Abstract
Cholangiocarcinoma (CCA) is an aggressive and multifactorial malignancy of the biliary tract. The carcinogenesis of CCA is associated with genomic and epigenetic abnormalities, as well as environmental effects. However, early clinical diagnosis and reliable treatment strategies of CCA remain unsatisfactory. Multiple compartments of the tumor microenvironment significantly affect the progression of CCA. Tumor-associated macrophages (TAMs) are a type of plastic immune cells that are recruited and activated in the CCA microenvironment, especially at the tumor invasive front and perivascular sites. TAMs create a favorable environment that benefits CCA growth by closely interacting with CCA cells and other stromal cells via releasing multiple protumor factors. In addition, TAMs exert immunosuppressive and antichemotherapeutic effects, thus intensifying the malignancy. Targeting TAMs may provide an improved understanding of, and novel therapeutic approaches for, CCA. This review focuses on revealing the interplay between TAMs and CCA.
Keywords: Tumor-associated macrophages, Cholangiocarcinoma, Tumor microenvironment, Tumor-promotion, Immunosuppression, Targeted therapy
Abbreviations: CCA, cholangiocarcinoma; TAMs, tumor-associated macrophages; iCCA, intrahepatic cholangiocarcinoma; pCCA, perihilar cholangiocarcinoma; dCCA, distal cholangiocarcinoma; eCCA, extrahepatic cholangiocarcinoma; TME, tumor microenvironment; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; IL, interleukin; MCP-1, monocyte chemotactic protein-1; CSF-1, colony-stimulating factor-1; VEGF-A, vascular endothelial growth factor-A; CSCs, cancer stem cells; EMT, epithelial-mesenchymal transition; CAFs, cancer-associated fibroblasts; TEMs, Tie2-expressing monocytes; PD-L1, programmed cell death ligand-1; PD-1, programmed cell death protein-1; CTL, cytotoxic lymphocyte; G-MDSCs, granulocytic-myeloid-derived suppressor cells; CD47, cluster of differentiation 47; SIRPα, signal regulatory protein α
1. Introduction
Cholangiocarcinoma (CCA) is a type of heterogeneous malignancy that occurs in the biliary tract, which may originate from different cell types, including hepatic stem cells, immature neural cell adhesion molecule positive (NCAM+) cholangiocytes, mature NCAM− interlobular cholangiocytes, peribiliary gland cells and hepatocytes [1]. Anatomically, CCA is classified into intrahepatic (iCCA; 10–20%), perihilar (pCCA; 50–60%) and distal (dCCA; 20–30%) CCA, whereby the latter two are collectively known as extrahepatic CCA (eCCA) [2], [3], [4]. Other characteristics such as cancer growth patterns (mass-forming, periductal infiltrating or intraductal), histology (mixed or mucinous) and tumor microenvironment (TME)-based subtypes (immune desert, immunogenic, myeloid or mesenchymal) can also be used for classification [5], [6], [7], [8]. The etiology of CCA remains obscure, while several conditions related to genetic mutations, epigenetic alternation and environmental factors have been identified to be involved in the pathogenesis of CCA. A variety of high-risk pathological states may drive the carcinogenesis of CCA, including cholestatic liver diseases (primary sclerosing cholangitis, fibropolycystic liver diseases and congenital hepatic fibrosis), infectious diseases (flukes and hepatitis B and C), biliary stone diseases (cholecystolithiasis and hepatolithiasis), metabolic disorders [diabetes, obesity and non-alcoholic fatty liver disease (NAFLD)] as well as drugs, toxins or chemicals (alcohol, smoking and thorotrast). Most pathological changes ultimately lead to chronic inflammation and cholestasis of the intrahepatic or extrahepatic bile duct, which possess a serious risk of transforming into premalignant lesions and even invasive cancer [3,4,9]. Although CCA is a rare type of cancer, it ranks the second-most common primary liver malignancy after hepatocellular carcinoma (HCC). Currently, Thailand, China and South Korea have the highest incidence of CCA (>6 cases per 100,000) worldwide, with continued increasing incidence and mortality rates [3]. Due to the lack of obvious early symptoms and reliable prognostic markers, patients are frequently diagnosed at an advanced stage. Compromised surgery and unsatisfactory chemotherapies offer dismal results and often along with a high recurrence rate. Hence, more effective treatment methods are urgently needed [10].
Tumor-associated macrophages (TAMs), a group of plastic immune cells that are infiltrated and activated at tumor sites, possess potent protumor and immunosuppressive properties [11,12]. Macrophages can integrate numerous environmental signals and shape their polarization manners accordingly. Historically, macrophages are polarized into two distinct states: classically activated (M1 type) and alternatively activated (M2 type) macrophages. M1 macrophages are activated by lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), and exhibit pro-inflammatory and antitumor properties. M2 macrophages are mainly stimulated by interleukin (IL)-4 or IL-13, and facilitate tumor-promoting effects. M1 macrophages are characterized by the surface marker cluster of differentiation (CD) 86, CD80, while M2 feature CD206 and CD163 [13], [14], [15]. However, macrophages polarization is a dynamic and fuzzy process, new definition and classification is needed for current investigation of TAMs. It is difficult to specifically define TAMs, since this M1/M2 division is too simplistic to comprise all heterogenous macrophages [16], [17], [18]. It is widely accepted that TAMs prefer the M2 phenotype, which are mainly driven from circulating monocytes induced by monocyte chemotactic protein-1 (MCP-1/CCL2), colony-stimulating factor-1 (CSF-1), vascular endothelial growth factor (VEGF)-A and other cytokines such as IL-1β, IL-10, IL-13 and IL-4 released by tumor and stromal cells [11,19,20]. TAMs participate in the progression of a number of malignancies including breast cancer, HCC and CCA [21], [22], [23].
The current understanding of CCA initiation and progression and the role of the TME can provide a fundamental description of CCA. Targeting the compartments of TME and the tumor itself together may provide ideal approaches in clarifying and treating CCA. This review summarizes the current knowledge of the interaction between TAMs and CCA.
2. CCA recruits and shapes macrophages to a protumor type
Tumor cells have the ability to release different cytokines and other factors to recruit and shape the pericellular environment to a protumor profile. A variety of cells such as fibroblasts [recruited and activated by platelet-derived growth factor (PDGF)-D], neutrophils [recruited and activated by C-X-C motif chemokine ligand (CXCL) 5), natural killer cells (deceived by MHC class I antigens) and tumor infiltrating lymphocytes (TILs) (dampened by immune checkpoint protein) could be shaped to a protumor phenotype by CCA cells [24], [25], [26], [27]. Apart from above these, macrophages are also the primary targets of CCA. TAMs mainly derive from circulating monocytes instead of resident Kupffer cells, which are one of the most abundant infiltrated and activated stromal cells [19,28]. They are guided to the tumor regions with the help of CCA cells and other components of the TME by releasing multiple factors, including MCP-1/CCL2, CSF-1 and VEGF-A [10,12,29,30]. Dwyer et al [30] revealed that TNF-like weak inducer of apoptosis (TWEAK)/fibroblast growth factor-inducible 14 (Fn14) is upregulated in CCA and can orchestrate the tumor niche by inducing MCP-1, CX3CL1, IL-6, IL-8, macrophage colony stimulating factor (M-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF) secretion in a NF-κB-mediated way. Of which, MCP-1 facilitate macrophages recruitment and correlated with increased CD206+ TAMs marker and worse tumor behavior. After infiltrating the tumor regions, macrophages deviate to a protumor type despite their original function.
