Abstract
BACKGROUND AND PURPOSE: To compare multisection CT angiography (CTA) analyzed with source/maximum intensity projection (MIP) images as well as semiautomated vessel analysis software with intra-arterial digital subtraction angiography (DSA) in detection and grading of carotid artery bifurcation stenosis.
METHODS: Consecutive patients with sonography evidence of a marked internal carotid artery stenosis underwent both carotid CTA and DSA (37 patients, 73 vessels). In CTA, the grade of stenosis was determined using axial source and MIP images as well as vessel analysis. The scans were blind-analyzed by 2 neuroradiologists using the NASCET criteria.
RESULTS: Correlation of CTA source/MIP images versus DSA estimates of stenosis (R = 0.95) was higher than for the vessel analysis method versus DSA (R = 0.89). Compared with DSA, CTA source/MIP images underestimated high (78.2% versus 86.4%, P < .05) and moderate grades of stenosis (57.3% versus 63.1%, P < .05) to a lesser extent than the vessel analysis method (68.5% versus 83.5% and 51.8% versus 63.1%, P < .05). For a high-grade stenosis, sensitivity and specificity of source/MIP image CTA were 75% and 96%, respectively, whereas for the vessel analysis method, they were 47% and 96%, respectively. For moderate stenosis, the source/MIP image CTA sensitivity and specificity were 88% and 82%, respectively, and for vessel analysis method, 62% and 82%, respectively. CTA detected all 4 occlusions.
CONCLUSION: In evaluation of carotid stenosis, CTA provides an adequate, less invasive alternative with a high correlation to conventional DSA, though it tends to underestimate clinically relevant grades of stenosis. Its accuracy is not improved by semiautomated analysis. The data support the use of CTA in confirming carotid occlusion.
Ischemic stroke is the most common cause of disability in adults and the third leading cause of mortality in developed countries.1 Roughly half are caused by atherothromboembolism and most of these by extracranial atheromatous lesions, most often involving narrowing of the internal carotid arteries (ICAs).2,3 Symptomatic patients with severe stenosis (70%–99%) benefit from carotid endarterectomy.4,5 It has been suggested that endarterectomy could also reduce the risk of stroke from moderate (50%–69%) stenosis.6 Therefore, imaging of the carotid arteries is indicated in patients with symptoms of cerebral ischemia. The imaging should be done as soon as possible because the benefit from surgery seems to be greatest within 2 weeks of the most recent ischemic symptoms, decreasing rapidly with delay.7 Duplex ultrasonography (US) is usually the first imaging method for carotid arteries and has many advantages as a fast, noninvasive, and easily available screening method. However, a confirmatory imaging method is necessary if an intervention is considered or if the degree of stenosis remains undetermined by US.
Digital subtraction angiography (DSA) has been the “gold standard” for diagnosis of carotid artery stenosis. Noninvasive MR angiography (MRA) and CT angiography (CTA) have partially replaced conventional angiography, which has up to a 1% risk of stroke, a 4% risk of transient ischemic attack (TIA), and nearly a 1% mortality rate.8–10 Multisection helical CT scanners enable fast and accurate vessel imaging, and CTA is increasingly used in assessment of carotid artery stenosis.11,12
The aim of our study was to compare the grade of carotid artery stenosis in DSA and CTA using a novel commercially available 3D vessel analysis software in evaluation of the maximal stenosis in CTA, along with axial source images and maximum intensity projections (MIP). Vessel analysis software is a semiautomatic system that provides intraluminal diameter measurements and cross-sectional areas of the carotid artery at selected anatomic points, calculating the maximum stenosis percentage in proportion to the chosen reference point. To our knowledge, no previous study comparing DSA with CTA in carotid stenosis has evaluated the use of both vessel analysis software and MIP and source images.
Methods
We enrolled 37 consecutive patients, 19 male and 18 female, with an average age of 67 years, referred to the radiologic department of a university hospital for examination of the carotid arteries. The number of vessels studied was 73, because 1 carotid artery catheterization was unsuccessful. All patients had hemispheric or retinal symptoms. They also had evidence of an ICA stenosis more than 50% on US13 or a stenosis that was difficult to grade because of extensive calcifications or limited visibility of vessel anatomy.
