Abstract
BACKGROUND AND PURPOSE: The aim of acute stroke interventions is to achieve recanalization of the target occluded artery. We sought to determine whether pretreatment cortical cerebral blood flow (CBF) was associated with vessel recanalization in patients undergoing intra-arterial therapy.
METHODS: This is a retrospective analysis of patients who underwent a quantitative xenon CT blood flow study and were noted to have a documented M1 middle cerebral artery (MCA) or carotid terminus occlusion less than 6 hours from symptom onset between January 1997 and April 2001. Twenty-three patients who underwent intra-arterial thrombolysis were included in the analysis. Univariate and multivariate analyses were performed to determine whether pretherapy CBF was correlated to the likelihood of recanalization.
RESULTS: A total of 23 patients were studied in this analysis with a median age of 69 (range 32–81) and median National Institutes of Health Stroke Score of 19 (range, 8–22). Twelve patients (52%) underwent combined intravenous/intra-arterial therapy, and 11 patients (48%) were treated with intra-arterial thrombolytics alone. Successful vessel recanalization (Thrombolysis in Myocardial Infarction classification 2 or 3 flow) occurred in 13 patients (57%). The only variable associated with recanalization in multivariate modeling was mean ipsilateral MCA CBF (odds ratio, 1.25; 95% confidence interval, 1.01–1.54; P = .035). A receiver operating characteristic curve was generated, and a mean ipsilateral MCA CBF threshold of 18 mL/100 g/min was found to be the threshold for successful recanalization.
CONCLUSIONS: Our study suggests that patients with higher mean ipsilateral MCA CBF are more likely to recanalize. The threshold for successful revascularization may be 18 mL/100 g/min. Further study is required to determine whether pretreatment CBF is related to recanalization success.
In acute ischemic stroke, abrupt vessel occlusion results in a drop in regional cerebral blood flow (CBF) leading to time-dependent compartmentalization of the ischemic brain into ischemic core, ischemic penumbra, and oligemic brain.1 Reversal of the pathophysiologic mechanisms triggered by this process with the ultimate goal of penumbral salvage through vessel recanalization is the aim of thrombolytic therapy. It has been shown that the earlier this occurs, the higher the likelihood of a good clinical outcome.2 Current thrombolytic modalities largely consisting of intravenous (IV) or intra-arterial (IA) pharmacologic lysis fail to achieve recanalization in a significant proportion of patients. With IA thrombolysis, the overall complete or partial recanalization rate of the M1 or M2 middle cerebral artery (MCA) segments ranges between 60% and 70%,3 whereas it is noted to be appproximately 40% when the thrombolytic effect of IV tPA is augmented by externally applied sonography.4 Moreover, the time to recanalization with both modalities may be lengthy, allowing large penumbral areas to be incorporated into the core, thus diminishing the benefit of treatment. Mechanical thrombolysis can potentially increase the time window for therapy and rates of recanalization, but carries a risk of vessel injury or perforation5,6 with potentially catastrophic consequences. Therefore, a need for better treatment strategies that are safe and result in higher and faster recanalization rates is clearly recognized. A thorough understanding of the factors affecting vessel recanalization is an essential requirement for progress in this area.
Mechanisms underlying thrombus lysis are complex and depend on many factors, presumed to be composition, age, size and location of the thrombus, type of thrombolytic agent used, and type of thrombolytic technique used.7,8 We sought to determine whether an association exists between pretreatment tissue perfusion and vessel recanalization in a homogeneous group of patients with M1 MCA occlusion who underwent quantitative cerebral blood flow measurement before undergoing IA thrombolytic therapy.
Patients and Methods
Patients
We retrospectively selected our patients from a prospective registry of 378 consecutive persons admitted to the University of Pittsburgh Stroke Service between January 1997 and April 2001 who underwent a xenon-CT-CBF study; of these, 160 patients were studied within 6 hours of symptoms onset. Fifty patients had a documented MCA or carotid terminus occlusion demonstrated on CT angiogram or conventional angiography. Fourteen of these 50 patients were excluded from further analysis because of excessive motion artifact. Another 13 patients were excluded because they were given IV tPA and did not undergo angiography after the xenon CT study. Thus, information with regard to recanalization was not available or they were just outside the 6-hour time window after the xenon CT was performed, and the protocols at that time were for patients to be in the angiography suite within 6 hours. Thus, 23 patients who underwent IA thrombolysis and xenon CT under 6 hours were included in our study. Twelve patients were candidates for IV tPA and were treated with 0.9 mg/kg of tPA, with 10% given as a bolus and the rest over 1 hour. These patients were taken for xenon CT and then angiography for IA therapy because it was believed that they had a persistent cerebral arterial occlusion. Before 2002 at our institution, the IA therapy protocol was to infuse tPA or urokinase within the thrombus without aggressive mechanical manipulation. A maximum dose of 22 mg of tPA (Genentech, San Francisco, Calif) was administered in the thrombus over 2 hours unless recanalization occurred before maximal dose administration. IA urokinase was administered in similar fashion: increments of 250,000 U every 15 minutes for a maximal dose of 2 million units over 2 hours. Intravenous heparin administration during the case occurred at the discretion of the operator. The end point of IA therapy was vessel recanalization or 2 hours, whichever came first (ie, the protocol at the time of this study period). Clinical and demographic data were obtained from either a prospectively acquired clinical data base or from medical records. All patients had angiographically confirmed MCA stem occlusion diagnosed within 6 hours of symptom onset, IA thrombolytic therapy allowing for determination of recanalization status within 2 hours of therapy initiation, and a preprocedure xenon cerebral blood flow CT (Xe-CBF-CT). All patients screened had a documented time of symptom onset and a documented time at which the CBF study was performed.
