Abstract
BACKGROUND AND PURPOSE: To report the initial experience by using a new liquid embolic agent (Onyx) for embolization of brain arteriovenous malformations (AVMs).
METHODS: Between May 2000 and December 2005, 44 patients with brain AVMs were embolized with Onyx. There were 18 women and 26 men with a mean age of 42.4 years (median 44, range 14–71 years). Clinical presentation included seizures in 26 patients (59%), hemorrhage from the AVM in 13 patients (30%), subarachnoid hemorrhage from a concomitant aneurysm in 3 patients (7%), visual disturbances in 1 patient (2.3%), and in 1 patient (2.3%) the AVM was an incidental finding. Mean estimated size of the AVM was 3.9 cm (median 4, range 2–7 cm).
RESULTS: In 44 patients, 52 embolization procedures were performed with 138 feeding pedicles embolized, ranging from 1 to 7 per patient. Average estimated size reduction was 75% (median 80%, range 40%–100%). Total obliteration was achieved in 7 AVMs (16%), and partial embolization was followed by surgery in 10 patients and by radiosurgery in 20 patients. Complications occurred in 6 patients, leading to death in 1 patient (mortality 2.3%) and to permanent disability in 2 patients (morbidity 4.6%).
CONCLUSION: Onyx is feasible and safe in the embolization of brain AVMs. Complete obliteration can be achieved in small AVMs. Large AVMs can be adequately reduced in size for additional surgical or radiosurgical treatment.
Modern treatment of brain arteriovenous malformation (AVM) comprises the following interventions alone or in combination: surgery, stereotactic radiosurgery, and embolization. Embolization is mainly used to reduce the size of large AVMs, to enhance the safety of surgery, or to make the AVM amenable to radiosurgery.1–4 The most commonly used embolic agent is the fast polymerizing liquid adhesive n-butyl cyanoacrylate (n-BCA). The use of n-BCA in brain AVMs requires experience and skills, because intranidal flow and polymerization of n-BCA are quick and largely unpredictable. Recently, a new liquid embolic agent became available: Onyx liquid embolic system (ev3, Irvine, Calif). Onyx is less adhesive and polymerizes slowly, which seems advantageous over n-BCA.5,6 In this paper, we report our initial experience with Onyx in the embolization of 44 brain AVMs.
Methods
Indications for Treatment
AVM treatment strategy and indications for embolization were assessed from MR imaging (MRI) and angiography in a joint meeting of 2 neurosurgeons with experience in radiosurgery, 2 neurologists, and 2 neuroradiologists. In general, AVMs larger than 3 cm were embolized to achieve size reduction, to enhance the safety of surgery, or to make the AVM amenable to radiosurgery (AVM < 3 cm) (Fig 1). In some small AVMs (≤3 cm) with angioarchitecture suitable for embolization (1 or 2 feeding pedicles), this approach was offered as a first-line treatment with the aim of complete obliteration of malformations (Fig 2). AVM size was estimated from MRI and angiography, and all AVMs were classified according to the Spetzler-Martin scale. After every embolization session, results were discussed in a joint meeting and additional treatment was planned. AVM size reduction after embolization was estimated and consensus was established during the joint meeting. In general, after embolization, additional radiosurgery was offered for AVMs that had never bled, and additional surgery was offered for accessible AVMs that had bled recently. The clinical status of patients was assessed at presentation, after embolization, and at follow-up visits by a neurosurgeon or a neurosurgical resident according to the Glasgow Outcome Scale.
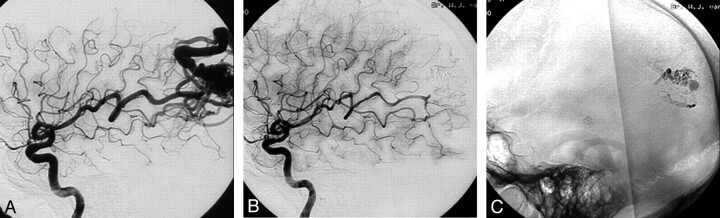
Fig 1.