They underwent both routine carotid DSA and CTA designed for research within 2 weeks. DSA and CTA were analyzed by 2 experienced neuroradiologists blinded to the results of the other technique and the clinical data. The degree of ICA stenosis was graded according to the North American Symptomatic Carotid Endarterectomy Trial Collaborators (NASCET). The ethics committee of the hospital approved the study protocol, and all patients gave their informed consent. US results were not incorporated into the study protocol because of the high interobserver variability of the technique.14
Our initial intention was to measure the absolute diameter of the stenotic part of the lumen from the surgical specimen, the plaque, and use these data for comparison with the radiology. After removal at the operation, the plaque was immediately covered with Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, Calif), frozen in liquid nitrogen, and kept in a freezer at −80°C until sectioning in a cryostat into sections of approximately 5 μm. However, it appeared early on that, especially with soft, hemorrhagic plaques, the manipulation and extraction of the plaque from the carotid bifurcation fractured the plaque and squashed the lumen. The outer surface of the plaque was rarely attenuated enough to protect the specimen during the manipulation; furthermore, it remained unclear whether the outer diameter of the specimen really represented the radiolucent angiographic diameter of the vessel, rendering the comparison potentially inadequate. Because of the methodologic uncertainty, the surgical measurement of the lumen was abandoned.
DSA Techniques
Conventional intra-arterial DSA was performed with an Integris V5000 angiography unit (Philips Medical Systems, Best, the Netherlands) as the standard procedure using transfemoral Seldinger technique. The contrast injections were performed with a power injector (Angiomat 6000 Digital Injection System; Liebel-Flarsheim Company, Cincinnati, Ohio) by using Hexabrix (200 mg of iodine/mL; Guerbet, Roissy, France). The aortic arch was imaged in left anterior-oblique and anteroposterior projections with 35–40 mL of contrast medium at an infusion rate of 15 mL/s. The common carotid arteries were selectively catheterized, and 6–10 mL contrast medium was injected at the rate 4–6 mL/s. Images from both carotid artery bifurcations were obtained in anteroposterior, lateral, and at least 1 but usually 2 oblique projections (−45° and +45°). The intracranial circulation was also included in anteroposterior and lateral projections. Rotational angiography was not used.
CTA Techniques
CTA was performed with a GE LightSpeed Ultra 8-row multisection helical scanner (General Electric, Milwaukee, Wis). Patients were placed in a supine position with the head tilted back as far as possible to avoid dental filling artifacts. Patients were instructed to breathe quietly without swallowing during the imaging. Nonionic contrast medium (100 mL of Omnipaque, 300 mg of iodine/mL; GE Healthcare, Little Chalfont, Buckinghamshire, UK) was injected with a power injector into an antecubital vein at a rate of 4 mL/s. The helical acquisition was initiated after the bolus reached the ascending aorta using a triggering system (Smart Prep; GE Medical Systems). Data were acquired from the aortic arch to the vertex. The section thickness was 2.5 mm, pitch was 1.35:1, field of view was large, 120 kV and 250 mA. Contiguous 2.5-mm axial raw data were reformatted into 1.25-mm axial sections.
Image Analysis
Both CTA and DSA were interpreted by 2 neuroradiologists (H.M.S., S.I.) unaware of the clinical information and the results of other modalities. The DSA images were read from films containing no patient information. The degree of ICA stenosis was analyzed according to NASCET criteria, and other possible stenoses were recorded.
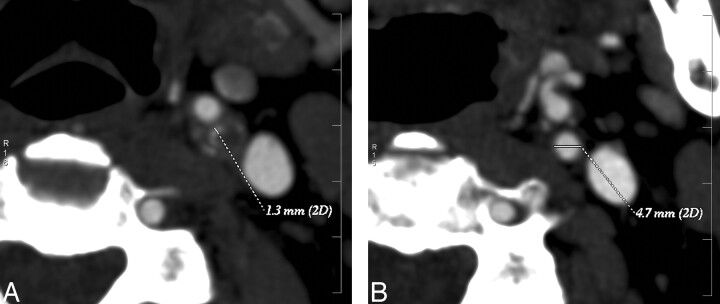
CTA data were stored on optical disks and transferred to a computer workstation (Advantage Workstation, AW 4.0; GE Medical Systems) for analysis. The reformatted 1.25-mm axial images (Fig 1), MIP reformats (Fig 2A), and vessel analysis software (Fig 2B) were used to determine the grade of ICA stenosis according to the NASCET criteria. The evaluating neuroradiologists performed the digital image processing to obtain both the MIP images and the vessel analysis.
