Abstract
BACKGROUND AND PURPOSE: Sacral insufficiency fractures are an infrequent but often disabling cause of severe low back pain. We report our results of a sacroplasty technique, using CT for needle placement and fluoroscopy to monitor the polymethylmethacrylate injection in a group of patients with sacral insufficiency fractures.
METHODS: All patients had a history of chronic back pain and had an osteoporotic sacral insufficiency fracture documented by imaging before the procedure. With the patient under conscious sedation, a bone biopsy needle was placed under CT guidance; the patient was then transferred to the fluoroscopy suite, where a polymethylmethacrylate mixture was injected into the sacrum under real-time fluoroscopy. Clinical outcome was assessed by telephone.
RESULTS: The procedure was performed on 13 female patients with an average age of 76 years (range, 60–88 years). A bilateral procedure was performed in 11 patients and a unilateral procedure was performed in 2 patients. An average of 4.1 mL of cement was injected for each treatment. There were no instances of cement extravasation into the central canal or sacral foramina. Long-term follow-up, averaging 15 months, was available in 6 patients. Five patients (83%) reported no symptoms of pain at all. The final patient, in whom a bilateral procedure was performed, was completely asymptomatic on the left side but reported persistent unilateral pain on the right.
CONCLUSION: Sacroplasty is a safe and effective procedure in the treatment of sacral insufficiency fractures that can provide substantial pain relief and lead to a better quality of life.
Sacral insufficiency fractures are an infrequent but often disabling cause of severe low back pain. At times, the pain can be so severe that it may cause the patients to become bedridden, placing them at risk for complications of immobility1,2 such as deep vein thrombosis, pulmonary embolus, muscle atrophy, decubitus ulcers, and bone demineralization.3 Until the development of the sacroplasty technique,3,4 there was no definitive treatment other than bed rest.
Sacral insufficiency fractures result from an axial loading mechanism4,5 on abnormal bone, such as osteoporosis or underlying neoplasm. Analogous to vertebroplasty, the purpose of sacroplasty is to provide stabilization to prevent painful micromotion at the fracture site.6 We report out results of a sacroplasty technique, using CT for needle placement and fluoroscopy to monitor the polymethylmethacrylate (PMMA) injection in a group of patients with sacral insufficiency fractures.
Materials and Methods
All patients presented with a history of severe low back pain that was minimally responsive to narcotic anesthesia. The average time to presentation for the procedure was approximately 11 days after the sacral insufficiency fracture was documented by either CT, MR imaging, or bone scan. Preprocedure imaging of patients is not standard as all patients have a CT of the region at the time of the procedure. There were no contraindications to sacroplasty based on the fracture pattern. In particular, extension of the fracture into the sacral foramina was not believed to place the patient at increased risk for cement extravasation. Patients with metastatic lesions involving the sacrum were excluded from the study.
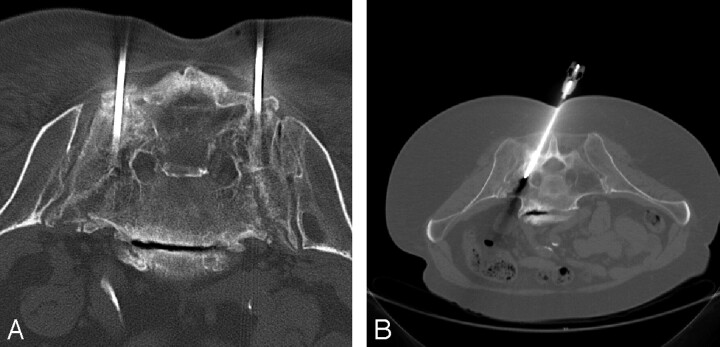
All patients received 1 g of cefazolin before the procedure to provide coverage against skin flora. Vancomycin was used if there was a penicillin allergy. Under conscious sedation, a bone biopsy needle, most commonly an 11 gauge, was placed under CT guidance with caudal-cranial angulation of the CT gantry. Every effort was made to place the needle within the fracture cleft, and the needles may be placed in a vertical or oblique orientation (Figure 1). The authors prefer an oblique orientation to the needle placement along the long axis of the sacrum because it allows a more precise placement of the needle in the fracture site. The patient was then transferred to the fluoroscopy suite, where a PMMA mixture (Codman cranioplasty slow-set MMC; Codman and Shurtleff, Rayham, Mass) was injected into the sacrum under real-time fluoroscopy (Figure 2) using 1-mL syringes. Slow-set rather than fast-set PMMA was used only because of author preference. The cement was injected as a toothpaste consistency, with the needle directed laterally to encourage the flow of cement away from the sacral foramen. The goal of the cement injection was to fill the fracture site and as much surrounding bone as possible without extravasation of cement.