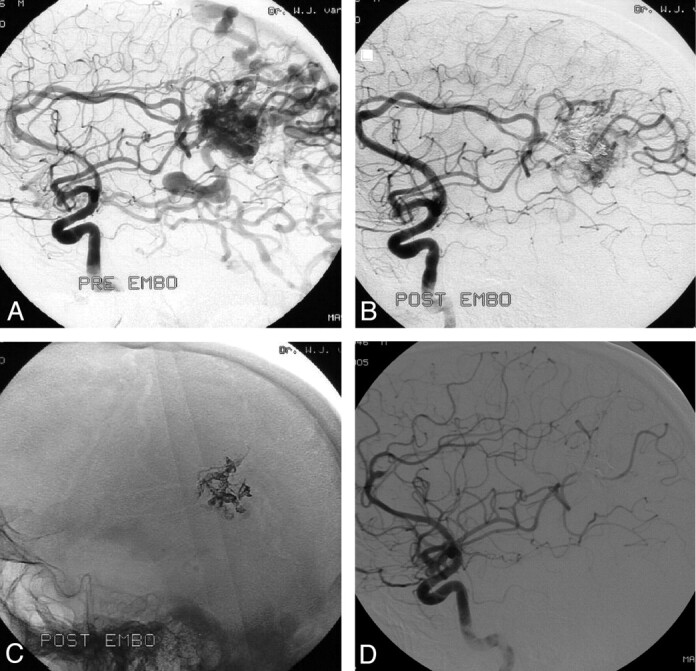
A 44-year-old man with seizures and a left parietal AVM.
A, A 3.5-cm left parietal AVM with superficial and deep venous drainage (Spetzler and Martin grade IV).
B, The AVM shows 80% obliteration with a single Onyx injection of 4.8 mL in 64 minutes.
C, Onyx cast after embolization.
D, Complete obliteration of the AVM 2 years after gamma knife radiosurgery.
Fig 2.
A 28-year-old man with a 3-cm right parietooccipital AVM.
A, The AVM has 2 main feeders.
B, Complete obliteration of the AVM with 2 injections of Onyx (total of 3.5 mL).
C, Cast of Onyx.
Onyx Liquid Embolic Agent
Onyx is a less adhesive liquid embolic agent and is supplied in ready-to-use vials. Each vial contains ethylene-vinyl alcohol copolymer, dimethyl sulfoxide (DMSO), and tantalum. Ethylene-vinyl alcohol copolymer is formed of 48 mol/L ethylene and 52 mol/L vinyl alcohol. The polymer is dissolved in DMSO and is prepared in 3 different concentrations: 6.0%, 6.5%, and 8.0%. Micronized tantalum powder (35% wt/vol) is added for radiopacity. The vials are kept on a shaker (Vortex-Genie, Scientific Industries, Bohemia, NY) for at least 20 minutes to ensure proper mixing of the tantalum powder. The lower the concentration of the copolymer, the less viscous the agent and the more distal penetration can be achieved. Viscosity of Onyx at 6.0%, 6.5%, and 8.0% is 18, 20, and 34 cP, respectively. Onyx is manufactured as Onyx 18, Onyx 20, and Onyx 34. Generally, Onyx 18 and 20 are used for embolization of a plexiform nidus, and Onyx 34 is used for embolization of large arteriovenous shunts in the AVM (Fig 3).
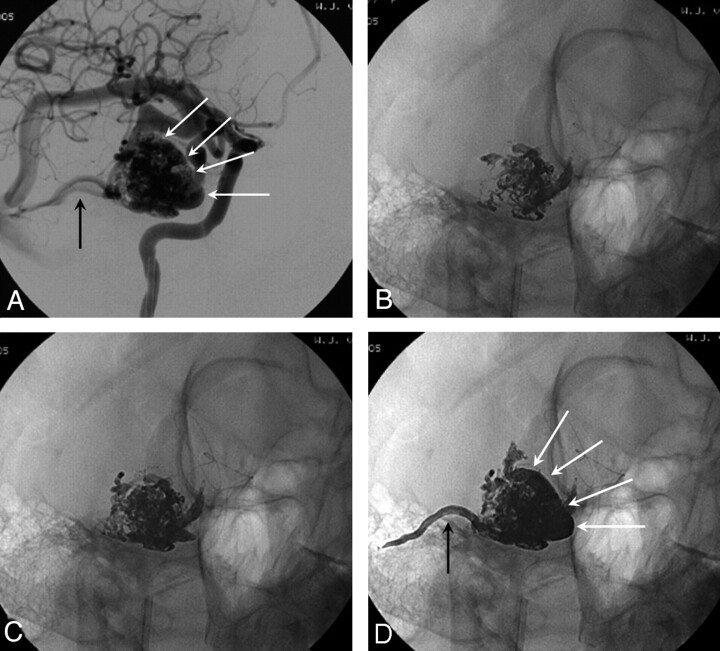
Fig 3.
