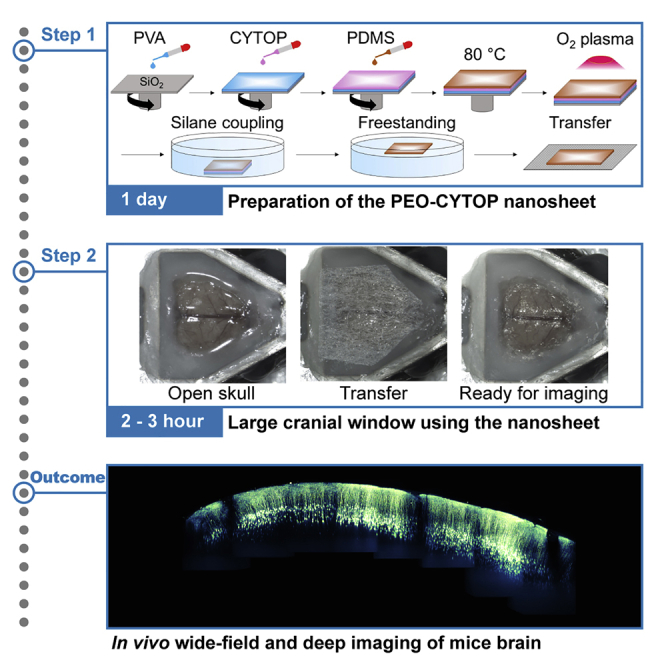
Summary
Large-scale optical measurements have revealed the anatomical and functional connectivity among brain regions underlying brain functions. Here, we describe how to construct a cranial window utilizing a polyethylene-oxide-coated CYTOP (PEO-CYTOP) nanosheet that suppresses bleeding on the brain surface of mice. We demonstrate in vivo two-photon imaging through the PEO-CYTOP nanosheet at the subcellular resolution in the parietal region of the mouse brain. This protocol improves the surgical procedure and expands the optically observable regions, thereby promoting understanding of brain function.
For complete details on the use and execution of this protocol, please refer to Takahashi et al. (2020).
Subject areas: Microscopy, Neuroscience
Graphical abstract
Highlights
-
•
Detailed protocol for constructing a vast cranial window for in vivo mouse brain imaging
-
•
Preparation and brain-sealing method of PEO-CYTOP nanosheet
-
•
Instruction to make a large cranial hole with a depressant for intracranial pressure
Large-scale optical measurements have revealed the anatomical and functional connectivity among brain regions underlying brain functions. Here, we describe how to construct a cranial window utilizing a polyethylene-oxide-coated CYTOP (PEO-CYTOP) nanosheet that suppresses bleeding on the brain surface of mice. We demonstrate in vivo two-photon imaging through the PEO-CYTOP nanosheet at the subcellular resolution in the parietal region of the mouse brain. This protocol improves the surgical procedure and expands the optically observable regions, thereby promoting understanding of brain function.
Before you begin
Prepare solutions for the fabrication of PEO-CYTOP nanosheet
Timing: 30–40 min
-
1.
Dissolve perfluoro(1-butenyl vinyl ether) polymer (commercially named CYTOP produced by AGC Inc.) in perfluorotributylamine at a concentration of 30 mg/mL.
Note: The amount of CYTOP solution depends on the size and number of nanosheets to be prepared. To prepare an approximately 40 × 40 mm2 piece of nanosheet, 0.5 mL of polymer solution is needed.
-
2.
Dissolve poly(vinyl alcohol) (PVA) in distilled water at 10 mg/mL. The volume of PVA solution may be the same as that of CYTOP solution.
-
3.
Mix the Sylgard 184 silicone elastomer base and curing agent at a 10:1 ratio by weight and dilute it in hexane at 1.25 wt% in a glass sample vial with a cap.
Note: To avoid an increase in concentration due to solvent evaporation, the solutions of CYTOP, PVA, and silicone should be stored at 4°C and used within one week after preparation. Please wear a mask when handling hexane as it is highly volatile and slightly neurotoxic. The volume of silicone solution may be the same as that of CYTOP solution.
