Abstract
BACKGROUND AND PURPOSE: Conventional MR imaging permits subcategorization of brain stem tumors by location and focality; however, assessment of white matter tract involvement by tumor is limited. Diffusion tensor imaging (DTI) is a promising method for visualizing white matter tract tumor involvement supratentorially. We investigated the ability of DTI to visualize and quantify white matter tract involvement in pontine tumors.
METHODS AND MATERIALS: DTI data (echo-planar, 1.5T) were retrospectively analyzed in 7 patients with pontine tumors (6 diffuse, 1 focal), 4 patient controls, and 5 normal volunteers. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) were calculated from the diffusion tensor in 6 regions of interest: bilateral corticospinal tracts, transverse pontine fibers, and medial lemnisci. Relationships between FA and ADC values and results of the neurologic examinations were evaluated.
RESULTS: The corticospinal tracts and transverse pontine fibers were affected more often than the medial lemnisci. The DTI parameters (FA and ADC) were significantly altered in all tracts of patients with pontine tumors (P < .05), compared with those values in the control groups. A marginally significant (P = .057) association was seen between the severity of cranial nerve deficit and decreased FA.
CONCLUSION: DTI provided superior visualization and quantification of tumor involvement in motor, sensory, and transverse pontine tracts, compared with information provided by conventional MR imaging. Thus, DTI may be a sensitive measure of tract invasion. Further prospective studies are warranted to assess the ability of DTI to delineate tumor focality and improve risk stratification in children with pontine tumors.
Diffuse pontine tumors comprise a group of malignant tumors with a much poorer prognosis than that of focal brain stem tumors. Pontine tumors account for about 15% of pediatric brain tumors and comprise approximately 20%–30% of posterior fossa tumors.1,2 Histopathologically, these diffuse pontine tumors are usually differentiated WHO grade II fibrillary astrocytomas or WHO grade III anaplastic astrocytomas at diagnosis and are known to infiltrate between normal axonal fibers.2 Although the appearance of focal brain stem tumors and that of diffuse brain stem tumors typically differ by conventional MR imaging, distinguishing focal from diffuse involvement is sometimes imprecise. The discovery of a method that distinguishes these 2 types of tumors is valuable because the treatment and prognosis for the brain stem tumors are substantially different.3 On T2-weighted MR images, focal tumors are typically well marginated, often enhance, may be exophytic, and occupy <50% of the axial diameter of the brain stem, whereas diffuse tumors are poorly marginated, rarely enhance, occupy more than 50% of the axial diameter of the brain stem, lack an exophytic component, and commonly engulf the basilar artery.1
Conventional MR imaging has demonstrated prognostic value in the treatment of brain stem tumors,2 but white matter appears homogeneous on MR images; therefore, this method cannot precisely define essential aspects of tract location, displacement, or invasion. In contrast, diffusion tensor imaging (DTI) detects anisotropic diffusion, thereby allowing the visualization of major fiber tracts in the brain stem. Thus, DTI may provide information about tumor involvement in white matter tracts. Recent work on the application of DTI in supratentorial tumors has explored quantitative values of the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) to characterize tumors,4–7 peritumoral involvement of tracts,8,9 and the appearance of tumor-altered white matter tracts.10 On the basis of these studies, DTI has become an important component of preoperative evaluation and treatment planning for patients with supratentorial tumors in certain institutions.8
Current management of suspected focal lesions in the brain stem involves stereotactic biopsy or resection,2 depending on the location and size of the tumor. Surgical options have an acceptable but real risk11–13 and are essential in determining therapy for focal brain stem lesions. To our knowledge, we recently reported the first application of DTI for surgical planning in cervico- and pontomedullary tumors.14
We report a study in which we tested the hypothesis that invasion of corticospinal, transverse pontine, and medial lemniscal tracts by pontine tumors can be demonstrated by DTI data showing reduced FA and elevated ADC values. We compare and contrast the differences when using DTI between the single patient with a focal tumor and those patients with more diffuse pontine tumors. Additionally, we evaluate the correlation between the degree of tract invasion and neurologic deficits.
Methods
Patient Demographics
DTI data were analyzed in a retrospective study of 3 groups. The experimental group consisted of 6 patients with diffuse pontine tumors and 1 with a focal pontine tumor. The mean age of the patient group was 7.7 years (range, 6–15 years). The second group consisted of 4 patients with cancer, who served as patient controls. The mean age of this group was 16.5 years (range, 12–20 years), and the sites and types of primary tumor were prostatic rhabdomyosarcoma, noncranial metastatic melanoma, pineal glioma, and parafalcine Rosai-Dorfman histiocytic lesion. Patients from these 2 groups underwent MR imaging and DTI procedures at St. Jude during 2002–2003. The third group consisted of 5 healthy adult volunteers who were nonsymptomatic graduate students and MR imaging technicians, whose ages ranged from 21 to 37 years. Clinical data were obtained by chart review, as approved by the institutional review board.