Fig 1.
Measurement of the internal carotid artery stenosis in CTA axial images according to NASCET.
A, Maximal stenosis in left ICA.
B, Normalized vessel diameter. The reference point was chosen so that diameters are the same over a distance.
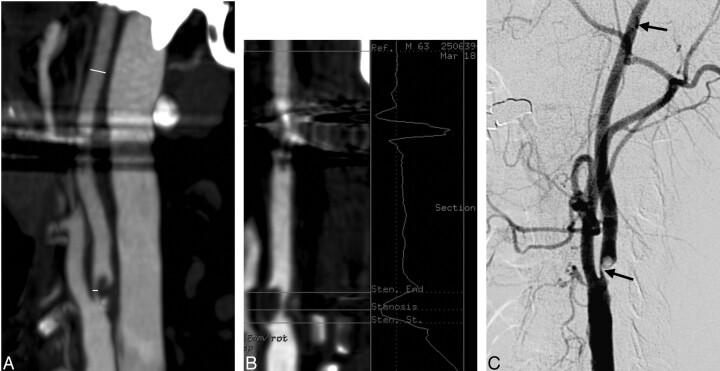
Fig 2.
A, Stenosis measurement levels marked to CTA MIP images according to NASCET. Dental filling artifacts are shown.
B, Vessel analysis method in determination of the grade of stenosis. Dental filling artifacts are shown.
C, DSA image.
Vessel analysis software (Advanced Vessel Analysis B7700SS; GE Medical Systems) is an optional software extension of the Volume Analysis application for AW systems. The operator has a variety of different 3D and reformatted images to choose from to perform analysis measurements. The user identifies the vessel to be analyzed by marking points inside the vessel. The starting point is placed at the distal common carotid artery and the ending point at the ICA to the level where the caliber of the vessel has normalized. One or more intermediate points can be added if required. The software automatically detects the vessel centerline and computes cross-sectional area and minimum, maximum, and mean diameters at each point. The user defines the key anatomic points of interest, which are the maximal stenosis and the reference point according to NASCET. The software calculates the percentage of stenosis.
In MIP images, instead of using constant windowing, the readers attempted to select windowing producing the best available edge detection for each vessel. Concentration of contrast medium in the arteries as well as the patient anatomy can have an effect on the selection of the best windowing. Vessel analysis results, MIP, and axial source images were then compared separately with DSA (Fig 2C).
Data Analysis
The average of the estimated stenosis by the 2 observers in DSA was considered the “gold standard” reference. Sensitivity and specificity were calculated for CTA methods in high-grade and moderate categories of stenosis. Linear regression analysis was applied with the DSA reference as the dependent variable to estimate the intermethod agreement by a regression coefficient, and scatterplots with regression lines were created. Interobserver and intermethod agreement was evaluated with Cohen weighted κ coefficients and Spearman rank correlation coefficient. Overall comparison of stenosis degrees within categories of stenosis was done with Wilcoxon matched pairs test. P values less than .05 were considered statistically significant.
