Abstract
BACKGROUND AND PURPOSE: Previous studies have shown microbleeds to be a risk factor for intracerebral hemorrhage and white matter hyperintensity (WMH) to be a risk factor for ischemic stroke. This study was performed to determine whether combinations of the presence or absence of microbleeds and advanced WMH are risk factors for subsequent recurrent stroke types.
METHODS: In 266 patients with stroke, microbleeds on T2*-weighted MR images were counted, and WMH on T2-weighted images was graded. Patients were divided into 4 groups by the combinations of the presence or absence of microbleeds and advanced WMH and were followed up for stroke recurrence.
RESULTS: During a mean follow-up period of 564.8 ± 220.5 days, 26 patients developed recurrent strokes, including 10 intracerebral hemorrhages and 16 ischemic strokes. Patients with microbleeds without advanced WMH (n = 42) developed only intracerebral hemorrhages (n = 8), and the recurrence rate of intracerebral hemorrhage in those patients estimated by the Kaplan-Meier method was the highest in the 4 groups (14.3% in 1 year and 21.2% in 2 years). In contrast, patients with advanced WMH without microbleeds (n = 39) developed only ischemic strokes (n = 6), and the estimated recurrent rate of ischemic stroke in those patients was the highest in the 4 groups (10.5% in 1 year and 17.4% in 2 years). Cox proportional hazards regression analysis revealed that microbleeds were associated with intracerebral hemorrhage (hazard ratio [HR], 85.626; 95% confidence interval [CI], 6.344–1155.649) and that advanced WMH was negatively associated with intracerebral hemorrhage (HR, 0.016; 95% CI, 0.001–0.258). Advanced WMH was associated with ischemic stroke (HR, 10.659; 95% CI, 2.601–43.678).
CONCLUSION: It appears that patients at high risk of subsequent intracerebral hemorrhage or ischemic stroke can be identified by combinations of the presence or absence of microbleeds and advanced WMH.
Gradient-echo T2*-weighted MR imaging is an extremely sensitive technique for detecting silent microbleeds, which are shown as signal-intensity loss, representing hemosiderin deposit.1,2 An association between the presence of microbleeds and the severity of white matter hyperintensity (WMH) has been revealed in many previous studies.3–8 Although both cerebral microbleeds and WMH are associated with common risk factors, the main one being hypertension, and are associated with small-artery diseases, the presence of microbleeds has been reported to be a risk factor for intracerebral hemorrhage,6–16 and WMH has been reported to be a risk factor for ischemic stroke.17–20 However, to the best of our knowledge, there have been no prospective studies focusing on combinations of microbleeds and advanced WMH as predictors for subsequent stroke types. Therefore, we performed the present study to determine whether cerebral microbleeds and advanced WMH are risk factors for certain types of subsequent stroke, by focusing on the combinations of the presence or absence of these 2 types of small-artery disease.
Methods
Subjects for the present study were enrolled from outpatients of our hospital who had acute stroke treated at our hospital, had been continuously followed up after discharge, and underwent MR imaging studies during the period from July 2002 to June 2004. Diagnosis of acute stroke was made on the basis of neurologic signs and symptoms and on the basis of results of neuroradiologic examinations. Stroke was classified into ischemic stroke and intracerebral hemorrhage, and ischemic stroke was further classified according to the criteria of the National Institute of Neurologic Disorders and Stroke as atherothrombotic infarction, cardioembolic infarction, and lacunar infarction.21 Among patients with ischemic stroke, those with lacunar infarction and atherothrombotic infarction were included and those with cardioembolic infarction or undetermined classification were excluded from this study. In addition, among patients with intracerebral hemorrhage, cases were restricted to those in which the hematoma was present in the pons, cerebellum, thalamus, or putamen. Cases in which the hematoma was present in the subcortical lesion and those in whom hematoma was not caused by spontaneous intracerebral hemorrhage (eg, caused by vascular malformation, trauma, cavernous angioma, or brain tumor) were excluded.
Hypertension was defined as systolic blood pressure of ≥140 mm Hg and diastolic blood pressure of ≥90 mm Hg and included patients currently undergoing medical treatment for hypertension. Diabetes mellitus was defined as a glycosylated hemoglobin A1c concentration of >5.8% and included patients currently using hypoglycemic agents. Hypercholesterolemia was defined as a total cholesterol level of ≥220 mg/dL and included patients currently undergoing cholesterol-lowering therapy.