A 37-year-old woman with small high-flow AVM (pial fistula).
A, Large arteriovenous shunt from anterior cerebral artery.
B, Microcatheter just proximal to the shunt; from this point Onyx 34 was slowly injected.
C, Onyx cast after injecting 3.4 mL in 16 minutes.
D, Complete closure of the fistula at 6 weeks follow-up angiogram.
Embolization Procedure
All embolizations were performed with the patient under general anesthesia on a biplane angiographic unit (Integris BN 3000, Philips Medical Systems, Best, the Netherlands) by the same operator (W.J.v.R.). Systolic blood pressure during the procedure was controlled between 100 and 110 mm Hg. Catheterization was performed with a transfemoral approach by using standard coaxial techniques. The guiding catheter was flushed via a pressure bag with saline containing 2500 U heparin/L. A DMSO-compatible microcatheter (UltraFlow 1.5F, ev3) was navigated to the nidus of the AVM with aid of an 0.008-inch guidewire (Mirage, ev3). Once the microcatheter tip was in the desired position, the injection of Onyx was carried out as follows: 1) the microcatheter was flushed with 5 mL of normal saline; 2) 0.25 mL of DMSO was injected into the microcatheter to fill the dead space; 3) Onyx was aspirated into a 1-mL syringe, and 0.25 mL of this amount was injected slowly for 40 seconds to fill the microcatheter and replace the DMSO in the dead space; 4) slow injection of the Onyx was then continued under fluoroscopy.
Single injections of Onyx were carried out for up to 90 minutes. Long injection times were possible because of the less adhesive nature of Onyx. During injections, we were able to pause, obtain an angiogram to assess nidus occlusion and the status of draining veins, and then continue with the injection. When any reflux of Onyx into the feeding pedicle or venous migration was noted, the injection was stopped for 1–2 minutes to allow for solidification, and then injection was continued. The Onyx then filled a different portion of the nidus, with no further reflux or filling of the vein. When reflux exceeded about 1.5–2 cm of the catheter tip, the catheter was withdrawn. For most AVMs, the goal of embolization was to reduce AVM nidus size to the point were radiosurgery would be possible, preferably in one embolization session. In some cases, where complete nidus occlusion was likely, Onyx was allowed to occlude the proximal parts of the draining veins at the end of the procedure (Fig 4). In cases with dural arteries feeding the AVM, we preferred occluding this dural supply with a mixture of 30% n-BCA and 70% lipiodol (Guerbet, Roissy, France). Dural feeders were not used as access to the nidus. Provocative testing was not performed.
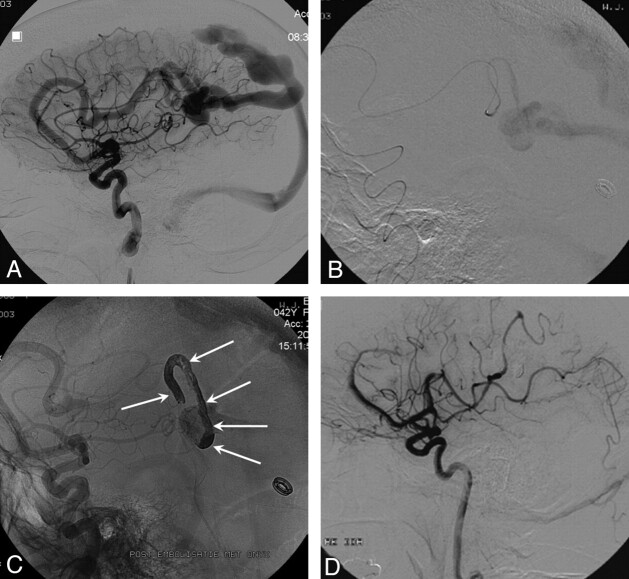
Fig 4.
