Highlights
-
•
Contemporary information on the pattern of disease recurrence in breast cancer provides useful information for planning clinical trials of novel adjuvant strategies.
-
•
Bone metastases remain the most frequent site for metastasis from stage II-III breast cancer.
-
•
The annual rates of disease recurrence and bone metastasis specifically are about 3% and 1% respectively in this intermediate to high-risk population.
-
•
Zoledronate reduces bone metastasis but has adverse effects on extra-skeletal recurrences in women who have not passed through menopause and/or have adverse histological features.
Keywords: Breast cancer, Bone metastases, Zoledronate, Patterns of metastasis, Menopausal status, Oestrogen receptor status
Abstract
Aim
The prognosis for women with breast cancer has improved markedly over recent decades. However, mortality from breast cancer remains high and, for those developing metastatic disease, curative therapy is not possible. Here, we report the frequency and distribution of disease recurrence(s) in a large population of women with AJCC stage II/III breast cancer and evaluate the impact of adjuvant treatment with the bisphosphonate zoledronate on clinical outcomes.
Patients and methods
In the context of the AZURE study (ISRCTN7981382), 3359 patients with histologically confirmed stage II/III breast cancer were randomised to receive standard adjuvant treatment ± zoledronate for five years. Patients were followed up for 10 years and all patients with recurrent disease in that time identified. The site of first recurrence, the first distant recurrence site(s) and bone metastasis at any time were recorded and outcomes in the control and zoledronate treatment groups compared. Survival after recurrence was also evaluated.
Results
In the study population as a whole, disease recurrence at a median follow-up of 117 months occurred in 1010/3359 (30%) women with a relatively constant rate of disease relapse of around 3% per year. 727 (72%) first recurrences were at distant sites, 178 locoregional (18%) and 105 (10%) both locoregional and distant relapses occurred synchronously. Bone was the most frequent first recurrence site occurring in 463 (14%) of all patients and was the only distant metastatic site in 265 (7.9%). 69% of the control group who developed recurrent disease had bone metastases identified. Bone metastases were more frequent in those with oestrogen receptor (ER) positive disease and recurrences overall, especially at visceral sites, were more likely with ER negative disease. Zoledronate reduced bone metastases in both ER subgroups but increased the proportion with extra-skeletal metastases, particularly in women who were not definitely postmenopausal at study entry. Adjuvant zoledronate also reduced bone metastases after recurrence at an extra-skeletal site.
Conclusions
This analysis provides contemporary information on the frequency and pattern of recurrences after treatment for stage II/III breast cancer that may be of value in planning future adjuvant trials. It confirms the ongoing importance of bone metastases and describes in detail for the first time the effects of adjuvant zoledronate on the pattern of metastasis.
1. Introduction
The outcomes for women with breast cancer have improved greatly over recent decades through a combination of earlier diagnosis and more effective treatment, especially through the incorporation of adjuvant systemic therapies alongside locoregional treatments for stage I–III disease, as defined by the American Joint Committee for Cancer (AJCC). However, considerable risks for breast cancer recurrence and subsequent death remain, that are determined by a mixture of disease burden, tumour biology and the host response. The majority of patients now present with stage I node negative disease and have an excellent prognosis. Nevertheless, for the minority of patients that present with AJCC stage II or III disease, the risk of recurrence remains relatively high and, despite many advances in the treatment of advanced breast cancer, metastatic disease remains incurable. Efforts are ongoing to improve the effectiveness of adjuvant systemic treatments with most approaches focusing on treating the cancer itself, either non-specifically through the use of adjuvant chemotherapy or by targeting the underlying biology of the disease with treatments targeted against oestrogen and/or HER2/neu receptors [1]. Targeting the host to prevent disease recurrence has been more challenging but adjuvant bisphosphonates have been shown to reduce bone recurrence and breast cancer mortality in the large postmenopausal subgroup of women with early breast cancer [2] and are recommended for use by a range of international treatment guidelines in this group of women if deemed to be at intermediate to high risk for breast cancer recurrence [3], [4], [5]. The effects of adjuvant bisphosphonates in premenopausal women, especially the very young, remain uncertain with suggestions from a number of trials that, while the risk for bone metastases may be reduced, the spread of disease to other organs appears to be increased and there is no overall benefit [2] and the possibility for harm [6], [7].
Several authors have explored breast cancer recurrence patterns, trying to identify clinical and/or pathological parameters that correlate with specific sites of relapse and clinical outcomes but many of them report on patients treated many decades ago and do not reflect the current population of women with the disease [8], [9], [10]. Follow-up was also often quite short and careful evaluation of sites of metastasis lacking. There is a need for information on contemporary rates of disease recurrence and the distribution of metastases in order to plan future suitably powered trials in early breast cancer as well as to understand the impact of treatments like bisphosphonates on the evolution of metastases across the spectrum of women presenting with early breast cancer. In this study we report on the frequency and patterns of metastasis over the first 10 years after diagnosis and treatment with contemporary adjuvant therapies and investigate the impact that adjuvant zoledronate, given within the context of the large AZURE trial (BIG 01/04) [11], had on the subsequent patterns of relapse according to menopause status and the expression of oestrogen receptors (ER).
AZURE patients were regularly reviewed during the 5-year treatment phase and then annually for up to 10 years, or until death, and during this period, both local and distant recurrences have been prospectively recorded and the first site of any distant recurrence and bone metastases at any time evaluated. Our study aims to describe the frequency and pattern of breast cancer recurrence in the modern treatment era. The correlations between menopausal status at the time of randomization, ER expression and sites of relapse are also described, as well as the impact of adjuvant zoledronate on clinical outcomes. Unusually, meta-analysis of the adjuvant bisphosphonate trials showed a somewhat greater effect on breast cancer mortality (HR = 0.82; 95%CI 0.73–0.93) than on disease recurrence (HR = 0.86; 95%CI 0.78–0.94) [2] suggesting that adjuvant bisphosphonates may influence survival after recurrence as well as prevent a proportion of patients from developing bone metastases. Therefore, as an additional exploratory endpoint, overall survival after BC recurrence was also evaluated, according to the sites of first relapse and the addition of zoledronate to standard adjuvant treatment.
2. Patients and methods
The AZURE trial was an academic, prospective, open label, randomised, controlled phase III international, multicentre, parallel-group trial. Eligibility has been reported previously [11] but, in summary, patients had to have histologically confirmed invasive breast cancer with either pathologically involved axillary lymph node metastasis or a T3/T4 primary tumour (AJCC Stage II/III). Prior complete resection of the primary tumour should have been performed or had to be planned if patients were treated with neoadjuvant chemotherapy. Patients were not eligible if there was clinical or imaging evidence of distant metastases prior to study entry or a history of prior cancer within the preceding five years. Staging imaging tests were performed in accordance with institutional protocols. All patients gave written informed consent. Prior to randomisation, haematological, renal and hepatic function tests were required.
