Abstract
BACKGROUND AND PURPOSE: Reteplase (RP) and urokinase (UK) are being used “off-label” to treat acute ischemic stroke. The safety and efficacy of intra-arterial RP or UK in the treatment of acute ischemic stroke, however, has yet to be proved. We aim to evaluate the safety and efficacy of RP compared with UK in acute ischemic stroke patients with large vessel occlusion.
METHODS: Retrospective analysis was conducted of cases from a prospectively collected stroke data base on consecutive acute ischemic stroke patients with large vessel occlusion by digital subtraction angiography treated with intra-arterial RP or UK. Thrombolytic dosage, recanalization rate, intracerebral hemorrhage (ICH), mortality, and outcome were determined.
RESULTS: Thirty-three patients received RP and 22 received UK (mean doses, 2.5 ± 1.4 mg and 690,000 ± 562,000 U, respectively). Vascular occlusions included 9 basilar arteries (BAs), 7 internal carotid arteries (ICAs), and 17 middle cerebral arteries (MCAs) with RP and 9 BAs, 4 ICAs, and 9 MCAs with UK. Median baseline National Institutes of Health Stroke Scales were as follows: 16 (range, 5–25; 81% ≥ 10) with RP and 17 (range, 6–38; 85%≥10) with UK. Mean time from symptom onset to thrombolytic initiation: 333 ± 230 minutes with RP and 343 ± 169 minutes with UK. Recanalization rates were as follows: 82% with RP and 64% with UK (P = .13). Symptomatic ICH rates were as follows: 12% with RP and 4.5% with UK (P = .50). The mortality rate was 24% with RP and 27% with UK (P = .8).
CONCLUSION: Although limited in statistical power, our study suggests that, although IA thrombolysis with RP shows a trend for higher recanalization rates and hemorrhage rates, IA thrombolysis with RP is not significantly different in recanalization, outcome, mortality, and ICH compared with that of UK or rates reported with IA pro-UK.
Since the National Institute of Neurologic Disorders and Stroke (NINDS) study, the use of intravenous (IV) recombinant tissue plasminogen activator (rtPA) in acute stroke patients presenting within 3 hours of symptom onset has become the standard of care.1 IV rtPA, however, has limitations in both efficacy and application for some patients. Fifty-seven percent of the patients in the NINDS trial and 58% in the Second European-Australasian Cooperative Acute Stroke Study (ECASS-II) did not show a favorable clinical response.1–3 In addition, the use of IV rtPA is limited by low recanalization rates, which have been shown to be associated with poorer outcome.4,5 We have previously reported complete recanalization in 30%, partial in 48%, and no recanalization in 22% of patients treated with IV rtPA when monitored by transcranial Doppler sonography (TCD).6,7 Recanalization rates are especially low with IV rtPA in patients with proximal, large-vessel occlusions.7–10 Furthermore, the benefits of rtPA application are limited by time. Many patients do not present for treatment within 3 hours of stroke onset. In such cases, intra-arterial (IA) thrombolysis has been reported to produce average recanalization rates as high as 70%.11–16
IA thrombolysis may provide many benefits in acute stroke treatment, including efficacy >3 hours, lower dose needed, direct administration, and combined use with mechanical clot disruption and/or IV thrombolytics in nonresponders.11,12,16–22 In the 1990s, the first prospective, randomized trial of IA thrombolysis, the Prolyse in Acute Cerebral Thromboembolism Trial (PROACT and PROACT II) began. In these trials, IA pro-urokinase (UK) was given to patients with acute middle cerebral artery (MCA) occlusion within 6 hours of stroke onset. IA pro-UK combined with heparin demonstrated partial/complete recanalization rates of 57% in PROACT, and 66% in PROACT II. In addition, treated patients showed increased functional independence at 3 months compared with control subjects.11,12
Reteplase (RP), a recombinant plasminogen activator derived from rtPA, has been shown to be safe and effective to recanalize coronary arteries in acute myocardial infarction.23–25 In addition, in 2 small case series of acute ischemic stroke patients, Quereshi and colleagues report an 84%–88% recanalization rate and 25% intracerebral hemorrhage rate with IA RP alone2 or in combination with mechanical clot disruption.26 Urokinase (UK), a direct plasminogen activator derived from human kidney cells, has also been shown to be safe and effective in the treatment of coronary artery occlusion as well as in the treatment of pulmonary embolism.27,28 Furthermore, UK has been reported in case series to improve outcome and recanalization rates in acute stroke patients.29,30 We describe our experience in the use of IA RP compared with UK in acute ischemic stroke patients, ineligible for intravenous rtPA, with large-vessel occlusion.