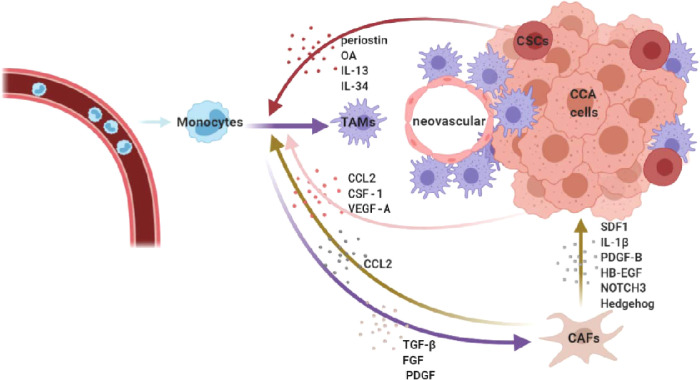
Cancer stem cells (CSCs) are a small subgroup of cancer cells with self-renewal ability, which are the leading force of tumor initiation, metastasis, recurrence and drug resistance [31]. In iCCA, the colocalization of CD163+ TAMs and CSC-related markers (CD44 or EPCAM) in the tumor front supported the potential association between infiltrating TAMs and the CSC-niche [17]. One of the most important ECM components, periostin [secreted by CSCs and cancer-associated fibroblasts (CAFs)], displays a potent ability to attract CD206+ TAMs [32], [33], [34]. Moreover, in a 3D CSC culture model, CCA sphere-conditioned medium acted as a strong monocytes attractor by releasing different stem-like secretomes, including IL-13, osteoactivin (OA) and IL-34, resulting in a mixed M1-M2 macrophage phenotype. It is worth noting that blood specimens of patients with CCA also showed elevated levels of IL-13, OA and IL-34, which are associated with stem-like genes. CSC-associated macrophages possess distinctive molecular features related to matrix remodeling and showed greater adhesion ability and better invasion capacity (Fig. 1) [17].
Fig. 1.
CCA recruits and shapes macrophages to a protumor type. TAMs are recruited from circulating monocytes and polarized to a protumor type by multiple factors released by CCA cells, CSCs, and CAFs. And they tend to gather in the invasive front and perivascular regions.
The aforementioned mechanisms result in a huge influx of TAMs, which is often correlated with worse tumor behavior, high metastasis and recurrence rates as well as poor prognosis. Interestingly, in CCA, TAMs tend to gather in the tumor invasive front and perivascular sites, implying that they may be a potential neovascular and tumor metastasis promoter (Table 1) [13,16,32,[35], [36], [37], [38], [39], [40]]. However, conflict results exist in the link between TAMs and tumor behavior, some studies demonstrated worse outcomes, while some found the opposite [41]. Meanwhile, it is worth noting that macrophages contribute scarcely to the most prevalent (47.3%) type of eCCA (mesenchymal class) [8]. More precisely evaluation and subgroup analysis based on tumor staging and CCA type may better explain the relationship between TAMs and CCA.
Table 1.
TAMs infiltrate in CCA sites and are correlated with prognosis.
| CCA type | Sample number | TAMs location | Marker | Prognostic value | References |
|---|---|---|---|---|---|
| CCA | 44 | Leading edge of tumor tissues and perivascular areas | MAC387 CD14 CD16 |
An expansion of the CD14+CD16+ monocyte in peripheral blood of CCA have tumor-promoting characteristics and was associated with tumor origin, high density of MAC387-positive TAMs and poor prognosis of patients | [35] |
| CCA | 43 | Tumor tissue | CD68 CD163 |
A high density of the M2-TAMs CCA in patients is significantly associated with the presence of extrahepatic metastases | [38] |
| CCA | 50 | Leading edge and perivascular areas of tumors | MAC387 | High densities of MAC387-positive cells were detected in more than 60% of the CCA tissues, and was significantly associated with poor prognosis parameters | [40] |
| CCA |
23 | Tumor front |
CD163 | CD163 progressively increased along with tumor grade (G2/G3 > G1) and was significantly associated with CCA pathological grade as well as CA19-9 serum marker | [17] |
| iCCA | 322 (tissue microarrays) | Tumor tissue | CD68 CD86 CD206 |
High CD86+ and low CD206+ TAMs infiltration was significantly correlated with certain favorable tumor clinicopathologic features and better prognosis in iCCA patients, when compared to low CD86+ and high CD206+ TAMs infiltration. CD86+/CD206+ TAMs model was an independent prognostic indicator for iCCA, especially in CA19-9 negative patients | [13] |
| iCCA | 39 | Tumor tissue | CD68 CD163 |
High counts of CD163+ macrophages showed poor disease-free survival | [39] |
| iCCA | 88 | Tumor invasive front | CD68 | high levels of TAMs in tumor invasive front or absence of histologic tumor necrosis are associated with a significantly improved recurrence-free and overall survival of patients with iCCA | [41] |
| hCCA | 47 | Tumor and tumor invasive front | Tie2 | TEMs in tumor and tumor invasive front correlated with increased survival and lower tumor recurrence | [16] |
| hCCA | 47 | Tumor invasive front | CD68 | Patients with high density of TAMs in tumor invasive front showed significantly higher local and overall tumor recurrence, decreased overall and recurrence-free survival | [37] |
| eCCA | 101 | Tumor tissue | CD163 | High number of tumor infiltrating CD163+ macrophages were significantly associated with poorly-differentiated histology and nodal metastasis | [36] |
3. The protumor effects of TAMs
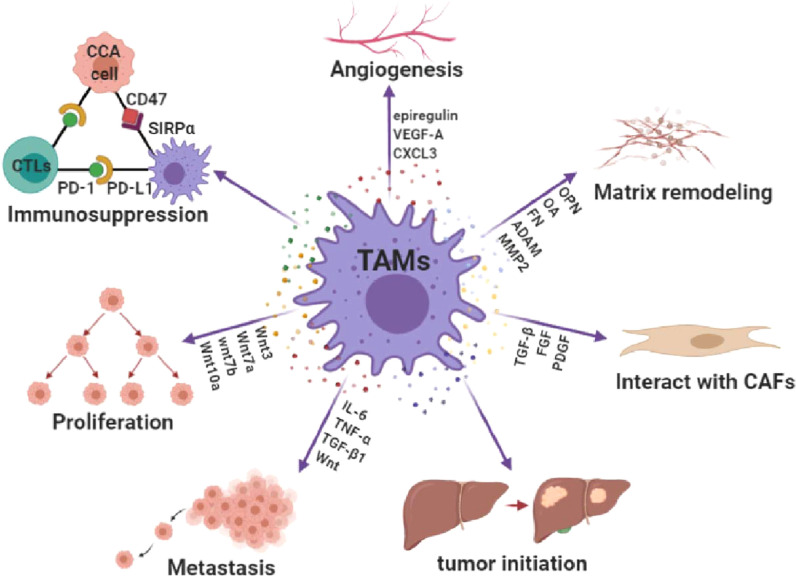
Following recruitment to CCA sites, TAMs participate in various processes of CCA progression, including tumor cell proliferation, metastasis and angiogenesis (Fig. 2).
Fig. 2.
Overview of the protumor effects of TAMs. TAMs promote CCA progression by participating in tumor initiation, proliferation, metastasis, angiogenesis, CAFs activation, matrix remodeling as well as immunosuppression.
