Abstract
BACKGROUND AND PURPOSE: We report abnormal high T2 signal intensity in the anterior lobe of the cerebellar vermis that we believe was the result of profound hypoxic ischemic encephalopathy in the perinatal period in term infants. We tested the hypothesis that this sign was associated with other signs of significant perinatal hypoxic damage.
METHODS: Thirty patients with clinically and radiologically confirmed perinatal profound hypoxia close to term were included in the study. The cranial MR images were reviewed by 2 pediatric neuroradiologists and were scored for the presence and severity of hypoxia/ischemia in the regions typically affected by profound hypoxia. The presence or absence of high T2 signal intensity in the vermis and other sites was correlated with the extent of damage in typically affected regions.
RESULTS: Eighteen of 30 patients had high T2 signal intensity in the vermis. The presence of vermian damage correlated positively with radiologic evidence of severe hypoxic damage and extremely poor (0 or 1) 1-minute Apgar scores.
CONCLUSIONS: High T2 signal intensity in the anterior lobe of the vermis probably represents gliosis secondary to hypoxia/ischemia and is related to the severity of damage in the term infant.
Perinatal hypoxic-ischemic encephalopathy (HIE) in the term infant is a catastrophic clinical event and may have considerable long-term patient care implications. The regions of the brain damaged by HIE depend on several factors, but most important is the degree and duration of the hypoxia and the maturity of the brain at the time of the insult. Short periods of very severe HIE in children born at or close to term has been termed “profound hypoxia” in the past. This produces a highly characteristic pattern of gliosis in the posterior putamen, ventrolateral thalamus, paracentral white matter, and hippocampus.1 Another region of selective vulnerability, the anterior lobe of the cerebellar vermis (ALV) is recognized; in this report, we describe the frequency and associations of that finding.2
Materials and Methods
Thirty consecutive patients who had been referred to our institution for MR examinations to investigate possible HIE in the perinatal period were included in this study. No patients were excluded from these consecutive referrals, but patients with severe HIE who failed to survive the first 9 months of life could not be included in this study population.
MR imaging showed patterns of damage distribution consistent with textbook descriptions of profound hypoxia and no patients had any suggestion of developmental brain pathology.1 All patients had MR imaging at 1.5T (Eclipse or Infinion; Phillips Medical Systems, Best, The Netherlands), and ages at the time of scanning ranged from 1 to 24 years. Imaging consisted of axial and coronal fast spin-echo T2-weighted, axial and sagittal spin-echo T1 images, and fast spin-echo fluid-attenuated inversion recovery (FLAIR) images in either the axial or sagittal plane.
Two experienced pediatric neuroradiologists (D.J.A.C., P.D.G.) reviewed the hard copy images, and a consensus opinion was attained without knowledge of the clinical information at the time of image review. The posterior putamen, ventrolateral thalamus, and paracentral white matter changes were graded 0 to 3 (0 for no change, 1 for signal intensity change but no volume loss, 2 for early volume loss and signal intensity change, and 3 for significant volume loss). A total score of these abnormalities was recorded. The presence of abnormal signal intensity in other regions was noted (eg, hippocampus or caudate) as well as the condition of the ALV.
The clinical information recorded included gestational age and birth weight, 1- and 5-minute Apgar scores, lowest recordable heart rate in the perinatal period (normally a cardiotocograph or scalp electrode reading) or at birth, age at MR imaging, and clinical symptoms. The Apgar score at 1 minute was categorized into those with a score of 0 or 1 (clinically severe) and those with a score of 2–6 (clinically moderate). The total score of putamen, thalamus, and paracentral white matter abnormality was also categorized into those with a combined score of 6 or more (radiologically severe) and those with a score of 5 or less (radiologically moderate). χ2 analysis was performed between ALV signal intensity change and 1-minute Apgar score, ALV signal intensity change versus low and high total scores of putamen, thalamic and paracentral white matter, ALV and hippocampal signal intensity change, and hippocampal signal intensity change and total score.
Results
All patients had Apgar scores of 6 or less 1 minute after birth (range, 0–6; median, 1). Cord pH measurements had been made in 18 of 30, and all were 7.1 or lower (15 below 7.0). Heart rate measurements were available in 22 of 30. The heart rate was not recordable in 8 patients, less than 100 beats per minute in 13 patients, and more than 100 beats per minute in 1 patient. All these children had long-term static neurologic deficits, either hemiplegia or quadriplegia, and 20 children had dyskinesia. All patients were born between gestational weeks 37 and 42 as calculated from last menstrual period and fetal sonography imaging data, and birth weights ranged from 2.3 to 4.2 kg. None of the patients included in this group had documented evidence of hyperbilirubinemia or hypoglycemia.