Conventional MR Imaging
MR imaging was performed on a 1.5T Siemens Symphony scanner (Siemens Medical Solutions, Malvern, Pa) by using a quadrature head coil. Conventional imaging included the following: fast low-angle shot T1-weighted images (TR/TE, 165–218/4 ms; field of view [FOV], 158 × 210; section thickness, 5/0-mm gap; matrix, 256 × 256; number of excitations [NEX], 2 in the sagittal, axial, and coronal planes; axial double-echo T2-weighted images (TR/TE, 4000–6000/16–109 ms; FOV, 210 × 210; section thickness, 5/0-mm gap; matrix, 512 × 512; NEX, 2; and axial fluid-attenuated inversion recovery images (TR/TE/TI, 9000–10,000/112/2400 ms; FOV, 158 × 210; section thickness, 5/0-mm gap; matrix, 144 × 256; NEX, 2.
Diffusion Tensor Imaging
Diffusion-weighted echo-planar images were acquired with a double spin-echo sequence15 (FOV, 230 × 230 × 200 mm; TR/TE, 10 000/100 ms; 4 acquisitions per series). Diffusion encoding was applied along 6 noncollinear directions (b = 1000), and 1 image was acquired without diffusion encoding. Image acquisitions were realigned by using the realignment tools within Statistical Parametric Mapping (SPM99, Wellcome Institute of Neurology, London, UK). Diffusion tensors were calculated by using the SPM diffusion toolbox software developed for SPM99 software.16 FA, ADC, and eigenvector maps were calculated (Figs 1 and 2). To aid in the visualization of the fiber tracts, we used an RGB-orientation color map to demonstrate fiber shape and direction.17 FA and ADC were evaluated in 6 regions of interest hand drawn under the supervision of experienced neuroradiologists (K.J.H., J.W.L.). The regions of interest were drawn around the bilateral corticospinal tracts, transverse pontine fibers, and medial lemnisci at the level of the middle cerebellar peduncles. Analyses of the DTI data were performed by authors blinded to the results of the patient’s neurologic evaluation.
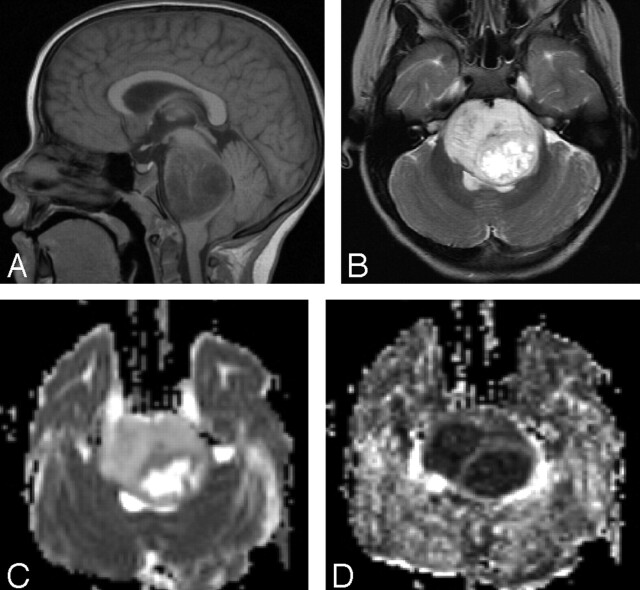
Fig 1.
MR imaging and DTI of a 15-year-old patient with diffuse pontine tumor.
A, Sagittal T1-weighted image. The pontine tumor is diffusely infiltrative. B, Axial T2-weighted image. The pons appears expanded and hyperintense with an area of focal necrosis. C, Axial ADC map. The tumor demonstrates elevated diffusion, and the necrosis is extremely hyperintense. D, Axial FA map. The tumor demonstrates diminished fractional anisotropy, and necrosis is hypointense.
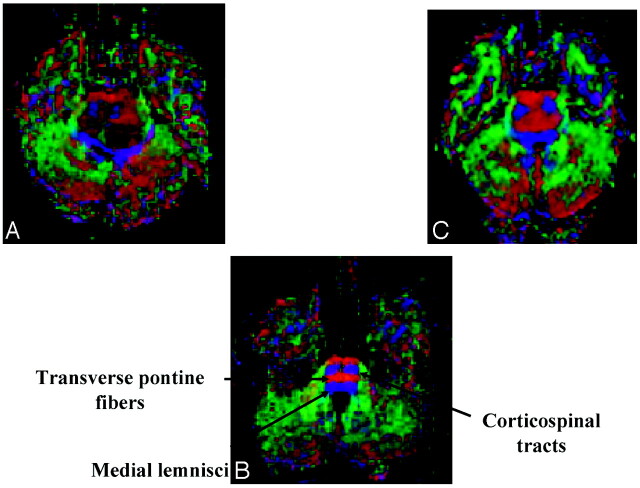
Fig 2.