3.1. Macrophages promote CCA initiation
In studies investigating the carcinogenesis of CCA, researchers revealed that macrophages could undergo multiple pretumor disease processes and accelerate cancer formation. Clonorchis sinensis (C. sinensis) infection is a major public health problem, especially in East Asian countries, and is closely associated with CCA formation [42]. In mouse C. sinensis infection models, macrophages showed polarization changes during different stages of infection. They tend to polarize to the M1 phenotype at an early stage, while dynamically shifting to the M2 phenotype during the late stage, especially at the fibrotic and cirrhotic stages of infection, contributing to fibrosis and the remodeling of bile ducts by direct contact with C. sinensis excretory-secretory products [43]. Similarly, the excretory-secretory product of the liver fluke Fasciola hepatica purified protein Fh12 is capable of modulating macrophages to an alternatively activated phenotype and up-regulates the expression of arginase-1 (Arg-1) and chitinase-3-like protein gene (CHI3L1), thereby creating an immunosuppressive environment that benefits CCA initiation [43,44]. Moreover, macrophages in liver steatosis may activate the Wnt signaling pathway, which maintains the survival and growth of tumor-initiating cells, and further promotes the carcinogenesis of CCA [45]. Mitochondrial dysfunction and reactive oxygen species (ROS) activation are common features of liver diseases [46]. Oxidative stress exists in the TME of both mouse iCCA models and human samples. By mimicking this hepatic mitochondrial dysfunction, heat shock protein family D (Hsp60) member 1 (Hspd1) deletion mice are used to create mitochondrial defect models and premalignant cholangiocellular lesions. Mitochondrial dysfunction triggers ROS accumulation and leads to the focal infiltration of TNF-producing macrophages, which creates a favorable environment for biliary proliferation via c-JunN-terminalkinase (JNK) signaling. Targeting the ROS/TNF/JNK axis or depletion of macrophages may provide promising therapeutic strategies for iCCA [47]. Furthermore, other CCA initiating diseases, such as congenital hepatic fibrosis caused by polycystic kidney and hepatic disease 1 (PKHD1) gene mutation and toxin-derived DNA damage also manifest macrophage participation [48,49]. Taken together, macrophages tend to facilitate CCA initiation in the late stages of precancerous lesions, and as the disease progresses, M2 macrophages gradually take predominant control and manufacture a tumor-promoting environment.
3.2. TAMs are associated with Wnt/β-catenin signaling
Canonical Wnt signaling is a complex core node that connects with numerous other signaling cascades and is often involved in multiple carcinomas, such as colorectal cancer, pancreatic cancer and lung adenocarcinoma. [50], [51], [52]. Activation of the Wnt/β-catenin pathway participates in the initiation, progression, epithelial-mesenchymal transition (EMT) and multidrug resistance of CCA via interaction with microRNAs, PI3K/AKT/PTEN/GSK-3β, retinoic acid receptors (RARs), dickkopf-1 (DKK1), protein kinase A regulatory subunit 1 α (RKAR1A) pathways, SRC-like adaptor protein (SLAP), liver kinase B1 (LKB1) and CXCR4 [50]. Wnt2, Wnt3a, Wnt5a, Wnt7b and Wnt10a are highly expressed in CCA compared with non-cancerous tissues [53], [54], [55]. The protumor effects of Wnt signaling are predominantly mediated by TAMs. Boulter et al [53] indicated that Wnt7b and Wnt10a are highly expressed in human CCA tissues as well as transgenic and thioacetamide-induced mice CCA models. Overexpression of Wnt7b and Wnt10a is accompanied by activating a series of genes that are relevant to cancer progression, including cell cycle, naive state and wound repair. Further experiments revealed that Wnt7b was expressed in the cytoplasm of CD68+ macrophages, which appears to have an M2 phenotype that simultaneously expresses CD206. The depletion or inhibition of macrophages results in a significant reduction of Wnt7b in animal models of CCA, thus reducing tumor burden and proliferation. Moreover, using phosphatase and tensin homolog deleted on chromosome ten (PTEN)-deletion or HCV/NS5A transgenic mice CCA models supplemented with a high-fat diet, Debebe et al found that Wnt signaling was induced in hepatosteatosis development and was essential for the expansion of tumor-initiating cells. Infiltrating CD68+ macrophages are a key source of steatosis-induced Wnt production. The depletion of macrophages reduced the expression of several Wnt ligands (Wnt3a, Wnt7a and Wnt10a) and resulted in smaller tumor nodules compared with the control group [45]. These studies indicated that TAMs assist CCA growth by producing Wnt ligands. Direct targeting of TAMs or modulation of Wnt signaling may be potential treatment strategies of CCA. Different types of treatments have been developed to target Wnt signaling in other malignancies, but these have little effects due to the complexity and multiplicity of Wnt pathways [51,56]. Therefore, focusing on Wnt-producing TAMs may provide a promising treatment strategy.
3.3. TAMs and CCA metastasis
EMT is an early metastasis event that enables tumor cells to gain invasive properties, thus causing them to widely spread [57]. Co-culture with M2-TAMs can enhance the EMT ability of iCCA cells, resulting in the enhancement of cell invasion and metastatic abilities via AKT3/PRAS40 phosphorylation [58]. This effect is also mediated by the secretion of a series of EMT-induced cytokines and chemokines, such as IL-6, TNF-α, transforming growth factor-1 (TGF-1) and Wnt, that may alter the microenvironment and promote EMT. This results in the downregulation of epithelial markers (E-cadherin and CK19) and increased expression of mesenchymal markers (S100A4, N-cadherin, vimentin, α-SMA, β-catenin and MMP9) [53,58,59]. This migration promotion effect can also be verified in Opisthorchis viverrini (OV)-induced CCA hamster models, where the conversion of macrophages and fibroblasts to TAMs and CAFs phenotype were observed, along with elevated metastatic potential [38]. To summarize, these studies indicated that TAMs play a vital role in CCA metastasis. The inhibition of such effects of macrophages may be a potential approach to suppress tumor metastasis.
3.4. TAMs promote angiogenesis
Neovascularization is a key step in benign-to-malignant transition. It maintains the nutrition of CCA tissues and further promotes CCA metastasis [60]. TAMs infiltrate the perivascular sites, and higher TAMs levels are associated with elevated levels of angiogenic factor-related genes such as epiregulin, VEGF-A and CXCL3 [35,39]. Conflicting data exist regarding the exact phenotype of angiogenesis-promoting macrophages [61]. Furthermore, a special subset of Tie2-expressing monocytes (TEMs) has previously been shown to be associated with tumor angiogenesis [62]. In patients with CCA, circulating CD14+CD16+ monocytes express Tie2, and high levels of growth and angiogenic factor-related genes (epiregulin, VEGF-A and CXCL3). At the same time, a high density of newly recruited MAC387+ macrophages were found in the leading edge of the tumor, especially within perivascular areas [35]. The later study further confirmed that TEMs and angiopoietins showed homogenous distribution in tumor-infiltrating fronts and perivascular areas, indicating that TEMs manifest angiogenic properties, of which its particular mechanism still requires further investigation [16]. It is worth noting that macrophages have also been show to induce lymphangiogenesis. Vascular endothelial growth factor receptor-3 (VEGFR3)-expressing macrophages serve as both the source and target of VEGF-C and VEGF-D. These two growth factors could not only upregulate reparative macrophage-related genes (mannose receptor and Fizz1), but also induce lymphangiogenic by activating VEGFR3 of lymphatic endothelial cells (LECs) [63,64]. Besides, podoplanin-expressing tumor-associated macrophages (PoEMs) have also been proved to attached to LECs thus stimulating lymphangiogenesis, and facilitate breast cancer cell lymphoinvasion [65]. However, the pro-lymphangiogenesis effects of macrophages have not been studied in cholangiocarcinoma, comprehensive view of distinctive effects on blood and lymphatic angiogenesis is worth being considered.