Of the 30 patients, 97% had putaminal, 90% had thalamic, and 87% had paracentral white matter changes (Table). Eighteen of 30 (60%) patients demonstrated high T2 signal intensity in the superior vermis. The total score of changes in the posterior putamen (0–3), ventrolateral thalamus (0–3), and paracentral white matter (0–3) was scored out of 9, with 15 patients scoring 5 of 9 or less and 15 scoring 6 of 9 or more. Those with high ALV T2 signal intensity were more likely to have a high total score (P = .02). There was no association between high total score and hippocampal or caudate abnormality (P > .05) or vermian and hippocampal abnormality (P > .05).
Patient clinical information and scores for brain damage secondary to hypoxic ischemic encephalopathy
| Case | Age (years) | Apgar |
Heart Rate (bpm) | Putamen | Thalamus | PCWM | Total Score* | Vermis | Others | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 minute | 5 minutes | |||||||||
| 1 | 4 | 6 | 6 | >100 | 2 | 2 | 1 | 5 | 0 | 0 |
| 2 | 2 | 1 | 3 | 80 | 3 | 3 | 2 | 8 | 1 | Hippocampus, caudate |
| 3 | 3 | 0 | 2 | 70 | 2 | 3 | 2 | 7 | 1 | 0 |
| 4 | 15 | 0 | 1 | UR | 1 | 3 | 2 | 6 | 1 | 0 |
| 5 | 4 | 1 | 7 | 60 | 3 | 3 | 3 | 9 | 1 | Hippocampus |
| 6 | 1 | 1 | 2 | 60 | 2 | 1 | 1 | 4 | 1 | Hippocampus |
| 7 | 1 | 1 | 5 | <20 | 3 | 2 | 2 | 7 | 1 | Hippocampus |
| 8 | 14 | 1 | 2 | UR | 2 | 2 | 2 | 6 | 1 | Hippocampus |
| 9 | 6 | 0 | 3 | UR | 2 | 1 | 1 | 4 | 0 | 0 |
| 10 | 7 | 6 | 9 | 2 | 1 | 2 | 5 | 0 | 0 | |
| 11 | 5 | 2 | 1 | 3 | 2 | 6 | 1 | Caudate | ||
| 12 | 5 | 3 | 5 | 1 | 2 | 1 | 4 | 1 | Hippocampus | |
| 13 | 5 | 0 | 4 | UR | 3 | 3 | 2 | 8 | 1 | 0 |
| 14 | 12 | 1 | 6 | 1 | 0 | 3 | 4 | 0 | 0 | |
| 15 | 3 | 1 | 5 | 40–60 | 2 | 2 | 1 | 5 | 1 | Hippocampus |
| 16 | 24 | 1 | 5 | 2 | 2 | 0 | 4 | 1 | 0 | |
| 17 | 1 | 3 | 5 | 3 | 0 | 2 | 5 | 0 | Caudate | |
| 18 | 5 | 1 | 0 | 3 | 3 | 3 | 9 | 1 | 0 | |
| 19 | 1 | 0 | 0 | UR | 2 | 2 | 1 | 5 | 0 | 0 |
| 20 | 2 | UR | 2 | 2 | 0 | 4 | 1 | Hippocampus | ||
| 21 | 2 | 2 | 3 | 70 | 2 | 3 | 2 | 7 | 0 | 0 |
| 22 | 2 | 5 | 6 | 80 | 3 | 3 | 3 | 9 | 1 | 0 |
| 23 | 1 | 1 | 1 | 60–80 | 2 | 2 | 2 | 6 | 1 | Hippocampus |
| 24 | 4 | 4 | 8 | 70 | 0 | 0 | 2 | 2 | 0 | Caudate |
| 25 | 2 | 4 | 6 | 60 | 2 | 1 | 3 | 6 | 0 | Caudate |
| 26 | 22 | 0 | 2 | UR | 1 | 2 | 0 | 3 | 1 | 0 |
| 27 | 15 | 6 | 7 | UR | 2 | 2 | 1 | 5 | 0 | 0 |
| 28 | 4 | 6 | 8 | 2 | 2 | 2 | 6 | 1 | Hippocampus | |
| 29 | 7 | 1 | 4 | 60 | 2 | 2 | 0 | 4 | 0 | 0 |
| 30 | 5 | 2 | 7 | <80 | 3 | 2 | 3 | 8 | 0 | 0 |
Note:—bpm indicates beats per minute; PCWM, paracentral white matter; UR, unrecordable.