Results
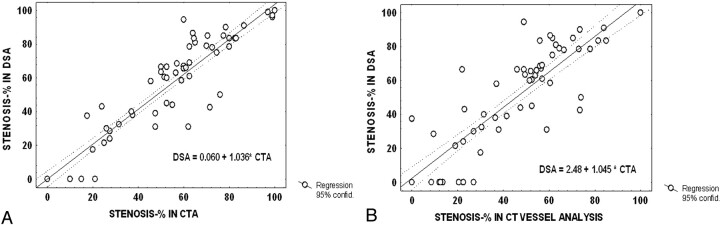
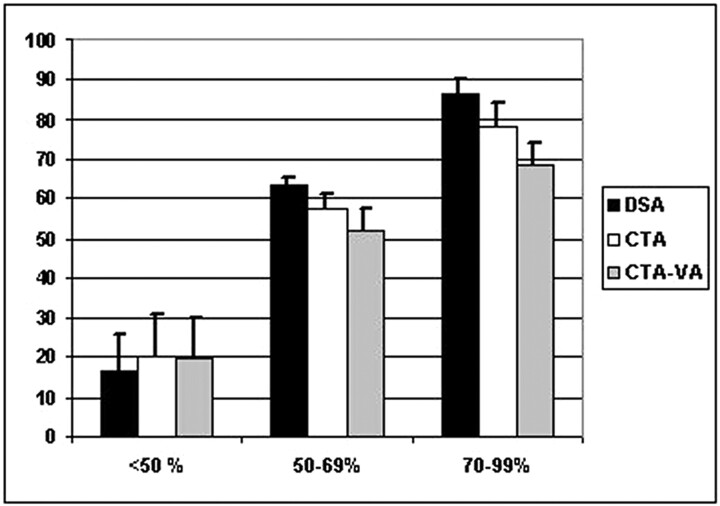
For DSA, 4 (5%) carotid arteries were occluded, 20 (27%) had a high-grade stenosis (70%–99%), and 16 (22%) had a moderate-grade (50%–69%) stenosis. There were 16 (22%) mild stenoses (<50%), and no stenosis was detected in 17 (23%). Three patients with carotid occlusion had experienced ipsilateral symptoms. Of the high-grade stenoses, 15 were symptomatic and 3 were defined as near occlusions. Of moderate stenoses, 9 were considered symptomatic. Degree of stenosis on CTA source/MIP images closely correlated with that for DSA (R = 0.95, regression analysis) (Fig 3A). With the vessel analysis method, the correlation was slightly lower (R = 0.89; P = .02 for paired comparison with DSA) (Fig 3B). In general, CTA underestimated high-grade and moderate stenosis compared with DSA (78.2% versus 86.4%, 57.3% versus 63.1%, P < .05; Wilcoxon matched pairs test). Underestimation was somewhat greater with the vessel analysis method (68.5% versus 83.5%, 51.8% versus 63.1%, P < .01) (Fig 4). Still, overall intermethod agreement of grading was high (Cohen weighted κ = 0.93, 95% confidence interval [CI], 0.90–0.96), and slightly lower for the vessel analysis method (weighted κ = 0.88, 95% CI 0.85–0.92). Interobserver correlation was equally high for manual and vessel analysis methods in CTA (R = 0.95, P < .0001, Spearman rank correlation). The mean magnitude of difference between observers was 3.9% (2.5–5.3%) in DSA, 5.9% in CTA (3.8–7.9%), and 5.8% with vessel analysis (3.8–7.8%).
Fig 3.
Scatterplot of degrees of stenosis in CTA (A) versus DSA (B) with regression line and 95% confidence intervals.
Fig 4.
Histogram of average degrees within different categories of stenosis by method of assessment.
CTA detected all 4 occlusions. For a high-grade stenosis, sensitivity of CTA was 0.75 (95% CI, 0.51–0.91) and specificity 0.96 (0.87–1.00). For moderate stenosis, the sensitivity was 0.88 (0.61–0.98) and specificity was 0.82 (0.69–0.92). For the vessel analysis method, sensitivity and specificity were lower, 0.47 (0.21–0.73) and 0.96 (0.87–1.00), respectively, for high-grade stenosis and 0.62 (0.35–0.85) and 0.82 (0.69–0.92) for moderate stenosis, respectively. All in all, in terms of surgical indication (50%–99%), only 1 case of moderate or high-grade stenosis on DSA was not detected by CTA, whereas 4 vessels within the surgical range on CTA were not within the surgical range on DSA, giving a positive predictive value (PPV) of 87.9% (95% CI, 75,8%–97.3%), a negative predictive value (NPV) of 97.1% (84.7%–99.9%), sensitivity of 0.97 (0.85 –1.00), and specificity of 0.89 (0.75–0.97). Using the vessel analysis method, 6 vessels in the surgical range on DSA were not correctly classified, whereas 3 within the surgical range on vessel analysis were not within the surgical range on DSA. The corresponding values for a diagnostic test were PPV 89.3% (71.8%–97.7%), NPV 84.6% (69.5%–94.1%), sensitivity 0.81 (0.62–0.92), and specificity 0.92 (0.78–0.98).
