Abstract
BACKGROUND AND PURPOSE: Neuroborreliosis is frequently indistinguishable from multiple sclerosis (MS) on both clinical and radiologic grounds. By using MR imaging, we assessed “occult” brain white matter (WM), brain gray matter (GM), and cervical cord damage in patients with neuroborreliosis in an attempt to achieve a more accurate picture of tissue damage in these patients, which might contribute to the diagnostic work-up.
METHODS: We studied 20 patients with neuroborreliosis and 11 sex- and age-matched control subjects. In all subjects, we acquired dual echo, T1-weighted, diffusion tensor (DT) and magnetization transfer (MT) MR imaging scans of the brain and fast short-τ inversion recovery and MT MR imaging scans of the cervical cord. T2-visible lesion load was measured by using a local thresholding segmentation technique. Mean diffusivity and fractional anisotropy histograms of the brain and cervical cord MT ratio histograms were produced. Normalized brain volumes (NBV) were measured by using SIENAx.
RESULTS: Brain T2-visible lesions were detected in 12 patients, whereas no occult damage in the normal-appearing WM and GM was disclosed by using MT and DT MR imaging. No macroscopic lesions were found in the cervical cord, which was also spared by occult pathology. NBV did not differ between patients with neuroborreliosis and control subjects.
CONCLUSION: This study shows that, contrary to what happens in MS, occult brain tissue damage and cervical cord pathology are not frequent findings in patients with neuroborreliosis. These observations might be useful in the diagnostic work-up of patients with neuroborreliosis and T2 brain lesions undistinguishable from those of MS.
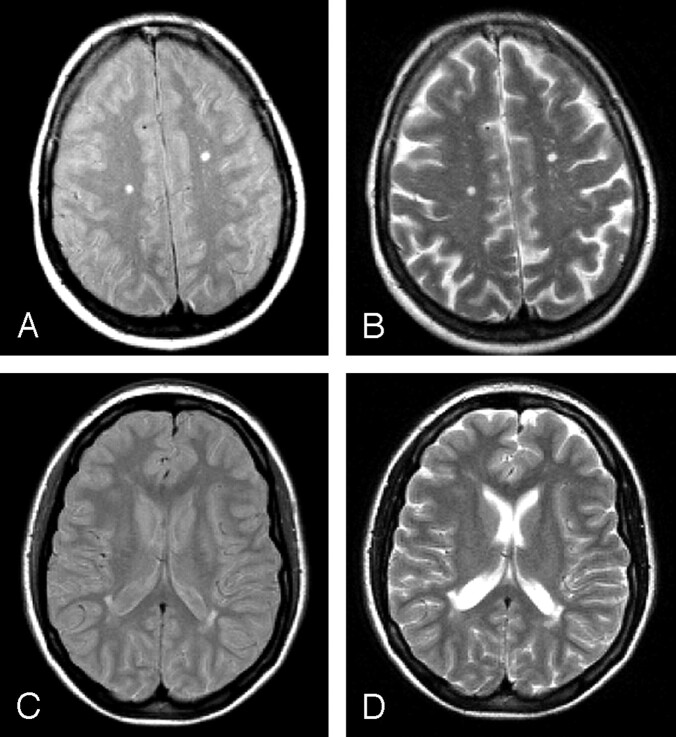
Lyme borreliosis is a tick-transmitted, multisystem disease caused by the spirochete Borrelia burgdorferi sensu lato.1 It is the most common vector-borne infection in United States and in some temperate regions of Canada, Europe, and Asia.1 In ≤10% of untreated patients, B burgdorferi may cause chronic neuroborreliosis, sometimes after long periods of latent infection.2 In neuroborreliosis, diagnostic work-up is challenging when central nervous system (CNS) involvement occurs in the absence of systemic manifestations and causes focal neurologic syndromes, such as focal or generalized seizures, hemiparesis, hemianopsia, aphasia, dysarthria, and, rarely, extrapyramidal symptoms.1–3 In addition, brain white matter (WM) abnormalities indistinguishable from those of multiple sclerosis (MS) have been reported on T2 (Fig 1) and fluid-attenuated inversion recovery (FLAIR) MR imaging scans of patients with neuroborreliosis.3–5
Fig 1.
Illustrative proton density (A and C) and T2 (B and D) axial MR images of the brain in a patient with neuroborreliosis and focal symptoms. Multiple hyperintense lesions are visible in the deep white matter (A and B) and around the lateral ventricles (C and D).