Total score of the abnormalities seen in putamen, thalamus, and paracentral white matter.
Seventeen of 30 infants had clinically severe HIE (1-minute Apgar score of 0 or 1). There was a significant (P = .02) association between low Apgar score (0 or1) at 1-minute and the presence of ALV high T2 signal intensity. There was however, no significant association between the 5-minute Apgar score and high signal intensity in the ALV (P > .05). One-minute Apgar score and total score were shown to have no direct association (P > .05).
Discussion
Cerebral palsy defines a group of neurologic conditions involving motor disorders with neurologic signs resulting from brain damage that is static and has occurred in early life.3 Patients with underlying metabolic disorders, hydrocephalus, brain tumors, and various other disorders by convention are excluded from the cerebral palsies. HIE in the perinatal period contributes a significant proportion of the total number of cases, and 3 “textbook” clinical and radiologic patterns are recognized. Hypoxia-ischemia severe enough to cause brain damage before gestational week 35 usually produces “periventricular leukomalacia”; the white matter immediately next to the ventricles is most affected. As the fetus matures, the pattern of involvement changes because of alterations in the site of the vascular watershed zones.1 For example, exposure to a long period of low-grade hypoxia in a 40-week-old fetus will cause damage to the parasagittal white matter. In contrast, exposure to acute severe hypoxia in a 40-week-old fetus will damage areas of the brain that are most metabolically active, including the putamen, thalamus, and paracentral white matter.
MR imaging has long been identified as the method that provides important information related to the timing and severity of HIE, with altering patterns of structural involvement depending on the age of the patient.4 Posterior putamen and ventrolateral thalamic gliosis is associated with extrapyramidal symptoms in cerebral palsy5 (Figs 1B and 3B). Paracentral lobule high T2 signal intensity change and volume loss is another site of involvement typical of HIE in a term infant (Figs 1A and 3A). The reason for the specific selection of these foci for damage in HIE is uncertain, but some authors believe that they may be selected because they are areas of active myelination in the term infant.6 Why the vermis is selectively injured in profound HIE may differ from the above active myelination.7 The vermis is rich in Purkinje cells, some of which may be deficient in aldolase C and excitatory amino acid transporter 4 (EAAT4). Aldolase C and EAAT4 deficiency means that these cells are unlikely to survive intense synaptic input after the restoration of blood flow.
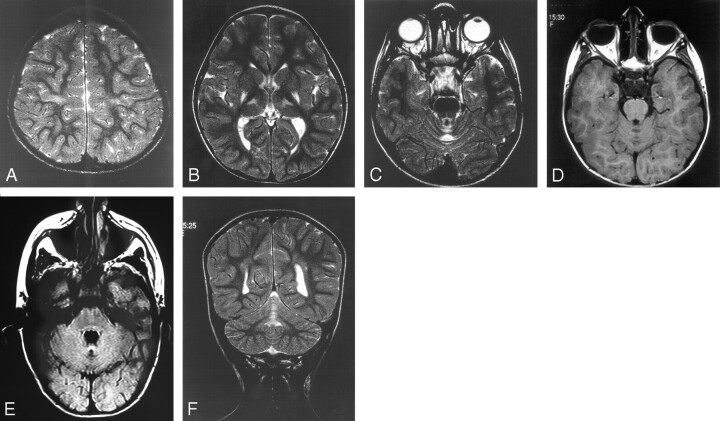
Fig 1.
Five-year-old girl with athetoid spastic quadriplegia.
A, Axial T2. Mild signal intensity change in the perirolandic white matter.
B, Axial T2. Typical signal intensity change in the posterior putamen and the ventrolateral thalamus bilaterally.
C, Axial T2. Typical signal intensity change in the anterior lobe of the vermis.
D, Axial T1. Low T1 signal intensity change in the vermis.
E, Axial FLAIR. High T2 signal intensity and cystic change.
F, Coronal T2. Anterior lobe of vermis high T2 signal intensity change.