Fabricate PEO-CYTOP nanosheet
Timing: 1 day
Note: The substrate for the spin-coating is a silicon wafer covered with a 200 nm silicon oxide layer, which is purchased from KST World Corp., Japan. The crystal structure of the silicon wafer is not specifically required. According to the size of the nanosheet, specifically the size of the cranial window to be prepared, the purchased wafer (4 inch diameter) is cut into an appropriately sized square using a diamond cutter and ruler by hand prior to use. The use of a precision diamond wafer scriber is better, if available.
-
4.
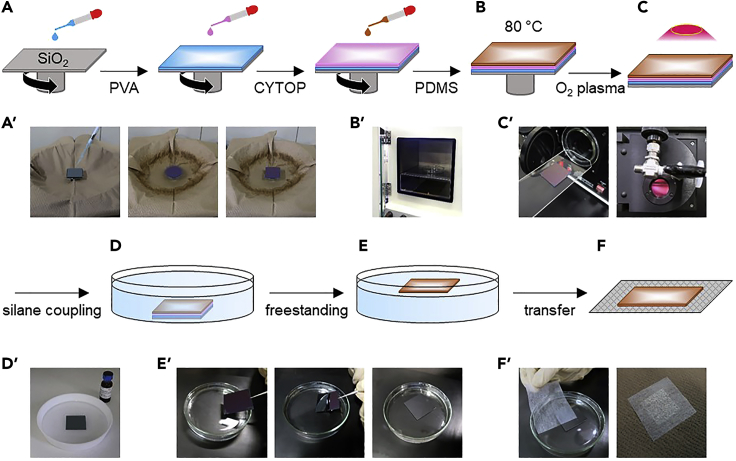
Drop PVA solution onto the silicon wafer using a glass dropper and then spin-coat PVA at 4000 rpm for 20 s to prepare a sacrificial layer (MS-A100, Mikasa Co., Ltd., Japan) (Figure 1A). Here, we use the term “sacrificial layer” to mean that this layer can be dissolved in water in the freestanding process, and the PEO-CYTOP nanosheet can then detach from the silicon wafer.
Note: There is no specific requirement for the volume of solution dropped onto the wafer, which indeed has no effect on the final thickness of the coating. However, to achieve a spatially uniform coating, full coverage of the solution on the wafer before spin-coating is recommended. Approximately 0.5 mL of solution is needed to coat a 40 × 40 mm2 silicon wafer. The interpretation of the volume of solution dropped on the wafer is applicable for steps 5 and 6 as well.
-
5.
Drop the CYTOP solution onto the PVA coated substrate and spin-coat it at 4000 rpm for 60 s.
-
6.
Drop the silicone solution onto the CYTOP coated substrate and spin-coat it at 4000 rpm for 20 s.
-
7.
Cure the composite at 80°C for 2 h (Figure 1B).
-
8.
Expose the composite to oxygen plasma at 11 W (medium operating power) for 60 s to obtain a hydrophilic surface (PDC-32G, Harrick Plasma, Inc., NY, USA) (Figure 1C).
Note: Oxygen plasma is a mixture of oxygen ions, radicals, ozone and neutral oxygen atoms, which is a general treatment to obtain a hydrophilic surface by introducing hydroxyl groups on polydimethylsiloxane (PDMS).
-
9.
Dissolve PEO-silane (2-(methoxy(polyethyleneoxy)propyl) trichlorosilane) in toluene at 2 mM in a Teflon Petri dish (ϕ: 150 mm, height: 30 mm, capacity: 300 mL, provided by AS ONE Corp., Japan).
-
10.
Place the substrate in a Teflon Petri dish filled with PEO-silane solution for 1 h to stabilize the hydrophilic surface for the long-term (Figure 1D).
Note: As PEO-silane is highly hydrolytic-sensitive, it should be dissolved just before use. While there is no specific requirement for the volume of PEO-silane solution, the processed substrates should be fully immersed in the solution. If one uses the above-described Teflon Petri dish, 100 mL of PEO-silane solution is sufficient for reacting with four pieces of 40 × 40 mm2 nanosheets. Note that PEO-silane may cause severe skin corrosion and eye damage. Please conduct this step in a fume hood while wearing the appropriate personal protective equipment, including gloves, clothing, eye protection, etc. Please refer to https://www.gelest.com/product/SIM6492.66/ for further safety information about PEO-silane.