Between September 2003 and February 2006, 3359 patients with histologically confirmed stage II/III breast cancer were randomised to receive standard adjuvant treatment ± zoledronate for five years. The majority of the patients were recruited from sites in the United Kingdom (n = 2710 [81%]) with others from Eire (n = 247 [7.4%]), Australia (n = 226 [6.7%]), Spain (n = 107 [3.2%]), Portugal (n = 32 [0.9%]), Thailand (n = 25 [0.7%]) and Taiwan (n = 13 [0.4%]). Surgical management, the use of adjuvant loco-regional external beam radiotherapy and the selection of systemic adjuvant treatments (chemotherapy and/or endocrine therapy) were decided in accordance with standard protocols at each participating institution. The addition of trastuzumab to chemotherapy was allowed in patients with Her-2 over-expressing tumours after its regulatory approval for adjuvant use in 2005.
As also described previously [11], a minimisation process to reduce possible imbalances in tumour and treatment characteristics was used. This took into account the treating centre, the number of involved axillary lymph nodes, clinical tumour stage, oestrogen receptor status, adjuvant systemic treatment modalities and timing in relationship to surgery, concomitant use of statins and the menopausal status of the patient at study entry. This was categorised as either not definitely postmenopausal (NPM), to include premenopausal women, those who would be considered as perimenopausal as they were within 5 years of previous regular menstruation, and those where menstrual status was difficult to ascertain or unknown e.g. post-hysterectomy, or those considered definitely postmenopausal (PM) in that greater than 5 years had elapsed since last menses.
Patients randomised to receive zoledronate were given a 4 mg intravenous infusion of zoledronate every 3–4 weeks for 6 cycles, then every three months for 8 doses, followed by 5 cycles on a six-monthly schedule for a total treatment duration of 5 years (19 doses). Patients stopped zoledronate on completion of 5 years treatment or following distant recurrence, unacceptable toxicity, three consecutively missed treatments, patient request or physician recommendation. Continuation of study medication was recommended after loco-regional recurrence, and was at the physician’s discretion following development of any new primary cancer.
The follow-up schedule was similar in both control and zoledronate arms of the study and included clinical assessment, adverse event monitoring and haematological, renal and hepatic function test measurements. Routine follow-up imaging, other than routine mammograms to screen for contralateral breast cancer and recurrence within a conserved treated breast, was not mandated, with investigations for possible recurrence clinically directed as deemed appropriate by the treating physician. Subjects were followed up on an annual basis after completion of the 5-year treatment phase (zoledronate or control) for disease recurrence, death, skeletal related events and adverse events of interest.
All first recurrences were classified as loco-regional or distant and the specific site(s) of all distant metastases recorded. If a patient developed an extra-skeletal first distant recurrence, any subsequent skeletal recurrence was collected as a post-distant recurrence. Recurrences developing after development of a bone metastasis were not systematically reported. Any two recurrence events occurring within 30 days of each other were considered as a single event to allow for the time necessary to perform the range of imaging tests (CT and/or MRI, bone scan) required to restage a patient with potential recurrent disease. Dates of relapse were backdated to the first clinical suspicion of recurrence rather than the confirmation date. Loco-regional relapses were classified by site: ipsilateral breast, chest wall, loco-regional lymph nodes or multiple sites. First distant relapse sites were classified as skeletal and/or extra-skeletal, with the latter being further categorized as visceral (liver, lung, pleura, central nervous system, adrenal gland, bowel, kidney, pancreas) or soft tissue (distant nodes, uterus, ovaries, peritoneum, mediastinum, skin, subcutaneous tissue) recurrences. To allow for interpretation of patients with multiple extra-skeletal metastatic sites and avoid double counting, non-osseous metastases were ascribed a hierarchy of likely clinical importance of brain +/− other sites, liver +/− other sites except brain, lung/pleura +/− other sites except for brain or liver metastases and soft tissue +/− other sites except for brain, liver and lung/pleura metastases.
Recurrence information was confirmed by on site or telephone-based review of clinical notes and imaging tests in 91% of patients. Recurrence dates and site classification were double-checked by two independent operators SD and SN); in case of disagreement, data were reanalysed with the lead author (RC) until all discrepancies were eliminated.
2.1. Statistical analysis
Descriptive statistics were used to show the patterns of recurrence. Risk ratios (RR) were used to compare subgroups. All analyses should be considered exploratory and as such no P values were ascribed to differences between groups. Survival analyses were investigated using Kaplan-Meier survival curves and hazard ratios (HR) with 95% confidence intervals (CI) for these data are presented.
3. Results
Key patient characteristics are shown in Table 1 reflecting a population of patients at intermediate to high risk of recurrence due to lymph node involvement or larger tumour size. Additional patient characteristics can be found in earlier reports of the AZURE trial. [11], [12] The patient and tumour characteristics were very similar across the two treatment groups. 3207/3359 (95%) of patients received adjuvant chemotherapy with or without endocrine treatment depending on ER status. 1776/2634 (67%) of women with ER positive tumours received an aromatase inhibitor at some time following study entry; 665/780 (85%) and 1111/1854 (60%) who were PM and NPM at study entry respectively. Fewer than 5% of premenopausal women received ovarian suppression as part of their adjuvant endocrine treatment programme. Because of the timeframe when patients were recruited, HER2 status was unknown in 1693/3359. (50%) of patients. Of the 1666 with known HER2 status at study entry, 415 (25%) were HER2 positive and 277 (67%) of these women received adjuvant trastuzumab. In addition, a further 169 patients, not known to be HER2 positive at study entry, received trastuzumab at some time during follow-up.
Table 1.
Allocation Standard Treatment Alone Standard treatment + ZOL.