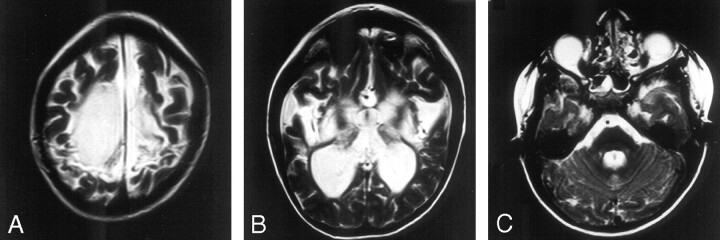
Fig 3.
Seven-year-old boy with severe spastic quadriplegia.
A, Axial T2. Severe signal intensity change and volume loss in the perirolandic white matter.
B, Axial T2. Severe putaminal and thalamic volume loss and high T2 signal intensity bilaterally.
C, Axial T2. Mild vermian high T2 signal intensity change.
Recent studies have assessed the use of new MR techniques such as diffusion-weighted imaging and MR spectroscopy in the investigation of patients with HIE. Diffusion-weighted imaging has been shown to demonstrate abnormalities in the thalami and internal capsule in the first day of hypoxic ischemic brain injury, when conventional MR imaging is normal.8 Diffusion-weighted imaging, however, underestimates the extent of the long-term injury,8,9 which may be explained by delayed neuronal and oligodendroglial cell death due to apoptosis in areas with lower metabolic demand.8 Diffusion-weighted images obtained between the 2nd and 4th days of life reflect the extent of injury more reliably. By day 7, diffusion MR is less sensitive to perinatal brain injury compared with conventional MR because of transient pseudonormalization of diffusion images.10
Barkovich et al11 used single-voxel MR spectroscopy centered on the basal ganglia or watershed vascular region in asphyxiated term neonates to predict developmental status at 12 months. High lactate (lactate/choline ratio) correlated with poor neuromotor outcome. Kuenzle et al12 have demonstrated an association between changes seen on MR imaging (including diffuse brain injury and basal ganglia/thalamic signal intensity changes) in the first week after delivery and poor developmental outcome. However, normal results on CT or MR imaging performed in the first few weeks of life do not preclude later neurologic dysfunction.13 The optimal timing and technique for MR imaging to assess for HIE is therefore uncertain. MR imaging in the first few days of life allows clinicians to provide families with information on prognosis. MR imaging after the age of 2 years, when myelination is complete, will allow more complete assessment of HIE-related brain damage.
We have described ALV high T2 signal intensity for HIE in the term infant. ALV high T2 signal intensity is most easily seen on coronal images. In more severe cases, there may be volume loss. This sign may assist in the correct diagnosis of HIE birth injury and should not be interpreted as showing a second pathologic condition. It should be appreciated that spurious high signal intensity change can sometimes be seen in the cerebellum on T2-weighted images if there is loss of brain volume and a partial volume effect from CSF within the voxel is encountered. We are confident that this is not the case in our patients because the high signal intensity is also seen on proton attenuation and FLAIR images. The T2 prolongation is probably due to gliosis as in the other regions of the brain affected by profound HIE.
In adults, injury to the vermis normally results in truncal ataxia. A larger study than this would be required to assess whether this radiologic sign is associated with specific symptoms and signs that can be attributed to injury to the vermis in patients who often have significant neurologic deficits. However, it should be noted that the extensive involvement of the other regions of the brain might make attributing functional implications of ALV damage difficult or impossible.
Levene et al14 and Jouvet et al15 stated that severity of encephalopathy in HIE could be graded retrospectively on the basis of a set of clinical signs. MR changes of HIE in the pediatric population have been shown to correlate with neurologic outcome.16 There is an association in our study of ALV high T2 signal intensity with both lower 1-minute Apgar scores and with a higher overall score for radiologic evidence of HIE. The ALV high T2 signal intensity sign may be therefore be an independent predictor of HIE associated with significant neurologic disability. We must accept that the classification of putaminal, thalamic, and white matter HIE signal intensity and volume changes into mild, moderate, and severe may seem arbitrary but this was completed as a consensus opinion by 2 experienced neuroradiologists blinded to the clinical outcome data. The correlation of a high overall score for MR imaging change of HIE and vermian high T2 signal intensity marks this as a potentially important marker of significant neurologic disability.
Conclusion
The ventrolateral thalamus, posterior putamen, and paracentral white matter (perirolandic gyrus) are known areas of brain injury manifest as loss of volume and high T2 signal intensity in term infants with profound HIE in the perinatal period. We have described ALV high T2 signal intensity as a sign of profound HIE in the term infant. ALV high T2 signal intensity is associated with radiologic and clinical indicators of severe HIE in term infants.