4. TAMs interact with CAFs and remodel the TME
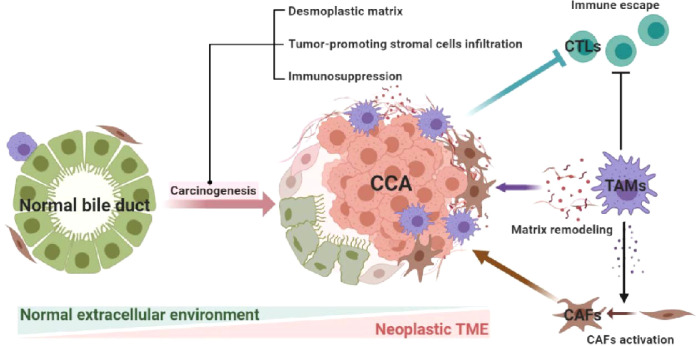
CCA, together with its environment, is a complete entity. TAMs help CCA to foster an environment that benefits tumor growth, which simultaneously requires close collaboration with other members of the TME. CAFs are a type of well-known stromal cell, which play an important role in CCA growth [66]. CAFs in iCCA could be divided to five subpopulations [vascular CAFs (vCAFs), matrix CAFs (mCAFs), inflammatory CAFs (iCAFs), antigen-presenting CAFs (apCAFs), EMT-like CAFs (eCAFs)] based on their diverse marker genes. Of which iCAFs is involved in immune modulation [67]. TAMs activate CAFs by releasing transforming growth factor-β (TGF-β), fibroblast growth factor (FGF) and PDGF [3,68]. CAFs can then secrete a number of signaling molecules such as stromal cell derived factor-1 (SDF1), IL-1β, PDGF-B, heparin-binding EGF-like growth factor (HB-EGF), Notch 3 and Hedgehog signals that promote the proliferation, metastasis and neovascularization of CCA [29,69,70]. Conversely, macrophage recruitment is also supported by CAFs-deriving factors such as periostin, IL-6, SDF1, M-CSF, CCL2, CHI3L1 [29,33,39]. Therefore, there exists a regulatory loop between CAFs and TAMs, whereby the two activate each other and amplify tumor-promoting effects.
Furthermore, TAMs may remodel the TME by releasing different extracellular matrix and adhesion molecules such as osteopontin (OPN), OA, fibronectin (FN), metalloproteinase ADAM (AD10, AD17) and matrix metalloproteinase 2 (MMP2) [17,33]. These components alter the CCA stroma and create a type of “desmoplastic matrix” of CCA, and are closely related to CCA metastasis and serve as promising prognostic indicators. Of which, OPN has been proven to be a reliable biomarker with a similar prognostic performance as existing biomarkers (CEA and CA19-9) for CCA. And could promote CCA metastasis in a mitogen-activated protein kinase 1 (MAPK1) and β-catenin dependent way (Fig. 3) [71,72].
Fig. 3.
TAMs interact with CAFs and remodel the TME. TAMs can activate CAFs, the two infiltrated to the CCA sites and exert tumor-promoting effects. TAMs could also suppress CTLs and release a sort of extracellular matrix thus creating neoplastic TME.
5. TAMs promote cancer immunosuppression
Immunotherapy is a promising strategy for future cancer treatment. The application of immune checkpoint blockade (ICB) which blocks the interactions between receptors and/or ligands, such as programmed cell death ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) involved in T-cell function, demonstrates potential clinical benefits in several tumors (including HCC, non-small-cell lung cancer and melanoma), while this has little effect in CCA [27,[73], [74], [75]]. The immunological environment varies in different tumor types. CCA may be classified into immune “hot” and “cold” types based on its cytotoxic lymphocyte (CTL) density. The immune “hot” type has a high density of CTLs and is associated with higher response rates to ICB, and vice versa [28,52]. Multiple immune escape mechanisms mediated by tumor and stroma cells weaken the effects of the antitumor defense in our body and immune targeted drugs. Hence, targeting TAMs warrant further investigation.
5.1. TAMs express PD-L1 and exert immunosuppressive effects
PD-L1 is a member of the B7 family molecules that are expressed on the surface of malignant cells and tumor-associated antigen-presenting cells. It facilitates immune evasion effects via its interaction with programmed cell death protein-1 (PD-1) on T cells [76]. Several cohort studies have investigated PD-L1 expression in CCA tissues. Due to the diversity of included sample sizes, cancer location, statistical cut-offs and different antibodies, the expression levels of PD-L1 showed high discrepancy between different studies. However, the consensus is that higher expression of PD-L1 is associated with worse tumor behavior, poor differentiation and prognosis. The percentage of PD-L1-positive expression in TAMs is much higher in CCA cells of both mice models and human tissues [77], [78], [79], [80], [81]. Higher PD-L1 expression in CCA cells is accompanied by abundant infiltration of tumor-associated neutrophils (TANs) and TAMs [79,82]. This indicated that TAMs may play a role in CCA immune escape by forming a barrier that dampen CTL attack through PD-1/PD-L1 interactions. Consequently, individualized ICB treatment should be applied to those who show a high PD-1/PD-L1 expression profile. Adaptive immune response components of TME present dynamic changes with CCA progression. And PD-L1 expression has been linked to an increase in apoptotic TILs. Single-cell RNA sequencing analysis (scRNA-seq) has shown advantages in revealing cellular diversity and intercellular interaction at single-cell resolution. It illuminates a comprehensive way to dissect the complex tumor and adaptive immune system landscapes, which would be of great help for our further exploration of CCA and TME immunity [83,84]. However, Loeuillard et al [80] found that inhibiting TAMs by CCR-2 deficiency or CSF-1R inhibition did not affect tumor burden, but instead promoted the compensatory infiltration of granulocytic-myeloid-derived suppressor cells (G-MDSCs), thus counteracting the potential antitumor effects of eliminating protumor macrophages in murine CCA models. These immunosuppressive elements, particularly TAMs and MDSCs, coordinate to foster an immune “cold” environment by suppressing CD8+ T cell infiltration, which facilitates a protumor effect. Besides, TAMs and MDSCs may also serve as guiding forces of cancer cell stemness which intensify resistance to anticancer treatments [85]. Therefore, eliminating existing TAMs together with a potential TAMs source (such as G-MDSCs) is necessary to potentiate anti-PD-1 therapy in CCA and has been proven to enhance the antitumor effects of anti-PD-1 therapy in CCA models. Besides, CD 40 on macrophages and dendritic cells (DCs) could drive the infiltration and activation of myeloid, CD4+ T, CD8+ T, and NK cells. Combination of CD40 agonist and anti-PD-1 therapy has shown significantly antitumor effect compared to control or either monotherapy groups [86]. Hence, a comprehensive understanding of the regulation network between macrophages and other immune components is needed when applying immunotherapy. However, it is still unknown as to why there is such a discrepancy in the PD-L1 expression profile. We speculate that a balance between cancer and immunity exists in CCA progression and this immunosuppressive trait can be inherited during proliferation of CCA cells.