All of the patients were examined by a 1T clinical MR unit (Siemens, Magneton Harmony, Siemens Medical Solutions, Malvern, Pa), and the whole brain was scanned with a section thickness of 5 mm and a 1.5-mm intersection gap. The imaging protocol consisted of axial T2-weighted spin-echo sequences (TR/TE, 4500/112; field of view, 201 × 230; matrix, 225 × 512) and axial T2*-weighted gradient-echo sequences (TR/TE, 800/26; flip angle, 20°; field of view, 230 × 230; matrix, 192 × 256). Microbleeds were defined as homogeneous round signal-intensity loss lesions on T2*-weighted MR images excluding lesions in the globus pallidus and in the subarachnoid space, which are likely to represent calcification and adjacent pial blood vessels, respectively. Intracerebral lesions with a hemorrhagic component were also excluded. The severity of WMH on T2-weighted images was graded by using the scoring system of Fazekas et al22 into 4 grades: grade 0, absent; 1, punctate; 2, early confluent; and 3, confluent. WMH of grade 2 or 3 was regarded as advanced WMH. MR images were evaluated by 2 of the authors (H.N., E.N.) separately without knowledge of the patients’ clinical profiles, and the number of microbleeds and the grading scores of WMH were determined by consensus. Patients were divided into 4 groups by the presence or absence of cerebral microbleeds and advanced WMH as follows: group A, patients with advanced WMH but without microbleeds; group B, patients with coexistence of microbleeds and advanced WMH; group C, patients without either microbleeds or advanced WMH; and group D, patients with microbleeds but without advanced WMH.
Follow-up of the patients started from the dates of their respective MR imaging studies. Patients were followed up until the recurrence of stroke or until March 2005. Intracerebral hemorrhage was diagnosed by CT. Acute ischemic stroke was confirmed by diffusion-weighted imaging and apparent diffusion coefficient maps. Whether the patients had received antiplatelet therapy after the ischemic stroke was recorded.
All values are expressed as means ± standard deviations. Among the 4 groups, the χ2 test for independence was used for comparison of sex ratio, stroke type ratio, antiplatelet therapy, hypertension, diabetes mellitus, and hypercholesterolemia, and 1-factor analysis of variance for age was also used. The Kaplan-Meier method was used to estimate the rates of recurrent stroke. Cox proportional hazards regression analysis was used to assess the relationships of subsequent intracerebral hemorrhage or ischemic stroke with the following variables: age, sex, stroke type, days from stroke onset to registration, hypertension, diabetes mellitus, hypercholesterolemia, antiplatelet therapy, advanced WMH, and microbleeds.
Results
The population in this study consisted of 266 patients (67.2 ± 11.5 years of age, 167 men and 99 women) with a history of stroke. The number of the patients in each group was as follows: 39 patients in group A, 52 patients in group B, 133 patients in group C, and 42 patients in group D. The baseline characteristics of the patients are summarized in Table 1. Cerebral microbleeds were found on T2*-weighted MR images in 94 (35.3%) of the patients.
Table 1:
Baseline characteristics of patients
| Group A | Group B | Group C | Group D | P | |
|---|---|---|---|---|---|
| Patients, n (M/F) | 39 (22/17) | 52 (34/18) | 133 (85/48) | 42 (26/16) | .8219 |
| Age, y (SD) | 74.7 (9.0) | 70.5 (10.1) | 64.3 (11.4) | 65.4 (11.3) | .0001 |
| Stroke types, n (ischemic/hemorrhagic) | 39 (33/6) | 52 (33/19) | 133 (97/36) | 42 (20.22) | .0018 |
| Antiplatelet therapy, n (%) | 32 (82.1) | 31 (59.6) | 96 (72.2) | 16 (38.1) | .0001 |
| Hypertension, n (%) | 26 (66.7) | 44 (84.6) | 81 (60.9) | 36 (85.7) | .0013 |
| Diabetes mellitus, n (%) | 10 (25.6) | 10 (19.2) | 42 (31.6) | 8 (19.0) | .2214 |
| Hypercholesterolemia, n (%) | 17 (43.6) | 14 (26.9) | 31 (23.3) | 9 (21.4) | .0698 |
Note:— Group A, patients with advanced white matter hyperintensity (WMH) but without microbleeds; group B, patients with coexistence of microbleeds and advanced WMH; group C, patients without either mcribleeds or advanced WMH; group D, patients with microbleeds but without advanced WMH.