Axial diffusion tensor color maps of the brain stem at the level of the middle cerebellar peduncles.
A, Image of patient with a pontine tumor showing destruction of the normal anisotropy of the corticospinal tracts and posterior displacement of the medial lemnisci.
B, Control image showing normal corticospinal tracts, transverse pontine fibers, and medial lemnisci.
C, Image of patient with a pontine tumor showing a diffusely infiltrating pattern.
Both conventional MR images (axial pre- and postcontrast T1-weighted and T2-weighted) and color-coded DTI maps were analyzed for obvious tract disturbance and areas of hemorrhage or necrosis. We used regions of interest of the white matter tracts on the color maps to obtain quantitative FA and ADC values. We also used corresponding T2-weighted images at the level of the middle cerebellar peduncles and adapted criteria developed by Witwer et al8 to classify fiber tracts into 1 of 5 categories: normal, displaced, edematous, infiltrated, or destroyed. In contrast to the study of Witwer et al, we did not use a contralateral “normal” brain image as a control because diffuse pontine tumors were most often bilateral. We also gathered quantitative ADC data for more in-depth analysis of white matter involvement, though we did not use that information to determine tract involvement, as per the adapted criteria of Witwer et al. Instead, we used the appearance and locations of the corticospinal, transverse pontine, and medial lemniscal tracts on the FA image and color maps.
Classification of Tract Involvement
Quantitative FA and ADC values from the volunteer group were used to define normal anisotropy and location of the tracts. Abnormal tracts displayed high signal intensity on T2-weighted images. “Displaced tracts” were defined as those that maintained normal anisotropy but were in abnormal locations, had an abnormal orientation on the color-coded map, or both. “Edematous tracts” were those that maintained normal anisotropy and orientation but demonstrated high signal intensity on T2-weighted images. “Infiltrated tracts” were those that demonstrated reduced anisotropy but were still identifiable on color-coded maps. “Disrupted tracts” were those that had markedly reduced anisotropy and no identifiable tracts on the color-coded map. Quantitative ADC and FA values from each region of interest in the pontine tumor group were compared with those values from the same region of interest in the patient and volunteer control groups.
Evaluation of Neurologic Deficits
We retrospectively reviewed the neurologic evaluations performed on each patient with pontine tumor before the MR imaging examination. Neurologic information for motor and cranial nerve findings were complete in all 7 patients, though sensory information was present in only 3 patients. DTI region of interest analysis was blinded to neurologic findings. Neurologic deficits were classified into 4 categories: corticospinal (assessment of motor strength, muscle tone, and reflexes), cranial nerve, sensory, and ataxia. The severity of deficit within each category (except sensory) was also graded as follows: absent if no deficit was present; mild if neurologic deficit resulted in no loss of function of the limb or, in the case of cranial nerves, <50% loss of function; moderate if neurologic deficit resulted in loss of function that restricted activity or, in the case of cranial nerves, >50% loss of function; and severe if there was marked loss of function prohibiting activity and complete paralysis in the case of cranial nerves. Sensory deficits were graded as absent if not present, mild if noted by the examiner only, moderate if the patient reported with no loss of function, and severe if loss of sensation affected function of the limb as well. Correlation analyses were then performed to determine whether the neurologic deficit scores were associated with the quantitative DTI measurements of the corresponding anatomic structures.
Statistical Analysis
The exact Kruskal-Wallis test was performed, and if a pair-wise comparison was necessary, the exact Wilcoxon-Mann-Whitney test was also used. All tests were performed by using StatXact five software (Cytel Software Corp, Cambridge, Mass), which was implemented by using SAS version 9.1 (SAS Institute, Inc, Cary, NC). A P value of ≤ .05 was considered statistically significant for the 3-group comparisons, and a P value of ≤ .0167 (ie, .05/3) was considered statistically significant for pair-wise comparisons. We combined data from the 2 control groups and compared the combined data with those from the pontine tumor group. Also, we combined datasets of the 2 lower grades (absent and mild) from individual neurologic deficit categories and compared the combined data with those from the moderate/severe grade of the same deficit by using the exact Wilcoxon-Mann-Whitney test; a P value of ≤ .05 was considered statistically significant for these comparisons.
Results
Conventional MR Imaging Evaluation
Tumors ranged in size. The axial dimensions ranged from 2.6 to 4.6 cm (median, 3.3 cm; mean, 3.2 cm), and the anteroposterior dimensions ranged from 1.8 to 4.7 cm (median, 3.6 cm; mean, 3.6 cm). One diffuse tumor had enhancement surrounding a region of necrosis. Tumors were moderately to severely hypointense on T1-weighted images and moderately to severely hyperintense on T2-weighted images. The focal tumor had both a nonenhancing infiltrative component and an enhancing exophytic component. Tumor mass effect was minimal (n = 4), mild (n = 1), or moderate (n = 2). Only 1 patient had hydrocephalus at the time of imaging, but another patient had previously received a shunt. No tumor hemorrhage or metastatic disease was detected (Table 1).