5.2. Other immune escape mechanisms
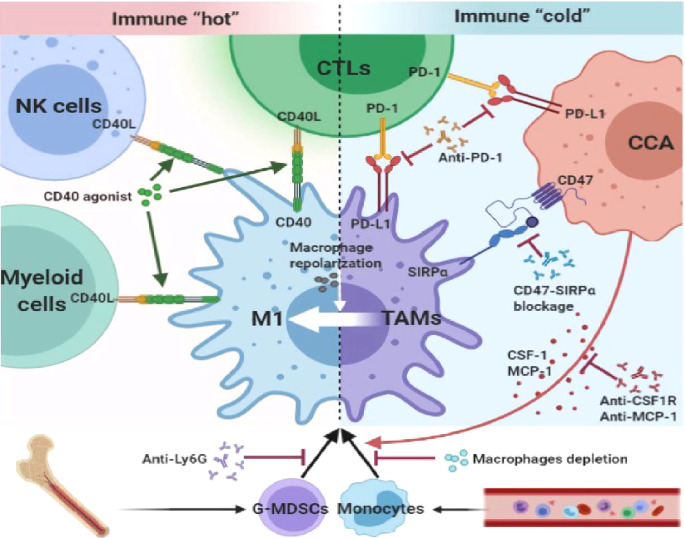
Cluster of differentiation 47 (CD47) is an antiphagocytic molecule that discriminates between host cells and damaged or foreign cells. It is a transmembrane glycoprotein that is ubiquitously expressed on the surfaces of various cell types. CD47 interacts with transmembrane protein signal regulatory protein α (SIRPα) on the surface of myeloid cells, such as macrophages and DCs [87]. CCA cells express higher CD47 than HCC and serve as a “don't eat me signal”, thus deceiving macrophages by binding to macrophages SIRPα. The blockage of CD47-SIRPα interactions by anti-CD47 results in increased macrophage infiltration and potentiated macrophage phagocytosis, leading to decreased cancer colonization in mice models [88]. Besides, macrophages have complex regulation effects on other immune cells of the TME. Hypoxia conditions augmented macrophage-mediated T-cell suppression in breast cancer via iNOS and arginase 1 (Arg1) releasing in a HIF-1α depend manner, which could be a potential immune regulation point in further investigations of TAMs and immune escape [89]. Other cytokines released by TAMs can also exert immune escape in CCA TME [90]. Some researchers showed that IL-10 released by TAMs can suppressing the activity of antigen presenting cell (APCs), DCs, cytotoxic T-cell and CD8+ T-cells [91]. TAMs-derived TGF-β contributed to exclusion of CD8+ T-cells and blocked the acquisition of the Th1 effector phenotype [92]. Thus, TAMs have potential and powerful immune suppression ability via interacting with other immune subtypes, which should be taken seriously in CCA immunomodulation. (Fig. 4).
Fig. 4.
TAMs exert an immune regulation effect and can be pharmacologically targeted. TAMs and CCA cells express PD-L1 which interact with PD-1 on CTLs thus dampen the anti-tumor immunity. The CD47-SIRPα interaction of TAMs and CCA can also help CCA to escape phagocytosis. Combination of TAMs reprogramming with immunotherapies may have a cooperative effect in CCA treatment.
6. Targeting TAMs in CCA treatment
The high heterogeneity CCA refers to its location and genetic mutation. Due to its complex classification and etiology, treatment cannot be generalized. In addition to palliative surgery, conventional chemotherapy is often accompanied with unpredictable results. Combination immunotherapy strategies have been launched in order to get positive treatment responses. By analyzing registered clinical trials on CCA, anti-PD-1 drugs plus anti-CTLA-4, ATR inhibitor, CSF-1 inhibitor, histone deacetylase inhibitor or vascular endothelial growth factor receptor 2 (VEGFR-2) inhibitor are ongoing (NCT04238637, NCT04298008, NCT04642664, NCT04301778, NCT03250273). Combination two kinds of ICB nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) in phase II clinical trial patients with advanced biliary tract cancers showed improved clinical outcomes [93]. Moreover, PD-1 monoclonal antibody camrelizumab in combination with VEGFR-2 inhibitor apatinib can also achieve controllable safety and good efficacy in primary liver cancer based on multicohort phase Ib/II trial [94]. As the revealing of protumor effects of TME components, especially, stable elements, such as TAMs, are comparative invariants and are regular visitors during cancer formation, which can thus be targeted.
Given the importance of macrophages in carcinogenesis and CCA promotion, corresponding methods have aimed at TAMs recruitment, inhibition, depletion or repolarization [95]. The aforementioned strategies include the intervention of toll-like receptor (TLR) agonists, a series of cytokines, antibodies, RNAs and other small molecules. And with the help of nanoscale drug carriers, targeting TAMs have shown promising effects on non-small cell lung cancer, breast, prostate and pancreatic cancer xenograft models [96], [97], [98], [99], [100], [101], [102], [103], [104]. In CCA mouse models, inhibiting CSFR1 activation prevented monocytes differentiation into macrophages and depleting macrophages in xenograft mice models reduced CCA cell proliferation and accelerated cell apoptosis, which relieved tumor burden in a Wnt7b-dependent manner [53]. Except CSFR1, MCP-1 is also a strong TAMs activator. By using multiple injections of anti-MCP-1 antibody, significantly smaller tumors were observed in SNU-1079-generated human CCA cell xenografts mice. TWEAK/Fn14 pathway is capable of inducing the releasing of MCP-1, CX3CL1, IL-6, IL-8, M-CSF and GM-CSF in a NF-kB dependent way, blocking the TWEAK/Fn14 pathway of CCA cells and CAFs resulted in a reduction of MCP-1 expression, thus reduced TAMs recruitment and CCA xenograft growth [30]. However, the aforementioned methods could also influence M1 macrophages, which have a positive effect [105]. Thereby, the pros and cons should be further weighed. Another strategy focusing on macrophage repolarization, which induces M2 macrophages to a pro-inflammatory phenotype, may exert an antitumor effect. Gao and colleagues provided a macrophage repolarization example by infusion of methotrexate-containing plasma-membrane microvesicles derived from apoptotic human tumor cells into the bile-duct lumen, which led to the abundant recruitment of antitumor neutrophils and the pyroptosis of eCCA cells, thereby relieving biliary obstruction in 25% of the patients. Treating macrophages with supernatants from pyroptotic CCA cells induced macrophages to produce proinflammatory cytokines IL-6 and CCL2, as well as neutrophil and T cell attractants including TNF, IL-1β, CCL3, CCL20 and CXCL5, triggering a secondary wave of neutrophil and naive T cell migration to the bile duct. In addition, TAMs could change their polarization states in response to eCCA pyroptosis and further promote TME transition to a proinflammatory state [106]. Besides, as mentioned above, TAMs showed a complex interaction with other immune cells, combination of TAMs reprogramming with other immunotherapies (such as ICB) may have a cooperative effect in treating malignancies, especially drug-resistant malignancies, such as CCA. However, these hypotheses are still in the experimental stage, with their relevant effects still remain unclear. Further studies are required to reveal the practicability of these strategies (Table 2).
Table 2.
Targeting TAMs in CCA treatment.
