesions are nearly always asymmetric.8 Evolution of the lesions usually takes weeks to months, and they disappear only after several months, because the imaging evolution of the disease lags behind the clinical evolution. Indeed, the white matter damage may be permanent. The reversible lesions in the SCC and peripheral white matter in 3 patients (patients 1–3) in this series and in the 2 previously reported patients4, 5 were symmetric and located centrally; they disappeared completely within a week. The reversible callosal and white matter lesions, therefore, are clinically and radiologically unlikely to represent a manifestation of ADEM.
The MR imaging studies of patient 4 showed lesions in other regions (the prefrontal cortex and white matter) of the brain; these ultimately resulted clinically in neurologic sequelae with frontal atrophy seen on imaging. Another patient with a reversible SCC lesion had clinical sequelae of involuntary movements and radiologic sequelae of irreversible lesions in corpus striatum (Hideo Yamanouchi, Department of Pediatrics, Dokkyo University School of Medicine, personal communication). Although this study details only a small number of patients, and many more patients will need to be evaluated to confirm this observation, it seems reasonable to speculate that a more severe clinical course and neurologic sequelae are more likely to develop if the MR imaging findings involve the brain beyond the SCC and symmetrical lesions in the frontoparietal white matter.
Acknowledgments
We appreciate the helpful advice of Masaharu Hayashi, MD, of the Department of Clinical Neuropathology, Tokyo Metropolitan Institute for Neuroscience, Fuchu, Japan.
References
- 1.Kim SS, Chang K-H, Kim ST, et al. Focal lesion in the splenium of the corpus callosum in epileptic patients: antiepileptic drug toxicity? AJNR Am J Neuroradiol 1999;20:125–29 [PubMed] [Google Scholar]
- 2.Mirsattari SM, Lee DH, Jones MW, et al. Transient lesion in the splenium of the corpus callosum in an epileptic patient. Neurology 2003;60:1838–41 [DOI] [PubMed] [Google Scholar]
- 3.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 2004;63:1854–58 [DOI] [PubMed] [Google Scholar]
- 4.Takanashi J, Barkovich AJ, Yamaguchi K, et al. Influenza encephalopathy with a reversible lesion in the splenium of the corpus callosum. AJNR Am J Neuroradiol 2004;25:798–802 [PMC free article] [PubMed] [Google Scholar]
- 5.Kobuchi N, Tsukahara H, Kawamura Y, et al. Reversible diffusion-weighted MR findings of Salmonella enteritidis–associated encephalopathy. Eur Neurol 2003;49:182–84 [DOI] [PubMed] [Google Scholar]
- 6.Dyken PR. Viral diseases of the nervous system. In: Swaiman KF, ed. Pediatric neurology. 2nd ed. St. Louis: Mosby;1994. :643–88
- 7.Barkovich AJ. Toxic and metabolic brain disorders. In: Barkovich AJ, ed. Pediatric neuroimaging. 3rd ed. New York: Lippincott Williams & Wilkins;2000. :71–156
- 8.Friese SA, Bitzer M, Freudenstein D, et al. Classification of acquired lesions of the corpus callosum with MRI. Neuroradiology 2000;42:795–802 [DOI] [PubMed] [Google Scholar]