Xe-CBF-CT Data Analysis
The stable Xe-CT-CBF technique9 and the mean MCA CBF calculation algorithm have been described previously.10 Four CT images of 1-cm section thickness were obtained along the orbitomeatal line. Although the duration of each study was not recorded, a standard head CT followed by a Xe-CT-CBF study typically require approximately 20 minutes for acquisition and 5 additional minutes for CBF calculation and display. Our analysis included the ipsilateral and contralateral outer 2 cm of cortical MCA territory at all 4 levels, which, for standardization purposes, was defined by anatomic templates that are part of the Xe-CT computer software (Diversified Diagnostic Products, Houston, Tex). In these areas, regional CBF values were computed by the xenon-CT software package yielding mean total MCA CBF values for each of the 4 levels. These values were then averaged into a single value representing mean ipsilateral cortical MCA CBF.
Other Imaging Data Analysis
Preprocedure and postprocedure cerebral angiograms as well as baseline CT scans were reviewed by a blinded neuroradiologist (S.Z.). All 23 patients had Thrombolysis in Myocardial Infarction classification (TIMI) 0 flow on the first angiographic run before initiation of IA thrombolysis. MCA recanalization status at 2 hours after angiography was categorized as absent (TIMI 0 and 1) or present (TIMI 2 and 3). Early CT changes were assessed and categorized as involving greater or less than a third of MCA territory.
Statistical Analyses
Statistical analyses were performed using the Intercooled Stata 7.0 (Stata, College Station, Tex) statistical software package. Univariate analyses of recanalization status at 2 hours according to several variables of interest were carried out (Table). Multivariate analysis of the relationship between recanalization and these variables of interest was performed in a stepwise logistic regression model in which entry was set at a univariate association with P ≤ 0.2.
Univariate analysis for predictors of recanalization after thrombolysis
| Recanalized (n = 13) | Not Recanalized (n = 10) | P Value | |
|---|---|---|---|
| Mean ipsilateral MCA CBF (ml/100 g/min) | 20.2 ± 6.3 | 14.7 ± 5.3 | .039* |
| Mean contralateral MCA CBF (ml/100 g/min) | 39.8 ± 9.5 | 38.5 ± 10.1 | .74* |
| Ratio | 0.53 ± 0.19 | 0.38 ± 0.09 | .019* |
| Platelet count (×109/L) | 234 ± 74 | 324 ± 188 | .12* |
| Age (years) | 66.7 ± 12 | 67.7 ± 11 | .69* |
| Time-to-treatment (min) | 212 ± 59 | 222 ± 32 | .7* |
| NIHSS | 18 ± 3 | 17.69 ± 4 | .74* |
| Thrombus at carotid terminus | Yes: 4/23 | Yes: 3/23 | .68† |
| No: 9/23 | No: 7/23 | ||
| Mean arterial pressure (mm Hg) | 102 ± 21 | 105 ± 17 | .39* |
| Admission glucose | 141.8 ± 60 | 131.3 ± 50 | .63† |
| Thrombolytic modality | IA: 6/23 | IA: 5/23 | .60† |
| IV/IA: 7/23 | IV/IA: 5/23 | ||
| Antiplatelet therapy before admission | Yes: 2/23 | Yes: 4/23 | .34† |
| No: 11/23 | No: 6/23 | ||
| Stroke cause | CE: 6/23 | CE: 5/23 | .59† |
| Non-CE: 7/23 | Non-CE: 5/23 | ||
| Symptomatic hemorrhage | Yes: 4 /23 | Yes: 2/23 | .66 |
| No: 9/23 | No: 7/23 |
Note:—MCA indicates middle cerebral artery; CBF cerebral blood flow; NIHSS, National Institutes of Health Stroke Score; IA, intra-arterial; IV, intravenous; CE, cardioembolic; Non-CE, noncardioembolic.