| Type of treatment | Target | Agent | Mechanisms | Experimental models | Major effects | References |
|---|---|---|---|---|---|---|
| TAMs Ablation | Macrophage depletion | Lipclod | Macrophages depletion | Xenograft model of human CCA cell lines, TAA-induced rat CCA model | Decrease CD68+ macrophages, reduced expression of Wnt7b and human pro-proliferation genes (BIRC5, CCND2, and CCNE), increased expression of apoptosis gene BAX1, increased apoptosis, less tumor burden | [53] |
| CSFR1 inhibition | W2580 or AZD7507 | Preventing macrophages recruitment and activation | Xenograft model of human CCA cell lines, TAA-induced rat CCA model | Decreased CD68+ and CD163+ macrophage, downregulation of Wnt7b, CTNNB1, cell cycle related genes (Ccnd1, Ctgf, Hedgehog receptors Ptch1 and Smo), progenitor phenotype genes (Jag1 and Klf5), reduced tumor load | ||
| Anti-MCP-1 antibody | 2H5 | Preventing macrophages recruitment and activation | SNU-1079-generated human CCA cell xenografts | Reduced F4/80+ and CD206+ macrophages, significantly smaller tumors | [30] | |
| TWEAK/Fn14/NF-kB pathway | Fn14 knockout | Reduction of MCP-1 expression | TAA-treated mice | Reduced PanCK+ tumor epithelia cells and F4/80+ and CD206+ macrophages | ||
| TAMs repolarization | Macrophage stimulation | Pyroptotic CCA cells supernatants induced by MTX–TMPs | Activate macrophages to release proinflammatory factors | Activate macrophages to release proinflammatory factors | Production of various cytokines (IL-6, CCL2, CCL3 and CCL20) and neutrophil attractants (CXCL1, CXCL5 and TNF) | [106] |
| CCA patients’ biliary drainage analysis | Upregulated production of CCL2, CCL3, CXCL1, CXCL5, IL-1β and IL-6 | |||||
| G-MDSCs and TAMs inhibition combined with anti-PD-1 therapy | G-MDSCs inhibition | Clone 1A8 or GW3965 | Elimination immunosuppressive G-MDSCs | YAP-driven mouse CCA model | Reduced G-MDSCs and TAMs in the tumor site, increased CD8+ T cell infiltration and activation, significantly lessen tumor burden, prolonged the survival of mice bearing SB tumors | [80] |
| Anti-CSF1R | AFS98 | Preventing macrophages recruitment and activation | ||||
| Anti-PD-1 antibodies | G4 | Promote anti-tumor immunity of CTLs | ||||
| CD40 agonist and anti-PD-1 therapy | Agonistic anti-CD40 | Clone FGK4.5 | Induction of effective anti-tumor immune responses | Subcutaneous and intrahepatic tumor injection model, AKT 250 and YAP-driven mouse CCA model | Reduced of tumor growth and improved survival, increased CD4+, CD8+ and NK cell infiltration, enhanced response to chemotherapy | [86] |
| Anti-PD-1 antibodies | Clone 29F.1A12 | Promote anti-tumor immunity of CTLs | ||||
| Cytotoxic agents | Gemcitabine and Cisplatin | Cell cycle inhibition and DNA replication inhibition | ||||
| CD47-SIRPα blockage | Anti-CD47 antibodies Anti-SIRPα |
B6H12.2 SE5A5 |
Enhance macrophage phagocytosis | Transplenic intrahepatic metastasis mouse model | Reduced cancer colonization, increased phagocytic indices of resting, M1, M2, and TAM-like MDMs | [88] |
7. Outstanding questions
TAMs play an important role in tumor progression via complicated interplay with CCA and other TME components. They play an important role in carcinogenesis, including cancer cell proliferation, metastasis, angiogenesis and immune suppression. Due to highly malignancy and severe drug-resistance, seeking more efficient treatment strategies for CCA are urgently needed. As the revealing of diversity protumor effects of TME, a new era of anti-tumor therapy has arrived. Targeting TME and tumor as a whole should be regarded as an integral therapy policy [107]. In particular, TAMs in tumor have shown great plasticity and tumor-promoting potential. It should be of great importance to suppress tumor cells and shape macrophages in the meantime. Future investigations targeting TAMs will likely clarify the comprehensive understanding and novel therapy of CCA. However, there remains several problems to be solved. First, the mechanisms of the interaction between TAMs and CCA has not been elucidated, and currently available studies are relatively rare and cannot provide us a complete network system for the interaction between TAMs and CCA. The regulation network of tumor and its TME covers a very wide range includes cytokines, exosomes, non-coding RNAs, as well as metabolites [108], [109], [110]. There still exist many unknown fields to be explored. Second, since studies targeting TAMs and CCA are relatively small than other tumors, whether the role of TAMs in other tumor processes has the same effects in CCA remains to be further determined. Third, the definition of TAMs may need to be improved. Although the M2 type does have tumor-promoting effects, as aforementioned, apart from M2, the atypical phenotypes of macrophages that may encourage CCA growth. And new markers such as TREM-1+, TREM-2+, GATA6+, VEGFR3 and podoplanin emerge as the in-depth study of TAMs in liver diseases [63,65,[111], [112], [113]]. Jumping out the stereotype of M2 macrophages equals TAMs may help to explain current dilemma and find out more about TAMs and CCA. Thus, further effort should be made to clarify the interaction between TAMs and CCA before targeting this interaction in clinical practice.
Search strategy and selection criteria
Data for this review were identified by searches of MEDLINE, PubMed, and references from relevant articles using the search terms “macrophage”, “cholangiocarcinoma”, and “tumor microenvironment”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in English between 2010 and 2020 were included.
Originality of figures
The authors confirm originality of all figures of the manuscript. These figures have not been published previously.
Contributors
Menghua Zhou wrote the manuscript, Chaoqun Wang, Shounan Lu, Yanan Xu, Zihao Li, Hongchi Jiang, Yong Ma reviewed and modified the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
This work was supported by the Research Fund of the First Affiliated Hospital of Harbin Medical University (2019L01, HYD2020JQ0007), Outstanding Youth Training Fund from Academician Yu Weihan of Harbin Medical University (2014), Chen Xiaoping Foundation for the Development of Science and Technology of Hubei Province (CXPJJH11900001-2019349), Natural Science Foundation of Heilongjiang Province of China (QC2013C094, LC2018037), and the National Natural Scientific Foundation of China (81100305, 81470876 and 81270527). The funders had no role in paper design, data collection, data analysis, interpretation, writing of the paper .
Contributor Information
Hongchi Jiang, Email: jianghc2013@163.com.
Yong Ma, Email: mayong@ems.hrbmu.edu.cn.
References
- 1.Cardinale V, Carpino G, Reid L. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J Gastrointest Oncol. 2012;4:94–102. doi: 10.4251/wjgo.v4.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raggi C, Invernizzi P, Andersen JB. Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: molecular networks and biological concepts. J Hepatol. 2015;62:198–207. doi: 10.1016/j.jhep.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Banales JM, Marin JJG, Lamarca A. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banales JM, Cardinale V, Carpino G. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 5.Nakanuma Y, Kakuda Y. Pathologic classification of cholangiocarcinoma: new concepts. Best Pract Res Clin Gastroenterol. 2015;29:277–293. doi: 10.1016/j.bpg.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Kendall T, Verheij J, Gaudio E. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):7–18. doi: 10.1111/liv.14093. [DOI] [PubMed] [Google Scholar]
- 7.Job S, Rapoud D, Dos Santos A. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2019;72:965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montal R, Sia D, Montironi C. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73:315–327. doi: 10.1016/j.jhep.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labib PL, Goodchild G, Pereira SP. Molecular pathogenesis of cholangiocarcinoma. BMC Cancer. 2019;19:185. doi: 10.1186/s12885-019-5391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malenica I, Donadon M, Lleo A. Molecular and immunological characterization of biliary tract cancers: a paradigm shift towards a personalized medicine. Cancers (Basel) 2020;12:2190. doi: 10.3390/cancers12082190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabris L, Perugorria MJ, Mertens J. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):63–78. doi: 10.1111/liv.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun D, Luo T, Dong P. CD86+/CD206+ tumor-associated macrophages predict prognosis ofpatients with intrahepatic cholangiocarcinoma. PeerJ. 2020;8:e8458. doi: 10.7717/peerj.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 16.Atanasov G, Hau HM, Dietel C. Prognostic significance of TIE2-expressing monocytes in hilar cholangiocarcinoma. J Surg Oncol. 2016;114:91–98. doi: 10.1002/jso.24249. [DOI] [PubMed] [Google Scholar]