In patients with MS, brain magnetization transfer (MT) and diffusion tensor (DT) MR imaging have shown tissue damage of the normal-appearing WM (NAWM) and gray matter (GM), which goes undetected when using conventional MR imaging.5 Macroscopic lesions and abnormal MT ratio (MTR) have also been frequently detected in the cervical cord from patients with MS.6–7
In this multiparametric MR imaging study, we investigated the extent of brain and cervical cord damage of patients with neuroborreliosis with isolated CNS involvement to achieve a more complete picture of the pathologic changes associated with this condition and, ultimately, to increase diagnostic confidence in those patients with multiple brain T2-weighted abnormalities.
Materials and Methods
We studied 20 consecutive patients with isolated neuroborreliosis (11 women and 9 men; mean age, 41.0; age range, 22–55 years) in whom diagnosis was confirmed by IgG Western blot.1 All patients were younger than 50 years of age at disease onset and all had a full clinical recovery after treatment with ceftriaxone (2 g/day for 30 days). They also had no other clinical attack or new MR imaging lesions after a mean follow-up period of 4.7 years. Fifteen patients presented with focal neurologic syndromes (13 had sensorimotor deficits, one had optic neuritis, and one had a brain stem syndrome), whereas 5 patients presented with nonfocal symptoms (3 had fatigue and sleep disorders and 2 a meningoencephalitis). CSF analysis was performed in all patients; modest pleocytosis and increase of proteins was found in the 2 patients with meningoencephalitis. Eleven healthy volunteers (6 women and 5 men; mean age, 40.9 years; age range, 32–53 years) served as control subjects. The local medical ethics committee gave its approval and written informed consent was obtained from all subjects.
The following sequences were obtained with the use of a 1.5T scanner:
Brain
(1) Dual-echo (DE) turbo spin-echo (TSE) (retention time [TR]/echo time [TE] = 3300/16–98); (2) T1-weighted SE (TR/TE = 768/15); (3) 2D gradient echo (GE) (TR/TE = 640/12, flip angle = 20°), with and without an off-resonance radio-frequency saturation pulse (offset frequency = 1.5 kHz; Gaussian envelope duration = 7.68 ms; flip angle = 500°); (4) pulsed gradient SE (PGSE) echo-planar (interecho spacing = 0.8; TE = 123), with diffusion gradients applied in 8 noncollinear directions.8
For the DE, T1-weighted, and GE scans, 24 contiguous, 5-mm-thick axial sections were acquired with 256 × 256 matrix and 250 × 250-mm field of view (FOV). The sections were positioned to run parallel to a line that joins the most inferoanterior and inferoposterior parts of the corpus callosum. For the PGSE scans, 10 axial, 5-mm-thick sections with 128 × 128 matrix, and 250 × 250-mm FOV were acquired, with the same orientation as the other scans, with the second-to-last caudal section positioned to match exactly the central sections of the other image sets.
Cervical Cord
(1) Fast short-τ inversion recovery (STIR) (TR/TE = 2288/60; inversion time [TI] = 110; matrix size = 264 × 512; FOV = 280 × 280 mm; number of sections = 8; sagittal plane; section thickness = 3 mm; intersection gap = 0.3 mm) and (2) 2D GE with the same acquisition scheme used for the brain.
All MR imaging postprocessing was performed by a single observer blinded to subjects’ identities. Brain lesions were identified on the DE images and cervical cord lesions on the fast-STIR scans. T1-weighted images were used to measure normalized brain volumes (NBV), by using the SIENA software (Fmrib, Oxford, UK).9 After coregistration of the 2 brain GE scans, MTR images were derived pixel by pixel, as described elsewhere.10 Extracerebral tissue was removed from MTR maps, and the resulting images were coregistered with the T2-weighted images.10 PGSE images were corrected for distortion induced by eddy currents and mean diffusivity (MD) and fractional anisotropy (FA) derived for every pixel, as described elsewhere.10 The diffusion images were interpolated to the same image matrix size as the DE and the b = 0 step of the PGSE scans coregistered with the DE T2-weighted images.10 Lesion outlines on the DE images were superimposed onto the coregistered MTR, MD, and FA maps, and average lesion MTR, MD, and FA calculated.10 By using SPM99 and maximum image in-homogeneity correction,11 brain GM, WM, and CSF were automatically segmented from the DE images. Each pixel was classified as GM, WM, or CSF, depending on which mask had the greatest probability at that location. This generated mutually exclusive masks for each tissue, which were superimposed onto the MTR, MD, and FA maps (on which hyperintense lesions were masked out previously), and the corresponding MTR, and MD histograms of the NAWM and GM produced.10 FA histograms were derived only for the NAWM, because no preferential water molecular motion is expected to occur in the GM. Only the average MTR, MD, and FA were a priori chosen to enter the analysis, to minimize the number of comparisons and hence reduce the risk of type I error. MTR maps were derived from and MTR histograms of the entire cervical cord produced from the 2 cervical cord GE images, as described elsewhere.6 As for brain, only average cervical cord MTR entered the analysis. Statistical analyses were performed by using 2-tailed Student t tests for nonpaired data.
