Highlights
-
•
Various skin lesions have been reported in systemic E. rhusiopathiae infection.
-
•
This study describes a case of E. rhusiopathiae bacteremia with generalized annular plaques consistent with Sweet syndrome.
-
•
This report originally demonstrates Sweet syndrome associated with E. rhusiopathiae bacteremia.
Keywords: Annular eruption, Eysipelothrix rhusiopathiae infection, Eysipelothrix rhusiopathiae bacteremia, Neutrophilic dermatoses, Sweet syndrome
Abstract
Erysipelothrix rhusiopathiae is a gram-positive bacillus causing three clinical syndromes in humans, including localized cutaneous infection, diffuse cutaneous form, and systemic infection. Various skin lesions in systemic form have been reported; however, no comprehensive study has been conducted. Here we report a case of a 60-year-old woman who suffered from E. rhusiopathiae bacteremia with distinct generalized annular purplish plaques. Negative microbiological studies of the lesional skin sample combined with the histopathological study showing diffuse neutrophilic infiltration confirm the diagnosis of Sweet syndrome. This study documents Sweet syndrome as one of the cutaneous manifestations in systemic E. rhusiopathiae infection.
Introduction
Erysipelothrix rhusiopathiae is a gram-positive bacillus commonly found in both wild and domestic animals including swine, fish, poultry and sheep. Direct contact with them or their excretions is of paramount importance to be an infective risk factor [1,2]. There are three distinct clinical presentations of E. rhusiopathiae infection; localized cutaneous infection (erysipeloid of Rosenbach), a diffuse cutaneous form, and systemic infection [2,3]. Interestingly, various skin lesions distant from the portal of entry have been described in the systemic form. However, the pathogenesis of these rashes has never been elucidated [1,[3], [4], [5], [6], [7], [8], [9], [10]]. Here we report a case of systemic E. rhusiopathiae infection with generalized purpuric annular plaques consistent with Sweet syndrome.
Case presentation
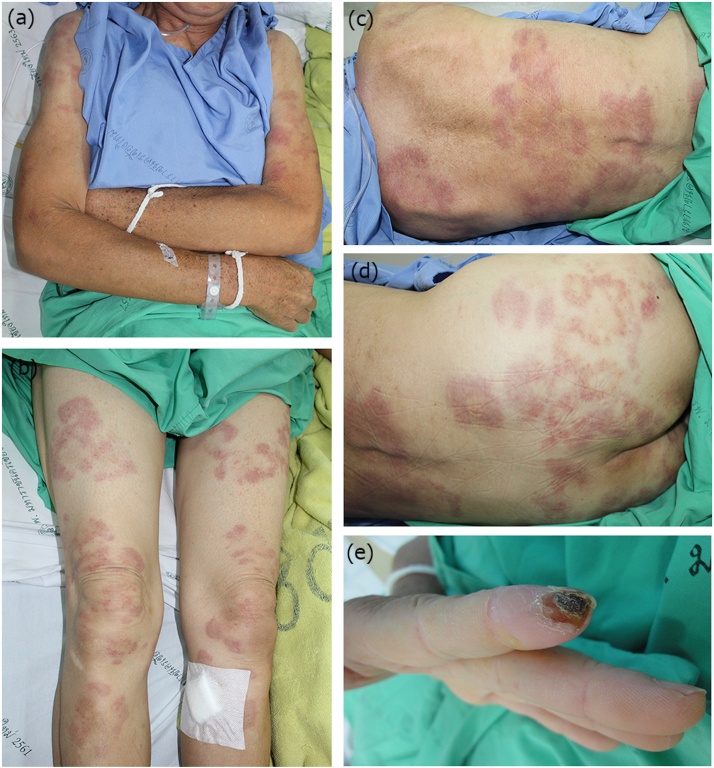
A 60-year-old unemployed woman presented with persistent high-grade fever and rash three days prior to admission. The skin eruption was initially noticed on her face, spreading to the trunk and all extremities overnight. The patient reported that she accidentally cut her left index finger with a cooking knife one month previously, resulting in an inflamed wound and a low-grade fever. At that time, she took an over-the-counter oral antibiotic and the wound was markedly improved within one week. Her past history included stable limited cutaneous systemic sclerosis. Physical examination showed a temperature of 39.7°c and the patient appeared acutely ill. No heart murmur was detected. Dermatological examination revealed multiple annular erythematous plaques on her face, back and extremities (Fig. 1a–d). Focusing on the fingertip of her left index in which the cutting wound was previously reported, showed the healing wound with a hemorrhagic crust (Fig. 1e). Multiple telangiectasia on her upper chest corresponding with underlying systemic sclerosis were noted, albeit without cutaneous sclerodermal change. Other findings were unremarkable.
