Abstract
SUMMARY: A 41-year-old man suspected of having lead poisoning was evaluated with MR imaging before and after British antilewisite therapy. The MR imaging findings showed bilateral symmetric involvement of the occipital lobe, affecting predominantly the gray-white matter junction and the subcortical white matter. A right cerebellar lesion was noted, with focal hyperintensities involving the gray-white matter. Similar lesions were seen in the temporal, parietal, and frontal regions. These lesions resolved after chelation therapy.
Lead encephalopathy is rare,1, 2 but lead encephalopathy due to ayurvedic medicines is even rarer. In this report, we discuss the MR imaging findings in a patient with lead encephalopathy due to ayurvedic medicines (Ayu-Life). Lead poisoning affects multiple organs: specifically bones (lead bands at the epiphyses of the long bones), blood (punctate basophilic stippling of erythrocytes and microcytic hypochromic hemolytic anemia), brain (brain edema and demyelination of the cerebral and cerebellar white matter), peripheral nervous system (demyelinating neuropathy), gingiva (lead lines), and kidneys (chronic tubulointerstitial disease). In the central nervous system, the lesions are thought to be the result of vascular injury.3 Lead acts as a cellular toxin by inhibiting mitochondrial respiration. The increased resistance of the adult to encephalopathy and ataxia is believed to be caused by the capacity of the mature brain to sequestrate lead away from its mitochondrial site of action within the cerebral and cerebellar neurons.4
Case Report
A 41-year-old man came to hospital with short-term memory loss, loss of appetite, disinterest in his surroundings, and loss of balance. The patient had hypertension and had been consuming ayurvedic medicines (Mahayogaraj-gugul)5 for the past 6 months. On clinical examination, the patient showed memory loss, mild disorientation, and weakness in the limbs with bilateral hypertonia and hyperreflexia, plantar upgoing, and spastic gait. The blood lead level was 161 μg/dL, more than 15 times the accepted normal level. Electroencephalography showed mild increased theta activity. The patient was evaluated with MR imaging, which showed bilateral symmetric involvement of the parasagittal occipital lobes and similar lesions in the temporal, parietal, and frontal regions (Fig 1). Theses lesions had resolved to a great extent on the repeat MR imaging after lead chelation therapy (Fig 2). The chelation therapy was given as 5 mg/kg of British antilewisite immediately, followed by 3 mg/kg 3 times a day for 2 days, then once daily for 10 days. Six months later, the patient is doing well clinically. We are awaiting a report of his recent blood lead levels.
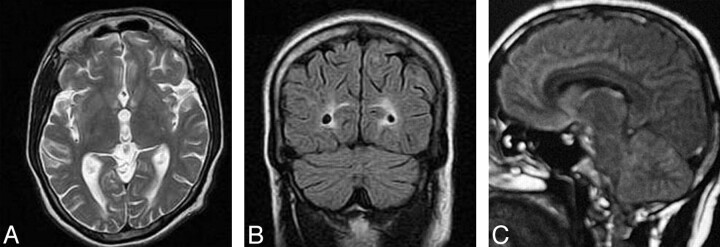
Fig 1.
Prechelation MR imaging.
A–C, T1- and T2-weighted axial and FLAIR (respectively) coronal MR images show cortical gray matter and subcortical white matter lesion in the occipital lobe with edema and sulcal effacement.
D, FLAIR sagittal image shows involvement of subcortical white matter in the frontal regions, parieto-occipital lobe, body of the corpus callosum, and cerebellum.
