Abstract
BACKGROUND AND PURPOSE: Distal embolism during carotid angioplasty with stent (CAS) can be protected by a flow-reversal device. Diffusion-weighted MR imaging was used to evaluate this protective procedure and perform a comparison with the control.
METHODS: Cases of CAS with protection procedures were included in this study. Sixty-five men (68 procedures) and 5 women (5 procedures), with an average age of 68.8 years, having severe carotid stenosis were treated in our department between 2002 and 2004. Eleven cases were treated with the Parodi Anti-Emboli System, with which the internal carotid blood flow is reversed by simultaneous occlusion of the proximal common carotid artery and external carotid artery. Diffusion-weighted MR imaging was performed within 1–3 days after CAS. As controls, data from diffusion-weighted MR imaging in 26 patients who had diagnostic angiography were included.
RESULTS: Diffusion-weighted MR imaging in diagnostic angiography showed 11.5% appearance of ischemic spots after procedures. In the Parodi Anti-Emboli System, this value was 18.2%. In the CAS group, ischemic lesions appeared only in the hemisphere ipsilateral to carotid stenosis. There were no ischemic lesions in the opposite carotid or vertebrobasilar territory. The appearance rate of new ischemic spots was not significantly different between the control group and the group of CAS with Parodi Anti-Emboli System (χ2 test, P = .6227, Fisher exact method).
CONCLUSIONS: Protection results obtained with the Parodi system were excellent and comparable with conventional angiography.
Severe carotid stenosis is one of the major causes of cerebral infarction, and carotid endarterectomy (CEA) is the gold standard treatment for stroke prevention.1–4
Along with technological improvements in interventional neuroradiology, some cases of carotid stenosis have been treated by using carotid angioplasty with stent (CAS), especially for patients with higher risk factors of CEA, such as cardiac insufficiency, chronic occlusive pulmonary disease, and contralateral carotid occlusion.5 A recent randomized controlled trial, SAPPHIRE (Stent Placement and Angioplasty with Protection in Patients at High Risk for Endarterectomy Trial), showed almost the same efficacy of CAS as compared with CEA in high-risk patients.6,7 Although many advantages of CAS have been reported, one of its disadvantages is the considerably high occurrence of distal emboli during CAS, even though they are subclinical; asymptomatic or “silent” ischemic lesions were detected by diffusion-weighted MR imaging more often in CAS than CEA.8
Several protective devices for distal embolism are available, such as blocking balloon catheters, guidewire with balloon, proximal occlusion balloon systems, and filters. Efficacy of protection methods used to eliminate debris is the key for wider treatment indications of CAS. In a previous study,9 we compared the results of 2 protection procedures of distal embolism caused by embolic debris during CAS by using diffusion-weighted MR imaging. We performed single distal protection of the internal carotid artery (ICA) with a PercuSurge GuardWire (Medtronic AVE, Santa Rosa, Calif) during CAS in 20 cases. In addition, we performed simultaneous temporary occlusion of the ICA and the external carotid artery (ECA) by using a PercuSurge GuardWire and a Sentry balloon catheter (Boston Scientific Japan, Tokyo, Japan) in 25 cases. Ischemic spots were observed in 11 of 20 cases in the group undergoing CAS with protection of the ICA by using a single balloon (55.0%) and in only 9 of 25 cases receiving protection of both the ICA and the ECA (36.0%). These differences were significant (P = .0068). We have attempted to determine the best protection system. Unfortunately, filter devices are not available in Japan. Therefore, we have focused on flow-reversal systems. With such systems, it is possible to stop and reverse blood flow before inserting the guidewire through a stenotic lesion. With regard to prevention of ischemic lesions by reversed carotid blood flow, the Parodi Anti-Emboli System (ArteriA Medical Science, San Francisco, Calif) was examined with diffusion-weighted MR imaging. Diffusion-weighted MR imaging data of patients undergoing diagnostic angiography were used as controls.
Materials and Methods
Between 2002 and 2004, 11 patients underwent 11 CAS procedures at Mie University Hospital, in which distal emboli were protected by using the Parodi Anti-Emboli System. All 11 patients were men. Symptomatic carotid stenosis of >70% was observed in 7 cases, and asymptomatic carotid stenosis of >60% was observed in 4 cases (Table 1).