Patients and Techniques
From November 1996 to January 2005, 156 acute stroke patients underwent emergent cerebral angiography for possible IA thrombolysis. Vascular neurologists and fellows comprising an experienced stroke team at a Joint Commission on Accreditation of Healthcare-certified, university-based, tertiary care center were responsible for the assessment and treatment of all patients. This team in conjunction with an interventional neuroradiologist or interventional neurologist determined patient eligibility for IA thrombolysis. On admission, the vascular neurologist assessed neurologic status by using the National Institutes of Health Stroke Scale (NIHSS) to quantify neurologic impairment. A cerebral CT scan was performed in all cases before treatment with thrombolytics. IA thrombolysis was considered according to an institutional review board–approved protocol after obtaining informed consent from a responsible family member. Following protocol, patients considered for IA thrombolytics had to meet the following criteria: (1) presentation >3 hours from symptom onset; (2) minimal ischemic changes on brain CT scan (<1/3 of the MCA territory); (3) disabling neurologic deficit (NIHHS ≥ 6, or complete aphasia); (4) evidence on TCD of large vessel arterial occlusion or stenosis; (5) no evidence of intracranial hemorrhage on initial brain CT. Per protocol, IA therapy was also considered in selected patients who received IV TPA within 3 hours of symptom onset and also met criteria 2–5. The subset of IA patients who met the following criteria was included in this analysis: (1) did not receive IV TPA; (2) acute occlusion or stenosis (thrombolysis in cerebral ischemia scale [TICI] < 1) of the BA, ICA, or MCA by digital subtraction angiography; and (3) received IA RP or UK.
The choice of thrombolytic drug was determined by drug availability. UK was available from 1996 to 1999, RP was used from 2001 to 2003, and both drugs were used from 2003 to 2005 at the discretion of the treatment team. IA thrombolysis was delivered by using a multidisciplinary approach. The treatment team consisted of interventional neuroradiologists, an interventional neurologist, and the treating vascular neurologist working in various combinations with at least 2 physicians present making consensus treatment decisions. Diagnostic cerebral angiography was performed via femoral artery approach. After initial diagnostic angiography to identify the culprit lesion, patients were anticoagulated with IA heparin (1000–2000U). A 6F guiding catheter was placed proximal to the occlusion site. A microcatheter and microguidewire were advanced through the guide catheter and navigated to the occluded vessel segment in proximity to the thrombus. The microcatheter tip was embedded into the thrombus for thrombolytic infusion. RP was manually infused by slow hand injection at an approximate rate of 0.1-U aliquots diluted in 1 mL normal saline over 1–2 minutes. UK was injected in the same manner at a rate of 50,000–200,000 U given at 10-minute intervals. Control angiography was performed approximately every 10 minutes to evaluate the status of recanalization. The microcatheter was repositioned as needed to maintain placement within the thrombus. Thrombolytics were administered until either recanalization was obtained, 6 hours from symptom onset occurred (except for BA), or maximum dose limits were achieved (RP 6U, UK 2,000,000 U). Aggressive mechanical clot disruption defined as the use of at least one of the following interventional techniques: (1) aggressive microcatheter/microwire clot maceration; (2) use of a snare device was permitted with failure to attain TICI ≥ 2 with thrombolytic therapy alone. A J loop or a coiled loop at the distal end of the microwire was formed and cautiously advanced through the thrombus in resistant or persistent clot. Balloon angioplasty and stent were reserved for underlying hemodynamically significant stenosis of the parent artery, performed after the prioritized reopening of the intracranial occlusion.
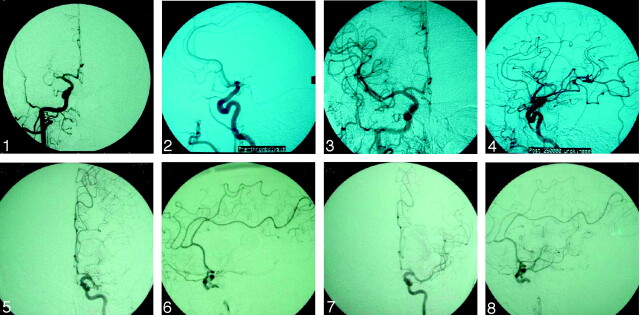
Angiograms were analyzed by either an interventional neurologist or interventional neuroradiologist. For the purpose of this study, we used a TICI scale based on the modified thrombolysis in myocardial ischemia (TIMI) criteria to define cerebral perfusion. Immediate recanalization was defined as TICI grades 2 or 3 achieved immediately after intervention (Fig 1).