Right temporal AVM in a 28-year-old woman with seizures. Complete occlusion occurred with a single 62-minute Onyx injection of 7.8 mL.
A, Oblique right internal carotid angiogram shows AVM fed by the anterior temporal artery and draining via a large superficial vein (white arrows) and a small superficial vein (black arrow).
B, Onyx cast after 12 minutes slow injection.
C, Onyx cast after 28 minutes.
D, Onyx cast after 62 minutes. The AVM is completely occluded, including the proximal parts of the draining veins (same arrows as in A).
Radiosurgery
Radiosurgery was performed with a Leksell Gamma Knife (Elekto, Stockholm, Sweden) from 2000 to 2001 at an institution in Krefeld, Germany, and from 2002 onwards at our own institution. After mounting a stereotactic head frame, angiography at 6 frames/sec. was performed in 2 directions, followed by MRI. Treatment planning, including delineation of the nidus remnant, was performed by a neurosurgeon and a neuroradiologist by using separate and merged images of angiography and MRI. Target volume and radiation dose were recorded. After radiosurgery, follow-up consisted of yearly MRI. When MRI suggested complete obliteration of the nidus, angiography was performed to confirm or refute this finding. When the AVM was not obliterated after 2 years, MRI was repeated 1 year later. If after 3 years the AVM was still not obliterated, repeated radiosurgery was considered.
Patients
From May 2000 to December 2005, 44 patients with an AVM were embolized with Onyx. During the same period, 9 additional patients with an AVM were embolized with n-BCA, mainly micro-AVMs and AVMs with “en passage” feeders only. The mean age of the 44 patients embolized with Onyx was 42.4 years (median 44, range 14–71 years). There were 18 women and 26 men. Clinical presentation included seizures in 26 patients (59%), hemorrhage from the AVM in 13 patients (30%), subarachnoid hemorrhage from a concomitant aneurysm in 3 patients (7%), visual disturbances in 1 patient (2.3%) with an occipital AVM, and in 1 patient (2.3%) the AVM was an incidental finding after head trauma. Of 44 patients with a brain AVM, 22 had involvement of the left cerebral hemisphere and 20 of the right hemisphere. Two AVMs were located in the cerebellum. There were no deep located AVMs in the basal ganglia with perforator feeders and no brain stem lesions. Mean estimated size of the AVM was 3.9 cm (median 4, range 2–7 cm). Two patients had previous partial embolizations of their AVMs at other institutions 8 and 11 years earlier. Classification according to the Spetzler and Martin scale7 was grade I in 4 patients, grade II in 11, grade III in 17, grade IV in 10, and grade V in 2 patients.
Results
Anatomic Results
The 44 patients with brain AVMs underwent an aggregate total of 53 embolization procedures: 35 patients were embolized once and 9 patients were embolized twice. The 44 patients had an aggregate total of 138 feeding pedicles embolized, ranging from 1 to 7 per patient. In 2 patients dural feeders were embolized with n-BCA. Average estimated size reduction was 75% (median 80%, range 40%–100%). Total obliteration, confirmed at follow-up angiograms after 6–12 weeks, was achieved in 7 AVMs (16%), all of which were small (Spetzler and Martin grades I and II).
Complications of Embolization
Complications of embolization occurred in 6 patients (13.6%) and are summarized in the Table. Complications led to death in 1 patient (mortality 2.3%) and to permanent neurologic deficit in 2 patients (permanent morbidity 4.6%). In 2 patients a microcatheter was glued in the AVM and was cut off in the groin without clinical sequelae.