-
11.
Immerse the substrate in water to dissolve the sacrificial layer and float the PEO-CYTOP nanosheet on the water's surface (Figure 1E).
-
12.
Transfer the PEO-CYTOP nanosheet to a nonwoven fabric with the hydrophilic side facing outwards (Figure 1F).
Note: One can easily differentiate the hydrophobic and hydrophilic sides by adding a drop of water to the surface. If the water forms a spherical droplet, this indicates the hydrophobic side; otherwise, if it wets the surface well, this indicates the hydrophilic side.
-
13.
Dry the PEO-CYTOP nanosheet on nonwoven fabric overnight (at least over 12 h).
Figure 1.
Procedures for preparing PEO-CYTOP nanosheet (schematics and photos taken at each step)
(A and A') Spin-coating.
(B and B') Thermal cure in an oven.
(C and C') Oxygen plasma treatment.
(D and D') Silane coupling.
(E and E') Freestanding in water.
(F and F') Transfer to the nonwoven fabric.
Prepare equipment for open-skull surgery
Timing: 5–10 min
-
14.
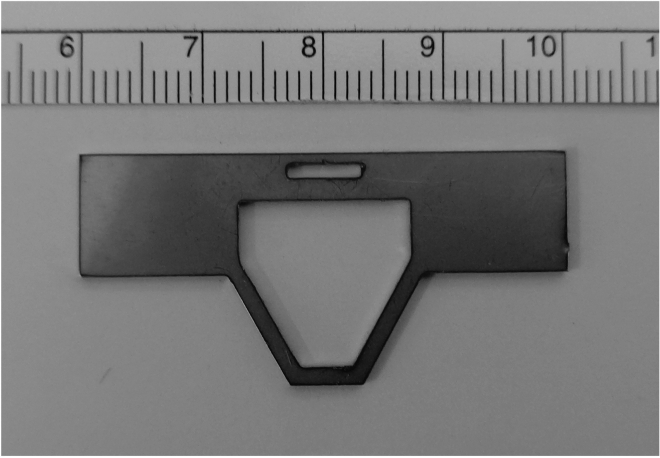
Manufacture the head plate (Figure 2) from an aluminum plate (1 mm thick) using machine tools or at the machine shop.
CRITICAL: The hole of the head plate must be larger than the region of the exposed skull. In this protocol, we use a custom-made head plate with a trapezoidal hole. Other shapes and head plates will work if the size requirements are met.
-
15.
Sterilize surgical tools and equipment, including scalpel, scissors, tweezers, drill bit, the head plate, and head holder with alcohol according to your protocol approved by the Institutional Animal Care and Use Committee (IACUC).
-
16.
Prepare the GLYCEOL Injection (anti-edema agent) (15 μL per body weight (μL/g)) and 200 μL xylocaine (local anesthetic) in individual syringes.
Figure 2.
