Abstract
BACKGROUND AND PURPOSE: Functional MR imaging (fMRI) is playing an important role in investigations of cortical development and maturation. Functional MR imaging in young children or infants frequently involves measuring a clinical population under sedation or anesthesia. We examined the effect of depth of anesthesia on the extent and amplitude of the blood oxygen level–dependent (BOLD) response.
METHOD: We performed BOLD-based fMRI on a visual stimulus flickering at 8 Hz at sevoflurane concentrations of 0.5 minimum alveolar concentration (MAC), 0.75 MAC, and 1.0 MAC, on 16 children at least 5 years of age. We determined the extent of activation by counting the number of activated voxels and assessed the change in the local deoxyhemoglobin concentration by comparing ΔR2*.
RESULTS: The number of activated voxels of the positive BOLD response was higher at 0.75 MAC than at 0.5 MAC or 1.0 MAC. The magnitude of their mean ΔR2* steadily declined as the level of sevoflurane was increased from 0.5 MAC to 1.0 MAC. The extent of activation of the negative BOLD response declined progressively from 0.5 MAC to 1.0 MAC. The magnitude of their mean amplitude of the ΔR2* did not change with sevoflurane concentrations. The change in the extent of activation and the magnitude of ΔR2* when the concentration of sevoflurane increased from 0.5 MAC to 0.75 MAC was due to its vasodilative property. The change in the extent of activation and the amplitude of ΔR2* following the increase in the concentration of sevoflurane from 0.75 MAC and 1.0 MAC was due to its anesthetic property. This was the case for both the positive and negative BOLD response.
CONCLUSIONS: Careful adjustment of anesthetic depth can be used advantageously when performing BOLD-based fMRI measurements in children.
Understanding how neuronal processes are modified as a result of maturational and developmental changes to the CNS can provide important insights into the central mechanisms underlying perceptual and cognitive mechanisms. By studying the changes in brain activity accompanying normal maturation, it becomes possible to detect and interpret deviations from the normal course of development and adopt strategies to remedy any detrimental influences early. To follow the developmental changes in brain activity, it is necessary to perform functional imaging in very young children.
Computer-assisted imaging methods such as functional MR imaging (fMRI), single-photon emission CT (SPECT), or positron-emission tomography (PET) provide a means of visualizing the neuronal activation associated with perceptual or cognitive processing. Functional MR imaging is currently the most widely used method for visualizing brain activity. The reason for this is that it can make use of the blood oxygenation level–dependent (BOLD) signal intensity.1 The BOLD signal intensity acts as an internal contrast agent, making it possible to detect neuronal activation in a noninvasive manner. This makes fMRI the ideal method for investigating the development of neuronal correlates of function as it is safe to use on children repeatedly.2
Functional imaging involving very young children or infants almost invariably has to take place while the child is sedated or under anesthesia.3 The depth of anesthesia is assessed by using the end-tidal concentration of the anesthetic gas on the minimum alveolar concentration (MAC) and conversion charts. The charts themselves are based on the incidence of a reflex reaction to a stimulus at a given concentration of the anesthetic agent.4–6 One MAC is defined as the concentration of an anesthetic agent at which 50% of patients still elicit a reaction to a noxious stimulus, usually the skin incision at the onset of surgery.
To assess the maturational influence on the fMRI data, the data obtained from very young children have to be compared with fMRI data obtained from older children. A number of studies have reported that the incidence of a negative BOLD response to a visual stimulus is higher in infants and very young children than in older children.2,7–9 By the age of 5 years, the BOLD response obtained from children to a visual stimulus is identical to that of an adult.10 Because the fMRI data from young children more often than not have to be obtained while the child is placed under sedation or anesthesia the influence of the anesthetic agent needs to be compensated for. The influence of a sedative or anesthetic agent on the BOLD signal intensity is poorly understood. At a concentration of 0.5 MAC, the anesthetic agent sevoflurane has been shown to have no significant influence on the BOLD signal intensity amplitude of children older than 5 years of age.11
The aim of our study was to investigate how changes in the concentration of the anesthetic agent sevoflurane influence the BOLD response in children older than 5 years of age. We expected to gain an insight into how the BOLD response changes with depth of anesthesia and so be able to discern its influence from the maturational influence on the BOLD response. Furthermore, by comparing the extent of activation and the magnitude of the BOLD signal intensity amplitude we were also able to test the predictions of the recently proposed standard model.12 Classically, neuronal activity is associated with the frequency of the action potentials. The standard model divides the neuronal activity into 2 components: the discharge activity and neuronal recruitment. The former is linked to the vascular response, the latter with the oxygen consumption. Under the classic definition of neuronal activity, the BOLD response should vary linearly with the concentration of the anesthetic agent. Under the standard model definition of neuronal activity, the BOLD response need not vary linearly with the concentration of the anesthetic agent. To test the predictions of the standard model against the classic predictions we considered the following hypotheses. The null hypothesis predicts a linear decline in the BOLD response with increasing concentration of sevoflurane. The alternative hypothesis predicts no linear relationship between the BOLD response and the concentrations of sevoflurane.
