Abstract
Background and PURPOSE: To determine which MR imaging sequences are necessary to assess for spinal metastases.
METHODS: Hypothetical MR imaging interpretations and management plans were made prospectively for consecutive adult cases acquired retrospectively. Standardized MR imaging protocols were independently interpreted by 2 neuroradiologists. MR imaging protocol types varied: 1) T1-weighted images only; 2) T1-weighted and T2-weighted images; 3) T1-weighted and postcontrast T1-weighted images; and 4) T1- and T2-weighted images and postcontrast T1-weighted images. Hypothetical management plans were created by 2 radiation oncologists. Logit model was used to investigate the effect of MR imaging protocol type on the probability of recommending radiation therapy (RT). Mixed effect models were used to investigate whether median spinal level or total number of spinal levels of planned RT was associated with MR imaging protocol type.
RESULTS: Thirty-one subjects were evaluated, each with multiple scan interpretations. Logit model showed that neither MR imaging protocol type nor neuroradiologist reader affected the probability that the oncologist would recommend RT (all P > .50). Mixed models showed that neither ML nor NL was affected by MR imaging protocol type or by neuroradiologist reader (all P > .12).
CONCLUSION: Although MR imaging is known to be the most useful diagnostic test in suspected spinal cord compression, which particular MR images are necessary remain unclear. Compared with T1-weighted images alone, the additional use of T2-weighted and/or postcontrast T1-weighted sequences did not significantly affect the probability that RT would be recommended or the levels that would be chosen for RT in our study. Our data suggest that unenhanced T1-weighted images may be sufficient for evaluation of possible cord compression.
The spinal column is the most common osseous site for metastatic deposits.1 Of those with spinal involvement, symptomatic spinal cord compression is seen in approximately 10%–20%.1 The most common tumors to metastasize to the spine are lung, breast, prostate, and renal cell.2 Pain is the presenting symptom in most (>90%) of cases.3-5 The pain is usually gradual in onset and progressive, and it typically worsens at night.4 Motor weakness (76%) and paresthesias (50%) are also common at presentation.3 As cord involvement develops, symptoms generally progress from pain to paraparesis, loss of sensation, and finally to autonomic dysfunction.3,5 The goal of therapy is palliation and preservation of function to optimize quality of life in most cases; treatment is rarely curative.2
MR imaging has long been recognized to have substantial impact on the evaluation and management of spinal tumors.6-7 Specific relevant diagnostic information that neuroradiologists can glean from MR imaging of the spine includes the diagnosis of metastasis, the characterization of the levels of involvement, and the diagnosis of any associated cord compression. Both bony involvement and neural compression from epidural tumor are demonstrable by MR imaging.
In clinical practice, there is disagreement regarding which routine sequences are necessary for clinical assessment in these cases. Therefore, some imagers routinely include only nonenhanced T1-weighted images in cases of clinically suspected cord compression. Other imagers variably obtain T2-weighted images as well. Others routinely administer contrast in this setting. Some administer contrast only when a lesion is suspected on the unenhanced images, and some do so only when no lesion is seen on the unenhanced images. Imagers need a rational standardized approach to imaging of the spinal axis for this clinical indication. Patients with this presentation frequently have difficulty lying still for prolonged periods on rigid MR imaging tables, so that shortening the duration of the scans for these patients is a desirable goal. In addition, multiple new, innovative sequences are currently being tried for spinal neoplasm imaging; some will probably be shown to provide additional clinically useful diagnostic information. Rather than adding the new (or investigational) sequences to a large, inclusive basic protocol, it would be useful to determine which conventional sequences are necessary at a minimum, so that newer sequences could be added with minimal increase in scan time.
The purpose of this study was to determine how often the addition of T2-weighted or postcontrast T1-weighted images to unenhanced T1-weighted images affects urgent treatment plans in cases of clinically suspected spinal metastases, especially plans for radiation therapy. The hypothesis was that the use of the more limited MR imaging protocols—1) unenhanced T1-weighted images only, 2) T1-weighted plus T2-weighted images, or 3) T1-weighted plus postcontrast T1-weighted images—would not affect the urgent clinical decision to recommend radiation therapy or the levels of planned therapy compared with the use of the reference standard MR imaging protocol4 including T1- and T2-weighted images and postcontrast T1-weighted images.