Table 1:
Comparison of conventional MR imaging and diffusion tensor imaging measures in 7 pediatric patients with pontine tumors
| Patient No. | Tumor Characteristics |
Conventional MR Imaging |
Diffusion Tensor Imaging |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Size (cm) | T1W | T2W | Enhancement | Focality | Necrosis | Mass Effect | Hydrocephalus | Corticospinal Tracts | Transverse Pontine Tracts | Sensory Tracts | |
| 1 | Midbrain/pons | 4.6 × 3.6 | Hypo | Hyper | No | No | No | Moderate | Shunt in place | Bilateral sev exp, distinct CB | Sev exp of left tract | Posterior displ, noninfiltrated |
| 2 | Pons | 3.0 × 3.4 | Hypo | Hyper | No | No | No | Minimal | No | Mild exp, lateral displ of right tract | Sev bilateral exp | Posterior displ, noninfiltrated |
| Mild reduction, medial deviation, and slightly isotropic left tract | ||||||||||||
| 3 | Pons | 2.8 × 3.1 | Hypo | Hyper | No | No | No | Minimal | No | Bilateral sev exp, minimal posterior displ of right tract | Mild bilateral exp | Normal |
| 4 | Pons | 3.2 × 4.3 | Sev hypo | Sev hyper | No | No | No | Minimal | No | Bilateral sev exp | Sev bilateral exp | Bilateral grossly posterior displ (left > right) |
| 5 | Pons | 3.8 × 4.5 | Sev hypo | Sev hyper | Surrounding necrosis | No | Yes* | Moderate | Mild | Bilateral sev exp, distinct CB | Sev bilateral exp, necrosis | Bilateral grossly posterior displ (left > right) |
| 6 | Pons | 3.9 × 4.7 | Sev hypo | Sev hyper | No | No | No | Mild | No | Bilateral sev exp, posterior displ, distinct right CB | Sev prominently anterior exp | Bilateral grossly posterior displ (left > right) |
| 7† | Pons | 1.6 × 1.0 | Hypo | Hyper | Minimal focal | Yes | No | Minimal | No | Mild exp of left tract | Normal | Normal |
| 1.0 × 0.8 | ||||||||||||
Note:—hypo indicates hypointense; hyper, hyperintense; sev, severe; exp, expansion; CB, corticobulbar tracts; displ, displacement.
The necrotic region measured 2.2 × 2.5 cm.
This patient’s tumor had an exophytic component that measured 1.0 × 0.8 cm.
Neurologic Evaluation
Various combinations of the following symptoms were experienced by the 7 patients with pontine tumors at time of imaging: long tract signs (n = 3), cranial nerve deficits (n = 5), and ataxia (n = 4) (Table 3). Sensory involvement was noted as normal in 3 patients and not recorded in the evaluations of the other 4.
Table 3:
Neurologic deficits in 7 pediatric patients with pontine tumors
| Patient No. | Neurologic Deficit (Grade* and Location) |
|||
|---|---|---|---|---|
| Corticospinal | Cranial Nerves | Sensory | Ataxia | |
| 1 | Mild (left) | Mild (left) VI | N/A | Absent |
| 2 | Mild (right) | M/S (left) VI, VII, IX | N/A | Absent |
| 3 | Absent | Absent | N/A | Absent |
| 4 | Absent | M/S (bilateral) VI (left), VII, IX | Absent | M/S (bilateral), worse on left |
| 5 | Absent | M/S left gaze, VI, VII, and partial IX | Absent | Severe gait, moderate bilateral limb dysmetria |
| 6 | Mild (bilateral), worse on left | M/S (bilateral) VI, IX, worse on right | N/A | Mild gait, left limb dysmetria |
| 7 | Absent | Absent | Absent | Mild limb dysmetria |
Note:—N/A indicates not available.
Deficits were classified into 2 grades: absent/mild, and M/S, moderate/severe.
DTI Evaluation
Evaluation of the DTI color maps revealed several patterns of tumor infiltration. Overall, the diffuse pontine tumors appeared to expand the transverse pontine components and, to a lesser extent, the corticospinal tracts (Fig 2). In 3 patients with diffuse pontine tumors, at least 1 corticospinal tract was so infiltrated that both the corticospinal and corticobulbar components were visible (Fig 3A). The corticospinal tracts were displaced (n = 3) or remained in their expected locations (n = 3) with various degrees of expansion. In 1 patient, the left corticospinal tract was diminished in size and appeared slightly isotropic. In 5 of 6 patients, the sensory tracts (including the medial lemnisci and multiple cranial nerve nuclei) were posteriorly displaced, often thinned, and draped over the substance of the tumor; however, the color map of those tracts indicated that they maintained their anisotropy (Table 1). In contrast, the single focal tumor had both a slightly expanded lateral component involving a nondisplaced left corticospinal tract and an exophytic component outside the motor tracts (Fig 3B).