Fig 1.
Initial and postprocedural angiography in a 58-year-old woman treated with UK (initial TICI, 0; final TICI, 2c) and in a 71-year-old treated with RP (initial TICI, 0; final TICI, 2c).
All patients were admitted to the neurology/neurosurgery intensive care unit or stroke unit, where their care was managed by the University of Texas—Houston Stroke Treatment Team. Concomitant antithrombotic therapy was not used. All antiplatelet therapies were started 24 hours after the procedure and after completion and review of the 24-hour cerebral CT for evidence of hemorrhage. No patients were treated with GpIIb/IIIa inhibitors. The standard guidelines of blood pressure management for IV rtPA therapy were followed before and after the procedure. If successful recanalization was achieved (TICI ≥2), a systolic blood pressure goal of <160 was targeted. Standard orders included acetaminophen for temperature ≥100°F and regular insulin sliding scale with blood glucose evaluation every 4–6 hours. Repeat cerebral CT scan was performed at 24 hours following IA thrombolysis and at any time when the patient experienced neurologic deterioration (increase in NIHSS of ≥2). Hemorrhagic transformation (HT) was defined as areas of increased attenuation on nonenhanced brain CT scans interpreted by a vascular neurologist or a neuroradiologist. Symptomatic hemorrhage was defined as ≥2-point increase in NIHSS attributable to HT by the vascular neurologist. PH-2 was defined as an intracerebral hematoma that involved >30% of the infarcted area with substantial mass effect.31 Favorable outcome was defined as 7-day modified Rankin Scale <3.
During or after the reperfusion therapy, attempts were made to categorize the type of occlusion treated. In patients who had arrhythmia or confirmed cardiac source of emboli, the occlusion was considered as cardioembolic. The occlusions deemed not cardiac in nature and with evident underlying atherosclerotic changes of the parent artery, were considered atherothrombotic. The recanalization rates were correlated with both categories.
χ2 and Fisher’s exact tests (STATA statistical software StataCorp, College Station, Tex) were used to analyze differences between the 2 groups in the categories of immediate recanalization, symptomatic hemorrhage, and mortality rate. P value <.05 was considered significantly different.
Results
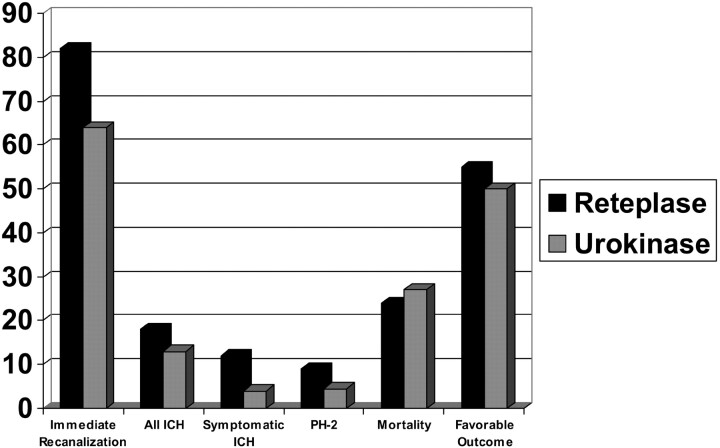
During the study period, 156 patients with acute ischemic stroke underwent emergent cerebral angiography for possible IA thrombolysis treatment, of whom 55 patients qualified for this analysis. No demographic differences between the 2 treatment groups were noted. The RP group contained 33 patients (20 men and 13 women; mean age, 63 ± 13 years; median age, 64 years; age range, 36–88 years). The UK group consisted of 22 patients (14 men and 9 women; mean age, 60 years ±15; median age, 62 years; age range, 25–83 years). Median baseline NIHSS values were 16 (range, 5–25; 81% ≥ 10) in the RP group and 17 (6–38; 85%≥10) in the UK group (Fig. 2).
Fig 2.