The head plate with the large trapezoidal hole for fixation on a stage of microscopy with a scale ruler (1 mm increments)
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| CYTOP | AGC | CTX-809SP |
| Perfluorotributylamine | AGC | CT-Solv.180 |
| PVA (Mw, 22,000) | Kanto Chemical | N/A |
| Sylgard 184 silicone elastomer and curing agent | Dow Chemical | N/A |
| Hexane | Kanto Chemical | 18041 |
| Toluene | Kanto Chemical | 40180 |
| 2-(Methoxy(polyethyleneoxy)propyl) trichlorosilane (Mw, ~538) | Gelest | SIM6492.66 |
| GLYCEOL Injection | TAIYO Pharma | 2190501A5064 |
| Xylocaine 2% | Dentsply Sirona | N/A |
| Isoflurane | FUJIFILM Wako Pure Chemical Corporation | N/A |
| Other | ||
| Silicon wafer (200 nm SiO2 coated) | KST World Corp. | N/A |
| Spin coater MS-A100 | Mikasa | N/A |
| Plasma Cleaner PDC-32G | Harrick Plasma | N/A |
| Glass dropper | AS ONE Corp. | 2-2045-01 |
| Teflon Petri dish | AS ONE Corp. | NR0213-004 |
| Thermal oven | AS ONE Corp. | AVO-250NB |
| Nonwoven fabric substrate (disposable tea filter bag made from polyethylene and polypropylene) | Daiso | H-070 No. 475 |
| Head holder for Mice SG-4N | NARISHIGE | http://products.narishige-group.com/group1/SG-4N/stereotaxic/english.html |
| Drill bit #140 ϕ1.4 | Minitor | N/A |
| Scalpel | FEATHER Safety Razor | No.10 |
| Scissors | Fine Science Tools | N/A |
| Tweezers | Fine Science Tools | 11251-20 |
| Aluminum plate (SUS304) | NANYO SHOKAI | N/A |
| Ultimate XL | NAKANISHI | Y141446 |
| Dental adhesive resin cement Super-Bond C&B Bulk-mix | Sun Medical | https://www.sunmedical.co.jp/english/index.html |
| Spongel | LTL Pharma | N/A |
| Anesthetic vaporizer MK-A110D | Muromachi Kikai | https://muromachi.com/en/archives/english/1738/ |
| Ionosit baseliner | DMG | N/A |
| Suction Pump SP30 | Markos Mefar | N/A |
| Disposable heating pad mini | Lotte | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: B6.Cg-Tg(Thy1-YFP)HJrs/J | The Jackson Laboratory | 003782 |
Step-by-step method details
CRITICAL: All animal experiments must be performed in accordance with the guidelines of your institution. For each step, the type of drug or anesthetic used should be modified according to the applicable guidelines.
Expose the skull of the mouse
Timing: 1–2 h
This section follows previous protocols with partial modifications (Holtmaat et al., 2009; Goldey et al., 2014).
-
1.
Anesthetize the adult mouse (over 8 weeks old, regardless of gender) with isoflurane using an inhaled anesthetic (MK-A110D, Muromachi Kikai, Japan).
Note: For induction of anesthesia, the concentration of isoflurane is 3%. After induction, the mouse is anesthetized with 1.5% isoflurane for the remainder of this protocol including the imaging session.
CRITICAL: Change the concentration of the isoflurane depending on the condition of the animal. For example, the concentration should be decreased if the mouse breathes with large motions.
-
2.
Wipe the scalp hair with cotton swabs soaked in 70% ethanol.
-
3.
Apply an eye ointment (Tarivid, Santen Pharmaceutical, Japan) to the eyes to protect against drying.
-
4.Remove the scalp hair (Figure 3A).
-
a.Gently shave the hair using a razor blade.Alternatives: Application of hair-removal cream (Veet hair-removal cream for sensitive skin, Reckitt Benckiser) can be performed instead of shaving with a razor blade.
-
b.Clean the remaining hair with cotton swabs soaked in 70% ethanol.
-
a.
-
5.
Set the head in the head holder (SG-4N, Narishige, Japan).
Note: any stereotaxic frame can be used for this protocol.
-
6.
Apply local anesthesia (to the skin, e.g., a few drops of xylocaine).
-
7.
Wait 3 min for induction of local anesthesia.
-
8.
Cut the skin from the whole dorsal part of the head using scissors. This includes the parietal area as well as the frontal, temporal, and occipital regions (Figure 3B).
-
9.
Apply a few drops of xylocaine to the skull.
-
10.
Wait 3 min for induction of anesthesia.
-
11.
Push away the temporal and occipitalis muscle from the skull using tweezers.
-
12.
Gently remove the periosteum on the skull surface using a scalpel or cotton swab (Figure 3C).
CRITICAL: The periosteum should be removed entirely. To create a large cranial window, the head plate must be strongly fixed. troubleshooting problem 1.