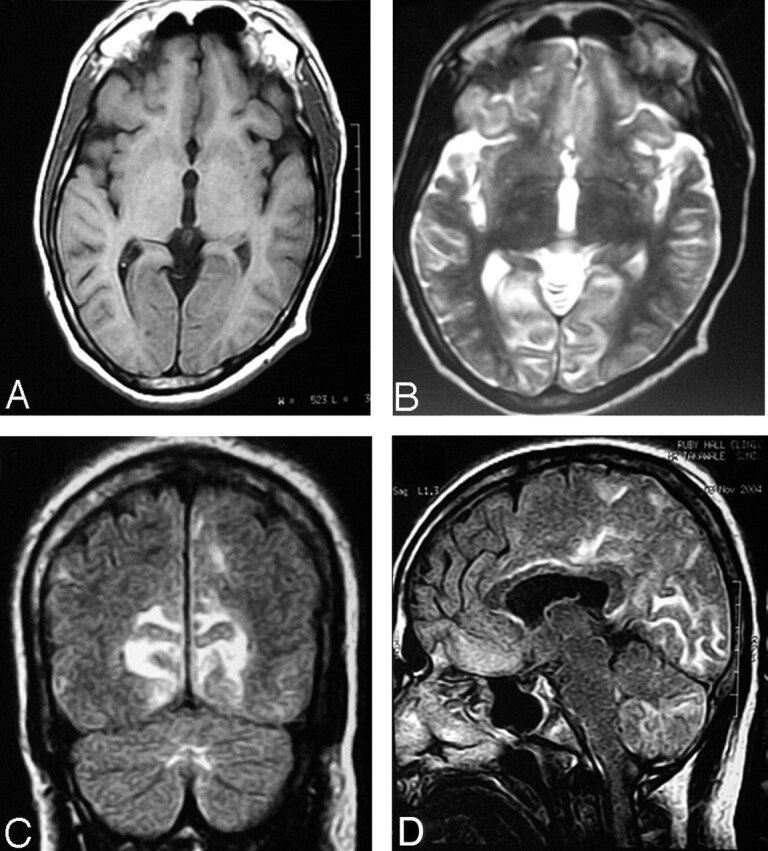
Fig 2.
Postchelation MR imaging.
A and B, T2-weighted axial and FLAIR coronal images show near-total resolution. Few focal subcortical white matter lesions are still seen in the occipital lobe.
C, FLAIR sagittal images show near-total resolution.
Discussion
Lead encephalopathy was first documented in 1925.1 It is a rare entity, even rarer in adults,2 and yet rarer from consumption of ayurvedic medicines. Normal lead levels should be below 10 μg/dL. In lead poisoning, the blood level is >40 μg/dL. Lead encephalopathy is almost always associated with a blood lead concentration of >100 μg/dL, but levels of lead as low as 70 μg/dL1,6 can also cause encephalopathy. Our patient had consumed ayurvedic medicine for more than 6 months, and the blood lead levels were 161.4 μg/dL prechelation therapy and 38.4 postchelation therapy.
The adult form of lead encephalopathy is rarer than the pediatric form.7 In adults, this encephalopathy can present with serious neurodeterioration and can sometimes be lethal.8
Lead encephalopathy can present in acute and chronic forms.9 Acute lead encephalopathy presents pathologically as cerebral edema,9 whereas in chronic lead encephalopathy, extensive tissue destruction with cavity formation and thickening of the veins with cellular disorganization of their walls (suggesting vascular insult in cerebral injury) is seen.6 All this can lead to edema, gliosis, hemorrhage, neuronal loss, and perivascular proteinaceous exudate.3 Lead acts as a cellular toxin by inhibiting mitochondrial respiration.4 Clinically, patients present with headache, vomiting, ataxia, convulsions, paralysis, stupor, and coma in acute forms,2, 7, 9, 10 whereas in chronic encephalopathy as in our case, patients present with loss of memory and concentration, depression, disorientation, headache, drowsiness, dizziness, lack of sensory perception, diminished libido, syncope, tremor, irritability, seizure, ataxia, restlessness, and behavioral abnormalities.1, 2, 6, 7, 9 In severe conditions, cerebral or cerebellar edema may develop; such patients may present with vomiting, apathy, stupor, coma, paralysis, and death.2, 9
In our patient, MR imaging showed symmetric involvement of the occipital lobe in the parasagittal region, affecting predominantly the gray matter, gray-white matter junction, and the subcortical white matter. Similar lesions were also seen in the temporal, parietal, and frontal regions. These lesions were bright on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images and hypointense on T1-weighted images. There was no significant edema associated with these lesions, except for that in the occipital lobe, where the cortical sulci were effaced. No enhancement was seen after intravenous contrast administration. Multiple tiny callosal lesions were also noted. Cerebellar lesions were seen, involving the right cerebellar hemispheres, predominantly in the posteroinferior cerebellar artery territory. A tiny brain stem lesion affecting the central pons was apparent.
The diffusion-weighted images and apparent diffusion coefficient maps did not show abnormal signals. The 2D time-of flight (TOF) angiogram obtained through the region of neck vessels and carotid bifurcations and the 3D TOF angiogram obtained through the circle of Willis revealed a normal appearance of all vessels. No significant intracranial calcification was seen in our patient. With these imaging findings, a diagnosis of primary central nervous system (CNS) angitis was considered. These lesions diminished and disappeared on posttreatment MR imaging. These findings were also supported by laboratory parameters and clinical improvement of the patient after chelation therapy.