Methods
Study Population
The subjects included consecutive adults (outpatients and inpatients 18 years of age or older) with known primary neoplasm and clinical suspicion of metastatic disease to the spinal axis. All patients were from a single large tertiary care center. All subjects had undergone spine MR imaging in the course of their clinical care. Names of all patients undergoing spine MR imaging were acquired from MR imaging logbooks over a 2-year period. All of the patients’ electronic medical records were reviewed. Patients with previous spinal irradiation or surgery were excluded because of the need for contrast in these cases related to the postsurgical findings (regardless of the presence of neoplasm). Records were specifically reviewed to identify any evidence of known primary malignancy; those without known primary malignancy were excluded. Three patients were excluded because they did not receive contrast (patient motion-related artifact was substantial, and contrast was deemed inappropriate at the time of scanning because images were nondiagnostic as a result of motion). One patient was excluded because of very poor quality images; all other patients, including those with MR imaging deemed by neuroradiologist interpreters to be of “satisfactory,” “borderline,” or “poor” quality, were included. Because we were interested in determining the sequences most useful in treatment planning for patients with MR imaging evidence of metastatic disease, we excluded 34 patients (of 65 consecutive patients) in whom MR imaging did not suggest the presence of any pathology other than degenerative disk disease. Clinical symptoms for the included subjects were obtained from the medical records, with recorded symptoms at the time of imaging ranging from back pain to paresthesias or paraparesis. The Institutional Review Board approved the study.
Design
This was a prospective study evaluating the effect of variations of MR imaging protocols on hypothetical treatment plans (an intermediate outcome) in which cases were acquired in a retrospective manner. After the identification of the cases, the MR images were prospectively and blindly interpreted with standardized reporting format (Neuroradiologist Report) by 2 attending neuroradiologists. One neuroradiologist had 33 years of experience and the other 2 years of experience since completion of a neuroradiology fellowship. Each neuroradiologist interpreted the cases independently, and each was provided only with clinical presentation at the time of imaging (eg, “50-year-old woman with history of breast cancer now presenting with back pain”). Neuroradiologists were blinded to other clinical information (eg, biopsy results, actual radiation therapy received, patient outcome).
Each patient’s spine MR imaging examination was interpreted 4 times by each neuroradiologist. The 4 interpretations reflected 4 different MR imaging protocols presented at distinct readout sessions: 1) T1-weighted images only; 2) T1- and T2-weighted images; 3) T1-weighted and postcontrast T1-weighted images; and 4) T1- and T2-weighted images and postcontrast T1-weighted images. Each readout session was separated by at least 1 month from the previous session for the same patient to decrease recall bias. Reports from previous readout sessions were not consulted, reviewed, or altered at subsequent sessions.
Hypothetical treatment plans were also determined prospectively. Each patient scenario was presented independently to 2 attending radiation oncologists who had 10 and 18 years of experience since completion of training. History and physical findings at the time of imaging and the Neuroradiologist Report were presented to each radiation oncologist, who was asked to provide a hypothetical treatment plan in a standardized format (Radiation Oncologist Questionnaire). Radiation oncologists were specifically required to indicate whether radiation therapy was recommended and, if so, for which specific spinal levels. Each patient’s case was considered for treatment planning multiple times by each radiation oncologist. Each time reflected a unique Neuroradiologist Report based on which neuroradiologist interpreted the scan and which of the 4 MR imaging protocol types was interpreted. Therefore, each case contributed up to 8 scenarios for each oncologist.
Statistical Analysis
A logistic model was used to investigate for an association between whether radiation therapy was recommended and the MR imaging protocol type used. The generalized estimating equation method was used in the model because each patient was repeatedly measured in the study. The effect of neuroradiologist identity and its interaction with MR imaging protocol type were investigated in the model. The frequency (and its SE) with which the oncologists recommended radiation therapy was summarized for each MR imaging protocol type, after adjusting for neuroradiologist effect in the logistic model. The other 3 protocols were compared with the reference MR imaging protocol (protocol 4: T1-weighted, T2-weighted, and postcontrast T1-weighted images) using Bonferroni multiple comparisons methods. P values less than 0.05 were considered statistically significant. κ statistics were used to investigate the agreement between the reference MR imaging protocol (protocol 4) and the other 3 more limited MR imaging protocols. The agreement was considered excellent if κ was 0.81–1.00, substantial if κ was 0.61–0.80, moderate if κ was 0.41–0.60, fair if κ was 0.21–0.40, and poor if κ was <0.21.