| Number | Percent | Number | Percent | |
|---|---|---|---|---|
| Lymph node involvement | 32 | 1.9 | 30 | 1.8 |
| 0 nodes involved | ||||
| One - three nodes involved | 1033 | 61.6 | 1042 | 62.0 |
| => four nodes involved | 607 | 36.2 | 604 | 35.9 |
| Unknown involvement | 6 | 0.4 | 5 | 0.3 |
| Tumour stage | 523 | 31.2 | 542 | 32.2 |
| T1 | ||||
| T2 | 867 | 51.7 | 850 | 50.6 |
| T3 | 228 | 13.6 | 228 | 13.6 |
| T4 | 59 | 3.5 | 58 | 3.5 |
| TX | 1 | 0.1 | 3 | 0.2 |
| ER status | 1315 | 78.4 | 1318 | 78.4 |
| ER positive | ||||
| ER negative | 356 | 21.2 | 350 | 20.8 |
| ER unknown | 7 | 0.4 | 13 | 0.8 |
| PR status | 699 | 41.7 | 725 | 43.1 |
| Positive | ||||
| Negative | 424 | 25.3 | 382 | 22.7 |
| Unknown/missing | 555 | 33.1 | 574 | 34.1 |
| HER2 status | 223 | 13.3 | 192 | 11.4 |
| Positive | ||||
| Negative | 604 | 36.0 | 648 | 38.5 |
| Unknown/missing/not measured | 851 | 50.7 | 841 | 50.0 |
| Histological grade | 141 | 8.4 | 146 | 8.7 |
| 1 | ||||
| 2 | 708 | 42.2 | 731 | 43.5 |
| 3 | 787 | 46.9 | 765 | 45.5 |
| Not specified/missing | 42 | 2.5 | 39 | 2.3 |
| Menopausal status | 1156 922 234 |
68.9 [80%] [20%] |
1162 932 230 |
69.1 [80%] [20%] |
| Not definitely postmenopausal (NPM) ER+ ER- | ||||
| More than 5 years since menopause ER+ ER- |
522 393 129 |
31.1 [75%] [25%] |
519 387 132 |
30.9 [75%] [25%] |
| TOTAL | 1678 | 100.0 | 1681 | 100.0 |
3.1. Natural history of stage II/III breast cancer
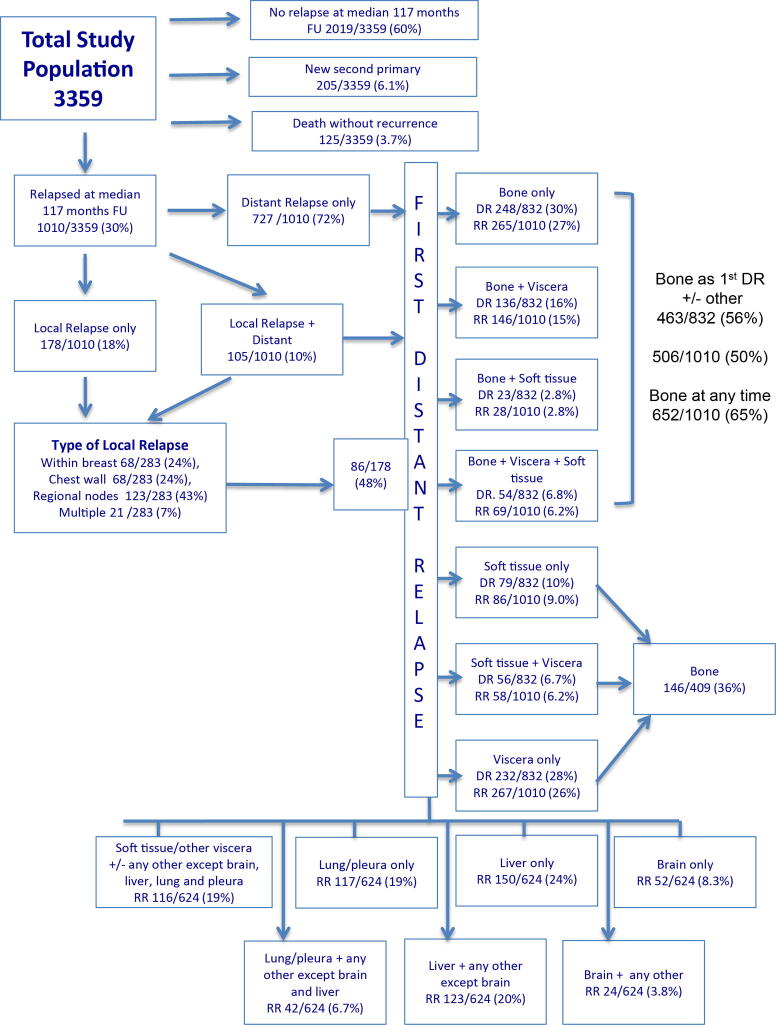
In the study population as a whole, disease recurrence at a median follow-up of 117 months (interquartile range 70–120 months) has occurred in 1010/3359 (30%) women with a relatively constant rate of disease relapse of around 3% per year. 727/1010 (72%) first recurrences were at distant sites, 178/1010 were locoregional (18%) and in 105/1010 (10%) both locoregional and distant relapses occurred synchronously (Fig. 1). Bone was the most frequent first recurrence site occurring in 463/3359 (14%) of all patients and was the only distant metastatic site in 265/3359 (7.9%).
Fig. 1.
Distribution and number of disease recurrences at 10 years of follow-up in the AZURE study of women with stage II/III breast cancer. DR, distant recurrences; RR overall recurrences; FU, follow up.
Although zoledronate had no impact on recurrence rates overall (control 508 patients, zoledronate 502: HR = 0.92, 95% CI 0.81–1.05) there were, as reported previously, [11], [12], [13] effects of adjuvant zoledronate within specific patient subgroups that are discussed below. As a result of these study treatment effects, the control group provides the most reliable data on the natural history of early breast cancer (Table 2). 508/1678 (30%) of control patients experienced disease recurrence (368/508 [73%] distant, 83/508 [16%] locoregional and 57/508 [11%] both distant and locoregional).
Table 2.
Type and site of first recurrence overall and by treatment allocation.
| Type and first site of recurrence | Recurrences | All patients |
|||
|---|---|---|---|---|---|
| % All first recurrences | [%]. Distant recurrence | Control | Zoledronate | ||
| n | 3359 | n = 1010 | n = 832 | 1678 | 1681 |
| All first recurrences | 1010 [30%] | [100%] | NA | 508 [30%] | 502 [30%] |
| Distant +/− locoregional recurrence | 832 [25%] | [83%] | [100%] | 425 [25%] | 407 [24%] |
| Distant recurrence only | 727 [22%] | [72%] | [87%] | 368 [22%] | 359 [21%] |
| Distant + locoregional recurrence | 105 [3%] | [10%] | [12%] | 57 [3.4%] | 48 [2.9%] |
| Locoregional +/− distant | 281 [8%] | [28%] | NA | 138 [8.2%] | 143 [8.5%] |
| Locoregional recurrence | 178 [5%] | [18%] | NA | 83 [4.9%] | 95 [5.7%] |
| Bone +/− other | 461 [14%] | [46%] | [56%] | 259 [15%] | 202 [12%] |
| Bone only recurrence | 248 [7.4%] | [25%] | [30%] | 144 [8.6%] | 104 [6.2%] |
| Viscera only | 232 [7%] | [23%] | [28%] | 99 [5.9%] | 133 [7.9%] |
| Soft tissue +/− other | 135 [4%] | [13%] | [16%] | 67 [4.0%] | 68 [4.0%] |
| Distant recurrence after locoregional recurrence | 86/178 (48%) | 36/83 (43%) | 50/95 (53%) | ||
| Bone recurrence after extrakeletal recurrence | 146/586 (25%) | 74/285 (26%) | 72/301 (24%) | ||
| Bone recurrence after locoregional recurrence | 44/178 (25%) | 19/83 (23%) | 25/95 (26%) | ||
Bone was the most frequent metastatic site, occurring as the site of first recurrence in 259/1678 control patients (51% of relapses) and the first site of distant recurrence in 278/450 (62%) patients with a distant recurrence including those with a first distant relapse in bone after a locoregional recurrence. Bone metastases occurred at any time during the 10 years of follow up in 352/508 patients (69%) with disease recurrence. 113/259 (44%) patients with first recurrence in bone had synchronous first metastases at other distant sites (viscera in 77, soft tissues in 11 and both viscera and soft tissues in 25 patients) while, in 144/1678 (8.6%) of all control patients and 144/259 (56%) of those with bone metastases, the disease appeared to be confined to bone as the only distant metastatic site with a further 7 patients developing bone only disease following a locoregional relapse. The annual rate of developing bone only metastases in this population did not exceed 1%.
