Abstract
BACKGROUND AND PURPOSE: Some reports of reconstructive management of carotid blowout syndrome (CBS) with stent-grafts are promising, but some are unfavorable. This study sought to evaluate the hemostatic efficacy, safety, and outcome of reconstructive, endovascular stent-graft placement in patients with head-and-neck cancers in association with CBS.
METHODS: Eight patients with head-and-neck cancers with CBS were treated with self-expandable stent-grafts. We evaluated the initial hemostatic results, complications, and outcomes by assessing the clinical and imaging findings.
RESULTS: Immediate hemostasis was achieved in all patients. Initial complications included stroke in 1 patient and asymptomatic thrombosis of the carotid artery in 2 patients. Delayed complications included rebleeding, delayed carotid thrombosis, and brain abscess formation. Rebleeding was noted in 4 patients and was successfully managed with a second stent-graft and embolization in 2 of them. Delayed carotid thrombosis with follow-up after 3 months was found in 3 patients, 1 of whom had associated brain abscesses.
CONCLUSION: Although stent-grafts achieved immediate and initial hemostasis in patients with head-and-neck cancers and CBS, long-term safety, stent patency, and permanency of hemostasis appeared unfavorable. This treatment may be for temporary or emergency purposes rather than serving as a permanent measure. We suggest its applications in patients with acute CBS that precludes performance of an occlusion test, as well as when carotid occlusion poses an unusually high risk of neurologic morbidity. We also propose prophylactic antibiotic treatment and combined embolization of pathologic vascular feeders to improve outcomes.
Carotid blowout refers to rupture of the carotid artery and its branches.1–4 It is one of the most devastating complications associated with therapy for head-and-neck cancers. The clinical signs and symptoms related to rupture of the carotid artery have been referred to as carotid blowout syndrome (CBS). Carotid blowout tends to occur in patients with head-and-neck cancers and radiation-induced necrosis, recurrent tumors, or pharyngocutaneous fistulas.2 The reported neurologic morbidity and mortality rates associated with this complication are 40% and 60%, respectively.3 Surgical management of carotid blowout is usually technically difficult because exploration and repair of the previously irradiated field are difficult. Endovascular therapy with either permanent balloon occlusion or stent deployment is reportedly a good alternative to surgery.1–5
As many as 15%–20% of patients with CBS who are treated with permanent balloon occlusion have immediate or delayed cerebral ischemia.1,6 A balloon occlusion test may be performed before threatened CBS is treated definitively, but this test is usually not possible in acute cases. Additionally, test occlusion may not help identify the small subset of patients in whom delayed hemodynamic ischemia develops after the internal carotid artery (ICA) is permanently occluded.1–3 Reconstructive endovascular management of CBS seems reasonable to achieve hemostasis and to prevent neurologic morbidity. However, some reports of limited cases show unfavorable long-term outcomes after the deployment of foreign bodies into a field with ongoing contamination.5,7 Such studies suggest preserving flow in the artery adjacent to ongoing cancer and infection can leave the patient at a higher risk of delayed complications (eg, subsequent bleeding or stent occlusion) than with carotid sacrifice. The purpose of this study, therefore, was to evaluate the hemostatic efficacy, safety, and outcome of endovascular reconstruction with self-expandable stent-grafts to manage carotid blowout in patients with head-and-neck cancer.
Methods
Patient Population
During a 2-year period, 8 patients with head-and-neck cancers with CBS were included in this study (Table). All had received radiation therapy or chemoradiotherapy. Radical surgical excision of the tumor and neck dissection had been done in 5 patients.
Summary of patients with carotid blowout syndrome treated with self-expandable stent-grafts
| Patient No./Age (y)/Sex | Cancer and Treatment History | Presentation | Blowout |
Stent-Graft (mm)* | Initial Complication† | Follow-Up | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Onset (y) | Group | Location | |||||||
| 1/35/M | Mixed, submandibular gland; wide excision, R/T, C/T | Recurrence, RN; lung, kidney metastasis | 5 | Acute | CBF | 8 × 50 | Asymptomatic acute ICA thrombosis and occlusion | Not applicable | 19 d: rebleed; died |
| 2/43/M | Tonsillar; wide excision, R/T, C/T | Recurrence, RN | 1 | Acute | CCA | 8 × 30 | None | Not applicable | 2 d: rebleed; died |
| 3/52/M | Laryngeal; total laryngectomy, R/T, C/T | Recurrence, RN, PCF | 4 | Acute | ICA | 7 × 30, 9 × 50 | Asymptomatic transient in-stent thrombosis | Rebleed wk 3, second stent-graft; patency on 1-mo CTA & 3-mo angiogram | 6-mo CT: asymptomatic carotid thrombosis; lived 20 mo |
| 4/49/M | Nasopharyngeal, R/T; second primary hypopharyngeal, R/T, C/T | Recurrent hypopharyngeal cancer, RN | 12, 1 | Impending | CBF | 8 × 50‡ | Embolic infarct, MCA territory | Stent patency on 1-mo CT, sonography | 2-mo: died from progression |
| 5/52/M | Hypopharyngeal; R/T, C/T | Slightly swollen soft tissue of neck | 1 | Impending | CBF | 8 × 50‡ | None | Stent patency on 2-mo CTA | 4-mo: septic carotid thrombosis, brain, abscess; lived 14 mo |
| 6/65/M | Laryngeal; R/T | RN, PCF | 4 | Acute | CCA, CBF | 8 × 50, 8 × 50 | None | Rebleed at 2-mo, second stent-graft; rebleed at 3-mo, direct percutaneous ECA puncture for embolization; stent patency at 3-mo CT | 5-mo CT: asymptomatic carotid thrombosis; lived 13 mo |
| 7/44/M | Hypopharyngeal; total laryngopharyngectomy, C/T, R/T | Recurrence, RN, PCF, lung metastasis | 1/2 | Threatened | CCA | 9 × 70 | None | Stent patency on 2-wk sonography | 1-mo: died from lung metastasis |
| 8/54/M | Lower esophageal, primary hypopharyngeal; esophagectomy, laryngectomy, C/T, R/T | Recurrence, RN, PCF | 1 | Threatened | CCA | 9 × 70 | None | Stent patency on 2-wk CTA | 1.5-mo: died from progression, mediastinitis |
Note.—CBF indicates carotid bifurcation; CCA, common carotid artery; C/T, chemotherapy; CTA, CT angiography; ECA, external carotid artery; MCA, middle cerebral artery; PCF, pharyngocutaneous fistula; RN, radiation necrosis; R/T, radiotherapy.