Fig. 1.
Cutaneous eruptions on day 1 of admission. Multiple annular erythematous plaques on her (a) arms, (b) thighs, legs, (c) back, and (d) buttocks. (e) Noted a single healing wound with hemorrhagic crust on the left index finger.
Complete blood count revealed hemoglobin of 8.5 g/dL, white blood cells of 15,230 cells/mm3 (neutrophils of 92.4 %, lymphocytes of 6.8 %) and platelets of 370,000/mm3. Renal and liver function tests, and urinalysis were unremarkable. E. rhusiopathiae was detected in the hemoculture taken on the first day of admission. An echocardiogram revealed no evidence of infective endocarditis.
Following informed consent from the patient, a skin biopsy of a lesion on the left thigh was performed. Papillary dermal edema with diffuse superficial and deep dermal neutrophilic infiltration with prominent nuclear dust were demonstrated. There were also endothelial swelling and sparse red blood cell extravasation without fibrinoid necrosis in the dermis. Inflammation of eccrine glands and mixed perivascular inflammatory cell infiltration in the subcutaneous layer were present. No septic emboli were seen. No organism was detected from gram, AFB, PAS or GMS staining. Tissue culture for bacteria, fungus, mycobacterium along with a 16S rRNA sequencing were all negative. Direct immunofluorescence of lesional skin showed negative staining for IgG, IgA, IgM, C3, C1q and fibrinogen. Intravenous ceftriaxone 2 g/day was empirically initiated on the first day of admission and continued for 10 days and then was switched to oral cefixime 200 mg twice a day. The total duration of antibiotics was four weeks. Colchicine was increased to 1.2 mg daily for one week in order to treat Sweet syndrome.
The patient became afebrile from day 2 after admission and the rash started to fade over time on day 3. At one month, the rash remained only as post inflammatory hyperpigmentation on the edges of lesions.
Discussion
There are various cutaneous manifestations in E. rhusiopathiae infection according to the form of disease. Firstly, the localized cutaneous form, the most common reported manifestation, is described as a local cellulitis resulting from the inoculation of pathogen to a break in the integrity of the injured skin. The skin lesion initially begins with a distinctive, well-demarcated, violaceous plaque with raised margins. It often involves the web spaces and dorsum of hands or fingers but does not progress beyond the wrist. When the borders expand, the central region clears without desquamation [1,11]. Secondly, the rarely described diffuse cutaneous form. The clinical course runs chronically, with the longest reported duration up to one year [3,12]. Widespread erythematous and edematous cutaneous lesions which appear at sites remote from the primary lesion with negative hemoculture are the hallmarks [4]. The rash is characterized by a violaceous wave-like band with an advancing border and central clearing exhibiting gyrated figures [3]. Lastly, the form of systemic infection is characterized by positive hemoculture highlighting the more severe entity [3]. Importantly, endocarditis should always be excluded. One third of the cases reported the antecedent or concurrent erysipeloid lesions [11].