Discussion
In the recent literature, the overall sensitivity and specificity for CTA in carotid artery stenosis is generally good.15–19 In many studies, however,20 apparently depending on the selection of patients and the imaging technique, the sensitivity and specificity vary widely, ranging from 65% to 100% and 63% to 100%, respectively.
Randoux et al17 found a significant correlation between CTA, gadolinium-enhanced MRA, and DSA in a study that consisted of 22 patients and 44 vessels. They had 13 high-grade and 5 moderate stenoses in DSA. In severe grades, the sensitivity and specificity were both up to 100% with CTA and 93% and 100% with MRA, respectively. No case of overestimation with CTA was found.
Anderson et al18 compared CTA and DSA in detection and quantification of carotid stenosis in 40 patients (80 carotid arteries). They found that CTA was unable to reliably distinguish between moderate and severe stenosis, the corresponding sensitivity levels being as low as 0.65 and 0.73. In mild stenosis and carotid occlusion, CTA performed well, with the values for sensitivity, specificity, and accuracy approaching 100%.
Josephson et al19 found CTA sensitivity to be as high as 100% and specificity 63% compared with DSA in <70% stenosis. The total number of vessels was 81, but only 5 vessels had ≥70% stenosis. There were originally 66 patients (132 vessels), but owing to 24 poor-quality CTA images and 1 DSA scan, they were left with a remainder of 81 vessels in the analysis. Of these, 70 stenoses were less than 50%, only 6 had a stenosis of 50%–69%, and 5 of a higher grade, and the sensitivity and specificity of CTA were 86% and 43%, respectively, in the groups.
In our study, no patients were excluded as a result of suboptimal image quality on CTA. Furthermore, the distribution of the patients in different categories of stenosis was uniform. The stenoses of 4 vessels were graded moderate in CTA and severe in DSA. Retrospective examination of these vessels showed that 2 of these had a subtle poststenotic collapse in the distal ICA overlooked in the initial evaluation, rendering the NASCET method of stenosis measurement inaccurate. In our study, the total number of subtle collapses was 4 and the number of near occlusions with string sign was 3. It is known that the diameter of the vessel can be markedly reduced distal to a severe stenosis. Reduced poststenotic pulse pressure is thought to be the main cause of the collapse of the vessel wall.21 In case of a poststenotic collapse, the stenosis is severe, and its measurement may not be feasible. The issue of poststenotic collapse and subtle near occlusion was recently discussed in the literature by Fox et al,22 who pointed out the importance of poststenotic ICA collapse in evaluating the grade of stenosis. The imaging criteria in DSA (ie, slow filling of the poststenotic vessel and evidence of collaterals) are not equally applicable in CTA; however, in CTA, both ICAs and external carotid arteries are seen in the same axial image and can easily be compared with each other. This can be considered one of the strengths of CTA.
Intermethod agreement appeared somewhat more variable than inter-rater variability. The stenosis of a vessel estimated as moderate in DSA was found mild (<50%) by one reader and moderate by the other reader in CTA. Considering the intermethod agreement, it appears that the stenoses of 3 vessels were consistently graded moderate in CTA and mild in DSA. In 1 patient, both vessels were graded as severely stenotic in CTA and moderate in DSA. It is noteworthy that US also suggested a bilateral high-grade stenosis, and in surgery, the left carotid artery turned out to be severely stenosed. This supports the view that the overestimation of stenosis in CTA compared with DSA is not in all cases real, because the limited and suboptimal projections of the conventional angiography may in fact lead to underestimation of the stenosis degree.23,24 Hirai et al25 demonstrated that the luminal morphology of the carotid artery stenosis may affect the assessment of maximum stenosis of ICA at conventional DSA, and 3D CTA can in some cases demonstrate severe stenosis better than DSA. It may even be hypothesized that this more versatile visualization of stenosis could explain some of the few “false-positive” readings in the surgical range, contrary to the general trend of CTA to underestimate stenosis compared with DSA.