Table 1:
Patients undergoing carotid angioplasty with stenting using the Parodi Anti-Emboli System in Mie University Hospital from 2003 to 2004
| Patient No./Age (y)/Sex | Side | Symptom before Treatment | Systemic Complication | Stenosis Ratio (%) | Postprocedural Stenosis (%) | Occlusion Time during Procedure (s) | Control ACT (s) | ACT during Procedure (s) | Prolonged ACT times | Predilation | Stent | MRI-DWI Findings after Procedure (High-Signal Spot) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/71/M | R | Asymptomatic | HTN | 90 | 10 | 418 | 148 | 222 | 1.50 | (−) | SMARTeR | (−) |
| 2/88/M | L | TIA | HTN | 90 | 30 | 660 | 150 | 318 | 2.12 | (+) | SMARTeR | (−) |
| 3/69/M | R | Asymptomatic | DM, HL | 90 | 15 | 964 | 161 | 304 | 1.89 | (+) | SMARTeR | (−) |
| 4/75/M | R | TIA (Amaurosis) | DM | 90 | 10 | 683 | 151 | 389 | 2.58 | (+) | SMARTeR | (−) |
| 5/62/M | R | Minor | (−) | 80 | 15 | 600 | 138 | 286 | 2.07 | (+) | SMARTeR | (−) |
| 6/69/M | L | Minor | HL | 90 | 5 | 420 | 133 | 250 | 1.88 | (−) | SMARTeR | (−) |
| 7/61/M | R | Asymptomatic | DM, HTN | 90 | 0 | 830 | 167 | 523 | 3.13 | (+) | SMARTeR | (−) |
| 8/63/M | L | Major | DVT | 70 | 5 | 624 | 167 | 344 | 2.06 | (+) | SMARTeR | (+) |
| 9/65/M | L | Asymptomatic | HTN, HL | 70 | 10 | 517 | 137 | 337 | 2.46 | (−) | SMARTeR | (+) |
| 10/77/M | L | Minor | CRF, OMI | 90 | 5 | 1354 | 144 | 428 | 2.97 | (+) | SMARTeR | (−) |
| 11/66/M | R | Minor | DM | 95 | 5 | 931 | 110 | 254 | 2.31 | (+) | Protege | (−) |
Note:—ACT indicates activated clotting time; MRI-DWI, magnetic resonance imaging–diffusion weighted imaging; TIA, transient ischemia attack; HTN, hypertension; DM, diabetes mellitus; HL, hyperlipidemia; DVT, deep venous thrombosis; CRF, chronic renal failure; OMI, old myocardial infarction.
Averages are as follows: age, 69.7 ± 7.8 years; stenosis ratio, 85.4 ± 8.5%; occlusion time, 722.5 ± 255.2 seconds; ACT (control), 146.1 ± 15.2 seconds; ACT (during procedures, 329.5 ± 80.7 seconds.
Angiographic observations by 2 of the authors engaged in CAS were used in selection of patients for treatment by using the Parodi system. Those patients with poor collateral circulation to the affected carotid territory were excluded from treatment with the Parodi system, and these patients underwent CAS with distal blocking balloons. Assessment by MR imaging could be performed before and after CAS in all 11 cases.
In all procedures, the date of angiography preceded that of the first baseline MR imaging before CAS. Baseline MR imaging was performed between 4 and 32 days before CAS (average, 12.5 days). The second MR imaging was performed 1-3 days after CAS (average, 1.36 days). No neurologic events were found between the first angiography and the second MR imaging after CAS. Newly appearing high-intensity spots were counted by comparing the first and second MR images. Neurologic examination was carried out 1 day before and 1 day after CAS by an experienced neurosurgeon (S. Matsushima) who was not involved in the CAS procedure.
Reversed ICA flow was made by simultaneous temporal occlusion of both the proximal common carotid artery (CCA) and ECA, before insertion of a guidewire through the stenotic lesions until the end of procedures, continuously. The system used in this protection was the Parodi Anti-Emboli System.
Twenty-six patients, who were not included in the CAS group but who underwent angiography for diagnosis of ischemic cerebrovascular disease, cerebral aneurysm, and brain tumors, were included in the present study. The average age of the control subjects was 58.3 ± 15.3 years. MR imaging was performed 1-7 days after angiography (Table 2). Diagnostic angiography was performed by same operators as CAS without any bolus injection of heparin via veins or use of filters between catheters and injection syringes. These operators were experienced specialists. During angiography and MR imaging, there were no events that might have affected neurologic status of the patients or might have elicited changes in the MR imaging findings.