Complications of embolization with Onyx in 6 of 44 patients
| Sex/Age (y) | AVM Location and Size | Estimated Size Reduction (%) | Complication | Additional Therapy | Outcome, GOS |
|---|---|---|---|---|---|
| M/31 | Right temporal, 3.5 cm | 95 | Hemorrhage 3 d after embolization | Acute surgical removal of hematoma and AVM | No deficit at 3 mo, GOS 5 |
| M/44 | Left temporal 3.5 cm | 80 | Partial occlusion M2 by reflux | Radiosurgery | Transient dysphasia, GOS 5 |
| M/51 | Right frontoparietal, 4.5 cm | 50 | Hemorrhage during embolization | Death, GOS 1 | |
| M/60 | Left parietal, 5 cm | 90 | Reflux in distal ACA branch | Radiosurgery | Transient paresis right leg, GOS 5 |
| M/27 | Right parieto-occipital, 4 cm | 90 | Hemorrhage 3 h after embolization | Acute surgical removal of hematoma and AVM | Quadrantanopsia, GOS 4 |
| F/44 | Right partietal, 7 cm | 40 | Reflux in distal ACA branch | Refused | Distal paresis left leg, GOS 4 |
AVM indicates arteriovenous malformation; ACA, anterior cerebral artery; GOS = Glasgow Outcome Score; M2, second segment of middle cerebral artery.
Case 1
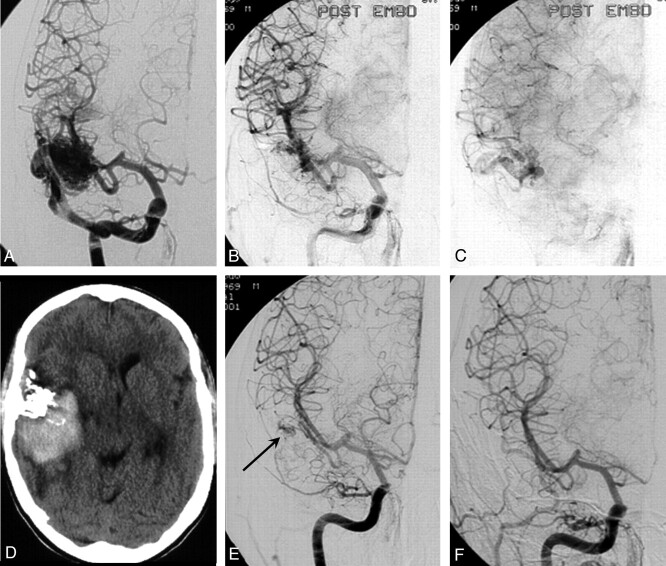
A 31-year-old man with a 3.5-cm right temporal AVM with superficial venous drainage (Spetzler and Martin grade II) was embolized with Onyx (Fig 5). The postembolization angiogram showed a remaining small part of the nidus with patency of the principal draining vein. The patient was discharged the following day. Three days later he was readmitted with severe headache, and a CT scan showed a large right temporal hematoma. Angiography demonstrated the small nidus remnant, but the principal draining vein was occluded. Emergency surgery followed with complete removal of the hematoma and AVM remnant. The patient made an uneventful recovery.
Fig 5.
A 31-year-old man with seizures and right temporal AVM, embolized with Onyx.
A, Right temporal AVM with superficial venous drainage.
B, C, Small nidal remnant with open draining vein after subtotal obliteration.
D, Hemorrhage 3 days after embolization.
E, Angiogram after hemorrhage shows nidal remnant with no apparent venous drainage.
F, Angiogram after surgery demonstrates complete removal.
Case 2
A 44-year-old man with a 3.5-cm left temporal AVM fed by the anterior temporal artery (Spetzler and Martin grade II) was embolized with Onyx. During injection of Onyx, reflux into an M2 branch occurred, resulting in partial occlusion of the branch. After embolization the patient had dysphasia, and an MRI showed a small infarction. Six weeks later the dysphasia had resolved, and the AVM remnant was treated with radiosurgery that resulted in complete obliteration 2 years later.
Case 3
A 51-year-old man with a 4.5-cm right frontoparietal AVM (Spetzler and Martin grade III) was embolized with Onyx, and 90% nidus obliteration was achieved. After embolization the patient did not wake up from general anesthesia and CT scanning showed a large hematoma adjacent to the AVM. He deteriorated rapidly and died 5 hours later. We postulate that a rupture was caused by the microcatheter or guidewire at the beginning of the procedure.