Extra-skeletal sites were the first site of recurrence in 166/1678 (10%). Table 3 shows the first site(s) of these extra-skeletal relapses, both at single sites and in combination with other extra-skeletal sites. In the control group, first distant recurrence in the brain only was seen in 26 (1.5%), in the liver only in 68 (4.1%), in the lung and/or pleura in 68 (4.1%) and in soft tissues in 38 (2.3%) of the 1678 patients in the control group.
Table 3.
Sites of first distant relapse by oestrogen receptor (ER) status and menopausal status. PM, postmenopausal; NPM, not postmenopausal; Zol, zoledronate.
| Site[s] of recurrence* | All patients | All patients | All patients | ER -ve | ER -ve | ER -ve | ER +ve | ER +ve/unk | ER +ve/unk | NPM | NPM | NPM | PM | PM | PM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distant recurrence* [%] | Control | Zol | All | Control | Zol | All | Control | Zol | All | Control | Zol | All | Control | Zol | |
| n = 1678 | n = 1681 | n = 707 | n = 356 | n = 350 | 2633 | n = 1322 | n = 1331 | 2318 | n = 1156 | n = 1162 | n = 1041 | n = 522 | n = 519 | ||
| All distant relapses | n = 888 | n = 450 [27%] | n = 438 [26%] | n = 239 [34%] | n = 118 [33%] | n = 123 [35%] | n = 649 [25%] | n = 334 [25%] | n = 315 [24%] | n = 620 [27%] | n = 301 [26%] | n = 309 [27%] | n = 276 (27%) | n = 150 [29%] | n = 126 [24%] |
| Bone +/− other distant site | 507 [57%] | 278 [62%] | 229 [52%] | 83 [35%] | 53 [45%] | 30 [24%] | 420 [65%] | 225 [67%] | 195 [62%] | 361 [58%] | 198 [66%] | 163 [43%] | 146 [53%] | 80 [53%] | 66 [52%] |
| Bone only | 266 [30%] | 151 [33%] | 115 [26%] | 37 [15%] | 23 [19%] | 13 [11%] | 230 [35%] | 128 [38%] | 102 [32%] | 196 [32%] | 111 [37%] | 85 [28%] | 72 [26%] | 40 [27%] | 32 [25%] |
| Bone + soft tissue | 28 [3.2%] | 14 [3.1%] | 14 [3.2%] | 6 [2.5%] | 4 [3.4%] | 2 [1.6%] | 22 [3.4%] | 10 [3.0%] | 12 [3.8%] | 20 [3.2%] | 10 [3.3%] | 10 [3.2%] | 8 [2.9%] | 4 [2.7%] | 4 [3.2%] |
| Bone + viscera | 144 [16%] | 82 [19%] | 62 [14%] | 3 [1.3%] | 21 [18%] | 9 [7.3%] | 117 [18%] | 61 [18%] | 56 [18%] | 99 [16%] | 55 [18%] | 44 [14%] | 48 [17%] | 27 [18%] | 21 [17%] |
| Bone + viscera + soft tissue | 69 [7.8%] | 31 [6.9%] | 38 [8.7%] | 11 [4.6%] | 5 [4.2%] | 6 [4.9%] | 51 [7.9%] | 26 [7.8%] | 25 [7.8%] | 46 [7.4%] | 22 [7.3%] | 24 [7.8%] | 18 [6.5%] | 9 [6.0%] | 9 [7.1%] |
| Brain +/− any other distant site | 76 [8.6%] | 39 [8.7%] | 37 [8.4%] | 41 [17%] | 21 [18%] | 20 [16%] | 35 [5.4%] | 18 [5.4%] | 17 [5.4%] | 52 [8.4%] | 26 [8.6%] | 26 [8.4%] | 29 [11%] | 12 [8.0%] | 17 [13%] |
| Brain | 52 [5.9%] | 26 [5.8%] | 26 [5.9%] | 31 [13%] | 15 [13%] | 16 [13%] | 21 [3.2%] | 11 [3.3%] | 10 [3.2%] | 37 [6.0%] | 19 [6.3%] | 18 [5.8%] | 16 [5.8%] | 6 [4.0%] | 10 [8.0%] |
| Brain + any other distant site | 24 [2.7%] | 13 [2.9%] | 11 [2.5] | 10 [4.2%] | 6 [5.3%] | 4 [3.3%] | 14 [2.2%] | 7 [2.1%] | 7 [2.2%] | 15 [2.4%] | 7 [2.3%] | 8 [2.6%] | 13 [4.7%] | 6 [4.0%] | 7 [5.6%] |
| Liver +/− other than brain | 273 [31%] | 131 [29%] | 142 [32%] | 65 [27%] | 34 [29%] | 31 [25%] | 208 [32%] | 97 [29%] | 111 [35%] | 187 [30%] | 86 [29%] | 101 [33%] | 86 [31%] | 45 [30%] | 41 [33%] |
| Liver | 150 [17%] | 68 [15%] | 82 [18%] | 38 [16%] | 21 [18%] | 17 [14%] | 111 [17%] | 47 [14%] | 65 [21%] | 108 [17%] | 47 [16%] | 61 [20%] | 42 [15%] | 21 [14%] | 21 [17%] |
| Liver + other | 123 [14%] | 63 [14%] | 60 [13%] | 27 [11%] | 13 [12%] | 14 [11% | 95 [15%] | 50 [15%] | 46 [15%] | 79 [13%] | 39 [13%] | 40 [13%] | 44 [16%] | 24 [16%] | 20 [16%] |
| Lung/pleura +/− other than brain or liver | 159 [18%] | 82 [18%] | 77 [17%] | 58 [24%] | 24 [21%] | 34 [28%] | 101 [16%] | 58 [17%] | 43 [14%] | 104 [17%] | 50 [17%] | 54 [17%] | 56 [20%] | 32 [21%] | 24 [19%] |
| Lung/pleura | 117 [13%] | 68 [15%] | 49 [11%] | 41 [17%] | 19 [17%] | 22 [18%] | 76 [12%] | 49 [15%] | 27 [8.6%] | 75 [12%] | 42 [14%] | 33 [11%] | 42 [15%] | 26 [17%] | 16 [13%] |
| Lung/pleura + other | 42 [4.7%] | 14 [3.1%] | 28 [6.4%] | 17 [7.1%] | 5 [4.4%] | 12 [9.8%] | 25 [3.9%] | 9 [2.7%] | 16 [5.1%] | 29 [4.7%] | 8 [2.7%] | 21 [6.8%] | 14 [5.1%] | 6 [4.0%] | 8 [6.3%] |
| Soft tissue +/− other than brain. liver, lung, pleura | 86 [9.7%] | 38 [8.4%] | 48 [11%] | 25 [10%] | 8 [7.1%] | 17 [14%] | 61 [9.4%] | 30 [9.0%] | 31 [9.8%] | 60 [9.7%] | 23 [7.6%] | 37 [12%] | 26 [9.4%] | 15 [10%] | 11 [8.7%] |
| Viscera not specified | 30 [3.4%] | 11 [2.4%] | 19 [4.3%] | 16 [6.7%] | 8 [7.1%] | 8 [6.5%] | 14 [2.2%] | 3 [0.89%] | 11 [3.5%] | 22 [3.5%] | 5 [1.7%] | 17 [5.5%] | 8 [2.9%] | 6 [4.0%] | 2 [1.6%] |
*Includes bone relapses after local recurrence.