Table 2:
MRI-DWI assessment after DSA in Mie University Hospital from 2002 to 2004 (control group)
| Patient No./Age (y)/Sex | Diagnosis | DSA Study (No. of vessels) | MRI-DWI Study after DSA | High-Intensity Spot on MRI-DWI |
|---|---|---|---|---|
| 1/71/M | Bilateral IC stenosis | 4 | 1 day after | (−) |
| 2/46/F | Unruptured aneurysm | 4 | 1 day after | (+) (1 spot, 2mm) |
| 3/29/F | Dural AVF | 6 | Same day | (−) |
| 4/31/F | Brain tumor | 3 | 6 days after | (−) |
| 5/76/M | Right IC stenosis | 4 | 10 days after | (−) |
| 6/39/M | Brain tumor | 4 | 1 day after | (−) |
| 7/59/F | Brain tumor | 6 | 1 day after | (+) (1 spot, 2mm) |
| 8/56/F | Brain tumor | 4 | Same day | (−) |
| 9/77/F | Unruptured aneurysm | 4 | 1 day after | (−) |
| 10/37/M | Brain tumor | 3 | 5 days after | (−) |
| 11/64/M | Right IC stenosis | 4 | 3 days after | (−) |
| 12/76/F | Right IC stenosis | 4 | 8 days after | (−) |
| 13/77/M | Left IC occlusion | 4 | Same day | (−) |
| 14/49/M | Left MC occlusion | 4 | Same day | (−) |
| 15/59/F | Left IC occlusion | 4 | 1 day after | (−) |
| 16/76/M | Right IC stenosis | 4 | 1 day after | (−) |
| 17/43/F | Unruptured aneurysm | 4 | 4 days after | (+) (2 spots, 2mm) |
| 18/58/M | Left IC stenosis | 4 | 10 days after | (−) |
| 19/53/F | Right VA dissection | 4 | 1 day after | (−) |
| 20/69/M | Right IC stenosis | 4 | 1 day after | (−) |
| 21/48/M | Unruptured aneurysm | 4 | 1 day after | (−) |
| 22/71/M | Right IC stenosis | 4 | 1 day after | (−) |
| 23/74/M | Left IC stenosis, Right IC occlusion | 4 | 1 day after | (−) |
| 24/74/F | Unruptured aneurysm | 4 | 1 day after | (−) |
| 25/59/M | Left VA occlusion | 4 | 1 day after | (−) |
| 26/44/M | Unconsciousness due to hypnotics | 4 | Same day | (−) |
Note:—MRI-DWI indicates magnetic resonance imaging–diffusion weighted imaging; DSA, digital subtraction angiography; within the DSA study column, 4 vessels, bilateral common carotid arteries and bilateral vertebral arteries, and 6 vessels, bilateral internal and external carotid arteries and bilateral vertebral arteries; IC, internal carotid artery; MC, middle cerebral artery; VA, vertebral artery; AVF, arteriovenous fistula. Average age was 58.3 ± 15.3 years.
CAS Procedure and Protection
CAS with the Parodi Protective Device.
Acetylsalicylic acid (100 mg) and ticlopidine (200 mg) or cilostazol (200 mg) were given from at least 7 days before the procedure. Twelve procedures (12/13 = 92.3%) were performed under general anesthesia. All procedures were performed via the percutaneous transfemoral route. The procedures were carried out by an experienced neurointerventional team.
After placement of sheath introducers, heparin (100 U/kg) was administered intravenously, and activated clotting time (ACT) was kept at an average of 2.25 times (range, 1.50-3.13 times) longer than the control ACT.
An 11F introducer was placed in the right femoral artery, and a 4F introducer was placed in the left femoral vein. A 10.5F Parodi Anti-Emboli System occlusion catheter was guided up to the ipsilateral CCA proximal to carotid stenosis. Angioplasty and stent deployment were performed during temporary balloon inflation. In patients with enough space for stent deployment, predilation was not performed. SMARTeR stent (Johnson & Johnson, Cordis, Tokyo, Japan) or Protege (eV3 Inc., Plymouth, Minn) was used.
Protection was performed with the Parodi Anti-Emboli System. Both the proximal CCA and the ECA were occluded. The occlusion catheter for the CCA had double lumens: one was used for inflation and deflation of the balloon, and the other, larger and inner, lumen was open at the tip of the catheter to allow blood flow backward to the outside of the body when the CCA and the ECA were occluded. The reversed blood flow passed through lines connected to a filter device outside the body. Debris were filtered, and cleansed blood flowed to the venous circulation via a sheath placed in the femoral vein.
This group included 11 cases. In all cases, blood flow reversal was confirmed by gentle injection of contrast medium into the distal lumen of the ICA after inflation of the 2 balloons. In one case in which the reversed blood flow was so low that it appeared to be insufficient to drain the contaminated blood remaining in the distal lumen of the carotid artery, about 30-40 mL of the blood was aspirated manually with a syringe attached to a 3-way stopcock distal to a filter device and then returned to the venous circulation (Figs 1 and 2). Blood flow reversal was continuously maintained during procedures.