The mean follow-up period was 564.8 ± 220.5 days. Three patients were lost to follow-up (2 patients in group C and 1 patient in group D), and 1 patient in group B died of a cause not related to stroke. During the follow-up period, 26 patients developed recurrent strokes, including 10 intracerebral hemorrhages and 16 ischemic strokes. Representative MR and CT images of patients with recurrent stroke are shown in Figs 1 and 2. Frequencies of the development of overall recurrent stroke, intracerebral hemorrhage, and ischemic stroke are shown in Table 2, Table 3, and Table 4, respectively. Development of intracerebral hemorrhage was the most frequently observed in patients of group D (19.0%). Analysis by the Kaplan-Meier method showed that the estimated recurrence rate of intracerebral hemorrhage was also the highest in patients in group D. The frequency of development of ischemic stroke was the highest in patients in group A (15.4%), followed by patients in group B (9.6%) and patients in group C (3.8%), whereas no patients in group D developed ischemic stroke. Patients in group A also showed the highest estimated recurrence rate of ischemic stroke in the 4 groups.
Fig 1.
MR and CT images obtained from a patient (70-year-old man) with intracerebral hemorrhage in the cerebellum, who had been treated with aspirin after the occurrence of lacunar infarction. A and B, Initial T2*-weighted gradient-echo images (TR/TE, 800/26; flip angle, 20°) show multiple microbleeds in the brain stem, cerebellum, basal ganglia, and cerebral hemispheres. C and D, T2-weighted spin-echo images (TR/TE, 4500/112) do not show advanced white matter hyperintensity. E and F, CT image (E) and T2*-weighted gradient-echo image (F) obtained 9 months after the lacunar infarction show occurrence of cerebellar hemorrhage.
Fig 2.
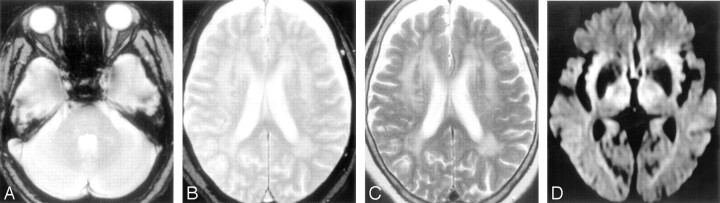
MR images obtained from a patient (85-year-old woman) with lacunar infarction in the right internal capsule after the occurrence of lacunar infarction in the right corona radiata. A and B, Initial T2*-weighted gradient-echo images (TR/TE, 800/26; flip angle, 20°) show no microbleeds. C, T2-weighted spin-echo image (TR/TE, 4500/112) shows advanced white matter hyperintensity. D, Diffusion-weighted image (single-shot echo-planar spin-echo sequence; TR/TE, 5300/135; b = 1000 mm2/s) obtained 23 months after the lacunar infarction shows a hyperintense lesion in the right internal capsule, consistent with acute infarction.
Table 2:
Frequency of development of recurrent stroke
| Recurrent Stroke, n (%) | Recurrence Rate by Kaplan-Meier Method |
||
|---|---|---|---|
| 1 y | 2 y | ||
| Group A (n = 39) | 6 (15.4) | 10.5 | 17.4 |
| Group B (n = 52) | 6 (11.5) | 9.6 | 14.9 |
| Group C (n = 133) | 6 (4.5) | 1.5 | 5.8 |
| Group D (n = 42) | 8 (19.0) | 14.3 | 21.2 |
Note:— Group A, patients with advanced white matter hyperintensity (WMH) but without microbleeds; group B, patients with coexistence of microbleeds and advanced WMH; group C, patients without either microbleeds or advanced WMH; group D, patients with microbleeds but without advanced WMH.