3.2. Impact of ER on patterns of disease recurrence
The recurrences by ER status in the control group of patients are shown in Table 4, Table 5. More relapses occurred in patients with ER− disease (RR = 1.43: ER− 143/356 [40%]; ER+ 363/1322 [28%]). Locoregional recurrences (RR = 3.62: ER− 41/356 [12%]; ER+ 42/1322 [3.2%]) and extra-skeletal recurrences, both with (RR = 2.84: ER− 83/356 [23%]; ER+ 107/1322 [8.1%]) and without (RR = 2.05: ER− 59/356 [17%]; ER+ 107/1322 [8.1%]) synchronous bone metastases were more frequent in patients with ER− disease. Bone only metastatic disease was associated with the presence of ER+ disease (RR = 1.79: ER+ 125/1322 [9.5%]; ER− 19/356 [5.3%]). ER status had greatest impact on the development of brain metastases, both as the only site of recurrence and in combination with other metastatic sites (RR = 3.30 (21/118 [ER− 18%]; ER+ 18/334 [5.4%]).
Table 4.
Impact of oestrogen receptor (ER) and menopausal status on patterns of recurrence in the control arm.
| First site[s] of recurrence | All control patients | ER −ve patients | ER +ve/unknown patients |
|---|---|---|---|
| 1678 | n = 356 | n = 1322 | |
| Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | |
| All first recurrences | 508 [30%] | 143 [40%] | 363 [28%] |
| Distant +/− local | 433 [26%] | 102 [29%] | 321 [24%] |
| Distant recurrence only | 368 [22%] | 83 [23%] | 285 [22%] |
| Distant + local Recurrence | 55 [3.3%] | 19 [5.3%] | 36 [2.7%] |
| Bone only distant recurrence | 144 [8.6%] | 19 [5.3%] | 125 [9.5%] |
| Extraskeletal distant recurrence +/− bone | 279 [17%] | 83 [23%] | 196 [15%] |
| Extraskeletal recurrence only | 166 [10%] | 59 [17%] | 107 [8.1%] |
| Local +/− distant | 138 [8.2%] | 60 [17%] | 78 [5.9%] |
| Local Recurrence | 83 [4.9%] | 41 [12%] | 42 [3.2%] |
| Postmenopausal status | Pre/peri and uncertain menopausal status | ||
| All | Control | Control | |
| n = 1678 | n = 522 | n = 1156 | |
| Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | |
| All first recurrences | 508 [30%] | 167 [32%] | 339 [29%] |
| Distant +/− local | 423 [25%] | 136 [26%] | 287 [25%] |
| Distant recurrence only | 368 [22%] | 116 [22%] | 252 [22%] |
| Distant + local Recurrence | 55 [3.3%] | 20 [3.8%] | 35 [3.0%] |
| Bone only distant recurrence | 144 [8.6%] | 36 [6.9%] | 108 [9.3%] |
| Extraskeletal distant recurrence +/− bone | 279 [17%] | 100 [19%] | 179 [15%] |
| Extraskeletal recurrence only | 166 [10%] | 61 [12%] | 105 [9.1%] |
| Local +/− distant | 138 [8.2%] | 51 [9.8%] | 87 [7.5%] |
| Local Recurrence | 83 [4.9%] | 31 [5.9%] | 52 [4.5%] |
Table 5.
Interaction between treatment arm and both oestrogen receptor (ER) and menopausal status on patterns of recurrence.