Case 4
A 60-year-old man with a 5-cm left parietal AVM (Spetzler and Martin grade III) was embolized with Onyx, and subtotal obliteration was achieved. During injection, the Onyx migrated via the nidus into a remote feeding branch of the anterior cerebral artery. After embolization, a distal paresis of the right leg was noted and MRI demonstrated a small infarction in the vascular territory of the anterior cerebral artery. At follow up 6 weeks later, the paresis had resolved. The AVM remnant was treated with radiosurgery.
Case 5
A 27-year-old man with a 4-cm right parietooccipital AVM (Spetzler and Martin grade III) was embolized with Onyx, and 90% of the nidus was obliterated. The principal draining vein remained open. Immediately after embolization the patient complained of headache. CT scanning was normal. However, the headache became worse and repeated CT scans 3 hours later showed a hematoma. Emergency surgery followed with complete removal of the AVM remnant and hematoma. The patient made an uneventful recovery and neurologic follow-up 6 weeks later demonstrated only a quadrantanopsia.
Case 6
A 44-year-old woman with a 7-cm right parietal AVM (Spetzler and Martin grade V) was embolized with Onyx for the second time. During Onyx injection, reflux via the nidus into another feeding pedicle from the anterior cerebral artery occurred and resulted in a small infarction with distal paresis of the left leg. At follow-up 6 weeks later, this distal paresis was unchanged. She refused further treatment of the partially embolized AVM.
Additional Therapy and Complications after Embolization
Of 44 AVMs, 7 were completely obliterated after embolization. One patient died of a hemorrhagic complication. Two patients with partially embolized AVMs emigrated to other countries and were lost for follow-up. One patient with a partially embolized AVM refused further treatment after a complication. Of the remaining 33 patients, 10 patients had additional surgery (including the 2 patients operated on following acute hemorrhagic complications from embolization), 20 patients had additional radiosurgery, and 3 patients are scheduled for radiosurgery.
All 10 patients who had operations had complete removal of AVMs, which was confirmed with angiography. Two patients (both with Spetzler and Martin grade IV AVMs) had a postsurgical hemorrhage that resulted in permanent neurologic deficits, requiring dependent care (Glasgow Outcome Scale 3).
No complications occurred after radiosurgery in 20 patients. One patient had a small interval hemorrhage 3 months after radiosurgery without permanent clinical sequelae. Follow-up MRI and angiography showed complete obliteration after radiosurgery in 5 patients. The remaining 15 patients are awaiting 2-year postradiosurgery results. Currently 22 of 44 AVMs (50%) have been obliterated completely.
Discussion
Our initial experience with use of Onyx for embolization of brain AVMs is encouraging, with an average size reduction of 75% and complete obliteration with embolization alone in 16%. In most patients, one embolization session was sufficient to obtain a complete obliteration or sufficient size reduction for subsequent surgery or radiosurgery. These results are comparable to other studies that have used Onyx.5,6
We think that Onyx has several advantages over n-BCA. The introduction of Onyx in most AVM embolizations in our practice has transformed the embolization of AVMs from a rather unpredictable intervention into a controlled procedure. Prolonged (>60 minutes) and controlled intranidal injection is possible with Onyx. When reflux in the feeding pedicle or early filling of draining veins is noted during injection, the injection can be paused for 1–2 minutes, thus allowing the Onyx to solidify. During this pause, angiographic control can be performed. Continued injection usually results in penetration of different portions of the nidus until a satisfactory result is obtained. Only when repeated reflux in the feeding pedicle occurs of more than 1.5–2 cm, the microcatheter is withdrawn. Although Onyx is less adhesive, microcatheters may rarely be glued in the nidus, as occurred in 2 of 138 injections.
We found the use of Onyx particularly useful for slow occlusion of large arteriovenous shunts inside the AVM, without the use of adjuvant embolic materials such as coils. For this purpose, injection is started with Onyx 34 until the fistula is closed, and injection is continued with Onyx 18 for subsequent filling of the nidus. In smaller AVMs with 1 or 2 feeding pedicles, complete obliteration may be accomplished, including occlusion of the proximal draining veins at the end of the procedure.