Further analysis was based on consecutive numbering of spinal levels from C1 to the sacrum, such that C1 was numbered 1, C2 = 2, C3 = 3 … and sacrum = 25. For cases in which the oncologist recommended radiation therapy, 2 parameters were used for a more detailed evaluation of the therapy recommended. The 2 parameters were the median level (ML) of recommended spinal radiation therapy and the total number of levels (NL) of recommended spinal radiation (eg, if radiation therapy was recommended for C4 through T5, this was numbered 4 through 12, so that the ML would be 8 and the NL would be 9). Mixed-effect models were used to investigate whether either ML or NL was associated with MR imaging protocol type. The effect of neuroradiologist identity and the interaction of this variable with MR imaging protocol type were also investigated in the model; the patients were the random effect. The mean of ML (or NL) of MR imaging protocol 4 was compared with those of the other 3 protocols by using Dunnett multiple comparison method. Pearson correlation coefficients were used to investigate the relationship of ML and NL between MR imaging protocol type 4 and the other 3 MR imaging protocols. Agreement of ML and NL values between protocol 4 and the other protocols was assessed by intraclass correlation coefficient (ICC).8
Results
A total of 31 consecutive subjects (Table 1) was evaluated. Multiple scan interpretations were obtained by MR imaging protocol type and neuroradiologist for each subject. MR imaging protocols 1, 2, and 3 showed no difference from the reference protocol 4 regarding the decision of whether to plan radiation therapy or not. The decision whether to recommend radiation therapy was also not affected by which neuroradiologist interpreted the scan. Table 2 summarizes the main and interaction effects in the logistic regression model. The frequencies with which the oncologists recommended radiation therapy did not vary significantly by MR imaging protocol type (Table 3).
Table 1:
Subjects
| Patient No./Age (y)/Sex | Primary Neoplasm | Other Metastatic Disease |
|---|---|---|
| 1/58/F | Breast | No |
| 2/52/F | Chronic myelogenous leukemia | No (after stem cell transplant) |
| 3/76/F | Breast | No |
| 4/70/F | Lung | Brain |
| 5/62/M | Colon | Liver, omentum |
| 6/75/M | Melanoma | No |
| 7/70/F | Merkel cell, lip | Liver |
| 8/56/M | Non-Hodgkin lymphoma | Brain, cranial nerve |
| 9/65/F | Multiple myeloma | |
| 10/59/F | Breast | Axillary nodes |
| 11/60/F | Breast | Liver, brain |
| 12/31/F | Breast | Brain |
| 13/28/F | Breast | No |
| 14/49/F | Breast | Lung, brachial plexus |
| 15/65/M | Melanoma | Liver, neck |
| 16/42/M | Melanoma | Questionable adrenal |
| 17/71/M | Prostate | No |
| 18/69/F | Lung | Brain |
| 19/62/M | Pancreas | Liver, rib |
| 20/33/M | Non-Hodgkin lymphoma | Cranial nerves |
| 21/50/F | Breast | No |
| 22/52/M | Floor of mouth | No |
| 23/54/F | Breast | No |
| 24/64/M | Lung | Mediastinum |
| 25/56/M | Multiple myeloma | |
| 26/85/M | Prostate | Liver, retroperitoneum |
| 27/68/F | Lung | Brain |
| 28/46/F | Non-Hodgkin lymphoma | Kidney |
| 29/71/M | Multiple myeloma | |
| 30/80/F | Lung | Brain |
| 31/64/M | Ureter | Mediastinum |
Table 2:
Association of radiation therapy being planned with other factors (logistic regression model)
| Factor | P Value* |
|---|---|
| MRI protocol type | 0.873 |
| Neuroradiologist | 0.761 |
| MRI protocol type × neuroradiologist | 0.724 |
Note:—* indicates that P values are from the logistic regression model.