Materials and Methods
Subjects.
Sixteen children (5 girls), at least 5 years of age were measured as part of a diagnostic MR imaging session, during which the child had to be placed under anesthesia for clinical reasons (eg, paradox reaction to a sedative agent or respiratory problems). The mean age of the children was 8.7 years, with a range of 5.1–17.4 years. The clinical indicators leading to a referral for diagnostic MR imaging included accident, cranial malformation, facial tumor, micro- or macroencephaly, nausea, persistent headache, and extracranial tumors in the extremity. We excluded children with anatomic anomalies involving the visual system and especially the occipital lobe and children with disturbances of the cerebral metabolism. The study was approved by the local ethics committee and written informed consent from a parent or a legal guardian was obtained in each case. Throughout the entire diagnostic and functional MR measurements the child was kept under constant video surveillance and was continuously monitored by the anesthetic team. By measuring each child at 3 different depths of anesthesia, the need for a control group was eliminated, as each child served as its own control.
Apparatus.
Measurements were performed on 2T Bruker Tomikon S200 whole body scanner (Bruker-Medical, Faehlanden, Switzerland). The use of a quadrature head coil was dictated by the need to obtain high-quality diagnostic MR images.
Stimulus.
A visual flicker stimulus at 8 Hz was applied through the closed eyelids by using a set of LED goggles (Grass Instruments, Quincy, Mass).
Functional MR Sequence.
A T2*-weighted, gradient-echo, echo-planar imaging (EPI) sequence was used to obtain BOLD contrast-sensitive images of the brain. The sequence parameters were TR of 2000 milliseconds; TEEffective of 58 milliseconds; spectral width of 100 kHz; and tip angle of 90°. We also used a fat-suppression pulse with a 350-Hz bandwidth. We used a section package of 14 slices with a thickness of 4.5 mm and an intersection distance of 1 mm. The package was placed, with the sections horizontally, in such a manner that the second section from the bottom was located above the base of the occipital cortex. This was necessary to ensure that we covered the entire posterior occipital cortex, because the functional analysis tool we used discarded the first and the last section in the package. The field of view was 25 cm × 25 cm. Our recording matrix was 128 pixels in the read direction and 64 pixels in the phase direction. The images were then reconstructed to a resolution of 128 × 128 pixels, including zero filling. At the end of the last functional measurement we used a high-resolution, T2-weighted RARE sequence to image the brain with the same section locations used for the functional measurement. The functional data were then overlaid onto the high-resolution images.
Anesthetic Management.
All anesthetic procedures were carried out by the hospital’s anesthetist. The hospital guidelines on performing of MR imaging under anesthesia were adhered to at all times. Each patient was administered an oral premedication of midazolam (Dormicum, 0.5 mg/kg; maximum dose of 10 mg). This is a short-acting sedative and is used routinely to prepare patients for anesthesia. Anesthesia was initiated by using sevoflurane administered via a breathing mask. Nitrous oxide was avoided, so as not to have to contend with the influence of 2 anesthetic agents. Tracheal intubation followed. Breathing and anesthesia was thereafter controlled via a mechanical ventilator. Throughout the entire MR session, all vital signs—including rectal body temperature, respiratory rate, heart rate, blood pressure, blood oxygen saturation, and expiratory CO2—were constantly monitored by the anesthetist team. Expiratory CO2 levels were kept between 4.8% and 6.0%, and the blood oxygen saturation was kept at 97% while the child was anesthetized.
Functional Paradigm.