- 17.Raggi C, Correnti M, Sica A. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102–115. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Franklin RA, Liao W, Sarkar A. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabris L, Sato K, Alpini G, Strazzabosco M. The tumor microenvironment in cholangiocarcinoma progression. Hepatology. 2020;73:75–85. doi: 10.1002/hep.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan S, Zhao E, Kryczek I. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Liang Y, Song X, Li Y. LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol Cancer. 2020;19:85. doi: 10.1186/s12943-020-01206-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Cadamuro M, Nardo G, Indraccolo S. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58:1042–1053. doi: 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou SL, Dai Z, Zhou ZJ. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35:597–605. doi: 10.1093/carcin/bgt397. [DOI] [PubMed] [Google Scholar]
- 26.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 27.Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110. doi: 10.1186/s13046-018-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binnewies M, Roberts EW, Kersten K. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziani L, Chouaib S, Thiery J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front Immunol. 2018;9:414. doi: 10.3389/fimmu.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwyer BJ, Jarman EJ, Gogoi-Tiwari J. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Wu HJ, Chu PY. Role of cancer stem cells in cholangiocarcinoma and therapeutic implications. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20174154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng J, Liu Z, Sun S. Tumor-associated macrophages recruited by periostin in intrahepatic cholangiocarcinoma stem cells. Oncol Lett. 2018;15:8681–8686. doi: 10.3892/ol.2018.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabris L, Cadamuro M, Cagnin S. Liver matrix in benign and malignant biliary tract disease. Semin Liver Dis. 2020;40:282–297. doi: 10.1055/s-0040-1705109. [DOI] [PubMed] [Google Scholar]
- 34.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9:44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- 35.Subimerb C, Pinlaor S, Lulitanond V. Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161:471–479. doi: 10.1111/j.1365-2249.2010.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miura T, Yoshizawa T, Hirai H. Prognostic impact of CD163+ macrophages in tumor stroma and CD8+ T-Cells in cancer cell nests in invasive extrahepatic bile duct cancer. Anticancer Res. 2017;37:183–190. doi: 10.21873/anticanres.11304. [DOI] [PubMed] [Google Scholar]
- 37.Atanasov G, Hau HM, Dietel C. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer. 2015;15:790. doi: 10.1186/s12885-015-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thanee M, Loilome W, Techasen A. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac J Cancer Prev. 2015;16:3043–3050. doi: 10.7314/apjcp.2015.16.7.3043. [DOI] [PubMed] [Google Scholar]
- 39.Hasita H, Komohara Y, Okabe H. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subimerb C, Pinlaor S, Khuntikeo N. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep. 2010;3:597–605. doi: 10.3892/mmr_00000303. [DOI] [PubMed] [Google Scholar]
- 41.Atanasov G, Dietel C, Feldbrugge L. Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1331806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pak JH, Lee JY, BY Jeon. Cytokine production in cholangiocarcinoma cells in response to clonorchis sinensis excretory-secretory products and their putative protein components. Korean J Parasitol. 2019;57:379–387. doi: 10.3347/kjp.2019.57.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim EM, Kwak YS, Yi MH. Clonorchis sinensis antigens alter hepatic macrophage polarization in vitro and in vivo. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Figueroa-Santiago O, Espino AM. Fasciola hepatica fatty acid binding protein induces the alternative activation of human macrophages. Infect Immun. 2014;82:5005–5012. doi: 10.1128/IAI.02541-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debebe A, Medina V, Chen CY. Wnt/β-catenin activation and macrophage induction during liver cancer development following steatosis. Oncogene. 2017;36:6020–6029. doi: 10.1038/onc.2017.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 47.Yuan D, Huang S, Berger E. Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell. 2017;31 doi: 10.1016/j.ccell.2017.05.006. 771-789 e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locatelli L, Cadamuro M, Spirli C. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology. 2016;63:965–982. doi: 10.1002/hep.28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zong C, Kimura Y, Kinoshita K. Exposure to 1,2-dichloropropane upregulates the expression of activation-induced cytidine deaminase (AID) in human cholangiocytes co-cultured with macrophages. Toxicol Sci. 2019;168:137–148. doi: 10.1093/toxsci/kfy280. [DOI] [PubMed] [Google Scholar]
- 50.Zhang GF, Qiu L, Yang SL. Wnt/β-catenin signaling as an emerging potential key pharmacological target in cholangiocarcinoma. Biosci Rep. 2020;40 doi: 10.1042/BSR20193353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Tammela T, Sanchez-Rivera FJ, Cetinbas NM. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature. 2017;545:355–359. doi: 10.1038/nature22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulter L, Guest RV, Kendall TJ. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269–1285. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loilome W, Bungkanjana P, Techasen A. Activated macrophages promote Wnt/β-catenin signaling in cholangiocarcinoma cells. Tumor Biol. 2014;35:5357–5367. doi: 10.1007/s13277-014-1698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Liang J, Huang L. Characterizing the activation of the Wnt signaling pathway in hilar cholangiocarcinoma using a tissue microarray approach. Eur J Histochem. 2016;60:2536. doi: 10.4081/ejh.2016.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinhart Z, Pavlovic Z, Chandrashekhar M. Genome-wide CRISPR screens reveal a Wnt–FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nature Medicine. 2016;23:60–68. doi: 10.1038/nm.4219. [DOI] [PubMed] [Google Scholar]
- 57.Vaquero J, Guedj N, Claperon A. Epithelial-mesenchymal transition in cholangiocarcinoma: from clinical evidence to regulatory networks. J Hepatol. 2017;66:424–441. doi: 10.1016/j.jhep.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Sun D, Luo T, Dong P. M2-polarized tumor-associated macrophages promote epithelial-mesenchymal transition via activation of the AKT3/PRAS40 signaling pathway in intrahepatic cholangiocarcinoma. J Cell Biochem. 2020;121:2828–2838. doi: 10.1002/jcb.29514. [DOI] [PubMed] [Google Scholar]
- 59.Techasen A, Loilome W, Namwat N. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13:115–118. Suppl. [PubMed] [Google Scholar]
- 60.Simone V, Brunetti O, Lupo L. Targeting angiogenesis in biliary tract cancers: an open option. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartneck M, Schrammen PL, Mockel D. The CCR2(+) macrophage subset promotes pathogenic angiogenesis for tumor vascularization in fibrotic livers. Cell Mol Gastroenterol Hepatol. 2019;7:371–390. doi: 10.1016/j.jcmgh.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Palma M, Murdoch C, Venneri MA. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Nakamoto S, Ito Y, Nishizawa N. Lymphangiogenesis and accumulation of reparative macrophages contribute to liver repair after hepatic ischemia-reperfusion injury. Angiogenesis. 2020;23:395–410. doi: 10.1007/s10456-020-09718-w. [DOI] [PubMed] [Google Scholar]