Student t test.
Fisher exact test.
A receiver operating characteristic (ROC) curve was generated comparing mean ipsilateral CBF with recanalization. The area under the curve (AUC) is also reported.
Results
The median age, NIHSS, and time to treatment were 69 years (range, 32–81 years), 19 (range, 8–22), and 210 minutes (range, 150–360 minutes), respectively. Twelve patients underwent combined IV/IA therapy (11 patients with tPA and 1 patient with IV tPA followed by IA urokinase), whereas 11 patients underwent IA thrombolysis alone (9 patients with tPA and 2 patients with urokinase). Vessel recanalization (TIMI 2 or 3) at 2 hours was obtained in 13 of 23 cases (57%), but there was no statistical difference between patients treated with IA therapy alone and combination IV/IA therapy (Table). By univariate analysis, mean ipsilateral MCA CBF and the ratio of mean ipsilateral MCA CBF/ contralateral MCA CBF was significantly associated with recanalization (Table). This ratio was significantly correlated to the mean ipsilateral MCA CBF (Spearman correlation coefficient = 0.78, P < .0001). Mean ipsilateral MCA CBF remained significant in a stepwise logistic regression model incorporating the variables age, NIHSS, glucose and platelet count on admission, time to angiography, and thrombolytic technique (odds ratio, 1.25; 95% confidence intervals [CI], 1.01–1.54; P = .035) as an independent predictor of recanalization.
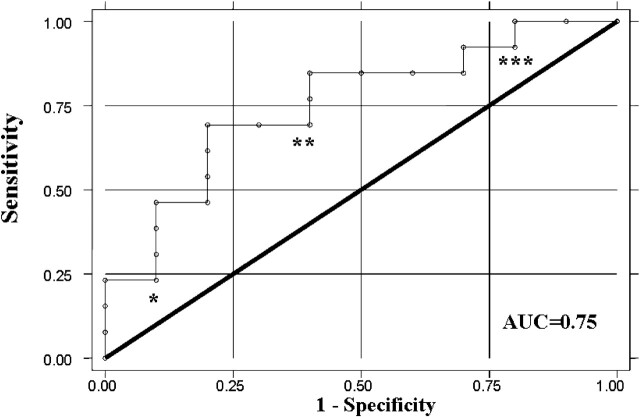
Figure 1 demonstrates the ROC curve when mean MCA ipsilateral CBF is plotted against recanalization. The AUC is 0.75 (95% CI, 0.55–0.96; P < .04).
Fig 1.
This graph represents the ROC curve for mean ipsilateral MCA CBF in relation to recanalization. ∗, This value represents a mean ipsilateral MCA CBF of 24 mL/100 g/min. ∗∗, This value represents a mean ipsilateral MCA CBF of 18 mL/100 g/min. ∗∗∗, This value represents a mean ipsilateral MCA CBF of 9 mL/100 g/min.
Discussion
This study demonstrates the relationship between pretreatment hemispheric perfusion assessed by quantitative CBF measurement techniques and vessel recanalization. Our findings indicate that recanalization in IA thrombolysis for MCA occlusion is affected by pretreatment hemispheric perfusion; the higher the regional CBF in the ipsilateral hemisphere, the higher the likelihood of successful thrombolysis.
An association between hemispheric hypoperfusion and vessel recanalization through impaired clearance of thrombi has been described previously.11 Our findings are in concordance with those reported by other investigators using different, nonquantitative modalities to assess ipsilateral hemispheric perfusion. Using continuous transcranial Doppler studies performed during and after administration of IV thrombolytics, Labiche et al12 have shown that if flow is partially established through a thrombus (and thus presumably milder degree of hypoperfusion), it has a greater chance of complete recanalization. Nighoghossian et al13 found that lower pretreatment perfusion-weighted imaging lesion volumes on MR imaging are correlated with a higher chance of recanalization. A possible explanation for this interesting phenomenon is that milder hemispheric hypoperfusion is likely to be the consequence of a lower clot burden, which in turn is more likely to be responsive to thrombolytic agents. Patients with MCA occlusion who have milder degrees of hypoperfusion determined by the pretreatment CBF study seem to be in an earlier stage of the process through which threatened brain tissue supplied by the occluded vessel is progressing toward infarction. Therefore, the ischemic vascular territory is composed predominantly by penumbral tissue, which is still perfused in the tissue viability range. Furthermore, most of the vascular bed supplying this area is still patent and the overall clot burden in the ischemic bed is lower. By contrast, patients with more severely hypoperfused hemispheres have a higher proportion of ischemic core in which, because of stagnant flow, the vessels supplying the area have a higher clot burden in the ischemic area with resultant lower recanalization rates.