We used a boxcar paradigm, starting with a period of visual stimulation lasting 20 seconds. This was followed by a rest period lasting 90 seconds. This cycle was repeated 5 times, resulting in an image series of 275 brain volumes. Three functional measurements were performed on each child. These measurements were performed at a sevoflurane concentration of 0.5 MAC (sevoflurane insp. = 1.26%: STD = 0.09; sevoflurane exp. = 1.22%: STD = 0.08), 0.75 MAC (sevoflurane insp. = 2.06%: STD = 0.18; sevoflurane exp. = 1.91%: STD = 0.13), and 1.0 MAC (sevoflurane insp. = 2.77%: STD = 0.25; sevoflurane exp. = 2.54%: STD = 0.19). In half of the children, the concentration of sevoflurane increased from 0.5 to 0.75 MAC and 1.0 MAC between measurements, whereas in the other half of the children the concentration of sevoflurane decreased from 1.0 MAC to 0.75 MAC and 0.5 MAC between measurements. The level of anesthesia was changed by 0.25 MAC at the end of each functional measurement. The next functional measurement did not commence until the end-tidal level of sevoflurane reached a steady state. This ensured equilibration between blood and central nervous concentration. During this period diagnostic MR imaging continued so as not to extend unnecessarily, the time during which the child was placed under anesthesia. Once the anesthetist signaled that the end-tidal anesthetic level had been stable for 15 minutes, we initiated the next functional measurement at the end of the ongoing diagnostic imaging sequence.
Data Analysis.
The functional image analysis was performed by using MEDx 3.4 (Sensor Systems, Sterling, Va). The functional image series were subjected to a motion correction by using the automated image registration (AIR) method. This was followed by a baseline correction and an intensity normalization to an arbitrary mean value of 1000. We located both activated voxels with a positive BOLD response and activated voxels with a negative BOLD response by using a cross-correlation analysis. Finally, the activated voxels ware subjected to a Bonferroni correction with an uncorrected P value of .05. The data from the activated voxels remaining were recorded. The correction threshold was obtained by convolving the uncorrected threshold (P = .05) with the number of voxels in a mask restricting the correction to the subject’s head. The x, y, and z coordinates of each corrected, activated voxel were noted.
The number of activated voxels at each concentration of sevoflurane was determined for each subject to obtain a measure of the extent of activation. The coordinates were used to extract the time series data, from which we determined the change in deoxyhemoglobin (HHb) concentration by calculating the change in R2*.11,13,14 A ΔR2* of zero indicated no change, a negative ΔR2* indicated a decrease, and a positive ΔR2* indicated an increase in the HHb concentration between the rest and the activated condition. The statistical analysis of the number of activated voxels and the ΔR2* was performed by using the general linear model for repeated measures of SPSS 11.5 (SPSS, Chicago, Ill), with level of “anesthesia” as our repeated measures factor. As is common practice, we will only provide details of the statistical analyses that yielded a probability value ≤.05. We included ETA2 as a measure of the size of the effect independent of sample size.
Results
Extent of Activation.
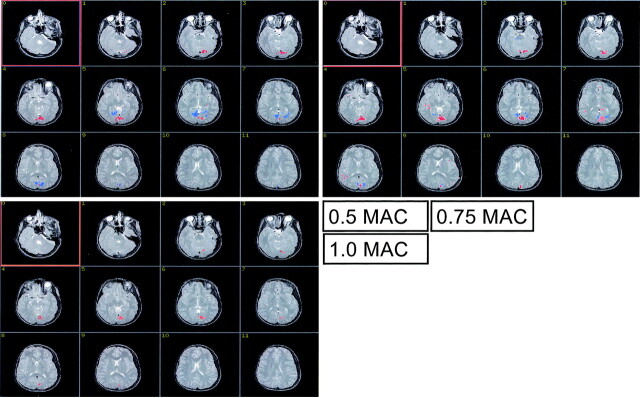
Figs 1A–C show the BOLD activation at the 3 levels of sevoflurane concentration in a single, representative subject. The positive (red) and the negative (blue) BOLD responses were spatially separated. The mean number of voxels with a positive or a negative BOLD signal intensity are shown in Figs 2A and 2B, respectively. The level of sevoflurane had a significant effect on the extent of activation of the positive BOLD response (FWilks = 4.572; df = 2 ,14; P = .03; ETA2 = 0.395). The data exhibited a significant quadratic trend (F = 8.040; df = 1; P = .013; ETA2 = 0.349). At 1.0 MAC we found activated voxels with a negative BOLD response in 3 subjects only. The extent of activation of the negative BOLD response failed to reach significance, though reduction between 0.5 MAC and 0.75 MAC was significant (F = 5.727; df = 15; P = .03; ETA2 = 0.276) and the data across all 3 concentration levels exhibited a significant linear trend (F = 7.033; df = 1; P = .018; ETA2 = 0.319).