We found axial source images the most reliable in grading the degree of carotid artery stenosis, but MIP reconstructions can be of help in certain situations. A horizontal or tortuous course of the vessel or a very short stenosis can render the assessment of the stenosis difficult on axial images. On the other hand, ring-like calcifications in the arterial wall can mask the open lumen in MIP reconstructions in every direction, and determination of the stenosis with MIP images may not be possible at all. Here we would like to point out the importance of learning and experience in evaluating the CTA images. In our study, 2 experienced neuroradiologists performed the image reformatting and analysis themselves on the workstation, and even then some mistakes could not be avoided in the initial evaluation, as pointed out earlier.
The accuracy of the images is influenced by imaging parameters, such as the section thickness and overlap. We used 2.5-mm section thickness in this study, but since then we have reduced the section thickness to 1.25 mm to improve the quality. The thinner the stenotic residual lumen, the higher the relative error of measurement that results from voxel size. In addition, the window settings and the vascular contrast medium attenuation influence the measurement accuracy in CTA.26 Further improvements on the technology, such as adding more detector rows to the equipment, may affect the accuracy of CTA.27
In our study, the semiautomatic vessel analysis did not improve the accuracy of assessment of stenosis in CTA. Zhang et al28 used a CTA vessel analysis program in carotid stenosis recognition and quantification and compared it with rotational DSA (rDSA). The intertechnique correlation between CTA and rDSA was 0.69 using automatic measurements and 0.81 with the manually corrected measurement. We did not use manual correction in our semiautomatic vessel analysis, and our results are consistent with those mentioned above.
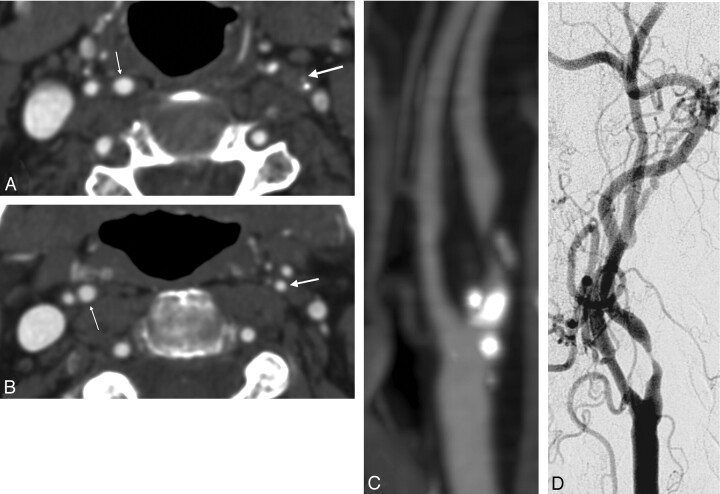
In our study, there were 4 total carotid occlusions in DSA, and CTA detected them all. The number is too small to allow conclusions of the reliability of CTA in this aspect. However, the finding is in accordance with previous reports.29–31 The ascending pharyngeal branch of the external carotid artery can be mistaken as a hairline open ICA. This pitfall can be avoided by following the proximal internal carotid artery into the petrous canal at the skull base.
It is not usually possible on US to distinguish total occlusion from near occlusion. Differentiation of total from near occlusion can affect patient management because patients with near occlusion can in some cases benefit from surgery, but total occlusion is treated medically. However, more evidence is needed before we know which patients really benefit from various lines of treatment. At this point, we cannot be sure whether the ability to differentiate between total and near occlusion is relevant for patient management. At the same time, more evidence would be necessary to find out whether the small differences in the performance of the diagnostic methods for carotid stenosis are important for patient outcome and in which cases DSA or a combination of noninvasive methods would produce the best result.12 The potential risks of DSA should be taken into consideration in weighing the diagnostic method of choice. After all, the present situation is notably different from the time of the major studies on carotid endarterectomy, with advances in the best medical treatment as well as the gradual advent of endovascular treatment options.
Conclusions
We found CTA to be a useful method for assessment of carotid stenosis. In most cases, it seems justifiable to replace the invasive DSA with CTA. Although underestimation of the stenosis seems more common than overestimation, misclassification is rare in careful assessment of images. At present, this is not improved by semiautomatic vessel analysis methodology. More study is needed to estimate in which cases recourse to DSA or a combination of methods is expected to have an impact on clinical decision-making or patient outcome.