Wallgraft; Boston Scientific Corporation.
Immediate hemostasis was achieved in all patients.
Plus 3 fiber coils in the ECA
CBS was classified into 3 types: threatened, impending, and acute.1,3 Threatened blowout was defined as exposure of the carotid artery because of wound breakdown or as angiographic findings consistent with neoplastic invasion of the carotid system and with nonhemorrhagic pseudoaneurysm. Rupture was almost inevitable if the exposed vessel was not promptly covered with healthy vascularized tissue. Impending carotid blowout consisted of short episodes of acute hemorrhage that resolved spontaneously or with simple surgical packing. Complete rupture was likely certain because the intermittent hemorrhage may have originated from a ruptured carotid artery with a pseudoaneurysm. Acute CBS was classified as an acute, profuse hemorrhage that was neither self-limiting nor controlled with surgical packing. The vessel was completely ruptured, and the patient’s condition would have deteriorated rapidly if immediate resuscitation and stabilization were not accomplished before definite management.
Associated clinical findings during interventional management included recurrent tumor in 6 patients, pharyngocutaneous fistula in 4 patients, and distant metastasis in 2 patients. All patients presented with various degrees of irradiation-induced change in the head-and-neck region, with findings ranging from slightly swollen soft tissue to large areas of radiation necrosis with ulceration or a sinus tract.
The interval between clinical diagnosis and the patients’ presentation with CBS was 0.5–12 years. Locations of pathologic vascular lesions, such as pseudoaneurysms, were recorded as the cervical ICA, carotid bifurcation, or common carotid artery (CCA).
Patients were treated with self-expandable stent-grafts if the circle of Willis was incomplete, if the contralateral carotid artery was occluded, if patients were in unstable clinical condition (eg, acute or impending CBS) that precluded balloon occlusion testing, and/or if patients could not tolerate a balloon occlusion test.6
Interventional Management
Patients with threatened CBS (patients 7 and 8) were given a premedication antiplatelet regimen consisting of orally administered aspirin (324 mg) and clopidogrel (300 mg) 1 day before treatment. Patients with acute or impending CBS (patients 1–6) were prophylactically given intravenous glycoprotein IIb/IIIa inhibitor (Aggrastat) during the interventional procedure. The inhibitor was diluted with 5% glucose water to a 41.6 mcg/mL solution. Adminstration began 10 minutes before the deployment of the stent-graft: intravenous infusion was started at 0.4 mcg/kg/min for 15–20 minutes, followed by continuous infusion at 0.1 mcg/kg/min for 4–6 hours.
During the procedure, we used a transfemoral arterial approach to obtain a complete neuroangiogram of the supra-aortic arteries and their branches, including the bilateral carotid, bilateral vertebral, and bilateral subclavian arteries. Meticulous evaluation of vascular causative lesions, such as focal luminal dilatation, disruption, pseudoaneurysm formation or extravasation, was focused on bilateral carotid arteries. We also evaluated the circle of Willis before preforming the intervention. An occlusion test was performed if the patient’s clinical status was stable.
When we decided to deploy a self-expandable stent-graft, we placed an 11F sheath through the right femoral artery. About 70–100 U/kg of heparin was injected IV to maintain an activated clotting time exceeding 250 seconds, as is standard in carotid angioplasty and stent placement. Patients with unstable vital signs (patients 1 and 2) were given heparin IV just before the stent-graft was deployed. A 5F diagnostic catheter (JB 2 or Simon 2; Cook, Bloomington, Ind) was superselected to the proximal CCA. Through the diagnostic catheter, a 0.014F marker wire (IQ guide wire; Boston Scientific, Natick, Mass) was advanced to the carotid artery. For achieving a precise vascular dimension, we carefully checked the markers of this wire in the straight segment of the target vessel as close to the pathologic lesion as possible. A magnified diagnostic angiogram was done. We measured the diameter and length of the carotid artery by reference to the marker wire. A self-expandable stent-graft (Wallgraft; Boston Scientific) was selected according to the dimensions of the diseased carotid artery. The ends of the stent-graft were deployed more than 1 cm over the margin of the pathologic vascular lesion. The diameter of the selected stent-grafts was at least 1 mm larger than the diameter of the carotid artery proximal to the pathologic lesion.