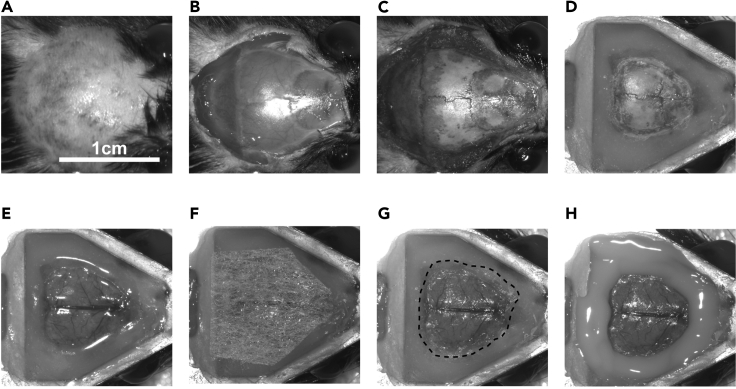
Figure 3.
Surgical procedures for a large cranial window utilizing the PEO-CYTOP nanosheet
(A) Remove the hair and clean the scalp. Scale bar: 1 cm.
(B) Remove the skin.
(C) Remove the periosteum.
(D) Fix the head plate with a super-bond.
(E) Remove the skull leaving the dura intact.
(F) Place the PEO-CYTOP nanosheet supported by the nonwoven fabric on the brain surface.
(G) Transfer the PEO-CYTOP nanosheet from the nonwoven fabric to the brain surface. The dotted line indicates the edge of the nanosheet.
(H) Fix the PEO-CYTOP nanosheet with an ultraviolet curable resin.
Fix the head plate
Timing: 30 min
This section describes how to fix the head plate on the skull.
-
13.
Place the head plate parallel to the transverse plane of the skull.
CRITICAL: If the head plate is non-parallel to the transverse plane of the skull, optical aberrations can occur, which may result in reduced image quality. To avoid this problem, the system reported by Kawakami et al. (2013) can be used to adjust the tilt of the head plate. Fix the head plate by applying a dental resin (Super-Bond C&B Bulk-mix, Sun Medical Company, Ltd., Japan) (Figure 1D).
CRITICAL: Cover the exposed skull entirely with dental resin since the fixation of the head plate is limited to a small area.
-
14.
Wait 15 min for the dental resin to cure.
Note: Ensure the head plate is firmly fixed to avoid the head plate detaching from the brain during imaging. troubleshooting problem 1.
Remove the skull
Timing: 1–2 h
This section describes how to expose the brain surface to create a large cranial window.
-
15.
Administer the GLYCEOL Injection (15 μL pe (15 μL per body weight (μL/g)) by intraperitoneal injection to reduce the brain tissue's intracranial pressure.
Note: This process makes it easier to remove the skull because the reduction in intracranial pressure increases the volume between the brain tissue and the skull.
Alternatives: 20% mannitol solution (15 μL per body weight (μL/g)) can be used instead of GLYCEOL Injection.
-
16.
Drill the skull to make the groove around the intentional craniotomy area using a drill bit (#140 ϕ1.4, Minitor, Japan). The skull should be drilled and thinned until the bone thickness can be easily pushed down by low pressure using the tweezers.
CRITICAL: After the begging of drilling, do not press the groove strongly with the drill bit vertical to the skull, as this can injure the brain tissue. Instead, move the drill bit in a circle along the edge of the intentional craniotomy area in a position horizontal to the skull, like scratching.
Note: The size of the window can be preferably defined. Unlike a glass coverslip, the PEO-CYTOP nanosheet can be easily cut to any size.
Note: Bone debris should be cleared away using a cotton swab or air.
-
17.Remove the skull (Figure 3E).
-
a.Soak the groove of the skull made by drilling with saline.
-
b.Insert the tweezers between the skull and the brain surface and gently remove the skull, leaving the dura intact.
-
a.
Note: The dura is sometimes stripped when the skull is removed. By reducing the intracranial pressure (step 15), the dura can be easily peeled from the skull.
CRITICAL: Keep the tweezers as parallel as possible to avoid injuring the dura and the brain tissue.
CRITICAL: Lifting the bone too quickly can tear the skull-connected blood vessels, which will severely injure the sagittal sinus. Therefore, it is important to slowly remove the skull while it is soaking in saline. troubleshooting problem 2.
-
18.
Stop the bleeding using a sponge hemostat (Spongel) soaked in saline.
CRITICAL: During this surgery, bleeding from the sagittal sinus is inevitable because the skull covering the sinus must be removed. However, a Spongel will stop the bleeding unless the blood vessels have been severely injured. troubleshooting problem 3.