Recanalization, intracerebral hemorrhage (ICH), mortality, and outcome in treatment groups.
Diagnostic angiography identified the involved vascular territories. Vascular distributions, occlusion etiology, mean time from symptom onset (when known), mean total dose of thrombolytic, and mechanical thrombolysis descriptions are shown in Table 1. Ten patients in the RP group and 6 patients in the UK group awoke with symptoms. These 10 patients met other study criteria for inclusion. Immediate recanalization (TICI ≥2) occurred in 82% of the RP group and in 64% of the UK group (P = .129). TICI scores are further described in Table 2.
Table 1:
Angiographic characteristics of treatment groups
| Reteplase (n = 33) | Urokinase (n = 22) | |
|---|---|---|
| Mean age, y (median, range) | 63 ± 13 (64, 36–88) | 60 ± 15 (62, 25–83) |
| Median NIHSS (range) | 16 (5–25, 81% ≥10) | 17 (6–38, 85% ≥10) |
| Occlusion territory | ||
| ICA | 7 (21%) | 4 (18%) |
| Location | 4 distal (3 “T” occlusions), 3 proximal | 3 distal (3 “T” occlusions), 1 proximal |
| Etiology | 3 thrombotic, 3 embolic, 1 unknown | 2 embolic, 2 unknown |
| MCA | 17 (52%) | 9 (41%) |
| Location | 10 M-1, 7 M-2 | 6 M-1, 3 M-2 |
| Etiology | 5 thrombotic, 11 embolic, 1 unknown | 4 thrombotic, 5 embolic |
| BA | 9 (27%) | 9 (41%) |
| Location | 6 proximal, 2 middle, 1 distal | 6 proximal, 1 middle, 2 distal |
| Etiology | 4 thrombotic, 4 embolic, 1 unknown | 1 thrombotic, 8 embolic |
| Mean, median, and range of time from stroke onset to IA treatment (minutes) | 333 + 230 (285, 148–1195) | 343 + 169 (305, 133–720) |
| Mean total IA thrombolytic dose | 2.5 ± 1.36 mg (2, 1–6) | 690,000 ± 562,000 U (575,000, 50,000–1,500,000) |
| Mechanical thrombolysis | ||
| Simple wire | 6 (1 BA, 4 MCA, 1 ICA) | 3 (1 MCA, 2 ICA) |
| PTA | 11 (2 BA, 8 MCA, 1 ICA) | 0 |
| PTA + stent | 2 (1 MCA, 1 ICA) | 2 (1 BA, 1 ICA) |
Note:—NIHSS indicates National Institutes of Health Stroke Scale; IA, intra-arterial; PTA, percutaneous transluminal angioplasty; BA, basilar artery; MCA, middle cerebral artery; ICA, internal carotid artery.
Table 2:
Immediate recanalization in treatment groups
| Reteplase (n = 33) | Urokinase (n = 22) | |
|---|---|---|
| Immediate recanalization (TICI ≥ 2)* | 82 | 64 |
| Final TICI | ||
| 0 | 15 | 27 |
| 1 | 3 | 9 |
| 2a | 21 | 14 |
| 2b | 43 | 23 |
| 2c | 15 | 27 |
| 3 | 3 | 0 |
| Immediate recanalization by artery | ||
| ICA | 71 | 25 |
| MCA | 76 | 67 |
| BA | 100 | 78 |
Note:—TICI indicates thrombolysis in cerebral ischemia scale; ICA, internal carotid artery; MCA, middle cerebral artery; BA, basilar artery.
P = .129.
Six ICH (2 asymptomatic, 4 symptomatic: 3 parenchymal hemorrhage type 2 [PH2]) occurred in the RP group and resulted in 3 deaths. Three ICH (2 asymptomatic, one symptomatic: one PH2); P = .727) occurred in the UK group leading to one death. Eight deaths (24%) occurred in the RP group, half of which were patients who did not recanalize. Six deaths (27%) occurred in the UK group, 2 of which remained occluded (P = .8). Favorable outcome was achieved in 55% (n = 18) of the RP group and in 50% (n = 12) of the UK group (P = .69).