Since the introduction of Onyx, we have used n-BCA only for micro-AVMs and for AVMs with a plexiform nidus and “en passage” feeders. In very small feeding pedicles the visibility of Onyx is less than that of n-BCA mixed with tantalum powder, and therefore reflux in these small pedicles may not be appreciated in a timely manner when Onyx is used.
A new phenomenon that may lead to complications relates to our observation that Onyx may reflux via the nidus into a remote feeder of the AVM. When this reflux is appreciated on fluoroscopy, injection is immediately stopped, but if the feeding pedicle is falsely interpreted as part of the nidus, infarction in the vascular territory of the feeding pedicle may occur, as happened in 2 of our patients. We therefore recommend biplane fluoroscopy during Onyx injection or frequent changes in the angle of the x-ray tube.
When the nidus is subtotally obliterated with patent draining veins, stagnant flow may cause thrombosis of these veins, leaving a part of the nidus without drainage. This situation may lead to postembolization hemorrhage, as occurred in 2 of our patients.
Our complication rate of 6.9% is in the range found in other studies that used Onyx, n-BCA, or particles as embolic agents.2,5,6,8–10 Embolization-induced hemorrhage, which occurs during embolization or hours to weeks afterward, is the most feared complication, leading to disability or death in a substantial proportion of cases. Subtotal nidus occlusion followed by immediate or delayed thrombosis of the draining veins is the most important cause. With the use of n-BCA, inadvertent migration of the glue into the draining veins may result in immediate hemorrhage, a phenomenon that is unlikely to occur with Onyx. During embolization with Onyx, with advancing occlusion of the nidus, it seems sensible either to leave a substantial part of the nidus open to assure sufficient flow in the draining veins or to proceed to complete occlusion. With growing experience, these decisions can be made with greater confidence.
A possible concern of prolonged intranidal injections of Onyx is the radiation exposure. Although we did not routinely record fluoroscopy times during embolization, 4 of our patients were the subject of a previous study concerning radiation exposure during neurointerventional procedures in 31 patients.11 In this study, the patient entrance dose to the skull did not exceed 2.3 Gy, and the highest entrance doses were in the lower part of the range in which nonstochastic effects may arise. We did not observe hair loss in any of our patients.
Conclusion
Embolization with Onyx of brain AVMs is feasible and safe and results in adequate nidus occlusion. As with other embolic agents, postembolization hemorrhage is the most important complication.
References
- 1.Richling B, Killer M. Endovascular management of patients with cerebral arteriovenous malformations. Neurosurg Clin N Am 2000;11:123–45 [PubMed] [Google Scholar]
- 2.Henkes H, Nahser HC, Berg-Dammer E, et al. Endovascular therapy of brain AVMs prior to radiosurgery. Neurol Res 1998;20:479–92 [DOI] [PubMed] [Google Scholar]
- 3.Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg 1996;85:19–28 [DOI] [PubMed] [Google Scholar]
- 4.Spetzler RF, Martin NA, Carter LP, et al. Surgical management of large AVM’s by staged embolization and operative excision. J Neurosurg 1987;67:17–28 [DOI] [PubMed] [Google Scholar]
- 5.Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery 2001;48:984–95 [DOI] [PubMed] [Google Scholar]
- 6.Pierot L, Januel C, Herbreteau D, et al. Endovascular treatment of brain arteriovenous malformation using Onyx: preliminary results of a prospective multicenter study. Interventional Neuroradiology 2005;11:159–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986;65:476–83 [DOI] [PubMed] [Google Scholar]
- 8.Taylor CL, Dutton K, Rappard G, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg 2004;100:810–2 [DOI] [PubMed] [Google Scholar]
- 9.n-BCA Trail Investigators. N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol 2002;23:748–55 [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich JO, Hartlieb S, Stendel R, et al. Bleeding complications after endovascular therapy of cerebral arteriovenous malformations. AJNR Am J Neuroradiol 2006;27:313–16 [PMC free article] [PubMed] [Google Scholar]
- 11.Kemerink GJ, Frantzen MJ, Oei K, et al. Patient and occupational dose in neurointerventional procedures. Neuroradiology 2002;44:522–28 [DOI] [PubMed] [Google Scholar]