A 300-cm exchange guidewire (Amplatz; Cook, Bloomington, Ind) was placed into the cervical ICA. The stent-graft was then advanced along this exchange guidewire to the carotid artery. If the causative lesion was in the proximal CCA or cervical ICA, we placed another diagnostic catheter (Simon 2) by means of transarterial approach into the contralateral femoral artery to provide an image for guiding the deployment of the stent-graft. For the pathologic lesion located in the carotid bifurcation or distal CCA, we took the carotid bifurcation on the corresponding level of the cervical spine as a landmark for stent deployment. Postdilation was performed in only patient 1, who had carotid stenosis. In patients 4 and 5, who had pseudoaneurysms in the carotid bifurcation, we placed fiber coils in the main trunk of the external carotid artery (ECA) and then deployed the stent-graft to avoid reconstitution of the collaterals from the branches of the ECA (Fig 1).
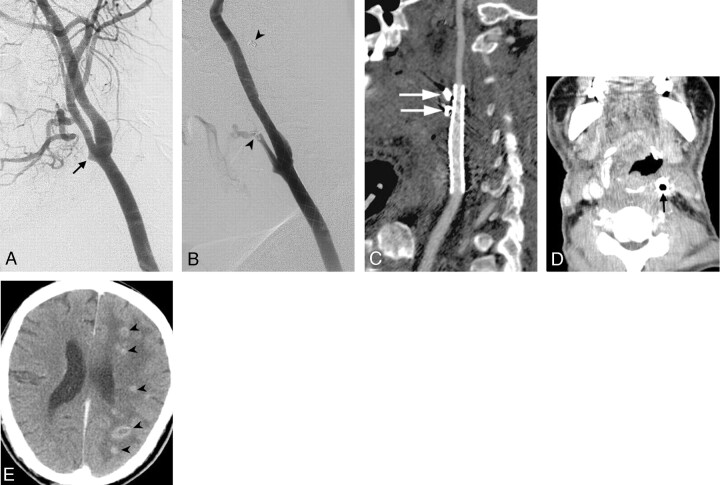
Fig 1.
Patient 5.
A, Left carotid angiogram shows a pseudoaneurysm in the bifurcation (arrow).
B, After 3 fiber coils (arrowheads) were placed in the main trunk of the ECA, an 8 × 50-mm stent was deployed in the left carotid artery.
C, Reconstructive CT angiography (curved multiplanar, reformatted images) of left carotid artery 2 months later shows complete obliteration of the pseudoaneurysm. Retained fiber coils in the thrombosed ECA with metallic artifact were also found (arrows).
D and E, Contrast-enhanced axial CT scans of the head and neck 4 months later show septic thrombosis of the stent-graft with gas collection (arrow in panel D) and several brain abscesses (arrowheads in panel E).
A control angiogram was obtained immediately and 15 minutes after deployment of the stent-graft to confirm its appropirate positioning as well as patency of the carotid artery. Interventional management was finished when clinical hemostatsis and adequate coverage of the pathologic lesion by the stent-graft were gained. This included complete obliteration of the lesions or progressively slow and stagnant flow of contrast media to the lesion on serial images. A daily antiplatelet regimen consisting of aspirin (324 mg) and clopidogrel (75 mg) was started soon after management. For patients with acute or impending CBS (cases 1–6), another single dose of clopidogrel (225 mg) was added to the antiplatelet regimen. After 1 month, this regimen was changed to aspirin (100 mg) for lifelong use. IV administration of the glycoprotein IIb/IIIa inhibitor was discontinued 4–6 hours after the procedure. Because patient 5 suffered from brain abscesses after the stent-graft deployment, we gave prophylactic antibiotic therapy to patients 6–8.
Patient Evaluation and Follow-Up
An otolaryngologist examined the patients for bleeding soon after stent placement to confirm immediate hemostasis. Patients were also examined for neurologic deficits. Patients without rebleeding underwent follow-up contrast-enhanced CT, CT angiography, sonography, or conventional angiography within the first month and then every 2–3 months. If rebleeding occurred, emergency angiography and interventional management were done. Follow-up lasted from 1 day to 20 months.
Results
The Table summarizes the technical and clinical results. Twelve therapeutic procedures, including the deployment of 10 stent-grafts, were performed in the 8 patients. Patients 3 and 6 received a second stent-graft because of recurrent bleeding. The diameters of the selected stent-grafts were 1.1–2.3 mm larger than the treated carotid arteries. Indications for carotid angioplasty and stent-graft placement were a critical clinical condition precluding a balloon occlusion test (patients 1–3, 5, 6), an incomplete circle of Willis (patients 4 and 8), and intolerance of the occlusion test (patient 7).
In all patients CBS was successfully managed by deploying one self-expandable stent-graft, with immediate hemostasis. Complete obliteration of the pathologic lesion immediately after deployment of the stent-graft was found in 4 patients (patients 1, 2, 6, and the first stent of patient 3). Obliteration of the pathologic lesion on the serial 15-minute angiogram was noted in 2 patients (patients 7 and 8). Progressive slow and stagnant contrast media leakage to the pathologic lesions on serial images and complete obliteration of them in the follow-up images were noted in 3 patients (patients 4 and 5 and the second stent of patient 3).