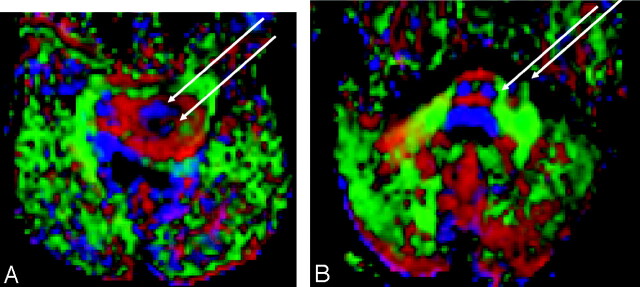
Fig 3.
Axial diffusion tensor color maps demonstrating tract invasion.
A, Image of a 6-year-old patient with a diffusely infiltrating pontine tumor. The left corticospinal tract is enlarged, compared with the right, and tumor infiltration separates the corticospinal (anterior arrow) and corticobulbar (posterior arrow) components.
B, Image of an 8-year-old patient with a focally exophytic pontine tumor. Mild lateral expansion of the left corticospinal tract (medial arrow), with focally exophytic tumor (lateral arrow).
White Matter Tract Involvement
In accordance with the criteria listed in the Methods section, we classified the status of the white matter tracks in each region of interest by using their appearance on T2-weighted images, the color maps, and FA values. The corticospinal tracts and transverse pontine fibers were more often infiltrated or destroyed than were the medial lemnisci (Table 2).
Table 2:
Color map assessment of diffusion tensor images of brain stem white matter tracts in 7 pediatric patients with pontine tumors*
| Patient No. | Corticospinal Tracts |
Transvers Pontine Tracts |
Medial Lemnisci |
|||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| 1 | D/I | I | I | I | D | D |
| 2 | I | X/I | I | I | D | D |
| 3 | I | I | I | I | D/I | D/I |
| 4 | I | I | X/I | X/I | D | D |
| 5 | X/I | X/I | X/I | X/I | I | I |
| 6 | I | X/I | I | I | N | I |
| 7 | I | N | N | N | N | N |
The condition of the tracts was classified as follows: D, displaced; E, edematous; I, infiltrated; N, normal; X, destroyed.
Quantitative DTI Analysis
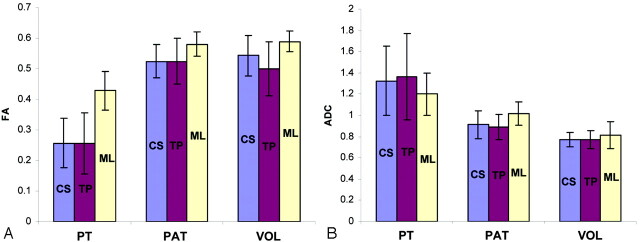
Median FA and ADC values of the pontine tumor cohort at the 6 regions of interest were compared with those in both control groups. The 3-way comparisons revealed that FA values of all 6 tracts were significantly decreased, and ADC values were significantly increased in patients with pontine tumors (Fig 4). In the pair-wise comparisons, the FA values of the pontine tumor group significantly differed from those in the patient controls in the following regions of interest: bilateral corticospinal tract, bilateral transverse pontine, and left medial lemniscus (Fig 4A). A marginal difference was observed in the ADC values of the left corticospinal and bilateral transverse pontine tracts (Fig 4B) and in the FA value of the right medial lemniscus. Interestingly, despite the differences in age and prior therapies in the patient control group, there was no significant difference in any of the regions of interest between the patient control and volunteer groups; only a marginal difference was observed in the ADC value of the right medial lemniscus. Consequently, a pair-wise comparison of data from the pontine tumor cohort and the combined control groups demonstrated significant differences in FA and ADC measures in all 6 regions of interest.
Fig 4.
Graphs show diffusion tensor imaging parameters for major white matter tracts in the brain stem.
A, Fractional anisotropy (FA). B, Apparent diffusion coefficient (ADC). Data are shown for the corticospinal (CS, blue bars), transverse pontine (TP, red bars), and medial lemnisci (ML, yellow bars). Subject groups included patients with pontine tumors, patient control (PAT), and healthy volunteer (VOL) groups.
Although we attempted to determine whether there was a correlation between the severity of neurologic deficits in the patients with pontine tumors and the median FA and ADC values of their corticospinal, transverse pontine, and sensory tracts, this analysis was limited by the small sample size because the neurologic examination data were gathered retrospectively and sensory data were incomplete. Despite these limitations, the comparison of severity of cranial nerve deficits with the average FA value of the transverse pontine tract showed a marginal difference (P = .057), which could be clinically significant. We compared data from patients with normal or mild cranial nerve deficits with those from patients with moderate/severe cranial nerve deficits. We performed a similar comparison by using data from patients with normal or mild ataxia and those with severe ataxia. There was no significant difference in any comparison.