Fig 1.
CT images from needle placement in 2 different patients with sacral insufficiency fractures illustrating the possible orientation for the bone biopsy needle(s) in the sacrum.
A, Axial image showing vertical orientation of the bone biopsy needles in a patient with bilateral fractures.
B. Axial image showing an oblique orientation, paralleling the sacroiliac joint of the bone biopsy needle, in a patient with a unilateral fracture.
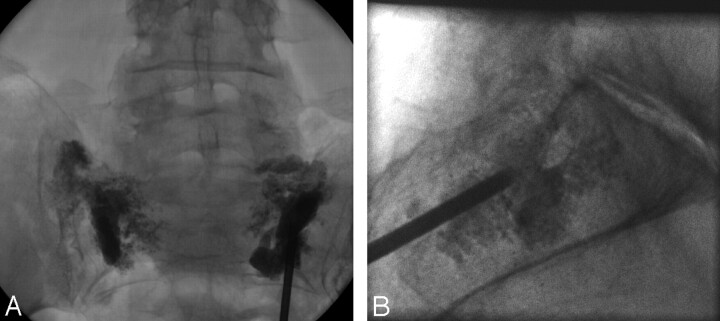
Fig 2.
Fluoroscopic spot films taken during the PMMA injection in a 65-year-old woman with bilateral sacral insufficiency fractures.
A, Frontal projection allows for monitoring of extravasation into the sacral foramina.
B, Lateral projection allows for monitoring for any cement extravasation into the soft tissues.
After the procedure, patients were observed for 3 hours before discharging home. Short- (within 2 weeks) and long-term (greater than 6 months) clinical follow-up was assessed by telephone.
Results
The procedure was performed on 13 female patients with an average age of 76 years (range 60–88 years). A bilateral procedure was performed in 11 patients, and a unilateral procedure was performed in 2 patients. An average of 4.1 mL of cement was injected via each cannula for a total of approximately 8 mL in bilateral procedures. There were no instances of cement extravasation into the central canal or sacral foramina. No repeat procedures were required, and no patients reported increase in pain.
On short-term clinical follow-up, averaging 12 days, 7 (64%) of 11 patients reported complete or moderate pain relief, 2 patients stated that their pain was slightly decreased, 1 patient was unsure how much pain relief had been achieved, and follow-up was unavailable in 2 patients. One patient reported no pain relief after the procedure.
Long-term clinical follow-up, averaging 15 months, was available in 6 patients. At the time of long-term clinical follow-up, 2 of the original 13 patients were deceased, and the 5 were lost to follow-up. In 5 (83%) of the 6 patients for whom long-term follow-up was available, they reported no symptoms of pain at all. One of these patients initially felt that she did not receive a lot of pain relief but currently was symptom-free. The final patient, in whom a bilateral procedure was performed, was completely asymptomatic on the left side but reported persistent unilateral pain on the right.