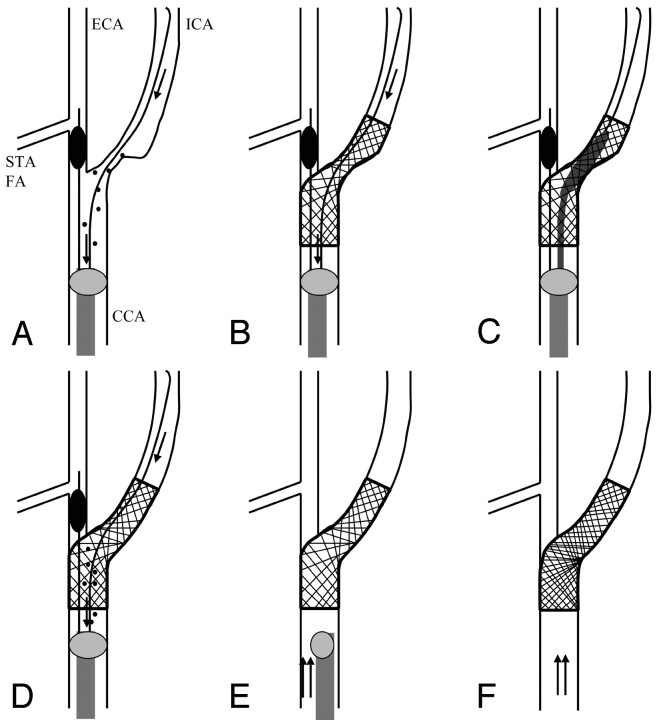
Fig 1.
Schema of the procedure by using the Parodi Anti-Emboli System. STA, superior thyroid artery; FA, facial artery; arrow, reversed blood flow; double arrow, normal blood flow; spot, debris
A, Flow reversal from the intracranial ICA by blockade of both the proximal CCA and ECA.
B, Placement of stent.
C, Postdilation.
D, Irrigation of debris by flow reversal.
E, Return to normal flow.
F, Final
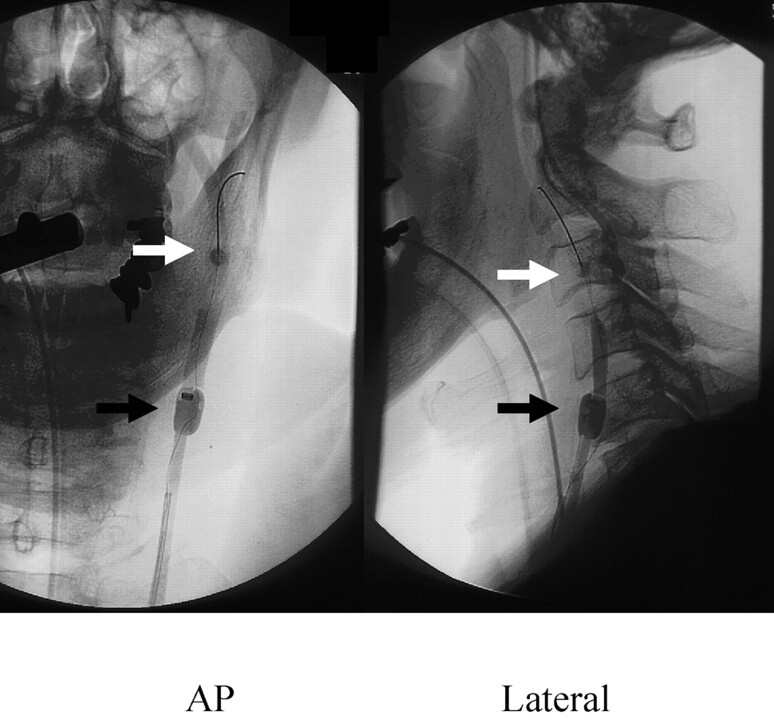
Fig 2.
Carotid angioplasty with stent by Parodi Anti-Emboli System (left carotid angiogram, anteroposterior and lateral view). White arrow, blocking balloon in the external carotid artery; black arrow, proximal blocking balloon in the common carotid artery.
Detection of Ischemic Lesion by MR Imaging.
The MR imaging used in the present study was a Signa Horizon Echospeed CV 1.5T (GE-Yokokawa Medical System, Tokyo, Japan). MR imaging studies consisted of axial T2 and diffusion-weighted images with the echo- planar method under the following conditions: TR/TE = 10000/69.2; section thickness = 5 mm; spacing = 1 mm; b value = 1000 seconds/mm2; field of view = 30 cm.