Discussion
We describe quantitative and significantly altered DTI measurements (FA and ADC) in the brain stem tracts of 7 patients with pontine tumors (P < .05), compared with those values in the 2 control groups. Pontine tumors comprise approximately 15% of pediatric brain tumors, and the most common subtype of pontine tumor is diffuse pontine glioma.18 Most children with apparent diffuse pontine gliomas die of the disease within 18 months of diagnosis, despite contemporary radiation therapy and chemotherapy.18–20 The current principal diagnostic technique for pontine tumors is MR imaging, and there are several key grading systems that use MR imaging findings to classify brain stem tumors by location and focality.3,19,21,22 There is still debate in the literature about whether the presence of enhancement is a useful prognostic sign2,21; however, there is no question that patients with focal tumors, as seen on conventional T2-weighted images, benefit from information obtained from the stereotactic biopsy.
A certain percentage of patients with focal pontine tumors have lower-grade histology; hence, they also have different treatment options and prognoses.19 Lesniak et al2 found that of 57 patients with enhancing brain stem tumors who were surgical candidates, 60% had low-grade histology (30 juvenile pilocytic astrocytoma, 3 ganglioglioma, and 1 oligoglioma), and 40% had higher-grade histology (12 glioblastoma multiforme, 10 fibrillary astrocytoma, and 1 primitive neuroectodermal tumor). Both location and focality as defined by MR imaging have substantial prognostic significance. The probability of 5-year overall survival of patients with tumors of the midbrain ranged from 72% to 100%; that of patients with tumors of the pons was 18%; that of patients with tumors of the medulla ranged from 50% to 64%; and that of patients with focal tumors ranged from 56% to 100%. The 5-year overall survival rate of patients with diffuse brain stem tumors ranged from 18% to 20%.21 Six of our 7 patients had classic diffuse pontine tumors, as determined by imaging; 1 patient had a focally invasive tumor with an enhancing exophytic component.
The general imaging features of diffuse and focal pontine tumors have been well described in the literature.3,21 We used quantitative DTI to explore the relationships between the involvement of the corticospinal, transverse pontine, and medial lemnisci brain stem tracts in 7 patients with pontine tumors (6 diffuse, 1 focal) with various degrees of invasion. Our analysis revealed both qualitative differences in the DTI color maps and statistically significant differences in the FA and ADC values of these tracts when compared with those of the 2 control groups. Therefore, DTI shows promise in furthering our understanding of the effects of brain stem tumors on nearby white matter tracts.
Stieltjes et al23 recently used the brain stem as a model to evaluate the ability of quantitative DTI to assess densely packed well-known fiber tracts in 6 healthy adults. They identified the corticospinal tracts; the superior, medial, and inferior cerebellar peduncles; and the medial lemnisci and evaluated their FA and ADC values. In our study, not only were these white matter tracts clearly seen in patients with pontine tumors, but also substantial differences in the FA and ADC values were found in a comparison of the patient and the control groups. Certainly the mean corticospinal tracts and medial lemnisci FA values and standard deviations for our patient control and volunteer groups compared favorably with data published by Stieltjes el al.
Sinha et al7 explored the regional mean diffusivity (ADC) values of high-grade gliomas in 9 adults. They found clear quantitative differences in ADC values of different tissues; the highest ADC was observed in the necrotic tumor core, followed by edematous brain, enhancing tumor core, enhancing tumor margin, and normal brain. A recent study comparing quantitative ADC values between 3 basic histopathologic pediatric tumor types (low-grade gliomas, embryonal tumors, and nonembryonal high-grade tumors) in 12 patients demonstrated that ADC is inversely correlated with tumor cellularity.4 This finding contributes another diagnostic aspect of tumor characterization. Although we did not examine the boundaries of the diffuse tumors, our finding that the tracts of the patients with pontine tumors had elevated ADC values and were hyperintense on T2-weighted images supports earlier findings of relatively low cellularity of these infiltrating tumors.2 Recent advances in DTI have permitted the evaluation of white matter tract anisotropy and the calculation of the principal eigenvectors of known white matter tracts.17,24 Pajevic and Pierpaoli17 and Pierpaoli et al25 developed methods to create directionally coded color maps of DTI data in which the intensity of the colors corresponds to the magnitude of the anisotropy in the white matter tracts. These and other advances were invaluable in taking DTI color mapping to the clinical arena.26,27
Witwer el al8 recently described preoperative DTI color mapping to augment surgical planning in 9 adult patients with supratentorial tumors. In that study, the authors examined the relationships between the primary tumor and characteristics of the nearby white matter tracts (ie, location, orientation, edema, infiltration, displacement, and disruption), compared with the contralateral normal hemisphere. Results from this study substantially improved preoperative planning, and the authors suggested that intact white matter tracts, including those displaced by the tumor but having normal FA values, are present in regions of the brain that appear abnormal on conventional MR images.