Fig 1.
The figure shows the cortical site of the positive BOLD response (red) and the negative BOLD response (blue) in a single child at the 3 levels of sevoflurane anesthesia. The extent of activation of the positive BOLD response is clearly largest, at 0.75 MAC. The extent of activation of the negative BOLD response can be seen to decline with increasing sevoflurane level.
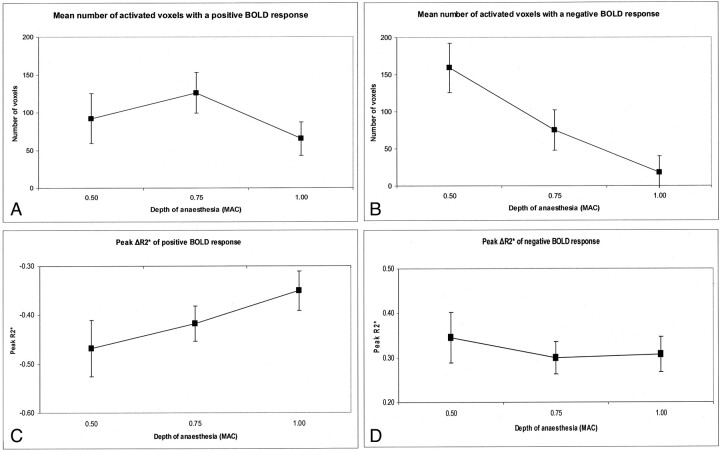
Fig 2.
A, The mean number of activated voxels with a positive BOLD response. B, The mean number of activated voxels with a negative BOLD response at the 3 levels of sevoflurane anesthesia. C, The mean, peak ΔR2* from the positive BOLD. D, The mean, peak ΔR2* from the negative BOLD response at the 3 levels of sevoflurane anesthesia. The error bars indicate the standard error of the mean.
We performed pairwise comparisons of the extent of activation and ΔR2*, by using the d value as our measure of effect. The d value is the difference between the 2 means, divided by the common SD.15 A d value ≥0.3 indicates a weak effect; a d value ≥0.5 indicates a moderate effect; and a d value ≥0.8 indicates a strong effect.
Positive BOLD Response.
The effect on the extent of activation of increasing the concentration of sevoflurane produced a moderate effect (d = 0.5) between 0.5 and 0.75 MAC, a strong effect (d = 0.8) between 0.75 MAC and 1.0 MAC, and a moderate effect (d = 0.5) between 0.5 MAC and 1.0 MAC.
Negative BOLD Response.
The effect on the extent of activation of increasing the sevoflurane concentration produced a moderate effect (d = 0.48) between 0.5 MAC and 0.75 MAC, a strong effect (d = 0.82) between 0.75 MAC and 1.0 MAC, and a very strong effect (d = 1.72) between 0.5 MAC and 1.0 MAC.
BOLD Response Magnitude.
Figures 2C and 2D show the peak ΔR2* values for the positive and the negative BOLD response, respectively. The peak ΔR2* served as our indicator of the local change in HHb concentration. Figure 2C shows that the peak ΔR2* of the positive BOLD response became steadily smaller as the concentration of sevoflurane increased (FWilks = 25.579; df = 2 ,6; P = .001; ETA2= 0.895). The data exhibited a significant linear trend (F = 6.863; df = 1; P = .034; ETA2 = 0.495).
At 1.0 MAC, we obtained a negative BOLD response from 3 subjects only, so our statistical analysis included only the peak ΔR2* at 0.5 MAC and 0.75 MAC. We found no significant difference in the peak ΔR2* of the negative BOLD response at 0.5 MAC and 0.75 MAC (Fig 2D).
We again examined the effect size of increasing sevoflurane on ΔR2*. With regard to positive BOLD response, the effect on the peak ΔR2* of increasing the concentration of sevoflurane from 0.5 MAC to 0.75 MAC was weak (d = 0.30), between 0.75 MAC and 1.0 MAC moderate (d = 0.5), and between 0.5 MAC and 1.0 MAC also moderate (d = 0.71). With regard to negative BOLD response, the effect on the peak ΔR2* of increasing the concentration of sevoflurane from 0.5 MAC to 0.75 MAC was weak (d = 0.34), none between 0.75 MAC and 1.0 MAC (d = 0.05), and between 0.5 MAC and 1.0 MAC also weak (d = 0.29).
