Abstract
SUMMARY: Symptoms of ipsilateral carotid artery compression secondary to an elongated styloid process or calcified stylohyoid ligament may be seen in Eagle syndrome. The patient will typically experience cervicofacial pain due to stimulation of the arterial nervous plexus. In addition, symptoms directly attributable to compression of the carotid artery may be seen, including visual symptoms and syncope. We report here the case of a patient who developed symptoms consistent with left hemispheric ischemia within 15 seconds of turning his head to the left. These symptoms were completely reversible on returning the head to the neutral position. No long-term sequelae were detected clinically or radiographically.
In 1937, Eagle described a pair of cases in which an elongated styloid process was associated with facial pain.1 Since then, it has been recognized that uncommonly face and neck pain may be associated with an elongated styloid process. More uncommonly, symptoms such as dysphagia, tinnitus, and otalgia may occur. Eagle syndrome is now thought to be due to 2 different subtypes,2 the classic form resulting from cranial nerve impingement and the second type from impingement of the carotid vessels.
Case Report
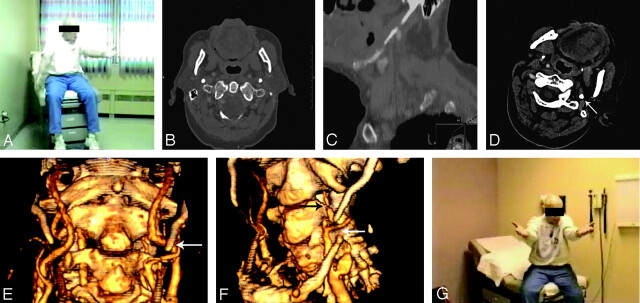
The patient was a 77-year-old man who presented with a 1-year history of progressive right-handed heaviness, clumsiness, and numbness and speech difficulties on turning his head to the left and downward. Findings of the neurologic examination were normal with the patient’s head in the neutral position. Within 15 seconds of turning his head to the left and downward, he developed right-sided weakness, numbness, and aphasia. This weakness involved the face, right arm, and right leg. These symptoms resolved within 5 seconds of returning the head to the neutral position. A single captured image of a digital video file of the patient’s initial clinical examination, with his head in symptomatic orientation, is shown in Fig 1A. Medical history was noncontributory.
Fig 1.
A, A single captured image of a digital video file shows the patient with head oriented to the left and downward and with the right arm drooping following 25 seconds in this orientation.
B and C, Axial CT image of the neck shows both styloid processes (B); whereas a sagittal reconstruction of the same CT scan shows a calcified stylohyoid complex (C).
D, Axial image from a CT angiogram obtained with the patient’s head in symptomatic orientation demonstrates the calcified styloid process (arrow). No definite flow is seen within the left ICA at this level.
E and F, 3D reconstructions of a CT angiogram obtained with the patient’s head in neutral position (E) show a patent left ICA flowing past the tip of the styloid process (arrow, E), whereas a 3D reconstruction of the CT angiogram obtained with the patient’s head in the symptomatic orientation shows severely diminished flow in the left ICA (black arrow, F) distal to its passage under the stylohyoid complex (white arrow, F).
G, A digitally captured image from a video file obtained after styloid process resection shows no arm droop deficit following 30 seconds in the same orientation as the preoperative picture (A).
On physical examination, there was a hard bulge to the left of the thyroid gland. Initial head CT showed only minor small-vessel ischemic disease. MR imaging/MR angiography of the brain likewise demonstrated no acute findings; white matter changes consistent with mild small-vessel ischemic disease were present. There was specifically no evidence of any lesion attributable to major vascular insult (ie, no lesions conforming to a specific vascular distribution were noted). No definite hemispheric asymmetry in the distribution of the white matter changes described previously was noted. Findings of carotid artery sonography performed with the head in both neutral and orientation to the left and downward were unremarkable.
Enhanced CT of the neck demonstrated a calcified left stylohyoid ligament, which appeared to abut the left internal carotid artery (ICA) (Fig 1B, -C). A CT angiogram of the neck (the patient refused conventional angiography) was obtained with the patient’s head in the neutral position and was repeated with the head oriented to the left and downward, in a position previously seen to elicit the patient’s symptoms. An axial image of the CT angiogram obtained with the patient’s head in the symptomatic orientation showed the left styloid process abutting the carotid artery; no definite flow was seen in the left ICA (Fig 1D). With the head in the neutral position, both ICAs were widely patent (Fig 1E). With the head in downward left orientation, CT angiography demonstrated decreased flow in the distal left ICA (Fig 1F). It is presumed that this reduction in flow was responsible for the patient’s striking symptoms. The patient developed right-sided weakness during the examination, which resolved on completion of the examination and the return of the head to a neutral position. The patient was carefully monitored during the examination to ensure that no permanent deficit developed.