Baseline MR imaging was performed in all patients after diagnostic angiography and before CAS. The average period between baseline MR imaging and CAS was 12.5 days, and during this period there were no new ischemic events, such as transient ischemic attack or stroke. The second MR imaging was performed 1-3 days after CAS.
By comparing the baseline MR imaging and the second MR imaging, the occurrence of newly appearing high-intensity spots in diffusion-weighted MR imaging could be counted. An experienced neuroradiologist (M. Maeda) and an experienced neurosurgeon (S. Matsushima), who were not involved in CAS, assessed these ischemic lesions.
Statistical Analysis
Statistical analysis was performed with StatView 5.0 (SAS Institute, Cary, NC). Differences between data for each category regarding the appearance rate of ischemic spots after procedures were analyzed by χ2 test (cell size ≥5) or Fisher exact (cell size <5) test for categorical data. A P value <.05 was considered statistically significant.
Results
Clinical Outcome
CAS was completed successfully in all patients. Stenosis was reduced to 10.0%. Eight patients needed predilation (Table 1). Angiography immediately after CAS showed no evidence of distal embolization in intracranial circulation in any of the present cases. There was no neurologic worsening after CAS. The average overall occlusion time was 12 minutes 7 seconds (ranging from 6 minutes 58 seconds to 22 minutes 34 seconds; Table 1).
In the control group, in which diagnostic angiography was performed, there were no symptomatic complications and no cases of angiographically confirmed embolism in the intracranial circulation.
Ischemic Lesions in Diffusion-Weighted MR Imaging
In the control group, the appearance rate of ischemic spots was 11.5% (Table 2). We found 4 newly appearing ischemic spots in 3 patients after diagnostic angiography. These 3 patients included 2 patients with unruptured aneurysm and one with brain tumor. These 3 patients never had ischemic symptoms before angiography.
Diffusion-weighted MR images obtained before and after CAS were compared and numbers of newly appearing ischemic lesions were counted. Table 3 shows newly appearing high-intensity spots, the average numbers, and area size in each case. The average number of spots was 2, and the diameter of each spot was 2 mm.
Table 3:
Newly appearing high-intensity spots after carotid angioplasty with stent on MRI-DWI
| Patient No. | Side | Findings on MRI-DWI |
||
|---|---|---|---|---|
| Position | No. of Spots | Size of Spots | ||
| 8 | Left | Ipsilateral frontal lobe | 1 | 2 mm |
| 9 | Left | Ipsilateral frontal lobe | 3 | 2 mm |
Note:— MRI-DWI indicates magnetic resonance imaging–diffusion weighted imaging.
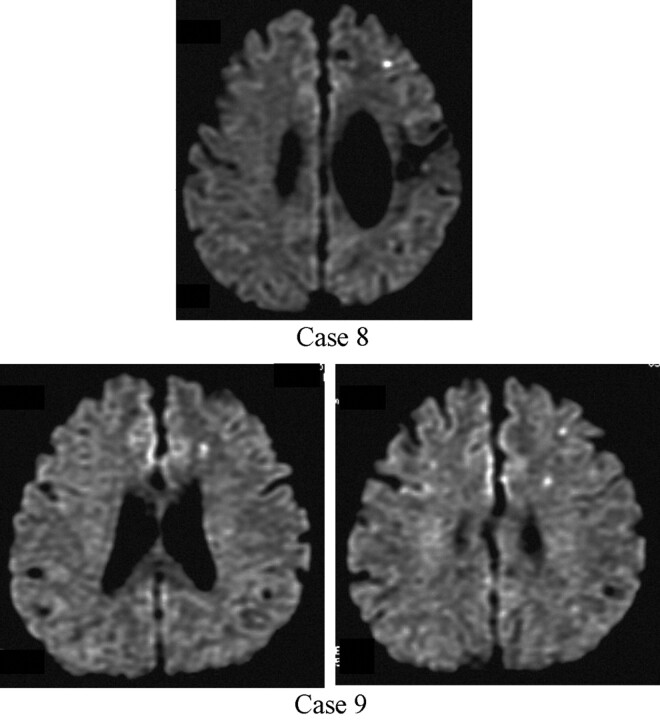
Newly appearing ischemic lesions were observed in 2 cases (18.2%) as shown in Figure 3. In case 8, only one newly appearing ischemic spot (maximum diameter, 2 mm) was detected in the ipsilateral carotid territory. In case 9, 3 newly appearing ischemic spots (maximum diameter, 2 mm) were detected in the ipsilateral carotid territory. In this study, we focused on not each step but total procedure of CAS.
Fig 3.