Results
Brain and cervical cord scans were normal in all control subjects and in the 5 patients with neuroborreliosis and nonfocal symptoms. Brain WM abnormalities were seen on the DE scans from 12 of 15 patients with neuroborreliosis with focal deficits (mean T2 lesion load = 1.36; range = 0–5.22 mL). These abnormalities were mainly located in the deep WM (7 patients) and periventricularly (9 patients) (Fig 1). In 2 patients, T2 lesions were seen in the infratentorial brain and in one patient in the corpus callosum (Fig 2). In 9 patients, some of these WM abnormalities were T1-hypointense (T1 mean lesion load = 0.58; range = 0–4.16 mL). In T2-visible lesions, average MTR was 41.3% (SD = 2.7%), average MD 0.99 × 10−3 mm2/s (SD = 0.10 × 10−3 mm2/s), and average FA 0.27 (SD = 0.02). These values did not differ from the corresponding values of the NAWM. Average MTR, MD, and FA of the NAWM, as well as MTR and MD of the GM obtained from patients with neuroborreliosis with focal deficits did not differ from the corresponding quantities obtained in control subjects (Table). Mean NBVs were 1561 mL (SD = 71 mL) in patients and 1562 mL (SD = 88 mL) in control subjects (P = not significant). In patients with neuroborreliosis with focal deficits, no macroscopic cervical cord lesions were found and average cord MTR did not differ from that of control subjects.
Fig 2.
Axial proton density (A) and T2-weighted (B) MR images of the brain from a patient with neuroborreliosis and focal symptoms. The lesions appear as hyperintense areas, suggestive of multifocal white matter pathology. One of these lesions is located in the corpus callosum.
Brain and cervical cord magnetization transfer and diffusion tensor MR imaging-derived metrics from patients with neuroborreliosis and from healthy volunteers
| MR Imaging Metrics | Healthy Volunteers (SD) | Patients with Neuroborreliosis (SD) |
|---|---|---|
| NAWM average MTR (%) | 43.2 (1.2) | 41.1 (1.9) |
| GM average MTR (%) | 38.4 (1.2) | 36.9 (1.5) |
| NAWM average MD (×10−3 mm2 s−1) | 0.82 (0.03) | 0.84 (0.04) |
| GM average MD (×10−3 mm2 s−1) | 0.10 (0.05) | 0.10 (0.07) |
| NAWM average FA | 0.29 (0.02) | 0.29 (0.02) |
| Cervical cord average MTR (%) | 40.6 (1.5) | 38.8 (2.7) |
Note:— NAWM indicates normal-appearing white matter; GM, gray matter; MTR, magnetization transfer ratio; MD, mean diffusivity; FA, fractional anisotropy.
Discussion
This study reports MTR and diffusion findings in a relatively large group of patients with neuroborreliosis. Although it is very challenging to pose a definite diagnosis of neuroborreliosis, we do think that our patients were affected by such a condition not only for their clinical and serologic profiles but also because of the dramatic response to antibiotic treatment and because they did not show any clinical and MR imaging sign of subsequent disease activity during a period of almost 5 years. It is clear that this is not the case for other conditions associated with white matter damage, such as vascular diseases and MS, where a florid disease activity is typically seen over very short periods. In agreement with previous findings,3 we detected brain WM abnormalities with MS-like features in approximately 75% of patients with neuroborreliosis with focal symptoms.