| First site[s] of recurrence | ER −ve patients | ER +ve/unknown patients | ||||
|---|---|---|---|---|---|---|
| All | Control | Zol | All | Control | Zol | |
| n = 706 | n = 356 | n = 350 | n = 2653 | n = 1322 | n = 1331 | |
| Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | |
| All first recurrences | 287 [41%] | 143 [40%] | 144 [41%] | 715 [27%] | 363 [28%] | 352 [26%] |
| Distant +/− local | 212 [30%] | 102 [29%] | 110 [31%] | 612 (23%) | 321 [24%] | 291 [22%] |
| Distant recurrence only | 175 [25%] | 83 [23%] | 92 [26%] | 546 [21%] | 285 [22%] | 261 [20%] |
| Distant + local Recurrence | 37 [5.2%] | 19 [5.3%] | 18 [5.1%] | 66 [2.5%] | 36 [2.7%] | 30 [2.3%] |
| Bone only distant recurrence | 31 [4.4%] | 19 [5.3%] | 12 [3.4%] | 217 [8.2%] | 125 [9.5%] | 92 [7.0%] |
| Extraskeletal distant recurrence +/− bone | 181 [26%] | 83 [23%] | 98 [28%] | 395 [15%] | 196 [15%] | 199 [15%] |
| Extraskeletal recurrence only | 145 [21%] | 59 [17%] | 86 [25%] | 222 [8.4%] | 107 [8.1%] | 115 [8.7%] |
| Local +/− distant | 114 [16%] | 60 [17%] | 52 [15%] | 169 [6.4%] | 78 [5.9%] | 91 [6.9%] |
| Local Recurrence | 77 [11%] | 41 [12%] | 34 [9.7%] | 103 [3.9%] | 42 [3.2%] | 61 [4.6%] |
| Postmenopausal status | Pre/peri and uncertain menopausal status | |||||
| All | Control | Zol | All | Control | Zol | |
| n = 1041 | n = 522 | n = 519 | n = 2318 | n = 1156 | n = 1162 | |
| Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | Number of recurrences [%] | |
| All first recurrences | 313 [30%] | 167 [32%] | 136 [26%] | 699 [30%] | 339 [29%] | 360 [31%] |
| Distant +/− local | 252 [24%] | 136 [26%] | 116 [22%] | 572 [25%] | 287 [25%] | 285 [25%] |
| Distant recurrence only | 221 [21%] | 116 [22%] | 105 [20%] | 500 [22%] | 252 [22%] | 248 [21%] |
| Distant + local Recurrence | 31 [3.0%] | 20 [3.8%] | 11 [2.1%] | 72 [3.1%] | 35 [3.0%] | 37 [3.2%] |
| Bone only distant recurrence | 66 [6.3%] | 36 [6.9%] | 30 [5.8%] | 182 [7.9%] | 108 [9.3%] | 74 [6.4%] |
| Extraskeletal distant recurrence +/− bone | 186 [18%] | 100 19%] | 86 [17%] | 394 [17%] | 179 [15%] | 215 [19%] |
| Extraskeletal recurrence only | 117 [11%] | 61 [12%] | 56 [11%] | 260 [11%] | 105 [9.1%] | 155 [13%] |
| Local +/− distant | 82 [7.9%] | 51 [9.8%] | 31 [6.0%] | 199 [8.9%] | 87 [7.5%] | 112 [9.6%] |
| Local Recurrence | 51 [4.9%] | 31 [5.9%] | 20 [3.9%] | 127 [5.5%] | 52 [4.5%] | 75 [6.5%] |
The rate of recurrence in the early years was faster in women with ER− tumours. After 24 months, invasive disease recurrence had occurred in 27% of ER− but only 8% of ER+ tumours. By 60 months 42% of ER− and 21% of ER+ women had developed recurrent disease. Thereafter the rates of recurrence were less than 1% and 3–4% per year from ER− and ER+ disease respectively.
3.3. Impact of menopausal status on patterns of disease recurrence
The risks for recurrence were similar across the menopausal subgroups (RR = 0.91: NPM 339/1156 [29%]; PM 167/522 [32%]). Table 5 shows these data including the 86 distant recurrences that developed after a local recurrence. Somewhat surprisingly first recurrence in bone (with or without other metastatic sites) appeared to be slightly more common (RR = 1.17) in NPM patients (198/1156 [17%]) compared with the PM subgroup (80/522 [15%]). However, this probably reflects the slightly higher proportion of ER− disease in PM patients (25%) recruited to the trial compared with the NPM subgroup (20%).
3.4. Effects of adjuvant zoledronate on disease outcomes
Although zoledronate had no statistically or clinically significant effect on recurrence rates overall (Table 5), it did reduce the proportion of patients developing bone metastases as the first site of recurrence both as the only metastatic site (RR = 0.72: zoledronate 104/1681 [6.2%]; control 144/1678 [8.6%]) and in combination with other metastatic sites (RR = 0.78: zoledronate 202/1681 [12%]; control 259/1678 [15%]). Bone recurrences were reduced in both menopausal subgroups and in those with either ER− or ER+ disease. However, extra-skeletal recurrences were more frequent in patients receiving zoledronate, particularly in NPM patients (RR 1.48: zoledronate 155/1678 [9.2%]; control 105/1681 [6.3%]) and those with ER− disease (RR 1.21: zoledronate 201/1681 [12%]; control 166/1678 [9.9%]).
Although the numbers are quite small, prior treatment with zoledronate also appeared to influence the development of bone metastases after recurrence of disease at an extra-skeletal site (RR = 0.76: zoledronate 72/211 [34%], control 74/166 [45%]) of patients relapsing first at an extra-skeletal distant site.
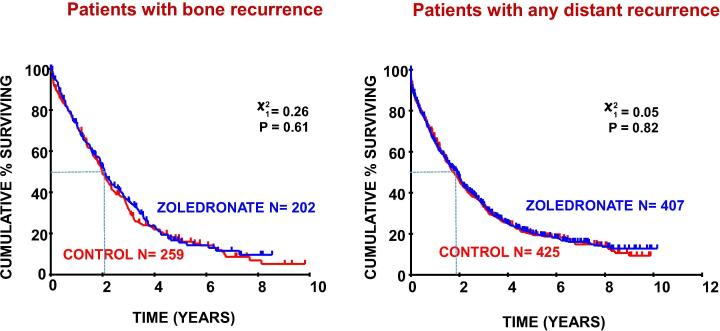
As reported previously, [6] overall survival was similar in the zoledronate and control groups (adjusted hazard ratio [HR] = 0.92; 95% CI 0.81–1.05) with a trend in favour of treatment with zoledronate in PM patients (HR = 0.84′ 95%CI 0.67–1.04). In this study we evaluated the impact of adjuvant zoledronate on survival after disease recurrence. Fig. 2 shows zoledronate had no effect on duration of survival after disease recurrence, either overall or in those specifically developing bone metastases.
Fig. 2.
Survival after recurrence by treatment group.
4. Discussion
In this descriptive analysis of the natural history of women with stage II/III breast cancer diagnosed between 2003 and 2006, the pattern of metastases with contemporary locoregional and adjuvant systemic treatments has been evaluated. Follow-up was performed systematically according to a clinical trial protocol throughout the 10 years and the site(s) of first recurrence and any subsequent development of bone metastases identified. Disease recurrence occurred in 30% of patients, illustrating the relatively low annual rate of around 3% per year in what would traditionally have been considered a population of patients at high risk for recurrence. 22% of patients had a distant recurrence, 5.3% an isolated locoregional relapse and 3.1% synchronous distant and locoregional recurrences at 10 years.
The treatment of patients reported in this analysis is representative of those presenting today other than with regard to the use of adjuvant HER2 targeted treatment, and may be of assistance to those designing adjuvant trials in helping with power calculations for likely recurrence rates. Because of the time frame of recruitment, evaluation of HER2 status was performed in only half of the patients. About 250/1692 patients with unknown HER2 status could be estimated to have had HER2+ disease and thus been eligible for adjuvant trastuzumab. In patients with HER2+ disease untreated with trastuzumab and with similar risk factors for relapse to our population, the proportion experiencing disease recurrence within 10 years of diagnosis in the control arms of the adjuvant trastuzumab trials was about 40% [13]. Based on the benefits seen in 10-year outcomes with the addition of trastuzumab reported in these trials, and subsequent refinement in HER2 targeted adjuvant therapy strategies [14], [15], around 20 recurrences (4%) in each treatment group might be expected to have been preventable with current HER2 targeted treatment approaches, thereby potentially reducing the 10-year rate of disease recurrence rate further from the 30% we report here to around 26%.