Both perfusion MR imaging and perfusion CT were available at this institution and were considered but were deemed impractical because of time constraints. These examinations require several minutes for image acquisition, but because the patient became symptomatic within 10–15 seconds of assuming a specific orientation of the head and neck, it was feared that maintaining such an orientation for the duration of either of these examinations would put him at risk for irreversible deficit.
Following these examinations, the patient underwent surgical resection of the left styloid process, with complete resolution of symptoms (Fig 1G).
Discussion
The association of cervicofacial pain and other symptoms with an elongated styloid process was described by Eagle in a pair of case reports,1 and this association is now known as Eagle syndrome. Subsequent investigation by Eagle2 and others revealed that pathology attributable to an elongated styloid process may be divided into 2 groups, with differing etiologies. The first of these, the classic Eagle syndrome, is typically seen in patients after tonsillectomy, though it can occur in patients without history of pharyngeal surgery. The patient generally presents with ipsilateral cervicofacial pain, often centered on the angle of the mandible, which may be referred to the ear and exacerbated on rotation of the head. Frequently, a mass or bulge may be felt on palpation of the ipsilateral tonsillar fossa, and this palpation may exacerbate the patient’s symptoms. Symptoms also frequently include dysphagia, the sensation of a foreign body in the throat, tinnitus, or otalgia.3 These symptoms are attributed to varying impingement of cranial nerves V, VII, IX, or X, all of which pass in close proximity to the styloid process. The appearance of this impingement following tonsillectomy or minor trauma has been hypothesized to be due to 1 or more of a number of causes, including entrapment in granulation tissue following surgery or minor trauma, direct irritation of tissues and structures surrounding the tip of the elongated styloid process, fracture of an ossified stylohyoid ligament due to sudden head movement and subsequent nonunion, and degenerative changes affecting the insertion of the stylohyoid ligament and leading to an insertion tendinosis.4
The second form of the syndrome, also described by Eagle, is attributed to impingement of the internal or external carotid artery by a laterally or medially deviated styloid process. This may be accompanied by referred pain along the distribution of the artery, caused by stimulation of the sympathetic nerve plexus associated with the artery. In the case of impingement of the internal carotid artery, pain is referred along the course of the ICA and includes eye pain as well as parietal headache. The patient may be diagnosed with cluster headache or migraine. Impingement and stimulation of the external carotid artery plexus causes pain in the face below the eyes. In addition, symptoms that can be attributed to interruption of blood flow within the affected artery, including aphasia, visual symptoms, weakness, and syncope, have been described. In the patient described here, the rapid onset and equally rapid resolution of symptoms directly attributable to the stemming of flow within the carotid artery are consistent with such an etiology, especially given the anatomic relationship of the styloid process with the left ICA. Incidents of carotid dissection related to close apposition of the styloid process have been described.5
The ability to visualize diminished flow within the affected carotid artery is limited in this case by the time required to perform the study using a given technique. As mentioned previously, perfusion CT and MR imaging studies require several minutes for image acquisition, and it was believed that maintaining the patient in a symptomatic orientation for this length of time would place him at significant risk of infarct. In contrast, CT angiography of the neck could be initiated after placing the patient’s head and neck in the symptomatic orientation and completed before significant risk of infarct occurred. Although it has the advantage of directly imaging flow within the affected arteries, there are a number of disadvantages to using MR angiography in this patient. Most important, the time required to obtain scout, mask, and angiographic images (required for optimum image quality) is greater than that required for CT angiography (>1 minute) and thus carries greater risk for the patient. This is assuming no difficulty achieving the required orientation while the patient is in a neurovascular coil and magnet. In addition, estimates of flow within the affected carotid artery by using noncontrast studies of the carotid arteries would be unreliable because of increasing turbulence as the diameter of the carotid artery decreased6; contrast studies would require additional time for bolus injection. Of course, a study performed in the absence of any symptoms of carotid stenosis, whatever the orientation of the head and neck, cannot either rule in or exclude a diagnosis of carotid artery syndrome.