Only one previous MTR study3 has investigated the presence of “occult” brain damage in patients with neuroborreliosis, and it found no difference between patients and control subjects. Our study confirms and extends previous findings by showing that not only MT MR imaging, but also DT MR imaging, does not disclose structural abnormalities in the NAWM and GM of these patients, thus possibly indicating that the multifocal perfusion abnormalities detected by using single-photon emission CT in patients with neuroborreliosis4 may not have a structural substrate. In addition, neither macroscopic lesions nor MTR changes were found in the cervical cord of patients with neuroborreliosis. All of these contrast with MR imaging findings in MS (including patients at the earliest clinical stage),12–14 where brain NAWM and GM as well as cervical cord are frequently and severely damaged.6–7 We also assessed the severity of tissue damage within T2-visible lesions of our patients and found that average lesion MTR, MD and FA were similar to those of NAWM, which suggests that, contrary to what happens in MS,6 the amount of tissue damage in T2-visible lesions of neuroborreliosis is relatively mild. Because diagnosing neuroborreliosis is challenging, especially in those patients with focal neurologic syndromes, absence of systemic manifestations, and multiple WM lesions, our findings suggest a role of modern MR imaging in the diagnostic work-up of these patients.
Conclusions
This study shows that, contrary to what occurs in MS, occult brain tissue damage and cervical cord pathology are not frequent findings in patients with neuroborreliosis. These observations might be useful in the diagnostic work-up of patients with neuroborreliosis and T2 brain lesions indistinguishable from those of MS.
References
- 1.Stanek G, Strle F. Lyme borreliosis. Lancet Neurol 2003;362:1639–47 [DOI] [PubMed] [Google Scholar]
- 2.Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med 1990;323:1438–44 [DOI] [PubMed] [Google Scholar]
- 3.Morgen K, Martin R, Stone RD, et al. FLAIR and magnetization transfer imaging of patients with post-treatment Lyme disease syndrome. Neurology 2001;57:1980–85 [DOI] [PubMed] [Google Scholar]
- 4.Logigian EL, Johnson KA, Kijewski MF, et al. Reversible cerebral hypoperfusion in Lyme encephalopathy. Neurology 1997;49:1661–70 [DOI] [PubMed] [Google Scholar]
- 5.Halperin JJ, Luft BJ, Anand AK, et al. Lyme neuroborreliosis: central nervous system manifestations. Neurology 1989;39:753–59 [DOI] [PubMed] [Google Scholar]
- 6.Filippi M. In-vivo tissue characterization of multiple sclerosis and other white matter diseases using magnetic resonance based techniques. J Neurol 2001;248:1019–29 [DOI] [PubMed] [Google Scholar]
- 7.Lycklama G, Thompson A, Filippi M, et al. Spinal-cord MRI in multiple sclerosis. Lancet Neurol 2003;2:555–62 [DOI] [PubMed] [Google Scholar]
- 8.Cercignani M, Bozzali M, Iannucci G, et al. Magnetisation transfer ratio and mean diffusivity of normal appearing white and grey matter from patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2001;70:311–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–89 [DOI] [PubMed] [Google Scholar]
- 10.Rovaris M, Gallo A, Valsasina P, et al. Short-term accrual of gray matter pathology in patients with progressive multiple sclerosis: an in vivo study using diffusion tensor MRI. Neuroimage 2005;24:1139–46 [DOI] [PubMed] [Google Scholar]
- 11.Ashburner J, Friston K. Multimodal image coregistration and partitioning: a unified framework. Neuroimage 1997;6:209–17 [DOI] [PubMed] [Google Scholar]
- 12.Iannucci G, Tortorella C, Rovaris M, et al. Prognostic value of MR and magnetization transfer imaging findings in patients with clinically isolated syndromes suggestive of multiple sclerosis at presentation. AJNR Am J Neuroradiol 2000;21:1034–38 [PMC free article] [PubMed] [Google Scholar]
- 13.Traboulsee A, Dehmeshki J, Brex PA, et al. Normal-appearing brain tissue MTR histograms in clinically isolated syndromes suggestive of MS. Neurology 2002;59:126–28 [DOI] [PubMed] [Google Scholar]
- 14.Gallo A, Rovaris M, Riva R, et al. Diffusion tensor MRI detects normal-appearing white matter damage unrelated to short-term disease activity in patients at the earlier stage of multiple sclerosis. Arch Neurol 2005;62:803–08 [DOI] [PubMed] [Google Scholar]