Discussion
Although the sacroplasty technique is similar to vertebroplasty, there are several technical considerations that are unique to sacroplasty. The technical challenges are inherent in the safe placement of the needle and prevention of cement extravasation. With fluoroscopy alone, it can be very difficult to know whether the needle has adequately traversed the outer cortex and has not breached the inner cortex on the pelvic side.2,3 If fluoroscopy alone is used, it can be difficult to visualize the sacral foramina; however, this can be overcome by placement of needles in the sacral foramina before cement injection, which will in turn help monitor for any potential migration of the cement medially that could compromise the exiting sacral nerve roots.4 Furthermore, in contrast to vertebroplasty, the cancellous bone in the sacrum is less attenuated than a vertebral body, which limits the tactile feedback during cannula placement.2 Even with proper needle placement and careful monitoring of cement injection, it can be difficult to determine whether the PMMA is extruding into the soft tissue of the buttock or pelvis or into the sacral foramina.2
These inherent difficulties led initial authors to explore alternative imaging guidance for needle placement and subsequent cement injection (Table). A report focused on the use of CT guidance in performing the procedure noted the higher precision in targeting the needle tip to the fracture plane within the bone and the better demonstration of the relationships within the treatment fields.1 Sacroplasty has also been described using Brain LAB (Brain LAB, Chicago, Ill) for image guidance and the modified balloon kyphoplasty technique before cement injection.2 Although balloon inflation may be useful in compacting the bone at the periphery of the fracture to reduce the incidence of cement extravasation, in contradistinction to vertebroplasty, balloon inflation will not result in any meaningful degree of height restoration or fracture reduction.2 A recent report focused on performing the procedure with CT fluoroscopy7 because it was believed that this technique might eliminate the need to perform venography, in that continuous fluoroscopic surveillance to assess for venous extension remains necessary during the cement injection. However, the authors stated that the cement extrusion is better assessed with conventional fluoroscopy than with CT fluoroscopy because the cement mixture usually distributes preferentially in the craniocaudal and lateral directions away from the needle tip. The authors advocated a combined approach with CT and conventional fluoroscopy.7 Thus, although there are many techniques that can be used to safely perform the procedure, the combined approach of CT with conventional fluoroscopy allows for precise needle placement as well as optimal monitoring of cement injection.
Previous studies of sacroplasty in patients with osteoporotic insufficiency fractures
| Needle Placement | Cement Injection | Complication(s) | Clinical Follow-Up | |
|---|---|---|---|---|
| Garant et al4 | Fluoroscopy under general anesthesia. Chiba needles placed in sacral foramina. | Fluoroscopy | None reported | Ambulatory and pain-free 9 months postprocedure |
| Brook et al1 | CT Guidance under general anesthesia in 2 patients. | Intermittent CT | Premature cement hardening | Pain-free and ambulating at 8 and 16 months |
| Pommersheim et al3 | Fluoroscopy in 2 patients. CT guidance in 1. Patients under conscious sedation. | Fluoroscopy in all | Cement extravasation in posterior soft tissues | Pain-free at 14 and 16 weeks. One patient lost to follow-up |
| Deen and Nottmeier2 | Kyphoplasty with fluoroscopy in 2 patients. Brain LAB in one patient. | Fluoroscopy in 2, Brain LAB in 1 | None reported | Decreased pain and increased function 3, 6, and 9 months after procedure |
| Butler et al7 | CT fluoroscopy under conscious sedation in 6 patients. | Fluoroscopy in 5, CT in 1 | Inadequate cement distribution, venous intravasation, and extension into SI joint | 2–8 weeks. Significantly reduced or eliminated pain in 4 patients |
Note:—SI indicates sacroiliac.
Potential complications of sacroplasty include venous intravasation with pulmonary embolus, infection, and extension and compromise of the sacral neural foramina.7 Venography can help confirm the trocar tips are within the marrow spaces and that there is no leakage of contrast into the presacral space, sacral spinal canal, or sacral iliac joint.4 In a study of 3 patients with painful metastatic lesions of the sacrum, Dehdashti et al8 observed a small venous leakage near the S1 radicular foramen in 1 patient. Butler et al7 observed a minimal venous intravasation of PMMA in 1 patient and a small amount of extension of PMMA into the sacroiliac joint in another patient; however, neither was felt to be clinically significant. Pommersheim et al3 observed a small amount of cement entering the posterior soft tissues without complication. Complications may also occur because of problems with the cement. Brook et al1 had to repeat the procedure in 1 patient on 1 side because only a small quantity of material could be administered as a result of cement hardening. This side was successfully treated on the second attempt. Butler et al7 described the use of sacroplasty in 1 patient with multiple myeloma; the patient required 2 treatments to the right side of the sacrum because there was insufficient distribution of the cement on the first treatment. A final possible complication is treatment failure, in which the patient experiences no pain relief. Butler et al7 had 1 treatment failure in 6 patients. We observed 1 case of treatment failure in our patient population and had no cases of premature cement hardening. Furthermore, no extravasation into the sacral foramina was observed at the time of cement injection. However, because patients are not imaged as a part of follow-up unless new neurologic symptoms have developed, a small amount of clinically insignificant extravasation may have occurred.