Table 3:
Frequency of development of intracerebral hemorrhage
| Recurrent Stroke, n (%) | Recurrence Rate by Kaplan-Meier Method (%) |
||
|---|---|---|---|
| 1 y | 2 y | ||
| Group A (n = 39) | 0 (0.0) | 0 | 0 |
| Group B (n = 52) | 1 (1.9) | 0 | 5.9 |
| Group C (n = 133) | 1 (0.8) | 0 | 1.5 |
| Group D (n = 42) | 8 (19.0) | 14.3 | 21.2 |
Note:— Group A, patients with advanced white matter hyperintensity (WMH) but without microbleeds; group B, patients with coexistence of microbleeds and advanced WMH; group C, patients without either microbleeds or advanced WMH; group D, patients with microbleeds but without advanced WMH.
Table 4:
Frequency of development of ischemic stroke
| Recurrent Stroke, n (%) | Recurrence Rate by Kaplan-Meier Method (%) |
||
|---|---|---|---|
| 1 y | 2 y | ||
| Group A (n = 39) | 6 (15.4) | 10.5 | 17.4 |
| Group B (n = 52) | 5 (9.6) | 9.6 | 9.6 |
| Group C (n = 133) | 5 (3.8) | 1.5 | 4.4 |
| Group D (n = 42) | 0 (0.0) | 0 | 0 |
Note:— Group A, patients with advanced white matter hyperintensity (WMH) but without microbleeds; group B, patients with coexistence of microbleeds and advanced WMH; group C, patients without either microbleeds or advanced WMH; group D, patients with microbleeds but without advanced WMH.
The detailed characteristics of the patients with recurrent stroke are summarized in Table 5. Only the development of ischemic stroke was observed in patients in group A. Development of ischemic stroke was observed in all except one of the recurrence patients in groups B and C. In contrast, patients in group D developed only intracerebral hemorrhage, and all of the 4 patients who developed intracerebral hemorrhage after ischemic stroke had been taking aspirin as an antiplatelet therapy.
Table 5:
Detailed characteristics of patients with recurrent stroke
| Group/Age (y)/Sex | Previous stroke | Microbleeds, n | WMH, Grade | Antiplatelet Therapy | Hyper-tension | Diabetes Mellitus | Hypercho-lesterolemia | Recurrent Stroke |
|---|---|---|---|---|---|---|---|---|
| A/84/F | Lacunar infarction | 0 | 2 | Cilostazol | (+) | (−) | (−) | Lacunar infarction |
| A/71/M | Lacunar infarction | 0 | 2 | Ticlopidine | (+) | (−) | (+) | Lacunar infarction |
| A/78/F | Atherothrombotic infarction | 0 | 2 | Ticlopidine | (−) | (−) | (+) | Atherothrombotic infarction |
| A/87/F | Lacunar infarction | 0 | 2 | Aspirin | (+) | (−) | (−) | Lacunar infarction |
| A/74/F | Lacunar infarction | 0 | 2 | Aspirin | (−) | (−) | (−) | Lacunar infarction |
| A/85/F | Lacunar infarction | 0 | 3 | Cilostrazol | (+) | (−) | (−) | Lacunar infarction |
| B/70/M | Atherothrombotic infarction | 3 | 3 | Ticlopidine | (+) | (−) | (+) | Atherothrombotic infarction |
| B/57/M | Intracerebral hemorrhage | 19 | 2 | (−) | (+) | (−) | (−) | Lacunar infarction |
| B/76/F | Lacunar infarction | 2 | 2 | Cilostazol | (+) | (−) | (+) | Lacunar infarction |
| B/66/M | Lacunar infarction | 1 | 2 | Aspirin | (+) | (+) | (+) | Lacunar infarction |
| B/55/M | Lacunar infarction | 2 | 2 | Cilostazol | (−) | (−) | (−) | Lacunar infarction |
| B/69/M | Lacunar infarction | 13 | 3 | Aspirin | (+) | (−) | (−) | Intracerebral hemorrhage |
| C/54/M | Lacunar infarction | 0 | 1 | Cilostazol | (+) | (+) | (−) | Lacunar infarction |
| C/61/F | Intracerebral hemorrhage | 0 | 1 | Aspirin + ticlopidine | (+) | (+) | (−) | Lacunar infarction |
| C/61/F | Atherothrombotic infarction | 0 | 1 | Aspirin + ticlopidine | (+) | (−) | (−) | Lacunar infarction |
| C/57/F | Lacunar infarction | 0 | 0 | Aspirin | (−) | (+) | (−) | Atherothrombotic infarction |
| C/54/M | Lacunar infarction | 0 | 1 | Aspirin | (−) | (+) | (−) | Lacunar infarction |
| C/74/M | Lacunar infarction | 0 | 1 | Aspirin | (−) | (+) | (−) | Intracerebral hemorrhage |
| D/77/M | Lacunar infarction | 13 | 1 | Aspirin | (−) | (−) | (−) | Intracerebral hemorrhage |