Fig 5.
Poststenotic collapse. A, Maximal stenosis in left ICA (big arrow). Calcification behind the open lumen. The right ICA is normal (small arrow).
B, The distal left ICA (big arrow) remains collapsed. Normal right ICA (small arrow).
C, MIP image of the left ICA.
D, DSA image of the left ICA.
Fig 6.
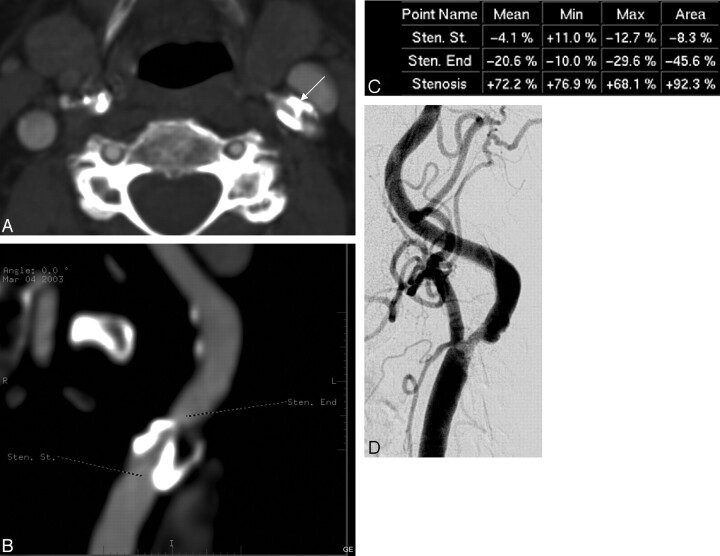
Underestimation of stenosis in DSA. A, An axial MIP image from the left ICA stenosis graded severe by CTA (arrow). According to the vessel analysis method (B, C) the stenosis is 77%. In DSA, the maximum stenosis is 50% (D). In surgery, the stenosis turned out to be severe.
Footnotes
This work was supported by grants from the Radiological Society of Finland and Helsinki University Central Hospital, Department of Radiology.
Presented as a poster at the 43nd Annual Meeting of the American Society for Neuroradiology; May 23–27, 2005; Toronto, Canada.
References
- 1.Bonita R. Epidemiology of stroke. Lancet 1992;339:342–44 [DOI] [PubMed] [Google Scholar]
- 2.Sandercock PA, Warlow CP, Jones LN, et al. Predisposing factors for cerebral infarction: the Oxfordshire community stroke project. BMJ 1989;298:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521–26 [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 5.Warlow C. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991;337:1235–43 [PubMed] [Google Scholar]
- 6.Barnett HJM, Taylor DW, Eliasziv M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998;339:1445–25 [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915–24 [DOI] [PubMed] [Google Scholar]
- 8.Hankey GJ, Warlow CP, Molyneux AJ. Complications of cerebral angiography for patients with mild carotid territory ischaemia being considered for carotid endarterectomy. J Neurosurg Psychiatry 1990;53:542–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies KN, Humphrey PR. Complications of cerebral angiography in patients with symptomatic carotid territory ischaemia screened by carotid ultrasound. J Neurol Neurosurg Psychiatry 1993;56:967–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston DC, Chapman KM, Goldstein LB. Low rate of complications of cerebral angiography in routine clinical practice. Neurology 2001;57:2012–14 [DOI] [PubMed] [Google Scholar]
- 11.Goddard AJP, Mendelow AD, Birchall D. Computed tomography angiography in the investigation of carotid stenosis. Clin Radiol 2001;56:523–34 [DOI] [PubMed] [Google Scholar]
- 12.Herzig R, Buřval S, Křupka B, et al. Comparison of ultrasonography, CT angiography, and digital subtraction angiography in severe carotid stenoses. Eur J Neurol 2004;11:774–81 [DOI] [PubMed] [Google Scholar]
- 13.Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis—Society of Radiologists in Ultrasound consensus conference. Radiology 2003;229:340–46 [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan J, Mayberg MR, Weiss DG, et al. Duplex accuracy compared with angiography in the Veterans Affairs Cooperative Studies Trial for Symptomatic Carotid Stenosis. Neurosurgery. 1995;36:648–55 [DOI] [PubMed] [Google Scholar]
- 15.Moll R, Dinkel HP. Value of the CT angiography in the diagnosis of common carotid artery bifurcation disease: CT angiography versus digital subtraction angiography and color flow Doppler. Eur J Radiol 2001;39:155–62 [DOI] [PubMed] [Google Scholar]
- 16.Hirai T, Korogi Y, Ono K et al. Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: combined MR angiography and CT angiography compared with digital subtraction angiography. AJNR Am J Neuroradiol 2002;23:93–101 [PMC free article] [PubMed] [Google Scholar]