Discussion
In this section, we will provide a cohesive account of the observed effect of sevoflurane on the BOLD response. In our argumentation, we will assume that the effect of a specific increase in the concentration of sevoflurane on a single mechanism will manifest itself as a similar effect on the BOLD response.
Our analysis showed that the extent of activation of the positive BOLD response actually increased when the concentration of sevoflurane increased from 0.5 MAC to 0.75 MAC but declined sharply as the level of sevoflurane was raised from 0.75 MAC to 1.0 MAC. The quadratic trend in the extent of activation data of the positive BOLD response is suggestive of 2 different mechanisms influencing the BOLD response. The extent of activation of the negative BOLD response declined in a linear manner as the level of sevoflurane was increased. This suggests that the positive and negative BOLD response may themselves be the product different mechanisms.16,17 The peak ΔR2* of the positive BOLD response declined linearly, whereas the peak ΔR2* of the negative BOLD response did not change with increasing sevoflurane concentration. This, too, suggests that different mechanisms may be giving rise to the 2 types of BOLD response.
Carryover Effects and the BOLD Response
Before engaging in an extensive discussion, we will consider the possible influence of order effects such as learning, fatigue, or habituation on our results. A previous study examining the influence of subclinical levels of anesthesia on the BOLD response during a motor learning task observed a carryover effect in the motor cortex but not in the visual cortex.18 Our experimental paradigm included a number of specific aspects that were designed to reduce any potential carryover effect on our measurements. The use of a featureless, flashed light source as a stimulus and the fact that all our subjects were measured in a state of induced sleep excluded any effects due to learning. To counter the possible effects of fatigue, we used a stimulation period of 20 seconds and introduced a rest period lasting 70 seconds between stimulations. This asymmetry in the stimulation and rest period has been used by several other investigators where it has been demonstrated to permit the system to recover to the baseline level.16,19 The effects of habituation were reduced by having successive stimulation blocks spaced ≥15 minutes apart. The BOLD response of the visual cortex has been reported to display a high level of reproducibility across session and subject.20,21
With the measures we have undertaken and the findings that the BOLD response from visual cortex is not subject to a “carryover effect,” we can be confident that our results are attributable to the influence of the anesthetic agent alone.
The Effect of Sevoflurane on the Extent of Activation
The effect on the extent of activation of increasing sevoflurane from 0.5 MAC to 0.75 MAC was smaller than the effect on the extent of activation of the identical increase from 0.75 MAC to 1.0 MAC. This was true for both the positive and the negative BOLD response. Increasing the sevoflurane level from 0.5 MAC to 0.75 MAC and from a 0.75 MAC to 1.0 MAC also had a comparable effect on the extent of activation on the positive and the negative BOLD response. These observations concur with our view that different mechanisms influenced the BOLD response at the 2 increases in sevoflurane level but that the same mechanism acted on the positive and the negative BOLD response.
The Influence of Sevoflurane on the BOLD Response
Our discussion of the influence of sevoflurane on the BOLD response will be based on the “standard” model.12 This model links the vascular response to the spiking activity and the oxygen consumption to the number of neurons activated. Increasing the concentration of sevoflurane will be assumed to attenuate the spiking activity more strongly than the number of neurons activated. We will briefly summarize the expected effect of sevoflurane on the neuronal response. In animal studies, sevoflurane has a minimal effect on spiking activity at concentrations of 0.5 MAC or lower.22 At concentrations >1.0 MAC, it suppresses the spiking activity in a dose-dependent manner.23–25 Sevoflurane does not influence the baseline cerebral blood flow26,27 or the blood flow velocity.28 In the peripheral vascular system, sevoflurane has been demonstrated to reduce vascular resistance and so lead to an increase in aortic blood flow.29 We interpret these findings to indicate that, at low concentrations, an increase in the concentration of sevoflurane modifies the BOLD response through its vasodilative property. At higher concentrations, it is the anesthetic property of sevoflurane that modifies the BOLD response.
Positive BOLD Response.