Sealing the brain surface with a PEO-CYTOP nanosheet
Timing: 20–30 min
This step describes how to seal the brain surface with PEO-CYTOP nanosheet.
-
19.
Use scissors to cut the PEO-CYTOP nanosheet and the nonwoven fabric to a size suitable for covering the exposed region of the brain.
CRITICAL: The cut piece should be slightly larger than the exposed brain area to allow the edges of the PEO-CYTOP nanosheet to be glued. It is not necessary to cut the nanosheet to a strictly defined size, because the size of the nanosheet can be adjusted after attachment if it is larger than the exposed region of the brain.
-
20.Seal the brain surface with the PEO-CYTOP nanosheet (Figures 3F and 3G, Methods video S1).
-
a.Add a few drops of saline to the brain surface. Remove any excess water and slightly dry the surface using a paper towel or a suction pump (SP30, MARKOS MEFAR, Italy).
 CRITICAL: Do not dry the brain surface completely. A wet surface is necessary for the successful attachment of the nanosheet. The wetting procedure is required to transfer the PEO-CYTOP nanosheet to the brain surface because of its hydrophilic adhesive surface. However, nanosheet transfer cannot be achieved if the brain surface is too wet. troubleshooting problem 4.
CRITICAL: Do not dry the brain surface completely. A wet surface is necessary for the successful attachment of the nanosheet. The wetting procedure is required to transfer the PEO-CYTOP nanosheet to the brain surface because of its hydrophilic adhesive surface. However, nanosheet transfer cannot be achieved if the brain surface is too wet. troubleshooting problem 4.
-
b.Place the PEO-CYTOP nanosheet on the brain with the hydrophilic side to the brain surface (Figure 3F). The hydrophilic surface enhances the strength of the adhesion to the surface of the mouse brain, resulting in the suppression of bleeding from the brain surface.
-
c.Attach the PEO-CYTOP nanosheet to the brain surface by gently pushing it via the nonwoven fabric with the tweezers.
 CRITICAL: Do not push the PEO-CYTOP nanosheet too firmly, as this can cause injury and bleeding.
CRITICAL: Do not push the PEO-CYTOP nanosheet too firmly, as this can cause injury and bleeding. -
d.After transferring the nanosheet, peel the nonwoven fabric away using the tweezers.
 CRITICAL: The PEO-CYTOP nanosheet can be peeled off by applying saline to the brain surface. To redo the transfer, remove the already transferred nanosheet and then return to step 19.
CRITICAL: The PEO-CYTOP nanosheet can be peeled off by applying saline to the brain surface. To redo the transfer, remove the already transferred nanosheet and then return to step 19.
Methods video S1. Transferring the PEO-CYTOP nanosheet to the brain surface, related to step 20Download video file (64.7MB, mp4) -
a.
-
21.
Adjust the area of the PEO-CYTOP nanosheet covering the skull by scratching the area with tweezers. Keep the edge of the nanosheet approximately 0.5 - 1 mm away from the exposed brain area so there is sufficient area to glue the nanosheet.
Note: Do not reduce the area of the PEO-CYTOP nanosheet covering the skull too much, as the resin should not make direct contact with the exposed brain.
-
22.
Fix PEO-CYTOP nanosheet by gluing its edges to the head plate (the surface covered with dental resin) using ultraviolet curable resin (Ionosit baseliner, DMG, Germany) (Figure 3H).
CRITICAL: The brain surface may swell because the intracranial pressure is too high. Prior to fixation, the intracranial pressure can be reduced by administrating GLYCEOL Injection (5 μL per body weight (μL/g)) via intraperitoneal injection. troubleshooting problem 5.
Post-surgery care for the imaging session
Timing: 5–10 min
-
23.
Fix the mouse to the stage with a heating pad (Lotte, Japan) to maintain the body temperature during the imaging session.
-
24.
Keep the mouse anesthetized with 1.5% isoflurane during the imaging session to reduce body movement.