Although the technical reports describing the variations of this procedure have a great deal of importance, the clinical outcomes of these patients, especially in the long term, are just as critical. Keller9 stated that outcomes research should focus on “patient-oriented” reports of the results of treatment because the reporting of process data (range of motion, radiographic results, etc) does not always correlate with outcomes relevant to patients (pain, daily function, quality of life, etc). Many of the patients in our patient population, and in those reported previously, have had chronic back pain that may not be limited to their sacral insufficiency fracture alone. Thus, it may difficult for these patients to objectively quantify how much the pain has improved from baseline.
The results of our study must be interpreted within the context of our study design. We performed a retrospective cohort study of a small group of patients treated by multiple interventional radiologists at a single institution. The sample size was small; however, to the best of our knowledge, this represents the largest series of patients yet published. A larger group of patients may reveal a higher complication rate, especially treatment failure. Furthermore, although our nonexperimental study design is more susceptible to the effects of bias, we believe it is a practical way to evaluate the efficacy of this technique because the opportunity to currently perform the procedure is so limited.
Conclusion
The current study supports and extends the work of previous authors showing that sacroplasty is a safe and effective procedure in the treatment of sacral insufficiency fractures. Sacroplasty can provide substantial pain relief leading to a decreased dependence on anesthesia and a better quality of life.1 Hopefully, with growing awareness of the procedure, further investigations can be performed to accurately quantify the complication rate and rigorously assess the clinical outcomes in patients with painful metastatic lesions or insufficiency fractures of the sacrum.
References
- 1.Brook AL, Mirsky DM, Bello JA. Computerized tomography guided sacroplasty: a practical treatment for sacral insufficiency fracture. Spine 2005;15:E450–54 [DOI] [PubMed] [Google Scholar]
- 2.Deen H, Nottmeier E. Balloon kyphoplasty for treatment of sacral insufficiency fractures. Neurosurg Focus 2005;3:1–5 [PubMed] [Google Scholar]
- 3.Pommersheim W, Huang-Hellinger F, Baker M, et al. Sacroplasty: a treatment for sacral insufficiency fractures. AJNR Am J Neuroradiol 2003;24:1003–07 [PMC free article] [PubMed] [Google Scholar]
- 4.Garant M. Sacroplasty: a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol 2002;13:1265–67 [DOI] [PubMed] [Google Scholar]
- 5.Leroux JL, Denat B, Thomas E, et al. Sacral insufficiency fractures presenting as acute low-back pain biomechanical aspects. Spine 1993;18:2505–06 [DOI] [PubMed] [Google Scholar]
- 6.Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol 2001;22:373–79 [PMC free article] [PubMed] [Google Scholar]
- 7.Butler CL, Given CA, Michel SJ, et al. Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. AJR Am J Roentgenol 2005;184:1956–59 [DOI] [PubMed] [Google Scholar]
- 8.Dehdashti AR, Martin JB, Jean B, et al. PMMA cementoplasty in symptomatic metastatic lesions of the S1 vertebral body. Cardiovasc Intervent Radiol 2000;23:235–41 [DOI] [PubMed] [Google Scholar]
- 9.Keller RB. Pro: outcomes research is cost effective and critical to the specialty. Spine 1995;20:384–86 [PubMed] [Google Scholar]