Ischemic lesions after carotid angioplasty with stent by Parodi Anti-Emboli System (diffusion-weighted MR image).
Top, Case 8 (after left carotid stent placement).
Bottom, Case 9 (after left carotid stent placement).
All spots were distributed on the ipsilateral hemisphere. There were no other areas of the brain that showed new ischemic spots.
The appearance rate of new ischemic spots was not significantly different between the control group and the group of CAS with Parodi Anti-Emboli System (χ2 test, P = .6227, Fisher exact method).
Discussion
There have been several studies concerning cerebral ischemia assessed by diffusion MR imaging after CAS,8,10–15 but, to the best of our knowledge, there have been no comparative analyses in relation to the Parodi System. We found one report16 of a transcranial Doppler study during CAS with filters and MO.MA system (Invatec, Roncadelle, Italy), which was a kind of flow-reversal system like the Parodi system. There was, however, no mention of MR imaging findings. Even in a recent report,15 newly appearing ischemic spots were found in 54% of CAS without protection devices. Therefore, we believe that protection devices are indispensable for CAS.
The average ages of the subjects were almost the same in the CAS with Parodi System group and in the control group. Thus, comparison between MR imaging results of the control group with those of patients who underwent CAS was thought to be appropriate. Bendszus et al17 found new ischemic lesions on diffusion-weighted MR imaging in 17 in 59 patients (29%) after diagnostic angiography. According to a prospective, randomized trial by the same group,18 17 of 150 patients (11%) showed newly appearing ischemic spots on diffusion-weighted MR imaging after cerebral diagnostic angiography. In another report,19 3 of 20 patients (15%) showed newly appearing ischemic spots on diffusion-weighted MR imaging after cerebral angiography. We found an incidence of 11.5% in our control population. This is as same as that of previous reports. The reason we performed 6 vessels study in only 2 cases was that 11 of 26 patients had cervical carotid stenotic lesions.
High-intensity spots in diffusion-weighted MR imaging are considered to be produced by ischemia and have been well documented in previous reports.20–22 Therefore, we should avoid making high-intensity spots in diffusion-weighted MR imaging during the CAS procedure, even if they cause no symptoms. From this perspective, we looked for the best protection system for distal embolism during CAS.
Appearance rates of subclinical ischemic spots after CAS of 21% to 54% have been reported with or without protection devices.8,10–15 The detection efficiency of ischemic spots in diffusion-weighted MR imaging is very different, depending on the MR imaging system used. Comparative analysis should be performed with clinical results observed by using the same MR imaging system with the inclusion of appropriate control data; however, comparisons based on the same MR imaging system have not been performed in previous studies. In the present study, all data were observed by using the same MR imaging system as used in our previous study.
In our previous study,9 we reported protection with a distal balloon occlusion device. When the ICA and the ECA were occluded simultaneously, high-attenuation ischemic spots appeared at a rate of 36.0%. When only the ICA was occluded, the rate of appearance of high-intensity spots in diffusion MR imaging was 55.0%. The present study was performed by using the same MR imaging, and the Parodi system showed a greater decrease in the appearance rate of high-attenuation spots. Although there was patient selection bias, the Parodi system showed a more thorough debris drainage effect as compared with distal balloon protection devices.
Features of the Ischemic Spots
As mentioned above, 4 newly appearing ischemic spots after CAS were observed in 2 patients. The maximum diameter of each spot was 2 mm, and all spots appeared in the ipsilateral carotid artery area.
Comparative Analysis with the Results of Previous Studies
Flach et al8 reported the appearance of high-intensity spots in diffusion-weighted MR imaging in 9 of 21 cases of CAS with filter protection devices and Parodi Anti-Emboli System (43%). They did not mention the numbers of cases protected by using filters or the Parodi System. Even in a recent report, Poppert et al15 reported the appearance of spots at a rate of 54% without protection.
The rates of newly appearing high-intensity spots in post-CEA were reported to be 7.8%,23 4%,24 and 4.2%,25 all of which were lower than the values obtained by diagnostic angiography. Intravascular manipulations are basic maneuvers in CAS, and the appearance rate may be decreased to as low as that of diagnostic angiography by application of various protection devices. The rates, however, are still higher than that of CEA.
High-Intensity Spots and Clinical Outcome
It is not yet known whether subclinical high-intensity spots in diffusion-weighted MR imaging cause permanent brain damage. Silent brain infarcts were reported to be associated with an increased risk of dementia and a steeper decline in cognitive function.26 Unfortunately, in the present study, we did not assess cognitive activity and thus could not draw any definitive conclusions in this regard. Fortunately, there were no cases of symptomatic infarction after CAS in our series.