| D/70/M | Lacunar infarction | 28 | 1 | Aspirin | (+) | (−) | (+) | Intracerebral hemorrhage |
| D/73/M | Atherothrombotic infarction | 1 | 1 | Aspirin | (+) | (−) | (−) | Intracerebral hemorrhage |
| D/80/M | Atherothrombotic infarction | 11 | 0 | Aspirin | (+) | (−) | (−) | Intracerebral hemorrhage |
| D/82/M | Intracerebral hemorrhage | 2 | 1 | (−) | (−) | (−) | (−) | Intracerebral hemorrhage |
| D/51/M | Intracerebral hemorrhage | 2 | 0 | (−) | (+) | (−) | (−) | Intracerebral hemorrhage |
| D/53/F | Intracerebral hemorrhage | 12 | 1 | (−) | (+) | (−) | (−) | Intracerebral hemorrhage |
| D/55/M | Intracerebral hemorrhage | 16 | 1 | (−) | (+) | (−) | (−) | Intracerebral hemorrhage |
Note:— Group A, patients with advanced white matter hyperintensity (WMH) but without microbleeds; group B, patients with coexistence of microbleeds and advanced WMH; group C, patients without either microbleeds or advanced WMH; group D, patients with microbleeds but without advanced WMH. Present is indicated by (+) and absent indicated by (−).
The results of Cox proportional hazards regression analysis showed that the presence of microbleeds was significantly and independently associated with subsequent intracerebral hemorrhage (hazard ratio [HR], 85.626; 95% confidence interval [CI], 6.344–1155.649), whereas advanced WMH had a negative association with subsequent intracerebral hemorrhage (HR, 0.016; 95% CI, 0.001–0.258) (Table 6). Advanced WMH was associated with subsequent ischemic stroke (HR, 10.659; 95% CI, 2.601–43.678) (Table 7).
Table 6:
Cox proportional hazards regression analysis for predicting subsequent intracerebral hemorrhage
| Variable | Hazards Regression | 95% CI | P |
|---|---|---|---|
| Increased age | 1.028 | 0.948–1.116 | .5024 |
| Male sex | 16.476 | 1.448–187.467 | .0239 |
| Stroke type (intracerebral hemorrhage) | 41.898 | 1.822–963.670 | .0195 |
| Microbleeds | 85.626 | 6.344–1155.649 | .0008 |
| Advanced leukoaraiosis | 0.016 | 0.001–0.258 | .0035 |
| Hypertension | 0.163 | 0.026–1.044 | .0555 |
| Diabetes mellitus | 0.83 | 0.092–7.461 | .868 |
| Hypercholesterolemia | 0.333 | 0.030–3.667 | .3689 |
| Antiplatelet therapy | 64.904 | 2.054–2050.683 | .0178 |
| Days from stroke onset to registration | 1.009 | 1.003–1.015 | .0017 |
Table 7:
Cox proportional hazards regression analysis for predicting subsequent ischemic stroke
| Variable | Hazards Regression | 95% CI | P |
|---|---|---|---|
| Increased age | 0.938 | 0.886–0.993 | .0269 |
| Male sex | 0.297 | 0.094–0.936 | .0381 |
| Stroke type (ischemic stroke) | 1.099 | 0.029–41.732 | .9596 |
| Microbleeds | 0.609 | 0.174–2.132 | .4378 |
| Advanced leukoaraiosis | 10.659 | 2.601–43.678 | .001 |
| Hypertension | 1.129 | 0.367–3.474 | .8327 |
| Diabetes mellitus | 0.821 | 0.277–2.434 | .7225 |
| Hypercholesterolemia | 0.609 | 0.200–1.849 | .381 |
| Antiplatelet therapy | 13.816 | 0.343–556.026 | .1636 |
| Days from stroke onset to registration | 0.987 | 0.971–1.003 | .106 |
Discussion
Although both microbleeds and WMH are associated with small-artery disease, their features are different. The presence of microbleeds, which pathologically represent hemosiderin deposit,1,2 is associated with the progression of bleeding-prone small-artery disease and with symptomatic intracerebral hemorrhage.6–16 A recent cohort study showed that the presence of microbleeds is a risk factor for subsequent intracerebral hemorrhage in patients with ischemic stroke.15 In contrast, the neuropathologic appearance corresponding to WMH (leukoaraiosis) is neuronal loss, ischemic demyelination, and gliosis,17 and WMH has been reported to be a risk factor for ischemic stroke.17–20 However, there have been no studies in which both microbeeds and WMH were evaluated as risk factors for subsequent stroke types in the same series of patients. The results of Cox proportional hazards regression analysis in the present study not only reconfirmed microbleeds as a risk factor for subsequent intracerebral hemorrhage and advanced WMH as a risk factor for subsequent ischemic stroke but also showed advanced WMH to be a negative risk factor for subsequent intracerebral hemorrhage.