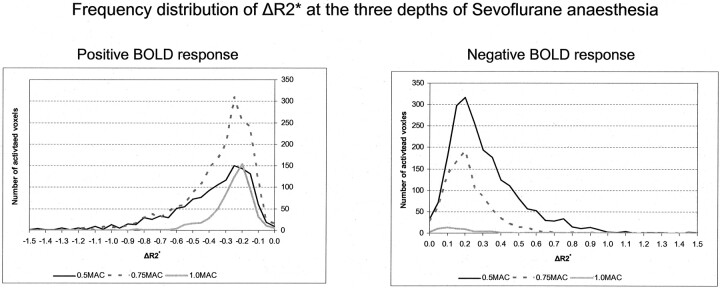
The peak ΔR2* at a sevoflurane concentration of 0.5 MAC is identical to that obtained in the awake state.11 Our analysis revealed that increasing the sevoflurane concentration from 0.5 MAC to 0.75 MAC attenuated the peak ΔR2* only slightly, while at the same time the extent of activation increased. The property of sevoflurane to augment the vascular response makes this paradoxical reaction understandable. The ability of sevoflurane to augment the vascular response not only reduces the local HHb concentration despite a decline in the spiking activity, but also increases the difference in the HHb concentration between the activated and the rest condition (ΔR2*), making it easier to detect activated voxels by means of statistical methods. The left panel of Fig 3 shows that the number of voxels with lower HHb concentration (ie, more negative ΔR2*) is larger at 0.75 MAC than at 0.5 MAC or 1.0 MAC.
Fig 3.
The 2 panels show the frequency distribution of the peak ΔR2* at the 3 levels of sevoflurane anesthesia. Left, the frequency distribution for the positive BOLD response. Right, the frequency distribution of the negative BOLD response. We selected to show the absolute values for each bin, rather than the relative values, to convey to the reader the difference in the number of activated voxels upon which the distribution is based.
Increasing the concentration of sevoflurane from 0.75 MAC to 1.0 MAC had a strong effect on the peak ΔR2* and strong effect on the extent of activation. Now however, both the extent of activation and the peak ΔR2* declined. The reduction in the peak ΔR2* indicates that the spiking activity was reduced, leading to a reduction in the vascular response and a rise in the local HHb concentration. Although sevoflurane at the higher concentration would still have augmented the vascular response, the reduction in the spiking activity would provide fewer regions for this to have a noticeable effect. This manifests itself in a reduction of the extent of activation and a decline in the number of voxels with a lower HHb concentration (ie, more negative ΔR2*).
Negative BOLD Response.
The reduction in the extent of activation observed with the negative BOLD response can also be accounted for by an augmentation of the vascular response by sevoflurane. A negative BOLD signal intensity indicates that the HHb concentration during stimulation rose above that of the rest condition. By augmenting the vascular response, sevoflurane reduced the local HHb concentration during activation. This reduced the difference in the local HHb concentration between the activated and the rest condition (ΔR2*), making it more difficult to detect activated voxels by statistical methods. The right-hand panel of Fig 3 shows that the number of voxels with a high HHb concentration (ie, more positive ΔR2*) declined when the sevoflurane concentration increased from 0.5 MAC to 0.75 MAC.
The increase in the sevoflurane concentration from 0.75 MAC to 1.0 MAC had a stronger effect on the extent of activation than the increase from 0.5 MAC to 0.75 MAC. At a concentration ≥1.0 MAC, sevoflurane suppressed the spiking activity in a dose-dependent manner, which precludes any vascular response. Figure 2D shows that there was no change in the peak ΔR2* when the sevoflurane concentration increased from 0.75 MAC to 1.0 MAC. Figure 3, shows that the ΔR2* values of the few voxels that were found were close to 0 and therefore represent the limit of the BOLD response that can be detected by using statistical methods.
By considering the vasodilative and the anesthetic property of sevoflurane we were able to account for the effect of increasing the sevoflurane concentration on the extent of activation and the peak ΔR2*. The same properties can also explain the change in the extent of activation and the ΔR2* of both the positive and the negative BOLD response.
Implications of the Use of Sevoflurane during Functional Measurements
The unexpected result of our investigation demonstrated that BOLD-based, functional imaging cannot only be performed with an anesthetized subject, but, contrary to expectations, anesthesia may even enhance the detection of activated brain regions when using BOLD–based functional imaging. Our study demonstrated that administering sevoflurane at a concentration <1.0 MAC may make it easier to detect activated brain regions during BOLD-based functional imaging, because at lower concentrations its vasodilative effect exceeds its anesthetic effect.