Expected outcomes
Using this protocol, we can implement a large cranial window with a size equal to that of the whole parietal region in the mouse brain by utilizing the flexibility of the PEO-CYTOP nanosheet. Through the window immediately after the surgery, we achieved in vivo two-photon imaging of neural structures in all cortical layers at a subcellular resolution (Methods video S2) and in vivo imaging of Ca2+ elevations with a vast field of view (Takahashi et al., 2020). The optical disturbance (e.g., refractive index mismatch) caused by the PEO-CYTOP nanosheet is almost negligible compared with a conventional cranial window using a glass coverslip because the thickness of the PEO-CYTOP nanosheet is ~10−3 smaller than that of the conventional coverslip (PEO-CYTOP nanosheet, ~130 nm; glass coverslip, ~170 μm). Moreover, re-bleeding does not occur because the PEO-CYTOP nanosheet firmly adheres to the brain surface, suppressing bleeding if the thickness of the PEO-CYTOP nanosheet used in the experiment is approximately 100 nm (Zhang et al., 2017). Importantly, the PEO-CYTOP nanosheet does not press against the brain tissue in the window; thus, there is no mechanical stress inside the brain and no disturbance of cerebrospinal fluid or blood flow. These advantages are effective to preserve the physiological conditions achieving in vivo imaging at a subcellular resolution. Besides, the absence of a coverslip enables to obtain an extra working distance for an objective lens, which is effective to expand optical observable depth especially in a high numerical aperture lens (Zhang et al., 2020).
Limitations
The PEO-CYTOP nanosheet cannot suppress motion artifacts because it does not press down on the brain surface. Thus, optical systems for real-time movement correction are required for imaging in awake mice (Karagyozov et al., 2018; Griffiths et al., 2020).
In a large cranial window, the curvature of the living brain surface can cause optical aberrations. Adaptive optical techniques proposed in previous studies can compensate for aberrations due to a curved surface (Matsumoto et al., 2018; Yamaguchi et al., 2020).
Troubleshooting
Problem 1
The head plate comes off during in vivo imaging (steps 12 and 14)
Potential solution
For strong fixation of the head plate, the periosteum should be perfectly removed. Additionally, slight drilling of the skull surface, the application of dental etchants, or both methods can be used to achieve a rough surface suitable for bonding.
Problem 2
Injure of the dura and the brain tissue during removing the bone (step 17).
Potential solution
To avoid Injure of the dura and the brain tissue during the bone removal, the bone needs to be thin enough. Large diameter drill bit used in this protocol is less likely to cause injure of the dura and the brain tissue than small diameter drill bits (0.3 – 0.6 mm in diameter) when thinning the skull.
Problem 3
Bleeding from the sagittal sinus does not stop (step 18).
Potential solution
Lift the skull from the dura and the sinus gradually and carefully, as described in step 17b. Although injury to the sagittal sinus cannot be avoided, the injured region and blood loss can be minimized. After removing the skull, place a piece of Spongel soaked in saline to the injured region until bleeding stops. Unless the sagittal sinus is severely damaged, the mouse should not die if proper hemostasis is applied. However, if the sinus is torn when the skull is removed, the mouse will certainly die.
Problem 4
PEO-CYTOP nanosheet transfer cannot be transferred from nonwoven fabric (step 20).
Potential solution
It is difficult to attach a PEO-CYTOP nanosheet to a dry surface. Make the brain surface wet by attaching a small piece of a Spongel soaked in saline. However, remove any excess water if the brain surface is too wet. Methods video S1 is helpful to know the degree of wetness.
Problem 5
Brain swelling appears during and/or after the surgery (step 22).
Potential solution
Swelling is caused by injury due to the craniotomy; thus, swelling does not begin during imaging. Administer GLYCEOL or mannitol via intraperitoneal injection during and/ or after the surgery (the dose of both cases is 5 μL per body weight (μL/g)) to reduce brain swelling. After administration swelling should gradually decrease, with the maximum effect reached after 15 min. Thus, the recovery time between the surgery and imaging session must be more than 15 min after injection. Notably, overdose can cause the brain surface to sink. In contrast, if the intracranial pressure is low, administer saline via intraperitoneal injection and promote drug metabolism.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to Tomomi Nemoto (tn@nips.ac.jp), who will fulfill all reasonable requests.
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate unique datasets and code.