In our study, the patterns of diffuse tumor involvement in 6 patients with pontine tumors were similar by conventional imaging, but their patterns of tract involvement were quite varied by DTI. As one would expect, the color map showed evidence of diffuse infiltration of the corticospinal and transverse pontine tracts. We noted bilateral enlargement of the corticospinal and transverse pontine tracts in many patients but frequent apparently random displacement of the individual corticospinal tracts and posterior displacement of the medial lemnisci. Patient 7 had a more focal lesion, which enlarged the left corticospinal tract, and an enhancing exophytic component, which had been stable for years. We also observed that the corticospinal tracts and transverse pontine fibers were more often infiltrated or destroyed than were the medial lemnisci. This finding supports the common clinical triad of presenting symptoms in children with pontine tumors (ie, long tract signs, ataxia, and cranial nerve deficits).18 Clinical features and conventional MR imaging are important elements of diagnosis and treatment planning for patients with pontine tumors,2,3,21 and our findings indicate that DTI evaluation may also contribute to clinical management of such cases. A large prospective study would be necessary to demonstrate a correlation between decreased FA values and severity of neurologic deficits and to determine if there is a threshold below which decreased FA values become clinically apparent.
Although stereotactic biopsy of suspected focal tumors has acceptable risks, attempted partial resections of low-grade tumors for tumor debulking and decompression can result in serious outcomes. Lesniak et al2 argued that despite the obvious benefits of careful resection of benign lesions, surgery has serious risks. Whereas most of the patients experienced improvement in neurologic deficits after their surgical procedure, significant morbidity was described (ie, 17.5% experienced moderate disability; 3.5%, severe disability; and 1.7%, vegetative state). We found that DTI (including color maps) provides a wealth of information that, until now, has not been available in surgical planning for patients with pontine tumors. It remains to be seen whether DTI can further our understanding of tract displacement and infiltration and facilitate surgical planning in patients with known focal lesions.
DTI technology is under rapid development, and the functional and clinical significance of the diffusion properties of brain tissue are unknown. Tropine et al (2004)28 demonstrated that DTI may not reliably distinguish tumor infiltration from vasogenic edema in supratentorial gliomas and glioblastomas. Nimsky el al (2005)29 discovered that though pre- and intraoperative DTI fiber tracking in supratentorial gliomas was possible, there was striking white matter tract shift, emphasizing the need for real-time intraoperative updates. Price et al (2004)30 examined peritumoral DTI white matter “signatures” at 3T field strength and concluded that more work is needed to establish the relationship between DTI parameters and tumor characteristics. Additionally, whereas adult gliomas are most often supratentorial and potentially surgically resectable, pontine gliomas are not. Although we have demonstrated the feasibility of imaging the largest pontine brain stem tracts, classic pontine gliomas are usually diffusely infiltrative and may involve small cranial nerve nuclei and their projecting nerve fibers. DTI in pontine gliomas may help better define the occasional questionable “focal” lesion. Furthermore, DTI in the pons may serve as a useful model system to investigate the relationship between tumor infiltration and diffusion parameters because of the rich structure of the major tracts in the pons.
Other important limitations of this retrospective study were discrepancy in age between the patient and control groups and limited sensory information in the neurologic evaluations in the patient group. Although an age-matched control group is generally the ideal in clinical studies, the median age of our patient group was 7 years. This is well above 2 years, the age of relative stability of FA and ADC values in central white matter pathways31; therefore, it is reasonable to assume that the differences in ages between the patient group and control groups in this study did not contribute to differences in the data. Although this study was limited by the small number of subjects, we found a potential clinically significant association between cranial nerve deficit severity and decreased FA.
Conclusion
To our knowledge, this study is the first to evaluate the quantitative DTI measures of FA and ADC in pediatric patients with diffuse and focal pontine tumors and in those with “normal” brain stem, as determined by conventional MR imaging. Our results indicate that DTI analysis can delineate tract invasion and displacement. These tools may help to better discriminate between diffuse and focal brain stem tumors in the future and may be useful for guiding surgical biopsies. DTI analysis also shows promise of providing quantitative measures of risk stratification, prognosis, and treatment response. We conclude that the results from this retrospective study support the expansion of this research in a large prospective study in pediatric patients with pontine tumors.
Footnotes
The research was supported by the American Lebanese Syrian Associated Charities.
Presented in part at the 42nd annual meeting of the American Society of Neuroradiology, Seattle, Wash, May 2004, and at the 90th scientific assembly and annual meeting of the Radiological Society of North America, Chicago, Ill, November 2004.