Initial complications included acute thromboembolism in 3 patients. Patient 1 had stenosis of the ICA that caused acute but asymptomatic thrombosis and occlusion of the carotid artery immediately after the stent-graft was deployed (Fig 2). Patient 3 had an acute, asymptomatic in-stent thrombosis and was subsequently lysed by IV glycoprotein IIb/IIIa inhibitor infusion in 10 minutes (Fig 3). Patient 4 had an acute stroke. He suffered from slurred speech and right hemiplegia soon after the deployment of the stent-graft in the left carotid artery. The muscle power of his right limbs decreased to grade 0–1. An embolus in the M1 segment of the left middle cerebral artery with total occlusion of the vessel was found on angiogram. We kept continuous IV infusion of glycoprotein IIb/IIIa inhibitor and advanced a microcatheter to the left middle cerebral artery for intra-arterial thrombolysis. After mechanical manipulation and intra-arterial infusion of urokinase (240 000 U), the embolus was partially lysed with improved flow to distal branches of the left middle cerebral artery. Unfortunately, the patient showed no immediate neurologic improvement and became stuporous and uncooperative. His blood pressure was elevated to 220/110 mm Hg, and we therefore decided to stop the procedure and consult a neurologist for medical treatment. IV infusion of glycoprotein IIb/IIIa inhibitor was given for 4 more hours, which was then changed to the oral antiplatelet regimen. After a 1-month admission, the patient’s neurologic symptoms improved: he demonstrated clear consciousness, had only slightly slurred speech, and the muscle power of his right limbs was grade 4–5.
Fig 2.
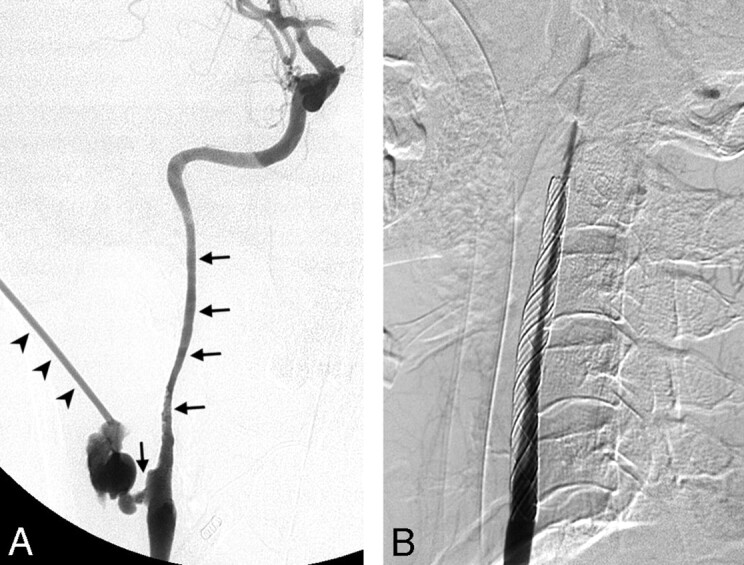
Patient 1.
A, Right carotid angiogram shows a ruptured carotid artery in the bifurcation (vertical arrows) and an active jet of extravasation (arrowheads) through a focal skin defect on the right side of the neck during the injection of contrast medium. Obvious long-segmental stenosis of the cervical ICA was also found (horizontal arrows).
B, After the deployment of an 8 × 50-mm stent from the ICA to the CCA, acute thrombosis with total occlusion of the carotid artery occurred immediately.
Fig 3.
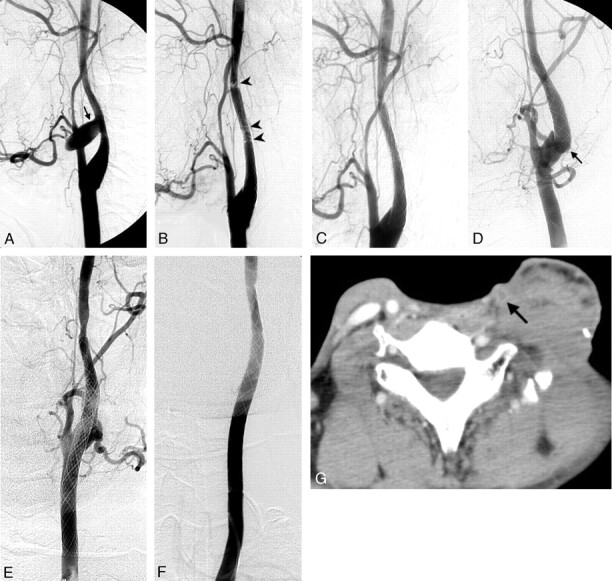
Patient 3. Left carotid angiograms. A, Pseudoaneurysm in the cervical ICA (arrow). B, Soon after a 7 × 30-mm stent was deployed in the cervical ICA, acute thrombosis was noted in the stent and its distal end (arrowheads). C, Acute thrombosis was lysed in 10 minutes by using an intravenous antiplatelet agent. D, Rebleeding due to a pseudoaneurysm in the carotid bulb was found 3 weeks later; the lesion (arrow) was just proximal to the first stent. E, A 9 × 50-mm stent was deployed in the carotid artery, which stopped the bleeding. F, Follow-up left carotid angiogram shows preserved carotid artery and complete obliteration of the pathologic lesion 1 week later. G, Six months later, axial contrast-enhanced CT scan of the neck shows thrombosis of the left carotid artery (arrow).