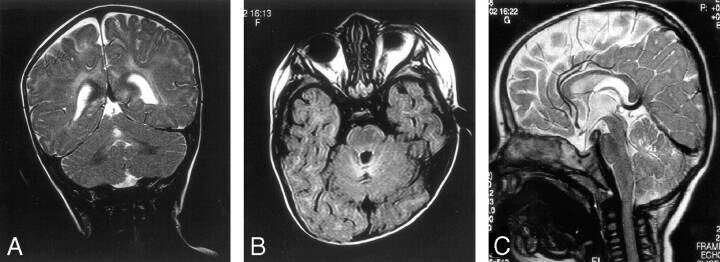
Fig 2.
Nine-month-old girl with dyskinetic cerebral palsy.
A, Coronal T2. Central lobule of anterior lobe of the vermis high T2 signal intensity.
B, Axial FLAIR. High T2 signal intensity and cystic change of anterior lobe of vermis.
C, Sagittal T2. Central lobule of anterior lobe of vermis high T2 signal intensity.
References
- 1.Barkovich AJ. Pediatric Neuroimaging, 3rd ed. Philadelphia: Lippincott, Williams and Wilkins;2000. :169–207
- 2.Sargent MA, Poskitt KJ, Roland EH, et al. Cerebellar vermian atrophy after neonatal hypoxic-ischemic encephalopathy. AJNR Am J Neuroradiol 2004;25:1008–15 [PMC free article] [PubMed] [Google Scholar]
- 3.Neville BGR. Paediatric neurology. In: Walton J, ed. Brain’s Diseases of the Nervous System, 10th ed. Oxford, UK: Oxford University Press;1993. :453–57
- 4.Barkovich AJ, Truwit CL. Brain damage from perinatal asphyxia: correlation of MR findings with gestational age. AJNR Am J Neuroradiol 1990;11:1087–96 [PMC free article] [PubMed] [Google Scholar]
- 5.Menkes JH, Curran J. Clinical and MR correlates in children with extrapyramidal cerebral palsy. AJNR Am J Neuroradiol 1994;15:451–57 [PMC free article] [PubMed] [Google Scholar]
- 6.Rademakers RP, van der Knaap MS, Verbeeten Jr B, et al. Central cortico-subcortical involvement: a distinct pattern of brain damage caused by perinatal and postnatal asphyxia in term infants. J Comput Assist Tomogr 1995;19:256–63 [PubMed] [Google Scholar]
- 7.Welsh JP, Yuen G, Placantonakis DG, et al. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol 2002;89:331–59 [PubMed] [Google Scholar]
- 8.Barkovich AJ, Westmark KD, Bedi HS, et al. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. AJNR Am J Neuroradiol 2001;22:1786–94 [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson RL, Ben-Sira L, Barnes PD, et al. MR line-scan diffusion-weighted imaging of term neonates with perinatal brain ischemia. AJNR Am J Neuroradiol 1999;20:1658–70 [PMC free article] [PubMed] [Google Scholar]
- 10.McKinstry RC, Miller JH, Snyder AZ, et al. A prospective longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 2002;59:824–33 [DOI] [PubMed] [Google Scholar]
- 11.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol 1999;20:1399–405 [PMC free article] [PubMed] [Google Scholar]
- 12.Kuenzle C, Baenziger O, Martin E, et al. Prognostic value of early MR imaging in term infants with severe perinatal asphyxia. Neuropediatrics 1994;25:191–200 [DOI] [PubMed] [Google Scholar]
- 13.Blankenberg FG, Loh N-N, Bracci P, et al. Sonography, CT, and MR imaging: a prospective comparison of neonates with suspected intracranial ischemia and hemorrhage. AJNR Am J Neuroradiol 2000;21:213–18 [PMC free article] [PubMed] [Google Scholar]
- 14.Levene ML, Kornberg J, Williams TH. The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev 1985;11:21–26 [DOI] [PubMed] [Google Scholar]
- 15.Jouvet P, Cowan FM, Cox P, et al. Reproducibility and accuracy of MR imaging of the brain after severe birth asphyxia. AJNR Am J Neuroradiol 1999;20:1343–48 [PMC free article] [PubMed] [Google Scholar]
- 16.Christophe C, Fonteyne C, Ziereisen F, et al. Value of MR imaging of the brain in children with hypoxic coma. AJNR Am J Neuroradiol 2002;23:716–23 [PMC free article] [PubMed] [Google Scholar]