Acknowledgments
We thank Prof. Junichi Nabekura, Dr. Masakazu Agetsuma, and Ms. Tomoko Kobayashi at the Division of Homeostatic Development, National Institute for Physiological Sciences, National Institutes of Natural Sciences, for the technical advice regarding the open-skull surgery. This work was supported in part by the Research Program of “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “Network Joint Research Center for Materials and Devices,” MEXT/JSPS KAKENHI grant number JP15H05953 (T.N., K.O.), JP18H04744 (Y.O.) “Resonance Bio,” JP16H06280 (T.N., J.N.) “Advanced Bioimaging Support,” JP20H00523 (T.N., K.O.) and JP20H05669 (T.N., K.O.), MEXT-Supported Program for the Strategic Research Foundation at Private Universities 2014–2018 (Y.O.), Brain/MINDS (AMED) JP20dm0207078 (T.N.), JP20dm0207087 (Y.O.), Core Research for Evolutional Science and Technology of the Japan Science and Technology Agency (CREST, JST) JPMJCR20E4 (K.O.), and the Cooperative Study Program (20-121) of National Institute for Physiological Sciences (Y.O.).
Author contributions
T.T. performed the surgery and imaging experiments. Y.O. and H.Z. fabricated PEO-CYTOP nanosheets. T.T., H.Z., K.O., Y.O., and T.N. designed and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100542.
References
- Takahashi T., Zhang H., Kawakami R., Yarinome K., Agetsuma M., Nabekura J., Otomo K., Okamura Y., Nemoto T. PEO-CYTOP fluoropolymer nanosheets as a novel open-skull window for imaging of the living mouse brain. iScience. 2020;23:101579. doi: 10.1016/j.isci.2020.101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldey G.J., Roumis D.K., Glickfeld L.L., Kerlin A.M., Reid R.C., Bonin V., Schafer D.P., Andermann M.L. Removable cranial windows for long-term imaging in awake mice. Nat. Protoc. 2014;9:2515–2538. doi: 10.1038/nprot.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A., Bonhoeffer T., Chow D.K., Chuckowree J., De Paola V., Hofer S.B., Hübener M., Keck T., Knott G., Lee W.C. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami R., Sawada K., Sato A., Hibi T., Kozawa Y., Sato S., Yokoyama H., Nemoto T. Visualizing hippocampal neurons with in vivo two-photon microscopy using a 1030 nm picosecond pulse laser. Sci. Rep. 2013;3:1–7. doi: 10.1038/srep01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Masuda A., Kawakami R., Yarinome K., Saito R., Nagase Y., Nemoto T., Okamura Y. Fluoropolymer nanosheet as a wrapping mount for high-quality tissue imaging. Adv. Mater. 2017;29:1–6. doi: 10.1002/adma.201703139. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yarinome K., Kawakami R., Otomo K., Nemoto T., Okamura Y. Nanosheet wrapping-assisted coverslip-free imaging for looking deeper into a tissue at high resolution. PLoS One. 2020;15:e0227650. doi: 10.1371/journal.pone.0227650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagyozov D., Mihovilovic Skanata M., Lesar A., Gershow M. Recording neural activity in unrestrained animals with three-dimensional tracking two-photon microscopy. Cell Rep. 2018;25:1371–1383.e10. doi: 10.1016/j.celrep.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths V., Valera A.M., Lau J.Y., Roš H., Younts T.J., Marin B., Baragli C., Coyle D., Evans G.J., Konstantinou G. Real-time 3D movement correction for two-photon imaging in behaving animals. Nat. Methods. 2020;17:741–748. doi: 10.1038/s41592-020-0851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N., Konno A., Inoue T., Okazaki S. Aberration correction considering curved sample surface shape for non-contact two-photon excitation microscopy with spatial light modulator. Sci. Rep. 2018;8:1. doi: 10.1038/s41598-018-27693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Otomo K., Kozawa Y., Tsutsumi M., Inose T., Hirai K., Sato S., Nemoto T., Uji-I H. Adaptive optical two-photon microscopy for surface-profiled living biological specimens. ACS Omega. 2020 doi: 10.1021/acsomega.0c04888. acsomega.0c04888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate unique datasets and code.