Overall delayed complications included rebleeding (in 4 patients), carotid thrombosis (in 3 patients), and brain abscess formation (in 1 patient). Rebleeding resulted from progression of the disease in 3 patients and was thought to be due to inadequate placement of the stent-graft over the pathologic lesion in one patient (patient 2) because of his critical clinical status. Rebleeding resulted in mortality in 2 patients: patient 1, whose family refused further intervention, and patient 2, who died from profuse bleeding and hypovolemic shock.
In patient 3, rebleeding occurred 3 weeks after the first procedure and was successfully treated with a second stent-graft (Fig 3). In patient 6, control of rebleeding was challenging. He presented with recurrent active extravasation 1 month after the first stent-graft was deployed (Fig 4). We embolized the abnormal vascular stains fed by the branches of contralateral superior and ipsilateral inferior thyroid arteries with 150–250 microns particles (Contour powder; Boston Scientific, Ireland) and achieved immediate hemostasis. Active bleeding recurred 1 month later, and another pseudoaneurysm in the carotid bulb just distal to the margin of the first stent-graft was found. We deployed a second stent-graft in the ICA and again achieved immediate hemostasis. However, rebleeding recurred 2 weeks later. Emergent subclavian angiography demonstrated a pseudoaneurysm in the branches of the right superior thyroid artery. The orifice of right ECA was occluded by the previously deployed stent grafts. Numerous collaterals from the right costocervical trunk anastomosed with the distal branches of the right ECA to feed the pseudoaneurysm (Fig 4). Because the branches of the right costocervical trunk may have anastomosed with the right vertebral artery, embolization of branches of the right costocervical trunk posed the risk of stroke. To target the source of bleeding, we performed direct percutaneous puncture of the main trunk of the right ECA by using a 21-gauge spinal needle. We contacted the right superior thyroid artery and embolized the pseudoaneurysm by slowly injecting 25% n-butyl cyanoacrylate (Histoacryl; Braun, Germany) and 75% lipiodol oil through the spinal needle. The control angiogram showed complete obliteration of the pseudoaneurysm, and no more bleeding was noted.
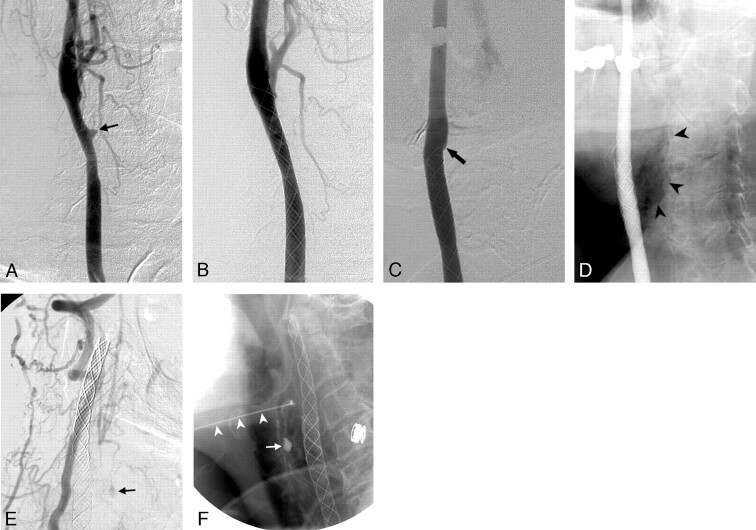
Fig 4.
Patient 6.
A, Right carotid angiogram shows a pseudoaneurysm in the right distal CCA (arrow).
B, An 8 × 50-mm stent was deployed from the right carotid bulb to the right CCA.
C, Another pseudoaneurysm in the right carotid bulb just distal to the distal end of the stent (arrow) was noted 2 months later.
D, A second 8 × 50-mm stent was deployed in the right cervical ICA to overlap the first stent. Mottled gas collection (arrowheads) in the soft tissue indicates radiation necrosis.
E, Right subclavian angiogram shows a pseudoaneurysm (arrow) in a branch of the right superior thyroid artery reconstituted from branches of the ipsilateral costocervical trunk via the ipsilateral ECA. The stent occludes the orifice of the right ECA.
F, Direct percutaneous puncture of the main trunk of the right ECA with a spinal needle (arrowheads) was done by using a roadmap image. The needle contacted the right superior thyroid artery and showed the small pseudoaneurysm (arrow). The pseudoaneurysm was successfully embolized with a slow injection of a mixture of liquid adhesives (n-butyl cyanoacrylate and lipiodol).
Patients 3–8 were followed up for 2 weeks to 3 months. In all of them, the carotid arteries and deployed stent-grafts were patent. In patients 3, 5, and 6, who were followed up for longer than 3 months, the treated carotid artery became thrombotic and occluded. One developed multiple brain abscesses due to septic thrombosis of the carotid artery (Fig 1), and 2 patients had asymptomatic thrombosis of the carotid artery (Fig 3). Five patients died: patients 1 and 2 from rebleeding, and patients 4, 7, and 8 from disease progression. Three patients were alive at the last follow-up of 13–20 months.