The styloid process is a slender outgrowth at the base of the temporal bone, immediately posterior to the mastoid apex. The styloid process arises embryonically from the second brachial arch, or Reichert cartilage. This cartilage can be divided into 4 sections based on the subsequent development of the stylohyoid complex. The most proximal is known as the tympanohyal and gives rise to the tympanic (proximal) portion of the styloid process as well as the stapes. The second portion is known as the stylohyal and forms the distal portion of the styloid process. The third portion, the ceratohyal, degenerates in utero, giving rise to the stylohyoid ligament. The fourth and most distal portion is known as the hypohyal and becomes the lesser cornu of the hyoid bone. Besides the stylohyoid ligament, the styloid process also serves as a point of attachment for the stylomandibular ligament as well as the styloglossus, stylohyoid, and stylopharyngeus muscles.
Within the population at large, the distribution of styloid process length falls into a bimodal distribution, with approximately one quarter of the surveyed processes measuring <20 mm and 3 quarters measuring >20 mm.7 According to the previously mentioned study by Lengele et al,7 this bimodal distribution could be explained by the presence or absence of ossification of the stylohyal portion or Reichert cartilage. Ossification and fusion of the stylohyal and tympanohyal portions of Reichert cartilage yield a long styloid process, whereas failure of stylohyal ossification yields a short styloid process. In terms of evoking symptoms, however, Eagle defined the length of a normal styloid process at 2.5–3.0 cm and placed the portion of the population with an elongated styloid process at 4%. Baugh and Stocks8 defined an elongated styloid process as being 5–7 cm. Only a minority of patients with an elongated styloid process have symptoms of Eagle syndrome. Eagle himself estimated this portion at 4%; other investigators have found symptoms in as many as 10.3% of patients with an elongated styloid process.9
The appearance of the carotid artery subtype of Eagle syndrome is not dependent merely on the existence of an elongated styloid process. Indeed, this subtype of the syndrome may occur with a styloid process of normal length. Instead, deviation of the tip of the styloid process, usually medially, is required. The tip of the styloid process must impinge the artery; hence, variations in vascular anatomy are important in the appearance of the syndrome as well. Also important in terms of the appearance of Eagle syndrome is the observation that ossification can occur at the distal aspect of the styloid process and the proximal stylohyoid ligament, leading to growth of the styloid process. In fact, growth of the styloid process has been observed following surgical resection.10 This observation clearly has implications for the appearance of the carotid artery subtype of Eagle syndrome in older patients.
References
- 1.Eagle WW. Elongated styloid processes: report of two cases. Arch Otolaryngol 1937;47:584–87 [Google Scholar]
- 2.Eagle WW. Elongated styloid process: further observations and a new syndrome. Arch Otolaryngol 1948;47:630–40 [DOI] [PubMed] [Google Scholar]
- 3.Eagle WW. Symptomatic elongated styloid process: report of two cases of styloid process-carotid artery syndrome with operation. Arch Otolaryngol 1949;49:490–503 [DOI] [PubMed] [Google Scholar]
- 4.Bafaqeeh SA. Eagle syndrome: classic and carotid artery types. J Otolaryngol 2000;29:88–94 [PubMed] [Google Scholar]
- 5.Zuber M, Meder JF, Mas JL. Carotid artery dissection due to elongated styloid process. Neurology 1999;53:1886–87 [DOI] [PubMed] [Google Scholar]
- 6.McKinney AM, Casey SO, Teksam M, et al. Carotid bifurcation calcium and correlation with percent stenosis of the internal carotid artery on CT angiography. Neuroradiology 2005;47:1–9 [DOI] [PubMed] [Google Scholar]
- 7.Lengele B, Dhem A. Length of the styloid process of the temporal bone. Arch Otolaryngol Head Neck Surg 1988;114:1003–06 [DOI] [PubMed] [Google Scholar]
- 8.Baugh RF, Stocks RM. Eagle’s syndrome: a reappraisal. Ear Nose Throat J 1993;72:341–44 [PubMed] [Google Scholar]
- 9.Rechtweg JS, Wax MK. Eagle’s syndrome: a review. Am J Otolaryngol 1998;19:316–21 [DOI] [PubMed] [Google Scholar]
- 10.Steinmann E. A new light on the pathogenesis of the styloid syndrome. Arch Otolaryngol 1970;91:171–74 [DOI] [PubMed] [Google Scholar]