Table 3:
Comparing frequencies of radiation therapy planned among MRI protocol types (logistic regression model)
| MRI Protocol | Frequency % (SE) |
|---|---|
| 1 | 45 (7) |
| 2 | 46 (6) |
| 3 | 49 (7) |
| 4 | 49 (7) |
The κ statistic to assess agreement between reference MR imaging protocol 4 and the other 3, more limited, MR imaging protocol types were all in the substantial agreement range (Table 4).
Table 4:
Agreement in plan for radiation therapy between the reference MRI protocol type and the other MRI protocols
| MRI Protocol Types | κ (SE) |
|---|---|
| 1 vs 4 | 0.76 (0.05) |
| 2 vs 4 | 0.73 (0.05) |
| 3 vs 4 | 0.76 (0.05) |
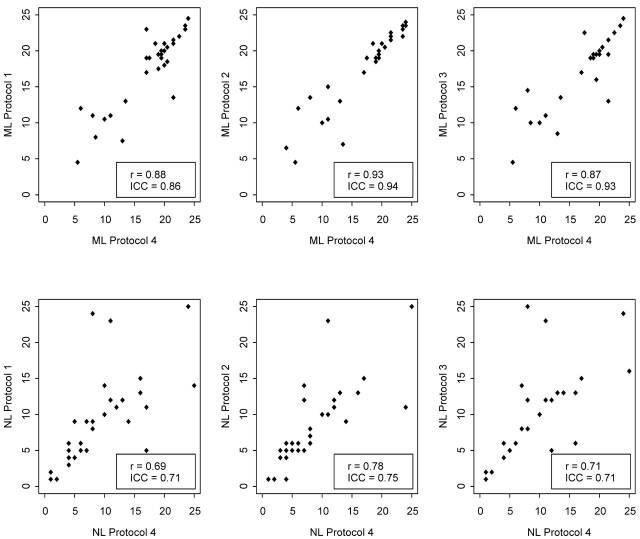
Neither the ML of radiation planned nor the NL of radiation planned was affected by which MR imaging protocol type was used. Which neuroradiologist interpreted the scan and created the Report had no significant effect on ML or NL (Table 5). MR imaging protocols 1, 2, and 3 showed strong relationships with the reference protocol 4 regarding both ML and NL (Fig), with similar Pearson correlation coefficients for all paired comparisons for ML (range, 0.87 to 0.93) and NL (range, 0.69 to 0.78). Intraclass correlations between protocol 4 and the other protocols ranged from 0.86 to 0.94 for ML and from 0.71 to 0.75 for NL.
Table 5:
Significance of factor effects on radiation therapy recommendations
| Effect | ML | NL |
|---|---|---|
| MRI protocol type | 0.780 | 0.686 |
| Neuroradiologist | 0.126 | 0.877 |
| MRI protocol type × neuroradiologist | 0.525 | 0.992 |
Note:—ML indicates median spinal level; NL, total number of spinal levels.
Values are P values are from mixed models.
Fig 1.
Scatterplots show the positive linear relationships between median spinal level (ML) and total number of spinal levels (NL) of radiation therapy planned by using reference MR imaging protocol 4 compared with the more limited MR imaging protocols 1, 2, and 3. The Pearson correlation coefficient, r, and intraclass correlation (ICC) are both indicated for each comparison.
Table 6 includes a summary of the mean ML and NL values determined using each protocol type. The mixed effects model showed that differences among these ML and NL values were not statistically significant (all P > 0.5).
Table 6:
Summary of median levels (ML) and total number of levels (NL) of recommended spinal radiation by MRI protocol type
| MRI Protocol Type | ML | NL |
|---|---|---|
| 1 | 17.10 (0.97) | 7.63 (1.05) |
| 2 | 17.18 (0.96) | 8.71 (1.02) |
| 3 | 17.58 (0.97) | 8.75 (1.04) |
| 4 | 17.04 (0.96) | 8.74 (1.00) |
Note:—Values are presented as mean (SE). Differences in means of ML and NL were not statistically significant at pairwise comparisons of any 2 protocol types.