Discussion
Carotid blowout, or rupture of the carotid artery, is a dreaded complication of head-and-neck surgery for malignant disease. Patients with CBS can have a variety of clinical presentations due to the carotid rupture, such as acute hemorrhage or exposure of part of the carotid artery.1,4,5 The reported incidence of carotid rupture after radical neck dissection is 4.3%.8 Risk factors for CBS in patients with head-and-neck cancers include pharyngocutaneous fistula, wound infection, exposure to saliva, radiation therapy, radical resection and stripping of the carotid sheath, flap necrosis with carotid exposure, and recurrent tumor.1,2 In patients with head-and-neck cancers, previous irradiation increases their risk of CBS 7.6-fold.1,2 The carotid artery ruptures if its wall is damaged as a result of ischemia, as it receives 80% of its blood supply from the adventitia.3 The formation of pseudoaneurysms has been reported 2–20 years after radical neck dissection and irradiation.9,10
Emergency surgical ligation of the ICA or CCA is typically used to treat CBS. This approach is associated with an average neurologic morbidity rate of 60%.3 Endovascular techniques, including permanent balloon occlusion of an affected ICA, substantially improve patient outcomes.1–4 However, as many as 15%–20% of patients with CBS who are treated with permanent balloon occlusion develop immediate or delayed cerebral ischemia.1,6 This complication results from factors such as an incomplete circle of Willis, thromboembolism from an acutely occluded carotid artery, or delayed failure of a collateral vessel. A balloon occlusion test may be performed before threatened CBS is treated definitively, but this is usually not possible in impending or acute cases. Additionally, test occlusion may not help in identifying the small subset of patients in whom delayed hemodynamic ischemia develops after the ICA is permanently occluded.1–3 Complications of this technique include acute pseudoaneurysm rupture during balloon inflation and delayed balloon rupture or deflation.1–3 Disease of the contralateral ICA may also preclude safe treatment by means of parent-artery occlusion.
Deployment of stent-grafts has been reported in the reconstructive management of arterial lacerations, dissections, pseudoaneurysms, and arteriovenous fistulas of the head-and-neck region.6,11,12 This treatment is especially useful in patients at high risk for occlusion of the carotid artery. In most, the vascular lesion is successfully managed and the parent artery is preserved. Few complications have been reported. However, the application of stent-grafts to manage carotid blowout in cases of head-and-neck cancers has had diverse results. In one series, CBS was safely managed with stent-assisted endovascular reconstruction (2 patients with stent-graft) without any procedure-related strokes or deaths.6 The authors reported that the use of self-expanding stent-grafts was promising in the management of recurrent CBS. However, others have mentioned that these self-expanding stent-grafts were useful only for the initial control of carotid bleeding and that they might be associated with delayed complications, such as rebleeding, thrombosis, or occlusion.5,7
The stent-graft that we used is an expandable metal mesh covered by polyethylene terephthalate. Several factors must be considered when this stent-graft is used to manage carotid blowout in patients with head-and-neck cancers. First, this self-expandable stent-graft requires favorable anatomic conditions, such as patent femoral and iliac arterial anatomy that permits the placement of a large-caliber (10–11F) vascular sheath, an aortic arch and supra-aortic branches that are simple and not tortuous, and a lack of coexistent carotid stenosis. For this stent, target vessels of 5–12-mm diameter are suggested. Associated carotid stenosis can hinder full expansion of the stent-graft, which may impede blood flow and cause acute thrombus formation (Fig 2). In patient 1, associated with long-segmental carotid stenosis, intimal injury could also occur in the region of distal end of the stent-graft when angioplasty was performed after the deployment of the stent-graft. This intimal injury may aggragate the acute thrombosis of the treated carotid artery. For this situation, the reconstructive management includes adequate predilation of the stenotic ICA before deployment of the stent-graft, use of a noncovered stent for the stenotic ICA and bridge with a covered stent proximally, and use of uncovered stent(s) with embolization of the pseudoaneurysm.6 Further technical improvement of the stent-graft to reduce the profile and increase the flexibility of the delivery system is important to widen its application in managing carotid blowout.
Furthermore, appropriate pathophysiologic conditions, such as tolerance to antiplatelet and anticoagulant medication and absence of a contaminated wound, are required. Premedication with antiplatelets is usually not possible in cases of acute or impending CBS. For patients in this situation, we administered IV glycoprotein IIb/IIIa inhibitor during the intervention and changed to an oral regimen after hemostasis was achieved. Glycoprotein IIb/IIIa inhibitor can achieve its thrombolytic effect in a few minutes and is effective in preventing acute thrombosis (Fig 3).13
Stent-grafts in general are more thrombogenic than are bare stents, and they therefore require more aggressive and prolonged antiplatelet therapy during and after management.14–16 The use of stent-grafts requires a minimum of 3 months of therapy with both aspirin and clopidogrel iclopidine.14 However, CBS in patients of head-and-neck cancers is not a simple vascular disease; instead, it is a systemic disorder. Such patients may have wound complications such as radiation necrosis or pharyngocutaneous fistula, or they may have systemic complications such as mucositis by previous chemoradiotherapy. Prolonged antiplatelet therapy carries the risk of bleeding or oozing from the extravascular lesions. Prolonged antiplatelet therapy may also interfere with further permanent management such as combined bypass surgery and carotid sacrifice, as we consider stent-grafts to be for temporary or emergency management of CBS. Most of our patients were younger than those in previous studies, and they were without associated diffuse atherosclerotic change and stenosis in the distal carotid artery and its branches. Accordingly, we shortened the time course of antiplatelet therapy of IV glycoprotein IIb/IIIa inhibitor to 4–6 hours and oral regimen of clopidogrel iclopidine to 1 month after the procedure. This inadequate adjuvant regimen that may have contributed to the long-term suboptimal outcomes of our patients. We suggest giving an extended time course of antiplatelet therapy for improving the patency rate of stent-grafts if patients can tolerate it.