- 64.Alishekevitz D, Gingis-Velitski S, Kaidar-Person O. Macrophage-induced lymphangiogenesis and metastasis following paclitaxel chemotherapy is regulated by VEGFR3. Cell Rep. 2016;17:1344–1356. doi: 10.1016/j.celrep.2016.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bieniasz-Krzywiec P, Martin-Perez R, Ehling M. Podoplanin-expressing macrophages promote lymphangiogenesis and lymphoinvasion in breast cancer. Cell Metab. 2019;30 doi: 10.1016/j.cmet.2019.07.015. 917-936 e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaquero J, Aoudjehane L, Fouassier L. Cancer-associated fibroblasts in cholangiocarcinoma. Current Opinion in Gastroenterology. 2020;36:63–69. doi: 10.1097/MOG.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 67.Ho DW, Tsui YM, Sze KM. Single-cell transcriptomics reveals the landscape of intra-tumoral heterogeneity and stemness-related subpopulations in liver cancer. Cancer Lett. 2019;459:176–185. doi: 10.1016/j.canlet.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 69.Brivio S, Cadamuro M, Strazzabosco M, Fabris L. Tumor reactive stroma in cholangiocarcinoma: the fuel behind cancer aggressiveness. World J Hepatol. 2017;9:455–468. doi: 10.4254/wjh.v9.i9.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guest RV, Boulter L, Dwyer BJ. Notch3 drives development and progression of cholangiocarcinoma. Proc Natl Acad Sci USA. 2016;113:12250–12255. doi: 10.1073/pnas.1600067113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Y, Zhou C, Yu XX. Osteopontin promotes metastasis of intrahepatic cholangiocarcinoma through recruiting MAPK1 and mediating Ser675 phosphorylation of β-catenin. Cell Death Dis. 2018;9:179. doi: 10.1038/s41419-017-0226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loosen SH, Roderburg C, Kauertz KL. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J Hepatol. 2017;67:749–757. doi: 10.1016/j.jhep.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 73.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slawinski G, Wrona A, Dabrowska-Kugacka A. Immune checkpoint inhibitors and cardiac toxicity in patients treated for non-small lung cancer: a review. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21197195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ribas A, Kefford R, Marshall MA. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou G, Sprengers D, Mancham S. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J Hepatol. 2019;71:753–762. doi: 10.1016/j.jhep.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 77.Walter D, Herrmann E, Schnitzbauer AA. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology. 2017;71:383–392. doi: 10.1111/his.13238. [DOI] [PubMed] [Google Scholar]
- 78.Yu F, Gong L, Mo Z. Programmed death ligand-1, tumor infiltrating lymphocytes and HLA expression in Chinese extrahepatic cholangiocarcinoma patients: possible immunotherapy implications. Biosci Trends. 2019;13:58–69. doi: 10.5582/bst.2019.01003. [DOI] [PubMed] [Google Scholar]
- 79.Kitano Y, Yamashita YI, Nakao Y. Clinical significance of PD-L1 expression in both cancer and stroma cells of cholangiocarcinoma patients. Ann Surg Oncol. 2020;27:599–607. doi: 10.1245/s10434-019-07701-4. [DOI] [PubMed] [Google Scholar]
- 80.Loeuillard E, Yang J, Buckarma E. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020;130:5380–5396. doi: 10.1172/JCI137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gani F, Nagarajan N, Kim Y. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016;23:2610–2617. doi: 10.1245/s10434-016-5101-y. [DOI] [PubMed] [Google Scholar]
- 82.Jing CY, Fu YP, Yi Y. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer. 2019;7:77. doi: 10.1186/s40425-019-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su M, Qiao KY, Xie XL. Development of a prognostic signature based on single-cell RNA sequencing data of immune cells in intrahepatic cholangiocarcinoma. Front Genet. 2020;11 doi: 10.3389/fgene.2020.615680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang M, Yang H, Wan L. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020;73:1118–1130. doi: 10.1016/j.jhep.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 85.Sica A, Porta C, Amadori A, Pastò A. Tumor-associated myeloid cells as guiding forces of cancer cell stemness. Cancer Immunol Immunother. 2017;66:1025–1036. doi: 10.1007/s00262-017-1997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diggs LP, Ruf B, Ma C. CD40-mediated immune cell activation enhances response to anti-PD1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yanagita T, Murata Y, Tanaka D. Anti-SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight. 2017;2:e89140. doi: 10.1172/jci.insight.89140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaeteewoottacharn K, Kariya R, Pothipan P. Attenuation of CD47-SIRPα signal in cholangiocarcinoma potentiates tumor-associated macrophage-mediated phagocytosis and suppresses intrahepatic metastasis. Translational Oncology. 2019;12:217–225. doi: 10.1016/j.tranon.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doedens AL, Stockmann C, Rubinstein MP. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ceci C, Atzori MG, Lacal PM, Graziani G. Targeting tumor-associated macrophages to increase the efficacy of immune checkpoint inhibitors: a glimpse into novel therapeutic approaches for metastatic melanoma. Cancers (Basel) 2020;12:3401. doi: 10.3390/cancers12113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ouyang W, O'Garra AJI. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. 2019; 50:871-891. [DOI] [PubMed]
- 92.Tauriello D, Palomo-Ponce S, Stork D et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. 2018; 554:538-543. [DOI] [PubMed]
- 93.Klein O, Kee D, Nagrial A. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. 2020;6:1405–1409. doi: 10.1001/jamaoncol.2020.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mei K, Qin S, Chen Z. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort A report in a multicenter phase Ib/II trial. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 96.van Dalen FJ, van Stevendaal M, Fennemann FL. Molecular repolarisation of tumour-associated macrophages. Molecules. 2018;24:9. doi: 10.3390/molecules24010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Q, Tong D, Liu G. Metformin inhibits prostate cancer progression by targeting tumor-associated inflammatory infiltration. Clin Cancer Res. 2018;24:5622–5634. doi: 10.1158/1078-0432.CCR-18-0420. [DOI] [PubMed] [Google Scholar]
- 98.Zhu Y, Knolhoff BL, Meyer MA. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, Cao Y, Sun X. Chloroquine (CQ) exerts anti-breast cancer through modulating microenvironment and inducing apoptosis. Int Immunopharmacol. 2017;42:100–107. doi: 10.1016/j.intimp.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 100.Parayath NN, Parikh A, Amiji MM. Repolarization of tumor-associated macrophages in a genetically engineered nonsmall cell lung cancer model by intraperitoneal administration of hyaluronic acid-based nanoparticles encapsulating microRNA-125b. Nano Lett. 2018;18:3571–3579. doi: 10.1021/acs.nanolett.8b00689. [DOI] [PubMed] [Google Scholar]
- 101.Xiao H, Guo Y, Li B. M2-like tumor-associated macrophage-targeted codelivery of STAT6 inhibitor and IKKbeta siRNA induces M2-to-M1 repolarization for cancer immunotherapy with low immune side effects. ACS Cent Sci. 2020;6:1208–1222. doi: 10.1021/acscentsci.9b01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Figueiredo P, Lepland A, Scodeller P. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2020 doi: 10.1016/j.actbio.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 103.Liao ZX, Fa YC, Kempson IM, Tseng SJ. Repolarization of M2 to M1 macrophages triggered by lactate oxidase released from methylcellulose hydrogel. Bioconjug Chem. 2019;30:2697–2702. doi: 10.1021/acs.bioconjchem.9b00618. [DOI] [PubMed] [Google Scholar]
- 104.Li K, Lu L, Xue C. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale. 2020;12:130–144. doi: 10.1039/c9nr06505a. [DOI] [PubMed] [Google Scholar]
- 105.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao Y, Zhang H, Zhou N. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4:743–753. doi: 10.1038/s41551-020-0583-0. [DOI] [PubMed] [Google Scholar]
- 107.Kubli S, Berger T, Araujo D. 2021. Beyond immune checkpoint blockade: emerging immunological strategies. [DOI] [PubMed] [Google Scholar]
- 108.Wang X, Luo G, Zhang K. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 109.Penny HL, Sieow JL, Adriani G. Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1191731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu JY, Huang TW, Hsieh YT. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 2020;77 doi: 10.1016/j.molcel.2019.10.023. 213-227 e215. [DOI] [PubMed] [Google Scholar]
- 111.Wu Q, Zhou W, Yin S. Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer. Hepatology. 2019;70:198–214. doi: 10.1002/hep.30593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Esparza-Baquer A, Labiano I, Sharif O. TREM-2 defends the liver against hepatocellular carcinoma through multifactorial protective mechanisms. Gut. 2020 doi: 10.1136/gutjnl-2019-319227. gutjnl-2019-319227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zindel J, Peiseler M, Hossain M et al. Primordial GATA6 macrophages function as extravascular platelets in sterile injury. 2021; 371. [DOI] [PubMed]