- 17.Randoux B, Marro B, Koswkas F, et al. Carotid Artery Stenosis: Prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology 2001;220:179–85 [DOI] [PubMed] [Google Scholar]
- 18.Anderson GB, Ashforth R, Steinke DE, et al. CT angiography for the detection and characterization of carotid artery bifurcation disease. Stroke 2000;31:2168–74 [DOI] [PubMed] [Google Scholar]
- 19.Josephson SA, Bryant SO, Mak HK, et al. Evaluation of carotid stenosis using CT angiography in the initial evaluation of stroke and TIA. Neurology 2004;63:457–60 [DOI] [PubMed] [Google Scholar]
- 20.Rothwell PM, Pendlebury ST, Wardlaw J, et al. Critical appraisal of the design and reporting of studies of imaging and measurement of carotid stenosis. Stroke 2000;31:1444–50 [DOI] [PubMed] [Google Scholar]
- 21.Wakhloo AK, Baruch B, Jaehoon S, et al. Hemodynamics of carotid artery atherosclerotic occlusive disease. J Vasc Interv Radiol 2004;15:5111–21 [DOI] [PubMed] [Google Scholar]
- 22.Fox AJ, Eliasziw M, Rothwell PM, et al. Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am J Neuroradiol 2005;26:2086–94 [PMC free article] [PubMed] [Google Scholar]
- 23.Schenk EA, Bond MG, Aretz TH, et al. Multicenter validation study of real-time ultrasonography, arteriography, and pathology: pathologic evaluation of carotid endarterectomy specimens. Stroke 1988;19:289–96 [DOI] [PubMed] [Google Scholar]
- 24.Elgersma OEH, Buijs PC, Wüst AFJ, et al. Maximum internal carotid arterial stenosis: assessment with rotational angiography versus conventional intraarterial digital subtraction angiography. Radiology 1999;213:777–83 [DOI] [PubMed] [Google Scholar]
- 25.Hirai T, Korogi Y, Ono K, et al. Maximum stenosis of extracranial internal carotid artery: effect of luminal morphology on stenosis measurement by using CT angiography and conventional DSA. Radiology 2001;221:802–09 [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Hopper KD, Mauger DT, et al. CT angiographic measurement of the carotid artery: optimizing visualization by manipulating window and level settings and contrast material attenuation. Radiology 2000;217:494–500 [DOI] [PubMed] [Google Scholar]
- 27.Kalra MK, Maher MM, Toth TL, et al. Multidetector computed tomography technology. Current status and emerging developments. J Comput Assist Tomogr 2004;28:S12–S16 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Berg MH, Ikonen AEJ, et al. Carotid artery stenosis: reproducibility of automated 3D CT angiography analysis method. Eur Radiol 2004;14:665–72 [DOI] [PubMed] [Google Scholar]
- 29.Lev MH, Romero JM, Goodman DNF, et al. Total occlusion versus hairline residual lumen of the internal carotid arteries: accuracy of single section helical CT angiography. AJNR Am J Neuroradiol 2003;24:1123–29 [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C-J, Lee T-H, Hsu H-L, et al. Multi-slice CT angiography in diagnosing total versus near occlusions of the internal carotid artery comparison with catheter angiography. Stroke 2004;35:83–85 [DOI] [PubMed] [Google Scholar]
- 31.Alvarez-Linera J, Benito-Leon J, Escribano J, et al. Prospective evaluation of carotid artery stenosis: elliptic centric contrast-enhanced MR angiography and spiral CT angiography compared with digital subtracion angiography. AJNR Am J Neuroradiol 2003;24:1012–19 [PMC free article] [PubMed] [Google Scholar]