The retrospective nature of this study did not allow us to quantify the extent of the thrombus; as such, this is merely an unconfirmed supposition. Future prospective studies, investigating the relationship between recanalization and hemispheric perfusion, should take into account the amount of thrombus present in the vascular bed of the affected territory to confirm our hypothesis.
Our findings may have therapeutic implications. As more treatment modalities for interventional acute stroke therapy become available, physicians are often faced with the choice between several endovascular therapies as first-line modalities. Our findings suggest that in patients with milder hemispheric hypoperfusion (pretreatment CBF values >20 mL/100 g/min), the likelihood of recanalization with pharmacologic lysis is high, justifying a first-line usage of this method. Conversely, in more severely affected hemispheres (mean MCA CBF in the 15–20 mL/100 g/min range), not only is progression to infarction more imminent but also the chance of recanalization is lower. In those situations, more aggressive interventions (angioplasty, mechanical embolectomy, stent placement) that are thought to have faster and higher recanalization rates,5,6 but are also more prone to complications, may be more appropriate as first-line treatment.
Our study has several limitations. The number of patients analyzed is small and the study design was retrospective. More importantly, different IA treatment modalities were used, which may confound our results. However, to our knowledge, no study has shown that one pharmacologic IA thrombolysis method (including IV/IA combination therapy) yields higher recanalization rates than another. In fact, most IA thrombolysis trials, regardless of the pharmacologic agent used, have reported recanalization rates of approximately 60%,3,14,15 which is what was achieved in our group of patients as well. It may therefore be possible that factors other than the type of thrombolytic agent are more important with regard to vessel recanalization. Our preliminary findings from this study indicating that pretreatment ipsilateral hemispheric perfusion may constitute one of these factors await confirmation from future, prospective studies in which, ideally, only a single treatment technique would be used.
Footnotes
This study was conducted with institutional review board approval (IRB #020178).
References
- 1.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke 1981;12:723–25 [DOI] [PubMed] [Google Scholar]
- 2.Christou I, Alexandrov AV, Burgin WS, et al. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial Doppler correlates with clinical recovery from ischemic stroke. Stroke 2000;31:1812–16 [DOI] [PubMed] [Google Scholar]
- 3.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute stroke thromboembolism. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 4.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 2004;351:2170–78 [DOI] [PubMed] [Google Scholar]
- 5.Gobin YP, Starkman S, Duckwiler GR, et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke 2004;35:2848–54 [DOI] [PubMed] [Google Scholar]
- 6.Nakano S, Iseda T, Yoneyama T, et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke 2002;33:2872–76 [DOI] [PubMed] [Google Scholar]
- 7.Urbach H, Hartmann A, Pohl C, et al. Local intra-arterial thrombolysis in the carotid territory: does recanalization depend on the thromboembolus type? Neuroradiology 2002;44:695–99 [DOI] [PubMed] [Google Scholar]
- 8.Pillai JJ, Lanzieri CF, Trinidad SB, et al. Initial angiographic appearance of intracranial vascular occlusions in acute stroke as a predictor of outcome of thrombolysis: initial experience. Radiology 2001;218:733–38 [DOI] [PubMed] [Google Scholar]
- 9.Johnson DW, Stringer WA, Marks MP, et al. Stable xenon CT cerebral blood flow imaging: rationale for and role in clinical decision making. AJNR Am J Neuroradiol 1991;12:201–13 [PMC free article] [PubMed] [Google Scholar]
- 10.Jovin TG, Yonas H, Gebel JM, et al. The cortical ischemic core and not the consistently present penumbra is a determinant of clinical outcome in acute middle cerebral artery occlusion. Stroke 2003;34:2426–33 [DOI] [PubMed] [Google Scholar]
- 11.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998;55:1475–82 [DOI] [PubMed] [Google Scholar]
- 12.Labiche LA, Malkoff M, Alexandrov AV. Residual flow signals predict complete recanalization in stroke patients treated with TPA. J Neuroimaging 2003;13:28–33 [PubMed] [Google Scholar]
- 13.Nighoghossian N, Hermier M, Adeleine P, et al. Baseline magnetic resonance imaging parameters and stroke outcome in patients treated by intravenous tissue plasminogen activator. Stroke 2003;34:458–63 [DOI] [PubMed] [Google Scholar]
- 14.Suarez JI, Sunshine JL, Tarr R, et al. Predictors of clinical improvement, angiographic recanalization, and intracranial hemorrhage after intra-arterial thrombolysis for acute ischemic stroke. Stroke 1999;30:2094–100 [DOI] [PubMed] [Google Scholar]
- 15.Jahan R, Duckwiler GR, Kidwell CS, et al. Intraarterial thrombolysis for treatment of acute stroke: experience in 26 patients with long-term follow-up. AJNR Am J Neuroradiol 1999;20:1291–99 [PMC free article] [PubMed] [Google Scholar]