Further analysis of the subset of patients who received mechanical thrombolysis showed immediate recanalization rates of 60% in the UK group compared with 85% in the RP group (P = .06), 15% rate of all hemorrhages versus 10% (P = .27), 5% symptomatic and PH2 hemorrhages versus 10%, (P = .39), 25% mortality versus 20% (P = .27), and 45% favorable outcome versus 50% (P = .26). Those patients without aggressive mechanical thrombolysis attained immediate recanalization rates of 60% in the UK group and 72% in the RP group (P = .52), 0% rate of all hemorrhages versus 18% (P = .29), 0% symptomatic and PH2 hemorrhages versus 18%, (P = .29), 0% mortality versus 27% (P = .42), and 100% favorable outcome versus 54% (P = .23). With cardioembolic occlusions, the recanalization rate was 61% and the hemorrhagic rate was 5% with RP, 60% and 6% with UK. Atherothrombotic recanalization was 90%, hemorrhage 29% with RP and 100%, 0% with UK.
Discussion
Our study suggests that IA thrombolysis with RP, with or without mechanical thrombolysis, is not significantly different in recanalization, ICH, mortality, or outcome compared with that of UK. Furthermore, our immediate recanalization rates of 82% of the RP group and in 64% of the UK group (P = .129) confirm the ability of IA thrombolysis to achieve recanalization in the large majority of patients.
Although a direct comparison between our study and previously reported IA studies is difficult because of differences in patient selection and occlusion location, our patient populations and treatment times are similar to those in the PROACT II trial and those in the case series of IA RP for acute stroke patients reported by Quereshi and colleagues14,26 In PROACT II, patients had a median NIHSS score of 17 compared with our median baseline NIHSS of 16 in the RP group and 17 in the UK group, whereas Quereshi and colleagues report a NIHSS median of 19.32 Our patient population, however, included multisegment and more extensive vascular locations, including the “T” internal carotid occlusion (involvement of the internal carotid bifurcation and of the horizontal segments of the anterior cerebral (A-1) and middle cerebral (M-1) arteries, whereas the PROACT II trial only treated the MCA territory. Our 5–5.5-hour time to treatment was also comparable with 5.3 hours reported in PROACT II and 5.6 hours reported elsewhere.11,32 Therefore, the patients in our series were representative of the population of patients enrolled in IA therapy studies and presently considered for IA therapy at most centers. Furthermore, our 2 treatment groups were very well matched for important variables that determine outcome such as age, time to treatment, NIHSS, and lesion location.
IA thrombolytic therapy has been reported to achieve recanalization rates of 45%–95% by allowing for direct administration of thrombolytics at the site of obstruction.11,12,33 Our recanalization rate of 82% with RP and 64% with UK (P = .129) parallels the 84%–88% recanalization rate reported by Quereshi et al with IA RP in combination with aggressive mechanical clot disruption.26 These rates are also slightly higher than the 57% and 66% recanalization rates reported in PROACT studies.11 Mechanical disruption of the clot, however, was not allowed in the PROACT trials, which may have contributed to our higher recanalization rates. These maneuvers yielded greater clot surface exposure for thrombolysis and permitted more distal drug saturation distal to the occlusions.
Initially, UK was the thrombolytic of choice for IA use at our center until it was temporarily removed from use in 1999. Although UK was recently reintroduced into the market, its application is still limited by availability. In part because of the difficulties obtaining UK and its expense, the use of alteplase in IA therapy gained in popularity; however, alteplase has the drawbacks of a short half-life (3–5 minutes) necessitating continuous infusion into the clot and strong binding to surface fibrin, which can cause decreased penetration into the clot matrix. RP has a longer half-life (15–18 minutes) allowing bolus dosing and does not bind as strongly to fibrin, which allows better clot penetration and improved fibrinolytic activity.34
Although higher rates of recanalization with IA therapy should be associated with better outcome, the occurrence of intracranial hemorrhage is a major concern after administration of thrombolytic therapy, either intravenously or intra-arterially. This risk may be higher with sudden dramatic reperfusion of severely ischemic brain tissue as may occur after delayed complete recanalization by IA therapy. Our overall hemorrhage rate between the RP group (18%) and the UK group (13%) was not significantly different (P = .727). Furthermore, these rates are similar to the 20% rate of hemorrhage reported in the low-dose heparin arm of PROACT11 and the 25% ICH rate reported by Quereshi et al.14 In addition, our symptomatic hemorrhage rate of 12% in the RP group is similar to the 10.9% reported in PROACT II, whereas our symptomatic hemorrhage rate in the UK group (4%) is somewhat lower.12 Our mortality rates between the RP (24%) and the UK group (27%) were not significantly different (P = .8). Furthermore, these rates are similar to the 26.9% rate reported in the PROACT trial11 and less than the 56% mortality rate reported by Quereshi et al.14
Although IA thrombolytic therapy offers the advantages of improved recanalization rates, the disadvantages of IA thrombolytic therapy must be acknowledged. This mode of therapy requires trained and dedicated vascular neurologists, neurologic interventionalists, and support staff who are not available at many facilities. In addition, this interventional team must be readily available with a system in place for rapid triage, evaluation, and transfer of potential candidates. Furthermore, there are many factors that can affect angiographic success and ultimate patient outcome such as drug used, dose given, time from onset, catheter type and position, and the use of additional mechanical disruption that can make comparisons between thrombolytic drugs difficult.