References
- 1.Barkovich AJ. Pediatric neuroimaging. In: Barkovich AJ. Intracranial, Orbital, and Neck Tumors of Childhood. 3rd ed. Philadelphia, Pa: Lippincott Williams & Wilkins;2000. :462–70
- 2.Lesniak MS, Klem JM, Weingart J, et al. Surgical outcome following resection of contrast-enhanced pediatric brainstem gliomas. Pediatr Neurosurg 2003;39:314–22 [DOI] [PubMed] [Google Scholar]
- 3.Barkovich AJ, Krischer J, Kun LE, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg 1991;16:73–83 [DOI] [PubMed] [Google Scholar]
- 4.Gauvain KM, McKinstry RC, Mukherjee P, et al. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. AJR Am J Roentgenol 2001;177:449–54 [DOI] [PubMed] [Google Scholar]
- 5.Tien RD, Felsberg GJ, Friedman H, et al. MR imaging of high-grade cerebral gliomas: value of diffusion-weighted echoplanar pulse sequences. AJR Am J Roentgenol 1994;162:671–77 [DOI] [PubMed] [Google Scholar]
- 6.Guo AC, Cummings TJ, Dash RC, et al. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 2002;224:177–83 [DOI] [PubMed] [Google Scholar]
- 7.Sinha S, Bastin ME, Whittle IR, et al. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol 2002;23:520–27 [PMC free article] [PubMed] [Google Scholar]
- 8.Witwer BP, Moftakhar R, Hasan KM, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg 2002;97:568–75 [DOI] [PubMed] [Google Scholar]
- 9.Clark CA, Barrick TR, Murphy MM, et al. White matter fiber tracking in patients with space-occupying lesions of the brain: a new technique for neurosurgical planning? Neuroimage 2003;20:1601–08 [DOI] [PubMed] [Google Scholar]
- 10.Field AS, Alexander AL, Wu YC, et al. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging 2004;20:555–62 [DOI] [PubMed] [Google Scholar]
- 11.Valdes-Gorcia J, Espinoza-Diaz DM, Paredes-Diaz E. Stereotactic biopsy of brain stem and posterior fossa lesions in children. Acta Neurochir (Wien) 1998;140:899–903 [DOI] [PubMed] [Google Scholar]
- 12.Cartmill M, Punt J. Diffuse brain stem glioma: a review of stereotactic biopsies. Childs Nerv Syst 1999;15:235–37 [DOI] [PubMed] [Google Scholar]
- 13.Boviatsis EJ, Voumvourakis K, Goutas N, et al. Stereotactic biopsy of brain stem lesions. Minim Invasive Neurosurg 2001;44:226–29 [DOI] [PubMed] [Google Scholar]
- 14.Phillips NS, Sanford RA, Helton KJ, et al. Diffusion tensor imaging of intraaxial tumors at the cervicomedullary and pontomedullary junctions. Report of two cases. J Neurosurg 2005;103 (6 suppl):557–62 [DOI] [PubMed] [Google Scholar]
- 15.Reese TG, Heid O, Weisskoff RM, et al. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med 2003;49:177–82 [DOI] [PubMed] [Google Scholar]
- 16.Poldrack R. Project: SPM Toolbox: Summary. Available at: http://sourceforgenet/projects/spm-toolbox. Accessed2005
- 17.Pajevic S, Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med 1999;42:526–40 [PubMed] [Google Scholar]
- 18.Jallo GI, Freed D, Roonprapunt C, et al. Current management of brainstem gliomas. Annals of Neurosurgery 2003;3:1–17 [Google Scholar]
- 19.Albright AL, Packer RJ, Zimmerman R, et al. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery 1993;33:1026–29 [DOI] [PubMed] [Google Scholar]
- 20.Allen JC, Siffert J. Contemporary chemotherapy issues for children with brainstem gliomas. Pediatr Neurosurg 1996;24:98–102 [DOI] [PubMed] [Google Scholar]
- 21.Fischbein NJ, Prados MD, Wara W, et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg 1996;24:9–23 [DOI] [PubMed] [Google Scholar]
- 22.Choux M, Lena G, Do L. Brainstem tumors. In: Choux M, DiRocco C, Hockley A, eds. Pediatric Neurosurgery. New York: Churchill Livingstone;2000;471–91
- 23.Stieltjes B, Kaufmann WE, van Zijl PC, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage 2001;14:723–35 [DOI] [PubMed] [Google Scholar]
- 24.Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–48 [DOI] [PubMed] [Google Scholar]
- 25.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 2001;13 (6 pt 1):1174–85 [DOI] [PubMed] [Google Scholar]
- 26.Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med 2000;44:625–32 [DOI] [PubMed] [Google Scholar]
- 27.Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A 1999;96:10422–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tropine A, Vucurevic G, Delani P, et al. Contribution of diffusion tensor imaging to delineation of gliomas and glioblastomas. J Magn Reson Imaging 2004;20:905–12 [DOI] [PubMed] [Google Scholar]
- 29.Nimsky C, Ganslandt O, Hastreiter P, et al. Preoperative and intraoperative diffusion tensor imaging–based fiber tracking in glioma surgery. Neurosurgery 2005;56:130–37 [DOI] [PubMed] [Google Scholar]
- 30.Price SJ, Pena A, Burnet NG, et al. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol 2004;14:1909–17 [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol 2002;23:1445–56 [PMC free article] [PubMed] [Google Scholar]