As expected [16], the proportion of patients developing disease recurrence was higher in those with ER− disease compared to those with ER+ tumours. Visceral, and especially brain metastases, were more frequent from ER− tumours and bone metastases were associated with ER+ disease. Interactions between ER and HER2 status on patterns of recurrence could not be assessed reliably due to missing HER2 status in one half of patients in the trial. Locoregional recurrence was also more frequent in those with ER− disease. The rates and distribution of disease recurrence in this study were similar in the PM and NPM subgroups. Premenopausal women are typically at higher risk of relapse due to the higher proportion with ER− disease [17] but, in this study, the proportions of patients with ER− disease were somewhat higher in the PM subgroup, likely reflecting patient selection for inclusion in a clinical trial of an intravenous treatment and the very high proportion in this study considered by their treating oncologist to require adjuvant chemotherapy.
As reported previously [12], adjuvant zoledronate did not improve disease outcomes for the overall study population, a finding that is consistent across the many trials evaluated by the Early Breast Cancer Trialists Collaborative Group (EBCTCG) meta-analysis [2]. However, treatment did reduce first recurrence of disease in bone, both as the only site of recurrence and occurring synchronously with other distant sites. This beneficial effect on bone metastases was seen in both ER− and ER+ tumours and in NPM as well as PM women. Extra-skeletal distant metastases however were more frequent in patients treated with adjuvant zoledronate, especially in NPM patients who would be expected to have higher levels of reproductive hormones in the bone microenvironment that zoledronate specifically targets. Indeed, in women aged less than 40 years, we have previously shown that this increase in risk for extra-skeletal metastases was associated with a 67% (95%C.I. 16–140%) increased risk of breast cancer death [6]. Metastasis is a highly complex, non-linear process with evidence, at least from animal models, that re-seeding by circulating tumour cells (CTC) after development of metastasis in one organ may occur at other distant and/or loco-regional sites [18]. Our observation that bone metastases after first recurrence at an extra-skeletal site were reduced in patients treated with zoledronate in the adjuvant setting suggests possible protection from re-seeding of CTC released from non-osseus sites.
Potential mechanisms underpinning the relationships between disease outcomes with adjuvant zoledronate and menopausal status and tumour biology are being actively studied. The relationships between menopause and outcome have been recapitulated in animal models, with zoledronate reducing metastases in young mice subjected to oophorectomy but not in those undergoing a sham operation [19]. More recently, the same group of researchers has evaluated the effects of zoledronate on the immune response to breast cancer and found that zoledronate causes a decrease in tumour suppression within the tumour microenvironment. This appeared to be mediated by a decrease in Treg infiltration and activity, an increase in macrophage polarisation towards an antitumour phenotype and an increase in γδ T cell antigen recognition [20]. These immune effects of a bisphosphonate may, at least in part, explain the difference in outcomes between adjuvant bisphosphonates and the even more potent inhibitor of osteoclast activity, denosumab. This agent failed to improve disease outcomes in stage II/III breast cancer [21] even though its effects on bone cell function are similar to a bisphosphonate, perhaps because it does not have the same immune modulating effects as the bisphosphonates.
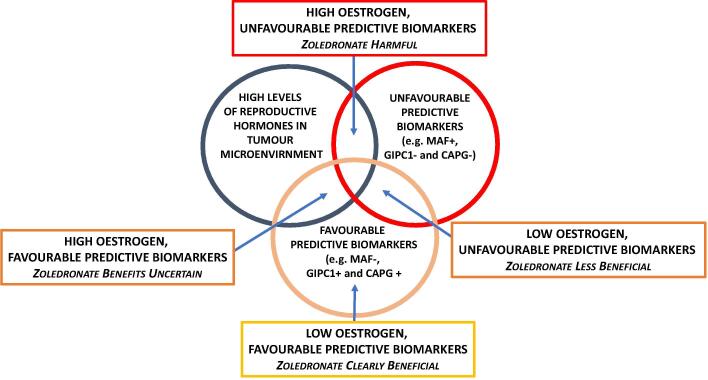
In terms of tumour biology, a number of biomarkers have been identified that, in addition to providing prognostic information [22], may also provide predictive information on likely response to adjuvant zoledronate and facilitate patient selection for treatment beyond the current criteria of recurrence risk and menopausal status [4], [5], [6]. These include immunohistochemical staining of the primary tumour for a number of bone homing peptides, including macrophage-capping protein (CAPG) and PDZ domain–containing protein member 1 (GIPC1) [23] and fluorescence in-situ hybridisation (FISH) analysis of the primary tumour for copy number of the transcription factor MAF [24]. MAF appears particularly promising as a predictive marker with benefits from zoledronate restricted to the 80% of patients with normal levels of MAF expression (MAF-) and occurring irrespective of menopausal status while, in those patients with amplification of MAF (MAF+), zoledronate treatment was associated with a marked excess of extra-skeletal metastases and worse overall survival [6], [25]. A schema illustrating the possible interactions between zoledronate treatment, menopausal status and tumour biology is shown in Fig. 3.
Fig. 3.
Schema representing interactions between menopausal status (microenvironment levels of oestrogen), tumour associated biomarkers and treatment with zoledronate. Use of adjuvant zoledronate in combination with premenopausal levels of reproductive hormones and unfavourable biomarker expression such as amplification of the transcription factor MAF is associated with adverse disease outcomes. Conversely, use of adjuvant zoledronate in combination with postmenopausal levels of reproductive hormones and favourable tumour biomarkers such as normal levels of MAF or/and expression of CAPG/GIPC1 is associated with improved disease outcomes [23], [25].
Survival after recurrence was unaffected by prior treatment with bisphosphonates despite the previously reported beneficial impact on skeletal complications after recurrence, especially those with relapse in bone [26]. Median survival after recurrence was only 22 months and it is salutary to note that, for those women who do develop recurrent disease, despite current adjuvant systemic and locoregional recurrences, survival after relapse has changed little over recent decades [27]. Recent developments beyond trastuzumab for those with HER2+ disease [28] and the use of CDK4 inhibitors in ER+ disease [29], neither of which were in routine use for the treatment of patients included in this population of patients, are improving survival but only by a matter of months and breast cancer remains the leading cause of cancer death in women in North America and Europe [30].
In conclusion, patients with stage II/III breast cancer can anticipate a relatively good prognosis with a 10-year recurrence rate of less than 30%. Bone remains the most common site for metastasis and, just as it did 3 decades ago [10], occurs in 70% of patients with advanced breast cancer. Adjuvant treatment with zoledronate changes the distribution pattern of metastases, reducing bone relapses but not recurrences at other sites and, in women with premenopausal levels of reproductive hormones and/or adverse tumour characteristics may promote disease spread to visceral sites. Novel bone targeted strategies are needed to further improve disease outcomes and reliable biomarkers identified to optimise patient selection for bone targeted treatments.