When stent-grafts are deployed to manage carotid blowout in patients with head-and-neck cancers, keeping foreign bodies away from the region of ongoing contamination is impossible. During follow-up of longer than 6 months, none of our 3 patients had a patent carotid artery. One presented with brain abscesses. The use of a stent-graft in a field with contaminated necrosis may result in persistent infection as infective organisms colonize the foreign material. This persistent infection may also inhibit neointimal formation inside the stent and ultimately cause thrombosis of the carotid artery. In a swine model, antibiotic prophylaxis was used during and after stent deployment to reduce the risk of local infection.17 For improved control of this local infection, we recommend prophylactic antibiotics before and after the intervention.
The other factor to consider when applying a stent-graft to manage carotid blowout is the location of the pathologic lesion. For lesions in the region of the carotid bifurcation, bleeding may recur because of reconstitution from the proximal branches of the ECA. Embolization of the main trunk of the ipsilateral ECA can reduce the possibility of this rebleeding (Fig 1). Four patients had pathologic lesions involving the carotid bifurcation. In patients 4 and 5, these were associated with embolization of the main trunk of the ECA; they had no rebleeding after 2- or 14-month follow-up. Patients 1 and 6, without combined embolization of the ECA, presented with rebleeding within 1 month.
The pathologic field of the head-and-neck cancer can show temporal and dynamic changes as a result of complex factors, such as tumoral recurrence, infection, and radiation necrosis. This ongoing pathologic process may progress over the area initially treated, especially as observed on long-term follow-up. In patients 3 and 6, recurrent bleeding caused by new pathologic vascular lesions extended beyond the first stent-graft (Figs 3 and 4). We suggest assessing the extent of the pathologic lesion on CT and the field of previous irradiation before stent placement is planned. A long, self-expandable stent-graft can fully cover the pathologic field, especially given the continuous shortening of the self-expanding stent-graft as it dilates and progressively accommodates to the carotid artery.
Typically, complete obliteration of the pathologic lesion soon after the deployment of a stent-graft is indicated to confirm the success of its management for carotid blowout. In 3 of our 10 stent-graft deployment procedures (patients 4 and 5 and the second stent of patient 3), complete obliteration of the pathologic lesions was not demonstrated by on-site angiogram but in the follow-up images. The reasons for not achieving immediate obliteration of the lesions in these cases include the use of IV antiplatelet medication (glycoprotein IIb/IIIa inhibitor) during and after the procedure, relatively poor compliance of the stent-graft to the carotid artery, and no postdilation after deployment of the stent-grafts. Similar to the design of Wallstent (Boston Scientific), the Wallgraft is a continuously braided, covered stent that has relatively poor conformity and compliance with vascular tortuosity and irregularity.18,19 An appropriately selected self-expandable stent-graft will slowly accommodate itself to the carotid artery and finally obliterate the pathologic lesion. It is possible to obtain obliteration of lesions if clinical improvement and progressively slower leakage of contrast media into the lesion are found on serial angiograms. Performing balloon angioplasty to these vulnerable carotid arteries, however, may risk vascular injury in the margin of the stent-grafts. This is especially true with the use of a large balloon, with the use of a short stent-graft with inadequate coverage of the lesion, or with the end of the stent-graft located in a curved carotid artery.
The poor long-term patency of the deployed stent-grafts and the delayed complications suggested that stent-graft placement may be a temporary rather than a permanent way to manage CBS in patients with head-and-neck cancers. However, the stent-grafts offered several benefits. First, immediate hemostasis was achieved in all patients, with only one instance of symptomatic stroke. This outcome highlights the efficacy of using self-expandable stent-grafts in emergency practice to achieve hemostasis and prevent neurologic morbidity in patients at high risk for carotid occlusion. The procedure also prolonged the time for further management to be planned, allowing clinicians to wait for the patients’ clinical status to stabilize enough for an occlusion test or allowing them to perform combined vascular bypass surgery and permanent carotid occlusion.
Second, the deployed stent-grafts showed good patency in 6 patients during the first 3 months of follow-up. This observation implies the temporary efficacy of self-expandable stent-grafts in achieving both hemostasis and vascular preservation in these patients. Moreover, progression of thrombosis in the carotid artery during several months may provide adequate time for reconstitution of the intracranial collaterals if persistent infection can be controlled with prophylactic antibiotic treatment. Selected patients with CBS, advanced disease, and a life expectancy of less than 3 months may benefit from this treatment. However, the rate of rebleeding (50%) of our patients was still too high, which limits application of the procedure. This high rate of rebleeding also indicates that preserving flow in the artery adjacent to ongoing contamination can leave the patient at higher risk of subsequent bleeding than with carotid sacrifice.