We report a female patient with the systemic form of E. rhusiopathiae infection, confirmed by the positive hemoculture, without any organ involvement other than generalized skin eruptions. The portal of entry is believed to be the cut wound on the left index finger. The skin lesions primarily appeared distant to the injury site, progressed rapidly and responded well to the antimicrobial treatment. Although the widespread nature of the rash in our case was similar to the rash in the diffuse cutaneous form, but it was different in (i) the positive hemoculture; (ii) the rapid onset and resolution of the skin eruptions; and (iii) the negative microbiologic studies from the skin biopsy, including tissue staining, culture and 16S rRNA sequencing method, all of which support the diagnosis of the systemic form with cutaneous involvement [3,12].
The negative microbiological analysis and the generalized distribution of the skin lesions favor a reactive basis. Together with the typical aforementioned histopathology, Sweet syndrome was the final diagnosis. After extensive review, we found only one patient who had the systemic form of E. rhusiopathiae infection and suffered from generalized skin eruptions, resembling our case, in terms of morphology and clinical progression. Unfortunately, neither histopathology nor microbiological study of the skin lesion was described [9].
Sweet syndrome is clinically characterized by abrupt onset of tender erythematous papules, plaques or nodules. The typical histopathological finding is papillary dermal edema with diffuse dermal neutrophilic infiltration. Regarding the inciting causes, it is categorized into classic, malignancy-associated, and drug-induced Sweet syndrome. Various etiologies have been reported in classic Sweet syndrome, of which, infection is the majority in about 17–45 %. The upper respiratory tract is the most common site of infection, while gastrointestinal, hepatobiliary, and genitourinary tract infection have also been described [13,14]. The commonly identified causative pathogens were nontuberculous mycobacteria, tuberculosis, HIV, Streptococcus spp., Yersinia spp., and Salmonella spp. [13,15]. A literature search in international databases, including Cochrane library, Embase, Proquest, PubMed, ScienceDirect, and Scopus produced no previous report of Sweet syndrome in a patient with E. rhusiopathiae infection.
Penicillin is the drug of choice for treatment of infection caused by E. rhusiopathiae, whereas cephalosporins are the most appropriate antibiotics among alternatives [2]. This patient is allergic to penicillin and received intravenous ceftriaxone followed by oral cefixime with good clinical response.
In conclusion, we originally report the case of a systemic form of E. rhusiopathiae infection in a patient with generalized erythematous annular plaques consistent with Sweet syndrome. This finding may expand our understanding of the pathogenesis involved in the cutaneous manifestations of systemic E. rhusiopathiae infection.
Author statement
Salin Kiratikanon: Data Curation, Writing - Original Draft.
Harit Thongwitokomarn: Data Curation, Writing - Original Draft.
Romanee Chaiwarith: Writing - Review & Editing.
Parichat Salee: Writing - Review & Editing.
Pongsak, Mahanupab: Writing - Review & Editing.
Sirinda Jamjanya: Writing - Review & Editing.
Panjit Chieosilapatham: Writing - Review & Editing.
Nayanunt Prinyaroj: Writing - Review & Editing.
Napatra Tovanabutra: Writing - Review & Editing.
Siri Chiewchanvit: Writing - Review & Editing, Supervision.
Mati Chuamanochan: Conceptualization, Writing - Review & Editing, Supervision, Project administration.
Sources of funding
This research was not funded by any organization.
Ethical approval
This study was reviewed by the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University (study code: MED-2563-07754).
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in Chief of this journal on request.
Contributions
Writing the original draft: Salin Kiratikanon, Harit Thongwitokomarn.
Editing the manuscript: Romanee Chaiwarith, Napatra Tovanabutra, Siri Chiewchanvit, Mati Chuamanochan.