Funding statement
No specific funding was involved for these analyses. The AZURE trial was funded through an unrestricted grant from Novartis and research support from the National Institute for Health Research Cancer Research Network in the United Kingdom and similar organisations in the other participating countries.
CRediT authorship contribution statement
Stella D'Oronzo: Formal analysis, Investigation, Writing - original draft, Writing review & editing. Walter Gregory: Formal analysis, Writing review & editing. Simon Nicholson: Investigation, Writing review & editing. Yuen Khong Chong: Investigation, Writing review & editing. Janet Brown: Investigation, Project administration, Writing review & editing. Robert Coleman: Conceptualisation, Formal analysis, Methodology, Project administration, Writing original draft, Writing review & editing.
Conflicts of interest statement
RC reports personal fees from Amgen, ITM for speaking engagements, consultancy fees from Amgen, Astra Zeneca, Biocon, Boehringer Ingelheim and Scancell, Advisory Board membership for Inbiomotion, ITM and Scancell and intellectual property rights and patent holder with Inbiomotion. JB reports personal fees from Novartis, Amgen, BMS, Ipsen, MSD, Sandoz and Bayer, with research grants from Amgen and Bayer. SO and SN have no conflicts to report
Funding statement: No specific funding was involved for these analyses. The AZURE trial was funded through an unrestricted grant from Novartis and research support from the National Institute for Health Research Cancer Research Network in the United Kingdom and similar organisations in the other participating countries.
References
- 1.Pondé N.F., Zardavas D., Piccart M. Progress in adjuvant systemic therapy for breast cancer. Nat. Rev. Clin. Oncol. 2019;16(1):27–44. doi: 10.1038/s41571-018-0089-9. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 3.Coleman R., Hadji P., Body J.J. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020;31(12):1650–1663. doi: 10.1016/j.annonc.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Dhesy-Thind S., Fletcher G.G., Blanchette P.S. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: A cancer care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017;35:2062–2081. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 5.Hadji P., Coleman R.E., Wilson C. Adjuvant bisphosphonates in early breast cancer: Consensus guidance for clinical practice from a European Panel. Ann. Oncol. 2016;27(3):379–390. doi: 10.1093/annonc/mdv617. [DOI] [PubMed] [Google Scholar]
- 6.Coleman R.E., Collinson M., Gregory W. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04) J Bone Oncol. 2018;13:123–135. doi: 10.1016/j.jbo.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saarto T., Vehmanen L., Virkkunen P., Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43(7):650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 8.Bartmann C., Wischnewsky M., Stüber T. Pattern of metastatic spread and subcategories of breast cancer. Arch. Gynecol. Obstet. 2017;295:211–223. doi: 10.1007/s00404-016-4225-4. [DOI] [PubMed] [Google Scholar]
- 9.Body J.-J., Quinn G., Talbot S. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit. Rev. Oncol. Hematol. 2017;115:67–80. doi: 10.1016/j.critrevonc.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Coleman R.E., Rubens R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer. 1987;55(1):61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman R.E., Marshall H., Cameron D. Breast cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 12.Coleman R., Cameron D., Dodwell D. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 13.Chumsri S., Li Z., Serie D.J., Mashadi-Hossein A. Incidence of late relapses in patients with HER2-positive breast cancerreceiving adjuvant trastuzumab: combined analysis of NCCTG N9831 (Alliance) and NRG Oncology/NSABP B-31. J. Clin. Oncol. 2019;37(35):3425–3435. doi: 10.1200/JCO.19.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron D., Piccart-Gebhart M.J., Gelber R.D. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denduluri N., Somerfield M.R., Chavez-MacGregor M. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO Guideline Update. J. Clin. Oncol. 2021 Feb 20;39(6):685–693. doi: 10.1200/JCO.20.02510. [DOI] [PubMed] [Google Scholar]
- 16.Ignatov A., Eggemann H., Burger E., Ignatov T. Patterns of breast cancer relapse in accordance to biological subtype. J. Cancer Res. Clin. Oncol. 2018;144:1347–1355. doi: 10.1007/s00432-018-2644-2. [DOI] [PubMed] [Google Scholar]
- 17.Harbeck N., Penault-Llorca F., Cortes J. Breast cancer. Nat. Rev. Dis. Primers. 2019;5(1):66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim M.Y., Oskarsson T., Acharyya S. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottewell P.D., Wang N., Brown H.K. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin. Cancer Res. 2014;20(11):2922–2932. doi: 10.1158/1078-0432.CCR-13-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George C.N., Canuas-Landero V., Theodoulou E. Oestrogen and zoledronic acid driven changes to the bone and immune environments: Potential mechanisms underlying the differential anti-tumour effects of zoledronic acid in pre- and post-menopausal conditions. J. Bone Oncol. 2020;25 doi: 10.1016/j.jbo.2020.100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman R.E., Finkelstein D., Barrios C.H. Adjuvant denosumab in early breast cancer: primary results from the international, multicenter, randomized, phase 3, placebo-controlled D-CARE study. Lancet Oncol. 2020;21(1):60–72. doi: 10.1016/S1470-2045(19)30687-4. [DOI] [PubMed] [Google Scholar]
- 22.D'Oronzo S., Brown J., Coleman R. The role of biomarkers in the management of bone-homing malignancies. J Bone Oncol. 2017;9:1–9. doi: 10.1016/j.jbo.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.J.A. Westbrook, D.A. Cairns, J. Peng, et al. CAPG and GIPC1: breast cancer biomarkers for bone metastasis development and treatment. JNCI J. Natl. Cancer Inst. 2016;108(4):djv360. [DOI] [PMC free article] [PubMed]
- 24.Pavlovic M., Arnal-Estapé A., Rojo F. Enhanced MAF oncogene expression and breast cancer bone metastasis. J. Natl Cancer Inst. 2015;107(12):djv256. doi: 10.1093/jnci/djv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman R., Hall A., Albanell J. Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. Lancet Oncol. 2017;18(11):1543–1552. doi: 10.1016/S1470-2045(17)30603-4. [DOI] [PubMed] [Google Scholar]
- 26.Wilson C., Bell R., Hinsley S. Adjuvant zoledronic acid reduces fractures in breast cancer patients; an AZURE (BIG 01/04) study. Eur. J. Cancer. 2018;94:70–78. doi: 10.1016/j.ejca.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 27.R.E. Coleman, P. Smith, R.D. Rubens, Clinical course and prognostic factors following bone recurrence from breast cancer. Br. J. Cancer 1998; 77(2), 336–340. [DOI] [PMC free article] [PubMed]
- 28.Cardoso F., Paluch-Shimon S., Senkus E. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann. Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piezzo M., Chiodini P., Riemma M. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: A systematic review and meta-analysis. Int. J. Mol. Sci. 2020;21(17):6400. doi: 10.3390/ijms21176400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOSCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]