Perelman et al8 reported MR imaging findings such as cerebellar edema, suggested by an enlarged cerebellum with the disappearance of the cerebellar fissure and protrusion. Mani et al6 reported high signal intensities in both thalami on T2-weighted MR images. Schroter et al11 reported high signal intensities in the periventricular white matter, basal ganglia insula, posterior thalamus, and pons on T2-weighted MR images. Tuzun et al12 reported low signal intensities on T1-weighted images and high signal intensities on T2-weighted images located bilaterally and symmetrically in thalami, lateral putamen, claustrum, and insula. There was no contrast enhancement.
Lead intoxication can cause intracranial calcification.3 Perelman et al8 reported an incidence of 84% cerebellar calcification at autopsy in a study of 44 adults with a history of chronic lead poisoning. Schroter et al11 reported that cranial CT scans showed bilateral symmetric calcification in the subcortical area of the cerebral hemispheres and basal ganglia. Reyes et al1 reported extensive intracranial calcification detected by CT in 3 adults with chronic lead poisoning. Tuzun et al12 reported bilateral symmetrical hypoattenuated lesions in the thalamus, lateral putamen, claustrum, and insula. Teo et al3 reported calcification that was extensive, symmetric, curvilinear, and specklike in the cerebellum, cerebral cortex (in the gray-white matter), thalamic regions, and basal ganglia. The pathogenesis of intracranial calcification has not yet been proved satisfactorily. In our patient, CT performed before MR imaging did not show any calcification.
In conclusion, lead poisoning is a possibility in patients with MR imaging indicative of subcortical white matter demyelination. Also differential diagnosis of primary CNS angitis, demyelination, edema, and hemorrhage should be considered. Clinical and laboratory work-up can aid in this differential diagnosis.13
References
- 1.Reyes PF, Gonzalez CF, Zalewska MK, et al. Intracranial calcification in adults with chronic lead exposure. AJR Am J Roentgenol 1986;146:267–70 [DOI] [PubMed] [Google Scholar]
- 2.Landrigan PJ, Todd AC. Lead poisoning. West J Med 1994;161:153–59 [PMC free article] [PubMed] [Google Scholar]
- 3.Teo JGC, Goh KYC, Ahuja A, et al. Intracranial vascular calcifications, glioblastoma multiforme, and lead poisoning. AJNR Am J Neuroradiol 1997;18:576–79 [PMC free article] [PubMed] [Google Scholar]
- 4.Holtzman D, DeVries C, Nguyen H, et al. Maturation of resistance to encephalopathy: cellular and subcellular mechanism. Neurotoxicology 1984;5:97–124 [PubMed] [Google Scholar]
- 5.Saper RB, Kales SN, Paquin J, et al. Heavy metal content of ayurvedic herbal medicine products. JAMA 2004;292:2868–73 [DOI] [PubMed] [Google Scholar]
- 6.Mani J, Chaudhary N, Kanjalkar M, et al. Cerebellar ataxia due to lead encephalopathy in an adult. J Neurol Neurosurg Psychiatry 1998;65:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bressler JP, Goldstein GW. Mechanisms of lead neurotoxicity. Biochem Pharmacol 1991;41:479–84 [DOI] [PubMed] [Google Scholar]
- 8.Perelman S, Hertz-Pannier L, Hassan M, et al. Lead encephalopathy mimicking a cerebellar tumor. Acta Paediatr 1993;82:423–25 [DOI] [PubMed] [Google Scholar]
- 9.Saryan LA, Zenz C. Lead and its compounds. In: Saryan LA, Zenz C, eds. Occupational Medicine. 3rd ed. St. Louis, Mo: Mosby;1994. :506–41
- 10.alKhayat A, Menon NS, Alidina MR. Acute lead encephalopathy in early infancy: clinical presentation and outcome. Ann Trop Paediatr 1997;17:39–44 [DOI] [PubMed] [Google Scholar]
- 11.Schroter C, Schroter H, Huffmann G. Neurologic and psychiatric manifestations of lead poisoning in adults (case report and review of the literature) [in German]. Fortschr Neurol Psychiatr 1991;59:413–24 [DOI] [PubMed] [Google Scholar]
- 12.Tuzun M, Tuzun D, Salan A, et al. Lead encephalopathy: CT and MR findings. J Comput Assist Tomogr 2002;26:479–81 [DOI] [PubMed] [Google Scholar]
- 13.Dahnert W. Radiology Review Manual. 4th ed. Baltimore, Md: Lippincott Williams & Wilkins;199X. :181–200