Interpretation of the results, discussion, review and approved the final version of the manuscript: All authors.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Grieco M.H., Sheldon C. Erysipelothrix rhusiopathiae. Ann N Y Acad Sci. 1970;174(2):523–532. doi: 10.1111/j.1749-6632.1970.tb45578.x. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J.E., Dolin R., Blaser M.J. In: Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 9 ed. Reboli A.C., editor. Elsevier; Philadelphia: 2020. [Google Scholar]
- 3.Klauder J.V. Erysipelothrix rhusiopathiae infection in swine and in human beings: comparative study of the cutaneous lesions. Arch Derm Syphilol. 1944;50(3):151–159. doi: 10.1001/archderm.1944.01510150003001. [DOI] [Google Scholar]
- 4.Barnett J.H., Estes S.A., Wirman J.A., Morris R.E., Staneck J.L. Erysipeloid. J Am Acad Dermatol. 1983;9(1):116–123. doi: 10.1016/s0190-9622(83)70116-7. [DOI] [PubMed] [Google Scholar]
- 5.Procter W.I. Subacute bacterial endocarditis due to Erysipelothrix rhusiopathiae. Report of a case and review of the literature. Am J Med. 1965;38:820–824. doi: 10.1016/0002-9343(65)90203-2. [DOI] [PubMed] [Google Scholar]
- 6.Mahavanakul W., Limmathurotsakul D., Teerawattanasuk N., Peacock S.J. Invasive Erysipelothrix rhusiopathiae infection in northeast Thailand. Southeast Asian J Trop Med Public Health. 2007;38(3):478–481. [PubMed] [Google Scholar]
- 7.Klauder J., Kramer W., Nicholas L., Kast C., Groskin L. Erysipelothrix rhusiopathiae septicemia: diagnosis and treatment: report of fatal case of erysipeloid. JAMA. 1943;122(14):938–943. doi: 10.1001/jama.1943.02840310030008. [DOI] [Google Scholar]
- 8.Gorby G.L., Peacock J.E., Jr. Erysipelothrix rhusiopathiae endocarditis: microbiologic, epidemiologic, and clinical features of an occupational disease. Rev Infect Dis. 1988;10(2):317–325. doi: 10.1093/clinids/10.2.317. [DOI] [PubMed] [Google Scholar]
- 9.Kositapantawong N., Siripaitoon P., Silpapojakul K. Erysipelothrix rhusiopathiae bacteremia with rare manifestation of diffused cutaneous skin lesions. J infect Dis Antimicrob Agents. 2011;28(4) [Google Scholar]
- 10.Borchardt K.A., Sullivan R.W., Blumberg R.S., Gelber R.H., Botch V., Crull S. Erysipelothrix rhusiopathiae endocarditis. West J Med. 1977;127(2):149–151. [PMC free article] [PubMed] [Google Scholar]
- 11.Kang S., Amagai M., Bruckner A., Enk A., Margolis D.J., McMichael A.J. In: Fitzpatrick’s dermatology. Norton S.A., CM A., editors. McGraw Hill; 2019. [Google Scholar]
- 12.Ehrlich J.C. Erysipelothrix rhusiopathiae infection in man; report of a case with cutaneous bullae, in which cure was achieved with penicillin. Arch Intern Med (Chicago, Ill: 1908) 1946;78(5):565–577. doi: 10.1001/archinte.1946.00220050070004. [DOI] [PubMed] [Google Scholar]
- 13.Marcoval J., Martín-Callizo C., Valentí-Medina F., Bonfill-Ortí M., Martínez-Molina L. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41(7):741–746. doi: 10.1111/ced.12899. [DOI] [PubMed] [Google Scholar]
- 14.Ratzinger G., Burgdorf W., Zelger B.G., Zelger B.G. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29(2):125–133. doi: 10.1097/01.dad.0000249887.59810.76. [DOI] [PubMed] [Google Scholar]
- 15.Heath M.S., Ortega-Loayza A.G. Insights into the pathogenesis of sweet’s syndrome. Front Immunol. 2019;10:414. doi: 10.3389/fimmu.2019.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]