Limitations of the Present Study
It was reported elsewhere27 that carotid plaque echolucency influences the risk of distal embolisms in carotid stent placement. One of the limitations of the present study is that we did not analyze the relationship between images obtained by duplex Doppler measurements before CAS and the appearance of diffusion MR imaging after CAS. The data and analysis are currently under evaluation in our department, but these data are in a different statistical population and thus were not included in this report. In addition, it was also reported28 that the median number of particles, their maximum diameter, and their maximum area were all significantly higher in aspirates obtained during procedures associated with periprocedural neurologic complications than in those obtained during procedures with no such associated complications. We did not, however, have a suitable method for accurate measurement of debris contained in the aspirated blood. We attempted to analyze both the amount and size of debris, but the error rates were high because of the small amounts of wet debris obtained.
An additional limitation of the present study was that the selection of protection procedures was not randomized, and thus it was possible that selection bias may have influenced the results. Patients with poor collateral circulation to the affected carotid territory were excluded from use of the Parodi system; these patients underwent CAS with distal blocking balloons.
Hyperfibrinogenemia has been reported to be a risk factor associated with complications of carotid atherosclerotic plaque.29 Another limitation of the present study was that we performed no analysis of hematologic factors, such as hyperfibrinogenemia.
In the United States and Europe, filter devices are mainly used for protection procedures. The appearance rate of high-intensity spots in CAS with filter devices was reported to be 15.0%.30 Zahn et al31 reported that no differences in clinical events rate between the 2 groups of protection devices (filters and occlusive balloons) were observed. Schmidt et al,16 however, reported that the MO.MA flow-reversal system led to significantly lower microembolic signals counts on a transcranial Doppler during CAS in comparison to a filter device. Unfortunately, filter devices are not commercially available in Japan, so filter protection could not be assessed by diffusion MR imaging in the present study.
The Parodi system may seem dangerous because of its large guiding catheter with a balloon; however, we have experienced no problems because of the guiding catheter. Of course, the Parodi system requires strict procedures during CAS for flow reversal by 2 balloons and one filter for the femoral vein. This may also seem to be complicated. Nevertheless, we have experienced neither procedure failure nor complications.
Conclusions
In this study, the Parodi system showed almost the same rates of appearance of new high-intensity spots as diagnostic angiography. This means that CAS with the Parodi system can be performed in patients with low embolic risk as diagnostic angiography. In conclusion, the Parodi System is very useful protection device for CAS.
Acknowledgments
We are grateful to Mrs. Ogawa, Mrs. Nakamura, Dr. Tanemura, and Dr. Miura for their valuable assistance in preparation of this manuscript.
References
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 2.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998;339:1415–25 [DOI] [PubMed] [Google Scholar]
- 3.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for a symptomatic carotid artery stenosis. JAMA 1995;273:1421–28 [PubMed] [Google Scholar]
- 4.European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991;337:1235–43 [PubMed] [Google Scholar]
- 5.CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Lancet 2001;357:1729–37 [PubMed] [Google Scholar]
- 6.Yadav JS. Carotid stenting in high-risk patients: design and rationale of the SAPPHIRE trial. Cleveland Clin J Med 2004;71:45–46 [DOI] [PubMed] [Google Scholar]
- 7.Mozes G, Sullivan TM, Torres-Russotto DR, et al. Carotid endarterectomy in SAPPHIRE-eligible high-risk patients: implications for selecting patients for carotid angioplasty and stenting. J Vasc Surg 2004;39:958–66 [DOI] [PubMed] [Google Scholar]
- 8.Flach HZ, Ouhlous M, Hendriks JM, et al. Cerebral ischemia after carotid intervention. J Endovasc Ther 2004;11:251–57 [DOI] [PubMed] [Google Scholar]
- 9.Asakura F, Kawaguchi K, Sakaida H, et al. Diffusion weighted magnetic resonance imaging in carotid angioplasty and stenting with balloon embolic protection devices. Neuroradiology 2006;48:100–12 [DOI] [PubMed] [Google Scholar]