This study also has limitations including retrospective design, lack of a control group, small sample size, possible selection bias, and lack of long-term outcome data. Furthermore, our sample size lacks statistical power to detect treatment differences. To reach a 90% power to detect a difference in reperfusion between the RP and UK groups, assuming a 2-sided test with an alpha level of 0.05, we would require a sample size of 252 (126 in each group). Finally, IA therapies were performed under the direction of a veteran stroke team and thus, may not be generalized to other settings. For all these reasons, our results and conclusions should be considered preliminary.
Conclusion
Although limited in statistical power, our study suggests that, though IA thrombolysis with RP shows a trend for higher recanalization and hemorrhage rates, IA thrombolysis with RP is not significantly different in recanalization, outcome, mortality, and ICH compared with that of UK regardless of whether mechanical thrombolysis was performed. Furthermore, these recanalization rates are slightly higher than rates reported with IA pro-UK, most likely because of the use of mechanical thrombolysis. Therefore, IA thrombolysis with RP appears to be a viable alternative for use if IA treatment of acute ischemic stroke is considered.
Acknowledgments
R.M.S. and H.M.S. were supported by a National Institutes of Health training grant T32NSO7412 to the University of Texas—Houston Medical School Stroke Program.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–87 [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Brott T, Caplan L, et al. Thrombolysis in acute ischemic stroke: controlled trials and clinical experience. Neurology 1999;53(suppl 4):s3–s14 [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Fieschi C, et al. Randomized double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–51 [DOI] [PubMed] [Google Scholar]
- 4.Labiche LA, Al-Senani F, Wojner AW, et al. Is the benefit of early recanalization sustained at 3 months? A prospective cohort study. Stroke 2003;34:695–98 [DOI] [PubMed] [Google Scholar]
- 5.Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol 1993;14:3–13 [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandrov AV, Demchuk A, Felberg R, et al. High rate of complete recanalization and dramatic clinical recovery during rPA infusion when continuously monitored with 2-mHz transcranial Doppler monitoring. Stroke 2000;31:610–14 [DOI] [PubMed] [Google Scholar]
- 7.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 2004;351:2170–78 [DOI] [PubMed] [Google Scholar]
- 8.Brott TG, Haley EC Jr, Levy DE, et al. Urgent therapy for stroke. Part 1. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke 1992;23:632–40 [DOI] [PubMed] [Google Scholar]
- 9.Von Krummer R, Hacke W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke 1992;23:646–52 [DOI] [PubMed] [Google Scholar]
- 10.Trouillas P, Nighoghossian N, Getenet JC, et al. Open trial of intravenous tissue plasminogen activator in acute carotid territory stroke: correlations of outcome with clinical and radiological data. Stroke 1996;27:882–90 [DOI] [PubMed] [Google Scholar]
- 11.Del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke 1998;29:4–11 [DOI] [PubMed] [Google Scholar]
- 12.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial pro-urokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 13.Ramee SR, Subramanian R, Felberg RA, et al. Catheter-based treatment for patients with acute ischemic stroke ineligible for intravenous thrombolysis. Stroke 2004;35:e109–e111 [DOI] [PubMed] [Google Scholar]
- 14.Quereshi AI, Ali Z, Suri MR, et al. Intra-arterial third generation recombinant tissue plasminogen activator (reteplase) for acute ischemic stroke. Neurosurgery 2001;49:41–48 [DOI] [PubMed] [Google Scholar]