No prospective studies have focused on combinations of microbleeds and advanced WMH as predictors for types of subsequent stroke. Kim et al6 reported that microbleeds are a predictor of intracerebral hemorrhage in patients with no or mild leukoaraiosis but that they appear similarly both in ischemic stroke and hemorrhagic stroke in patients with advanced leukoaraiosis. We performed the first prospective study aimed at determining whether cerebral microbleeds and advanced WMH are risk factors for types of subsequent stroke, by focusing on combinations of the presence or absence of these 2 types of small-artery disease. The results indicated that the presence of microbleeds appears to be a risk factor for subsequent intracerebral hemorrhage when the patient does not have advanced WMH. Patients with microbleeds but without advanced WMH developed only intracerebral hemorrhage, and all of the patients who developed intracerebral hemorrhage after ischemic stroke had been taking aspirin as antiplatelet therapy. As Wong et al9 reported, the presence of old silent microbleeds appears to be a risk factor for aspirin-associated intracerebral hemorrhage, and our results further suggest that the presence of cerebral microbleeds, but the absence of advanced WMH, might be a high risk for subsequent intracerebral hemorrhage.
Of course, it is possible that because patients with microbleeds but without advanced WMH had a high frequency of intracerebral hemorrhage as the initial stroke and hypertension, they showed a higher prevalence of intracerebral hemorrhage as the subtype of recurrent stroke. In fact, previous studies have shown that intracerebral hemorrhage or uncontrolled hypertension predict future intracerebral hemorrhage. However, the results of Cox proportional hazards regression analysis revealed that microbleeds were associated with intracerebral hemorrhage as the subtype of recurrent stroke independent of initial stroke type (intracerebral hemorrhage) or the presence of hypertension. The results of the present study also indicate that patients with advanced WMH, but without microbleeds, might be prone to the development of ischemic stroke, and even patients with coexistence of microbleeds and advanced WMH might be at higher risk for the development of ischemic stroke than for the development of intracerebral hemorrhage. Investigation of combinations of the presence or absence of cerebral microbleeds and advanced WMH might enable identification of patients who are at high risk for development of subsequent intracerebral hemorrhage or ischemic stroke, which would contribute to therapeutic strategies including antiplatelet therapy.
Of course, our study had some limitations. Because the baseline backgrounds of the patients in the 4 groups were not necessarily the same and because the number of patients in each group was small, the results may be controversial and may not be confirmed in studies with a larger number of patients. In addition, the present study was an observational study and causality has yet to be established because bias and confounding could not be eliminated in an observational study. Furthermore, the severity of hypertension and its control were not recorded in the present study. It remains to be determined whether the presence of microbleeds increases the risk of future intracerebral hemorrhage in patients with intracerebral hemorrhage whose hypertension is uncontrolled. We expect that our results will be confirmed by multicenter studies with large numbers of patients.