The 2 neuroradiologist readers were asked specifically whether each patient’s MR imaging had findings suggestive of dural, intradural, intramedullary, or conus neoplastic involvement not appearing to originate from bone. The 2 readers agreed that 4 patients had such MR imaging findings on the reference MR imaging protocol 4 (T1- and T2-weighted images and postcontrast T1-weighted images). However, when interpreting the more limited MR imaging protocol types, the nonbony disease was not uniformly identified: the more senior neuroradiologist detected all 4 cases when interpreting MR imaging protocols 1 (T1-weighted images only) and 3 (T1-weighted plus postcontrast T1-weighted images) but only 2 of 4 cases when interpreting protocol 2 (T1- plus T2-weighted images); the more junior neuroradiologist detected all 4 cases with protocol 3, 3 of 4 cases with protocol 2, and 2 of 4 cases with protocol 1.
Discussion
The most common indications to irradiate bone metastases include pain, risk for pathologic fracture, and cord compression. A number of clinical, prognostic, and therapeutic factors are considered in the treatment planning for palliative radiation therapy.9 Radiologic determination of the spinal levels involved with metastases is critical to radiation treatment planning. Although plain films, CT, myelography, and nuclear medicine have been used in the setting of spinal metastases, MR imaging is currently considered the imaging technique of choice for the detection of spinal tumors.4,5,10-16 Previous research has shown that noncontrast T1-weighted images are probably the most useful type of images in adult patients with clinically suspected cord compression, because vertebral metastases are most often appreciated with this MR imaging sequence.17-21 Bony metastases are typically well seen as hypointense foci within normal fatty marrow on T1-weighted images. The appearance of metastases on T2-weighted sequences varies, especially if fast spin-echo techniques are used.20 T2-weighted images have been seen by some investigators—but not by others—to be useful in this clinical situation14,17-21 and may be more useful than T1-weighted images in cases of multiple myeloma.22-23 Some bony neoplasms have been shown to demonstrate the greatest contrast on short τ inversion recovery (STIR) images; however, the signal intensity-to-noise ratio is lower for STIR than for fast spin-echo T2-weighted images. Patterns of neoplastic involvement tend to be similar for STIR and T2-weighted images.24 For the less common cases of dural or pial (nonbony) neoplastic involvement of the spinal axis, contrast enhanced images have been shown to provide additional independent information.18,25-26 Others have found that contrast allows for increased conspicuity of epidural tumor extension from bone.27
Given that previous studies have suggested that noncontrast T1-weighted images are the most useful type of images in adult patients with clinically suspected cord compression,17-21 we hypothesized that T1-weighted images alone would usually provide the same clinically relevant diagnostic information for urgent treatment planning as the reference MR imaging protocol. To test this hypothesis, we compared 3 different, limited MR imaging protocol types with the reference standard (T1-weighted, T2-weighted, and postcontrast T1-weighted images). The 3 limited protocol types were 1) T1-weighted images only, 2) T1- plus T2-weighted images, and 3) T1-weighted plus postcontrast T1-weighted images. We chose to investigate 2 aspects of the impact of the use of different types of MR imaging protocols in the setting of metastatic disease to the spine. First, does the choice of MR imaging protocol affect the decision of whether to recommend radiation therapy? Then, for those patients for whom radiation therapy is recommended, does MR imaging protocol affect which levels will be irradiated? We used the median spinal level of planned therapy and the total number of spinal levels of planned therapy to estimate this effect. We performed both univariate and multivariate analyses and found that use of T1-weighted images alone resulted in the same frequency of decisions to recommend radiation therapy, and that T1-weighted images alone agreed as well with the reference standard as did the other 2 limited protocols. All protocol types showed substantial agreement with the reference protocol. We further found that which MR imaging protocol type was used did not affect the median spinal level of planned radiation therapy or the total number of spinal levels of planned radiation therapy. Our results are in accord with the previous cited studies regarding the utility of unenhanced T1-weighted images in adult patients.
Regarding the much less common cases of nonbony neoplastic involvement of the spinal axis, previous studies have shown that intravenous contrast provides additional independent information.18,25-27 Our study population included only a small number of patients likely to have nonbony disease. However, our results showed that such nonbony disease may not be as readily detected, especially by less experienced neuroradiologists, without intravenous contrast administration. This agrees with previous research suggesting that postcontrast images do provide additional diagnostic information in cases with nonbony neoplastic disease. We note that these cases do not typically require urgent radiation therapy. Among the 4 such patients in our study, the oncologists recommended radiation therapy in only a single case, and that recommendation was made regardless of the MR imaging protocol used (ie, the unenhanced T1-weighted images led to the same recommendation as the reference protocol).