- 10.Jaeger HJ, Mathias KD, Drescher R, et al. Diffusion-weighted MR imaging after angioplasty or angioplasty plus stenting of arteries supplying the brain. AJNR Am J Neuroradiol 2001;22:1251–59 [PMC free article] [PubMed] [Google Scholar]
- 11.Schluter M, Tubler T, Steffens JC, et al. Focal ischemia of the brain after neuroprotected carotid artery stenting. J Am Coll Cardiol 2003;42:1007–13 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Sanchez S, Millan-Torne M, Capellades-Font J, et al. Ischemic brain lesions following carotid revascularisation procedures: a comparative study using diffusion-weighted magnetic resonance imaging. Rev Neurol 2004;38:1013–17 [PubMed] [Google Scholar]
- 13.Terada T, Tsuura M, Matsumoto H, et al. Results of endovascular treatment of internal carotid artery stenoses with a newly developed balloon protection catheter. Neurosurgery 2003;53:617–23 [DOI] [PubMed] [Google Scholar]
- 14.Roh HG, Byun HS, Ryoo JW, et al. Prospective analysis of cerebral infarction after carotid endarterectomy and carotid artery stent placement by using diffusion-weighted imaging. AJNR Am J Neuroradiol 2005;26:376–84 [PMC free article] [PubMed] [Google Scholar]
- 15.Poppert H, Wolf O, Resch M, et al. Differences in number, size and location of intracranial microembolic lesions after surgical versus endovascular treatment without protection device of carotid artery stenosis. J Neurol 2004;251:1198–203 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt A, Diederich KW, Scheinert S, et al. Effect of two different neuroprotection systems on microembolization during carotid artery stenting. J Am Coll Cardiol 16; 2004;44:1966–69 [DOI] [PubMed] [Google Scholar]
- 17.Bendszus M, Koltzenburg M, Burger R, et al. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet 1999;354:1594–97 [DOI] [PubMed] [Google Scholar]
- 18.Bendszus M, Koltzenburg M, Bartsch AJ, et al. Heparin and air filters reduce embolic events caused by intra-arterial cerebral angiography: a prospective, randomized trial. Circulation 2004;110:2210–15 [DOI] [PubMed] [Google Scholar]
- 19.Chuah KC, Stuckey SL, Berman IG. Silent embolism in diagnostic cerebral angiography: detection with diffusion-weighted imaging. Australas Radiol 2004;48:133–38 [DOI] [PubMed] [Google Scholar]
- 20.Minematsu K, Li L, Fisher M, et al. Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology 1992;42:235–40 [DOI] [PubMed] [Google Scholar]
- 21.Pierpaoli C, Righini A, Linfante I, et al. Histopathologic correlates of abnormal water diffusion in cerebral ischemia: diffusion-weighted MR imaging and light and electron microscopic study. Radiology 1993;189:439–48 [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Lo EH, Pierce AR, et al. Role of vasogenic edema and tissue cavitation in ischemic evolution on diffusion-weighted imaging: comparison with multiparameter MR and immunohistochemistry. AJNR Am J Neuroradiol 1995;16:1107–15 [PMC free article] [PubMed] [Google Scholar]
- 23.Tomczak R, Wunderlich A, Liewald F, et al. Diffusion-weighted MRI: detection of cerebral ischemia before and after carotid thromboendarterectomy. J Comput Assist Tomogr 2001;25:247–50 [DOI] [PubMed] [Google Scholar]
- 24.Feiwell RJ, Besmertis L, Sarkar R, et al. Detection of clinically silent infarcts after carotid endarterectomy by use of diffusion-weighted imaging. AJNR Am J Neuroradiol 2001;22:646–49 [PMC free article] [PubMed] [Google Scholar]
- 25.Barth A, Remonda L, Lovblad KO, et al. Silent cerebral ischemia detected by diffusion-weighted MRI after carotid endarterectomy. Stroke 2000;31:1824–28 [DOI] [PubMed] [Google Scholar]
- 26.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–22 [DOI] [PubMed] [Google Scholar]
- 27.Biasi GM, Froio A, Diethrich EB, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 2004;110:756–62 [DOI] [PubMed] [Google Scholar]
- 28.Tubler T, Schluter M, Dirsch O, et al. Balloon-protected carotid artery stenting: relationship of periprocedural neurological complications with the size of particulate debris. Circulation 2001;104:2791–96 [DOI] [PubMed] [Google Scholar]
- 29.Mauriello A, Sangiorgi G, Palmieri G, et al. Hyperfibrinogenemia is associated with specific histocytological composition and complications of atherosclerotic carotid plaques in patients affected by transient ischemic attacks.: Circulation 2000;101:744–50 [DOI] [PubMed] [Google Scholar]
- 30.Jaeger H, Mathias K, Drescher R, et al. Clinical results of cerebral protection with a filter device during stent implantation of the carotid artery. Cardiovasc Intervent Radiol 2001;24:249–56 [DOI] [PubMed] [Google Scholar]
- 31.Zahn R, Ischinger T, Mark B, et al. Embolic protection devices for carotid artery stenting: is there a difference between filter and distal occlusive devices? J Am Coll Cardiol 2005;45:1769–74 [DOI] [PubMed] [Google Scholar]