Conclusion
Combinations of the presence or absence of microbleeds and advanced WMH appear to enable identification of patients who are at high risk for the development of subsequent intracerebral hemorrhage or ischemic stroke, which would contribute to therapeutic strategies including antiplatelet therapy.
Acknowledgments
We thank Drs. Koji Nagao and Yoshio Suyama for their assistance in preparing the data for analysis.
Footnotes
This study was partially supported by research grants from the Ministry of Health, Labor and Welfare of Japan and from the Smoking Research Foundation of Japan.
References
- 1.Tanaka A, Ueno Y, Nakayama Y, et al. Small chronic hemorrhages and ischemic lesions in association with spontaneous intracerebral hematomas. Stroke 1999;30:1637–42 [DOI] [PubMed] [Google Scholar]
- 2.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–42 [PMC free article] [PubMed] [Google Scholar]
- 3.Kwa VIH, Franke CL, Verbeeten B Jr, et al. Silent intracerebral microhemorrhages in patients with ischemic stroke. Ann Neurol 1998;44:372–77 [DOI] [PubMed] [Google Scholar]
- 4.Roob G, Lechner A, Schmidt R, et al. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke 2000;31:2665–69 [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Izumiyama M, Izumiyama K, et al. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke 2002;33:1536–40 [DOI] [PubMed] [Google Scholar]
- 6.Kim DE, Bae HJ, Lee SH, et al. Gradient echo magnetic resonance imaging in the prediction of hemorrhage vs ischemic stroke: a need for the consideration of the extent of leukoaraiosis. Arch Neurol 2002;59:425–29 [DOI] [PubMed] [Google Scholar]
- 7.Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. AJNR Am J Neuroradiol 2003;24:88–96 [PMC free article] [PubMed] [Google Scholar]
- 8.Naka H, Nomura E, Wakabayashi S, et al. Frequency of asymptomatic microbleeds on T2*-weighted MR images of patients with recurrent stroke: association with combination of stroke subtypes and leukoaraiosis. AJNR Am J Neuroradiol 2004;25:714–19 [PMC free article] [PubMed] [Google Scholar]
- 9.Wong KS, Chan YL, Liu JY, et al. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology 2003;60:511–13 [DOI] [PubMed] [Google Scholar]
- 10.Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology 1996;46:1751–54 [DOI] [PubMed] [Google Scholar]
- 11.Greenberg SM, O’Donnell HC, Schaefer PW. MRI detection of new hemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology 1999;53:1135–38 [DOI] [PubMed] [Google Scholar]
- 12.Hermier M, Nighoghossian N, Derex L, et al. MRI of acute post-ischemic cerebral hemorrhage in stroke patients: diagnosis with T2*-weighted gradient-echo sequences. Neuroradiology 2001;43:809–15 [DOI] [PubMed] [Google Scholar]
- 13.Kidwell CS, Saver JL, Villablanca JP, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke 2002;33:95–98 [DOI] [PubMed] [Google Scholar]
- 14.Nighoghossian N, Hermier M, Adeleine P, et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke 2002;33:735–42 [DOI] [PubMed] [Google Scholar]
- 15.Fan YH, Zhang L, Lam WW, et al. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke 2003;34:2459–62. Epub 2003 Sep 4 [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Bae HJ, Kwon SJ, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology 2004;62:72–76 [DOI] [PubMed] [Google Scholar]
- 17.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–59 [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S, Okada K, Koide H, et al. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke 1997;28:1932–39 [DOI] [PubMed] [Google Scholar]
- 19.Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126–29 [DOI] [PubMed] [Google Scholar]
- 20.Strefler JY, Eliasziw M, Benavente OR, et al. Development and progression of leukoaraiosis in patients with brain ischemia and carotid artery disease. Stroke 2003;34:1913–17 [DOI] [PubMed] [Google Scholar]
- 21.Special report from the National Institute of Neurological Disorders and Stroke: classification of cerebrovascular diseases III. Stroke 1990;21:637–76 [DOI] [PubMed] [Google Scholar]
- 22.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987;149:351–56 [DOI] [PubMed] [Google Scholar]