A possible limitation of our study was the exclusion of patients whose MRIs showed no evidence of tumor. Our rationale for this exclusion criterion was that patients with such MRIs would not be candidates for urgent radiation therapy of the spinal axis in clinical practice. Ours was not an accuracy study with reference standard of pathologic confirmation. We chose instead to evaluate variations on an imaging test that is very often the major determining factor in deciding urgent management of patients with clinically suspected cord compression. We investigated whether this imaging test could be more limited than is commonly performed without negatively affecting urgent patient care; therefore, patients without evidence of spinal neoplasm would not be among the relevant patient population.
Regarding generalizability of our results, there are several issues we identify. Because ours is a tertiary care center, our results may not be generalizable to other types of health care centers or providers. All MRIs in our study were performed on 1.5T magnets; other field strength systems may not perform equally well. Our MR imaging interpreters were both fellowship-trained, CAQ-certified neuroradiologists; if other types of radiologists or nonradiologist physicians are interpreting spinal MR images in clinical practice, our results may not be relevant to that practice. For example, both radiation oncologist coauthors commented that they personally prefer T2-weighted images in their own reviews of scans in practice, suggesting that they find the sagittal T2-weighted series to most clearly show CSF and degree of cord compression. Such preferences may affect oncologist confidence levels or portals in actual treatment planning. Lastly, all of our patients were adults; pediatric patients have less fatty bone marrow (ie, darker on T1-weighted images), and it is possible that T1-weighted images alone are not as useful in these patients.
We chose to evaluate fast spin-echo T2-weighted images instead of STIR for several reasons. First, previous research has suggested that patterns of bony neoplastic involvement of the spine tend to be similar for STIR and T2-weighted images.24 In addition, an informal survey of colleagues at different sites in the United States at the time of our project suggested that most groups were using T2-weighted imaging in this clinical setting. Finally, we thought that a similar comparison would perhaps best be performed independently for MR imaging protocols including STIR (such as evaluating T1-weighted images alone versus T1-weighted and STIR images versus T1-weighted and diffusion-weighted images).
Conclusions
Although MR imaging is known to be the most useful imaging test in cases of clinically suspected spinal metastasis and cord compression, MR imaging protocols vary regarding the use of basic conventional sequences. We evaluated different combinations of sequences as interpreted by fellowship-trained, CAQ-certified neuroradiologists. Compared with unenhanced T1-weighted images alone, the addition of T2-weighted and/or postcontrast T1-weighted images did not significantly affect the probability that radiation therapy was planned or the levels chosen for radiation therapy. Unenhanced T1-weighted images may be sufficient as a minimum protocol in this setting, particularly for urgent treatment decisions.
Footnotes
This work was supported in part by a GE-AUR Radiology Research Academic Fellowship Award.
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology; May 21–27, 2005; Toronto, Canada.
References
- 1.Klimo P, Schmidt MH. Surgical management of spinal metastases. Oncologist 2004;9:188–96 [DOI] [PubMed] [Google Scholar]
- 2.Ratliff JK, Cooper PR. Metastatic spine tumors. South Med J 2004;97:246–53 [DOI] [PubMed] [Google Scholar]
- 3.Gilbert RW, Kim J-H, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol 1978;3:40–51 [DOI] [PubMed] [Google Scholar]
- 4.Walker MP, Yaszemsi MJ, Kim CW, et al. Axial metastatic bone disease. Metastatic disease of the spine: evaluation and treatment. Clin Orthop 2003;415S:S165–75 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg MS. Handbook of Neurosurgery, 4th ed. Lakeland, Fla: Greenberg Graphics, Inc;1997. :340–45