- 15.Quereshi AI, Ringer AJ, Suri MF, et al. Acute intervention for ischemic stroke: Present status and future directions. J Endovasc Ther 2000;7:423–28 [DOI] [PubMed] [Google Scholar]
- 16.Higashida RT, Furlan AJ, Roberts H, et al. Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–e137 [DOI] [PubMed] [Google Scholar]
- 17.The IMS Study Investigators. Combined intravenous and intra-arterial decimalization for acute ischemic stroke: the interventional management of Stroke Study. Stroke 2004;35:904–912 [DOI] [PubMed] [Google Scholar]
- 18.Noser EA, Shaltoni HM, Hall CE, et al. Aggressive mechanical clot disruption: a safe adjunct to thrombolytic therapy in acute stroke? Stroke 2005;36:292–96 [DOI] [PubMed] [Google Scholar]
- 19.Zadiat O, Suarez J, Santillan C, et al. Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke 2002;33:1821–27 [DOI] [PubMed] [Google Scholar]
- 20.Keris V, Rudnicka S, Vorona V, et al. Combined intraarterial/intravenous thrombolysis for acute ischemic stroke. AJNR AM J Neuroradiol 2001;22:352–58 [PMC free article] [PubMed] [Google Scholar]
- 21.Ringer AJ, Quereshi AI, Fessler RD, et al. Angioplasty of intracranial occlusion resistant to thrombolysis in acute ischemic stroke. Neurosurgery 2001;48:1282–90 [DOI] [PubMed] [Google Scholar]
- 22.Lee KY, Kim DI, Kim SH, et al. Sequential combination of intravenous recombinant tissue plasminogen activator and intra-arterial urokinase in acute ischemic stroke. AJNR Am J Neuroradiol 2004;25:1470–75 [PMC free article] [PubMed] [Google Scholar]
- 23.Smalling RW, Bode C, Kalbfleisch J, et al. More rapid, complete, and stable coronary thrombolysis with bolus administration of reteplase compared with alteplase infusion in acute myocardial infarction. Circulation 1995;91:2725–32 [DOI] [PubMed] [Google Scholar]
- 24.INJECT Study Group. Randomized, double-blind comparison of reteplase double-bolus administration with streptokinase in acute myocardial infarction (INJECT): trial to investigate equivalence. Lancet 1995;349–36 [PubMed]
- 25.Noble S, McTavish D. Reteplase: A review of its pharmacological properties and clinical efficacy in the management of acute myocardial infarction. Drugs 1996;52:589–605 [DOI] [PubMed] [Google Scholar]
- 26.Quereshi AI, Siddiqui AM, Suri MF, et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: a prospective study. Neurosurgery 2002;51:1319–29 [DOI] [PubMed] [Google Scholar]
- 27.Urokinase Pulmonary Embolism Trial Study Group. Urokinase-streptokinase embolism trial. Circulation 1974;229:1606–13 [PubMed] [Google Scholar]
- 28.Tennant SN, Dixon J, Venable TC, et al. Intracoronary thrombolysis in acute myocardial infarction: comparison of the efficacy of urokinase to streptokinase. Circulation 1984;69:756–60 [DOI] [PubMed] [Google Scholar]
- 29.Ionue T, Kimura K, Minematsu K, et al. A case-control analysis of intra-arterial urokinase thrombolysis in acute cardioembolic stroke. Cerebrovasc Dis 2005;19:225–28 [DOI] [PubMed] [Google Scholar]
- 30.Pettersen JA, Hudon ME, Hill MD. Intra-arterial thrombolysis in acute ischemic stroke: a review of pharmacologic approaches. Expert Rev Cardiovasc Ther 2004;2:285–99 [DOI] [PubMed] [Google Scholar]
- 31.Larrue V, Von Krummer R, Del Zoppo G, et al. Hemorrhagic transformation in acute ischemic stroke. Stroke 1997;28:957–60 [DOI] [PubMed] [Google Scholar]
- 32.Wardlaw JM, Warlow CT. Thrombolysis in acute ischemic stroke: does it work? Stroke 1992;23:1826–39 [DOI] [PubMed] [Google Scholar]
- 33.Mori E, Tabuchi M, Yoshida T, et al. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke 1988;19:802–12 [DOI] [PubMed] [Google Scholar]
- 34.Deitcher SR, Jaff MR. Pharmacologic and clinical characteristics of thrombolytic agents. Rev Cardiovasc Med 2002;3:s25–s33 [PubMed] [Google Scholar]