At a concentration of 0.5 MAC, sevoflurane diminishes the extent of activation, but the BOLD signal intensity amplitude is comparable with that of a waking subject.11 At a concentration of 0.75 MAC sevoflurane, the BOLD signal intensity amplitude is significantly lower than that of a waking subject but the extent of activation increases compared with a concentration of 0.5 MAC. At a concentration of 1.0 MAC, both BOLD signal intensity amplitude and extent of activation decline.
In summary, we found that, at low concentrations, sevoflurane influences the extent of activation and the ΔR2* through its vasodilative property. At higher concentrations, the extent of activation and the ΔR2* are influenced by the anesthetic property of sevoflurane. This was true for the positive as well as the negative BOLD response. In line with others,30 we find evidence against the “steal” effect,17 because the same mechanisms could be invoked to account for the influence of sevoflurane on the extent of activation and the ΔR2*.
The results of this study lead us to reject the null hypothesis in favor of the alternative hypothesis and, consequently, support for the “standard model.” The fact that the extent of activation and the BOLD signal intensity amplitude were affected in disparate manners by an change in sevoflurane concentration concurs with the view voiced by others, that the extent of activation (ie, number of activated voxels) is not a reliable measure of neuronal activation.21,31,32
Finally, where functional imaging needs to be performed under anesthesia, sevoflurane represents an ideal choice of an anesthetic agent, because its vasodilative property can be used in a manner that is advantageous for the detection of activated brain regions.
Conclusions
If the aim of performing BOLD-based fMRI measurements on a child under sevoflurane anesthesia is to compare the results with older children, the concentration of sevoflurane should not be >0.5 MAC. If the aim of the BOLD-based fMRI measurement is to demonstrate neuronal activity, a concentration of sevoflurane of 0.75 MAC is the most appropriate, because, at low concentrations, sevoflurane actually enhances the BOLD signal intensity, making it more likely that brain activation is detected. Careful management of the anesthetic agent sevoflurane can therefore be used advantageously during functional MR imaging of brain activity in children.
Footnotes
The authors were supported by a grant from the Swiss National Fonds (SNF: 31–59363.99) to V.L.M. and by the National Centre for Competence in Research on neural plasticity and repair.
This work was presented as an abstract to the annual conference of the Human Brain Mapping Society, Brighton, June 10–14, 2001.
References
- 1.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 1993;64:803–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin E, Joeri P, Loenneker D, et al. Visual processing in infants and children studied using functional MRI. Pediatr Res 1999;46:135–40 [DOI] [PubMed] [Google Scholar]
- 3.Martin E, Thiel T, Joeri P, et al. Effect of pentobarbital on visual processing in man. Hum Brain Mapp 2000;10:132–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eger EI 2nd, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology 1965;26:756–63 [DOI] [PubMed] [Google Scholar]
- 5.deJong R, Eger EI. AD50 and AD95 values of common inhalation anaesthetics on man. Anesthesiology 1975;42:384–89 [PubMed] [Google Scholar]
- 6.Stevens WD, Dolan WM, Gibbons RT, et al. Minimum alveolar concentrations (MAC) of isoflurande with and without nitrous oxide in patients of various ages. Anesthesiology 1975;42:197–200 [DOI] [PubMed] [Google Scholar]
- 7.Born AP, Miranda MJ, Rostrup E, et al. Functional magnetic resonance imaging of the normal and abnormal visual system in early life. Neuropediatrics 2000;31:24–32 [DOI] [PubMed] [Google Scholar]
- 8.Born P, Rostrup E, Leth H, et al. Change of visually induced cortical activation patterns during development. Lancet 1996;347:543. [DOI] [PubMed] [Google Scholar]
- 9.Yamada H, Sadato, N, Konishi, Y, et al. A rapid brain metabolic change in infants detected by fMRI. Neuroreport 1997;8:3775–78 [DOI] [PubMed] [Google Scholar]