Our study included a limited number of patients, many of whom had a short survival, thus preventing long-term follow-up. Additionally, the lack of experience with antiplatelet medications and prophylactic antibiotics might have affected safety and outcomes. Further research with improved prophylactic medications and interventional technique and devices is needed to improve the outcomes of reconstructive management of carotid blowout in patients with head-and-neck cancers.
Conclusion
Although deployment of stent-grafts can achieve immediate and initial hemostasis in patients with head-and-neck cancer and CBS, the long-term safety, stent patency, and permanency of hemostasis appear unfavorable. The use of stent-grafts in this context should be considered a temporary or emergency rather than a permanent method to manage CBS in these patients. We reserve application of this procedure for patients in critical clinical condition that preclude an occlusion test, and when carotid occlusion poses an unusually high risk of neurologic morbidity. We also recommend prophylactic antibiotic treatment and combined embolization of vascular feeders in the pathologic field to improve outcomes.
References
- 1.Chaloupka JC, Putman CM, Citardi MJ, et al. Endovascular therapy for the carotid blowout syndrome in head and neck surgical patients: diagnosis and managerial considerations. AJNR Am J Neuroradiol 1996;17:843–52 [PMC free article] [PubMed] [Google Scholar]
- 2.Macdonald S, Gan J, Mckay AJ, et al. Endovascular treatment of acute carotid blowout syndrome. J Vasc Interv Radiol 2000;11:1184–88 [DOI] [PubMed] [Google Scholar]
- 3.Citardi MJ, Chaloupak JC, Son YH, et al. Management of carotid artery rupture by monitored endovascular therapeutic occlusion (1988–1994). Laryngoscope 1995;105:1086–92 [DOI] [PubMed] [Google Scholar]
- 4.Chaloupka JC, Roth TC, Putman CM, et al. Recurrent carotid blowout syndrome: diagnosis and therapeutic challenges in a newly recongnized subgroup of patients. AJNR Am J Neuroradiol 1999;20:1069–77 [PMC free article] [PubMed] [Google Scholar]
- 5.Warren FM, Cohen JI, Nesbit GM, et al. Management of carotid “blowout” with endovascular stent grafts. Laryngoscope 2002;112:428–33 [DOI] [PubMed] [Google Scholar]
- 6.Lesley WS, Chaloupka JC, Weigele JB, et al. Preliminary experience with endovascular reconstruction for the management of carotid blowout syndrome. AJNR Am J Neuroradiol 2003;24:975–81 [PMC free article] [PubMed] [Google Scholar]
- 7.Simental A, Hohnson JT, Horowitz M. Delayed complications of endovascular stenting for carotid blowout. Am J Otolaryngol 2003;24:417–19 [DOI] [PubMed] [Google Scholar]
- 8.Maran AGD, Amin M, Wilson JA. Radical neck dissection: a 19-year experience. J Laryngol Otol 1989;103:760–64 [DOI] [PubMed] [Google Scholar]
- 9.Fonkalsrud EW, Sanchez M, Zerubavel R, et al. Serial changes in arterial structure following radiation therapy. Surg Gynecol Obstet 1977;145:395–400 [PubMed] [Google Scholar]
- 10.Ernemann U, Herrmann C, Plontke S, et al. Pseudoaneurysm of the superior thyroid artery following radiotherapy for hypopharyngeal cancer. Ann Otol Rhinol Laryngol 2003;112:188–90 [DOI] [PubMed] [Google Scholar]
- 11.Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg 2001;95:412–19 [DOI] [PubMed] [Google Scholar]
- 12.Alexander MJ, Smith TP, Tucci DL. Treatment of an iatrogenic petrous carotid artery pseudoaneurysm with a covered stent. Cardiovasc Intervent Radiol 2001;24:283–8511779022 [Google Scholar]
- 13.Topol EJ, Moliterno DJ, Herrmann HC, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the preventing of ischemic events with percutaneous coronary revascularization. N Engl J Med 2001;344:1888–94 [DOI] [PubMed] [Google Scholar]
- 14.Sovik E, Klow NE, Brekke M, et al. Elective placement of covered stents in native coronary arteries. Acta Radiol 2003;44:294–301 [DOI] [PubMed] [Google Scholar]
- 15.Tielliu IFJ, Verhoeven ELG, Zeebregts CJ, et al. Endovascular treatment of popliteal artery aneurysm: results of a prospective cohort study. J Vasc Surg 2005;41:561–67 [DOI] [PubMed] [Google Scholar]
- 16.Gercken U, Lansky AJ, Buellesfeld L, et al. Results of the Jostent coronary stent graft implantation in various clinical settings: procedural and follow-up results. Catheter Cardiovasc Intervent 2002;56:353–60 [DOI] [PubMed] [Google Scholar]
- 17.Paget DS, Bukhari RH, Zayyat EJ, et al. Infectibility of endovascular stents following antibiotic prophylaxis or after arterial wall incorporation. Am J Surg 1999;178:219–24 [DOI] [PubMed] [Google Scholar]
- 18.Tanaka N, Martin J-B, Tokunaga K, et al. Conformity of carotid stents with vascular anatomy: evaluation in carotid models. AJNR Am J Neuroradiol 2004;25:604–07 [PMC free article] [PubMed] [Google Scholar]
- 19.Berkefeld J, Turowski B, Dietz A, et al. Recanalization results after carotid stent placement. AJNR Am J Neuroradiol 2002;23:113–20 [PMC free article] [PubMed] [Google Scholar]