- 6.Han JS, Kaufman B, El Yousef SJ, et al. NMR imaging of the spine. AJNR Am J Neuroradiol 1983;4:1151–59 [Google Scholar]
- 7.Norman D, Mills CM, Brant-Zawadzki M, et al. Magnetic resonance imaging of the spinal cord and canal: potentials and limitations. AJNR Am J Neuroradiol 1984;5:9–14 [DOI] [PubMed] [Google Scholar]
- 8.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420–28 [DOI] [PubMed] [Google Scholar]
- 9.Janjan NA. Radiation for bone metastases. Cancer Suppl 1997;80:1628–45 [DOI] [PubMed] [Google Scholar]
- 10.Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med 2002;137:586–97 [DOI] [PubMed] [Google Scholar]
- 11.Carmody RF, Yang PJ, Seeley GW, et al. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology 1989;173:225–29 [DOI] [PubMed] [Google Scholar]
- 12.Algra PR, Bloem JL, Tissing H, et al. Detection of vertebral metastases: comparison between MR imaging and bone scintigraphy. Radiographics 1991;11:219–32 [DOI] [PubMed] [Google Scholar]
- 13.Avrahami E, Tadmor R, Dally O, et al. Early MR demonstration of spinal metastases in patients with normal radiographs and CT and radionuclide bone scans. J Comput Assist Tomogr 1989;13:598–602 [DOI] [PubMed] [Google Scholar]
- 14.Williams MP, Cherryman GR, Husband JE. Magnetic resonance imaging in suspected metastatic spinal cord compression. Clin Radiol 1989;40:286–90 [DOI] [PubMed] [Google Scholar]
- 15.Jordan JE, Donaldson SS, Enzmann DR. Cost effectiveness and outcome assessment of magnetic resonance imaging in diagnosing cord compression. Cancer 1995;75:2579–86 [DOI] [PubMed] [Google Scholar]
- 16.Ross JS. Lytic osseous metastases. In: Ross JS, ed. Diagnostic Imaging: Spine. Portland, Ore: Amirsys and Saunders;2004;IV-1-10–IV-1-13
- 17.Stimac GK, Porter BA, Olson DO, et al. Gadolinium-DTPA-enhanced MR imaging of spinal neoplasms: preliminary investigation and comparison with unenhanced spin-echo and STIR sequences. AJR Am J Roentgenol 1988;151:1185–92 [DOI] [PubMed] [Google Scholar]
- 18.Sze G, Krol G, Zimmerman RD, et al. Malignant extradural spinal tumors: MR imaging with Gd-DTPA. Radiology 1988;167:217–23 [DOI] [PubMed] [Google Scholar]
- 19.Smoker WRK, Godersky JC, Knutzon RK, et al. The role of MR imaging in evaluating metastatic spinal disease. AJR Am J Roentgenol 1987;149:1241–48 [DOI] [PubMed] [Google Scholar]
- 20.Sze G. Neoplastic diseases of the spine and spinal cord. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine, 3rd ed. Philadelphia: Lippincott Williams & Wilkins;2002. :1715–68
- 21.Mirowitz SA, Shady KL. Gadopentetate dimeglumine-enhanced MR imaging of the postoperative lumbar spine: comparison of fat-suppressed and conventional T1-weighted images. AJR Am J Roentgenol 1992;159:385–89 [DOI] [PubMed] [Google Scholar]
- 22.Rahmouni A, Divine M, Mathieu D, et al. Detection of multiple myeloma involving the spine: efficacy of fat-suppression and contrast-enhanced MR imaging. AJR Am J Roentgenol 1993;160:1049–52 [DOI] [PubMed] [Google Scholar]
- 23.Libshitz HI, Malthouse SR, Cunningham D, et al. Multiple myeloma: appearance at MR imaging. Radiology 1992;182:833–37 [DOI] [PubMed] [Google Scholar]
- 24.Baker LL, Goodman SB, Perkash I, et al. Benign versus pathologic compression fractures of vertebral bodies: assessment with conventional spin-echo, chemical-shift, and STIR MR imaging. Radiology 1990;174:495–502 [DOI] [PubMed] [Google Scholar]
- 25.Lim V, Sobel DF, Zyroff J. Spinal cord pial metastases: MR imaging with gadopentetate dimeglumine. AJNR Am J Neuroradiol 1990;11:975–82 [PMC free article] [PubMed] [Google Scholar]
- 26.Rothwell CI, Jaspan T, Worthington BS, et al. Gadolinium-enhanced magnetic resonance imaging of spinal tumours. Br J Radiol 1989;62:1067–74 [DOI] [PubMed] [Google Scholar]
- 27.Cuénod CA, Laredo J-D, Chevret S, et al. Acute vertebral collapse due to osteoporosis or malignancy: appearance on unenhanced and gadolinium-enhanced MR images. Radiology 1996;199:541–49 [DOI] [PubMed] [Google Scholar]