- 10.Marcar VL, Loenneker T, Schwarz U, et al. The influence of cortical maturation on the BOLD response: an fMRI study of visual cortex in children. Pediatr Res 2004;56:967–74 [DOI] [PubMed] [Google Scholar]
- 11.Marcar VL, Loenneker T, Strassle AE, et al. What effect does measuring children under anaesthesia have on the blood oxygenation level–dependent signal? A functional magnetic resonance imaging study of visual cortex. Pediatr Res 2004;56:104–110 [DOI] [PubMed] [Google Scholar]
- 12.Marcar VL, Loenneker T. The BOLD response: a new look at an old riddle. Neuroreport 2004;15:1–4 [DOI] [PubMed] [Google Scholar]
- 13.Bandettini PA, Kwong KK, Davis TL, et al. Characterization of cerebral blood oxygenation and flow changes during prolonged brain activation. Hum Brain Mapp 1997;5:93–109 [PubMed] [Google Scholar]
- 14.Kim SG, Rostrup E, Larsson HBW, et al. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med 1999;41:1152–61 [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press;1969
- 16.Harel N, Lee SP, Nagaoka T, et al. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab 2002;22:908–17 [DOI] [PubMed] [Google Scholar]
- 17.Shmuel A, Yacoub E, Pfueffer J, et al. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 2002;36:1195–210 [DOI] [PubMed] [Google Scholar]
- 18.Heinke W, Schwarzbauer C. Subanesthetic isoflurane affects task-induced brain activation in a highly specific manner: a functional magnetic resonance imaging study. Anesthesiology 2001;94:973–81 [DOI] [PubMed] [Google Scholar]
- 19.Janz C. Comparison of the hemodynamic response to different visual stimuli in single-event and block stimulation fMRI experiments. J Magn Reson Imaging 2000;12:708–14 [DOI] [PubMed] [Google Scholar]
- 20.Hagenbeek RE, Rambouts SARB, Van Dijk BW, et al. Determination of individual stimulus-response curves in visual cortex. Hum Brain Mapp 2002;17:244–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcar VL, Straessle A, Girard F, et al. When more means less: a paradox BOLD response in human visual cortex. Magn Reson Imaging 2004;22:441–50 [DOI] [PubMed] [Google Scholar]
- 22.Watts AD, Herrick IA, Shah A, et al. The effect of sevoflurane and isoflurane anesthesia on interictal spike activity among patients with refractory epilepsy. Anesth Analg 1999;89:1275–81 [PubMed] [Google Scholar]
- 23.Osawa M, Shingu K, Murakawa M, et al. Effects of sevoflurane on central nervous system electrical activity in cats. Anesth Analg 1994;79:52–57 [DOI] [PubMed] [Google Scholar]
- 24.Stucke AG, Stuth EA, Tonkovic-Capin V, et al. Effects of sevoflurane on excitatory neurotransmission to medullary expiratory neurons and on phrenic nerve activity in a decerebrate dog model. Anesthesiology 2001;95:485–91 [DOI] [PubMed] [Google Scholar]
- 25.Stucke AG, Stuth EA, Tonkovic-Capin V, et al. Effects of halothane and sevoflurane on inhibitory neurotransmission to medullary expiratory neurons in a decerebrate dog model. Anesthesiology 2002;96:955–62 [DOI] [PubMed] [Google Scholar]
- 26.Scheller MS, Nakakimura K, Fleischer JE, et al. Cerebral effects of sevoflurane in the dog: comparison with isoflurane and enflurane. Br J Anaesth 1990;65:388–92 [DOI] [PubMed] [Google Scholar]
- 27.Scheller MS, Tateishi A, Drummond JC, et al. The effects of sevoflurane on cerebral blood flow, cerebral metabolic rate for oxygen, intracranial pressure, and the electroencephalogram are similar to those of isoflurane in the rabbit. Anesthesiology 1988;68:548–51 [DOI] [PubMed] [Google Scholar]
- 28.Fairgrieve R, Rowney DA, Karsli C, et al. The effect of sevoflurane on cerebral blood flow velocity in children. Acta Anaesthesiol Scand 2003;47:1226–30 [DOI] [PubMed] [Google Scholar]
- 29.Larousse E, Asehnoune K, Dartayet B, et al. The hemodynamic effects of pediatric caudal anesthesia assessed by esophageal Doppler. Anesth Analg 2002;94:1165–68 [DOI] [PubMed] [Google Scholar]
- 30.Smith AT, Williams AL, Singh KD. Negative BOLD in the visual cortex: evidence against blood stealing. Hum Brain Mapp 2004;21:213–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcar VL, Loenneker T, Straessle A, et al. What the little differences between men and women tells us about the BOLD response. Magn Reson Imaging 2004;22:913–19 [DOI] [PubMed] [Google Scholar]
- 32.McGonigle DJ. Variability in fMRI: an examination of intersession differences. Neuroimage 2000;11:708–34 [DOI] [PubMed] [Google Scholar]