Abstract
SUMMARY: A 36-year-old woman presented with acute-onset right lower extremity paresthesias, dysarthria, right facial droop, and right hemiparesis. CT and MR imaging of the brain revealed extensive white matter disease and left basal ganglia infarction with dural and leptomeningeal enhancement. Differential considerations included vasculitis, granulomatous disease, and neoplasm. Chest, abdomen, and pelvis CTs were normal. Right temporal lobe biopsy revealed noncaseating granulomatous inflammation consistent with neurosarcoidosis.
Neurosarcoidosis has been described in 5% of patients with sarcoidosis.1–3 Imaging findings include dural thickening or mass, leptomeningeal involvement, enhancing and nonenhancing parenchymal lesions, cranial nerve involvement, and spinal or nerve root enhancement.4–9 Neurosarcoidosis also has a wide variety of clinical presentations; most commonly, it presents as cranial neuropathy, but presentation ranges from encephalopathy, meningitis, hydrocephalus, and seizure to spinal cord dysfunction, peripheral neuropathy, and myopathy.2, 3,4, 10 Only rare cases of neurosarcoidosis presenting with stroke have been described, none of which includes diffusion-weighted MR imaging (DWI).11–17 Furthermore, to our knowledge, none appears to have posed the diagnostic conundrum of focal neurologic symptoms and MR imaging findings as the sole presenting abnormality without systemic findings. We report an unusual case of isolated neurosarcoidosis presenting with infarction.
Case Report
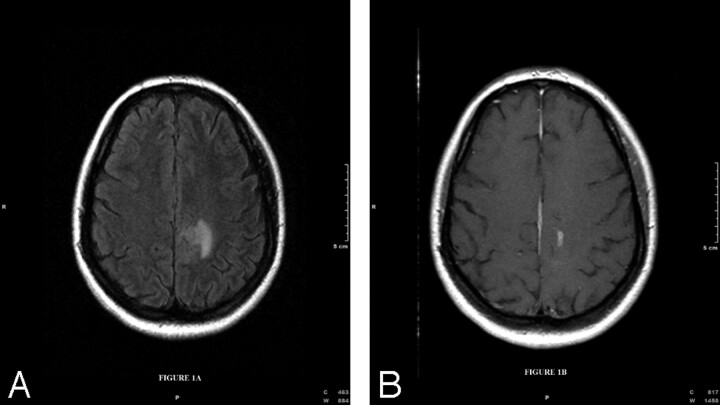
Three years prior, the patient, a 36-year-old woman, presented with blurred vision and headache, which was diagnosed as pseudotumor cerebri on lumbar puncture. MR imaging showed hyperintensity on T2-weighted fluid-attenuated inversion recovery images in the left frontal subcortical white matter (Fig 1A) and gadolinium enhancement in the left posterior frontal subcortical white matter (Fig 1B).
Fig 1.
A, Fluid-attenuated inversion recovery axial image of the brain (TR = 9000, TE = 110, TI = 2500) demonstrates hyperintensity in the left frontal subcortical white matter. B, T1-weighted axial image after gadolinium administration (TR = 65, TE = 15) demonstrates enhancement in the left posterior frontal subcortical white matter.
Follow-up MR imaging during several months demonstrated a waxing and waning pattern of T2 hyperintensity in the white matter, raising the possibility of multiple sclerosis. Again, there were no areas of restricted diffusion and no further work-up was ordered.
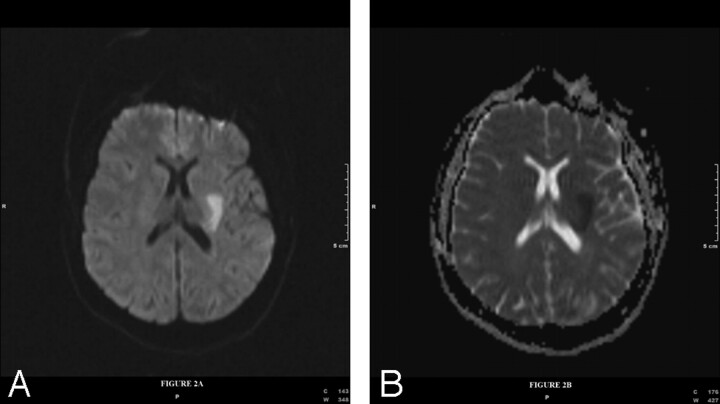
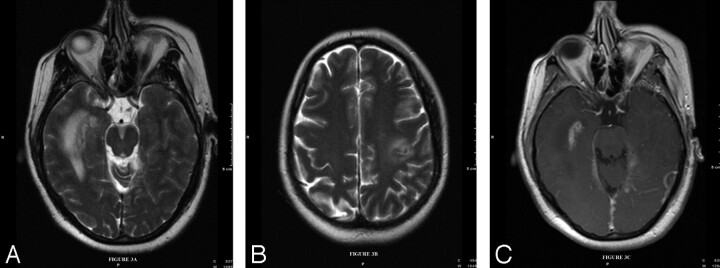
During this presentation, the patient experienced right leg paresthesias, slurred speech, right facial droop, and right upper and lower extremity weakness 2 hours after the first onset of symptoms. On neurologic examination, the patient had dysarthria, mild aphasia, and right upper and lower extremity weakness without cranial neuropathy. MR imaging of the brain with gadolinium enhancement and DWI (Fig 2A) with an apparent diffusion coefficient map (Fig 2B) showed an acute infarction. Additionally, there were also several areas of focal T2 hyperintensity (Fig 3A, -B). There was also extensive dural thickening and enhancement and nodular pial enhancement (Fig 3C). MR angiography of the neck and intracranial circulation was normal.
Fig 2.
Diffusion-weighted (TR = 3900, TE = 94, B = 1000, number of gradient directions = 90) imaging (A) with corresponding apparent diffusion coefficient map (B) shows restricted diffusion in the left basal ganglia extending into the deep periventricular white matter consistent with acute infarction.
Fig 3.
Axial T2-weighted images of the brain (TR = 6000, TE = 125) show multiple areas of hyperintensity within the subcortical white matter parenchyma in the medial right temporal lobe (A) and posterior left frontal lobe (B). T1-weighted (TR = 635, TE = 17) gadolinium-enhanced sequences show nodular dural and pial enhancement (C).
The differential diagnoses of vasculitis, with or without concomitant granulomatous disease, and neoplasm, such as lymphoma or metastatic disease, were queried. A chest radiograph and CT of the chest, abdomen, and pelvis were unremarkable.
Multiple laboratory tests, including determination of antibodies for rheumatoid factor, Sjogren syndrome, human immunodeficiency virus, double-stranded DNA, and anti-nuclear antibodies, as well as cytoplasmic and perinuclear staining for anti-neutrophil cytoplasmic antibodies, were negative. Additionally, endocrine panel, protein C and S, immunoglobulins G, A, and M, and CSF and serum antiotensin-converting enzyme levels were normal. The patient did have an elevated sedimentation rate of 35 (normal = 0–20). Cerebrospinal fluid studies showed elevated protein at 77 (normal = 12–60) and a mild lymphocytic pleiocytosis. The diagnosis was uncertain and the patient went to surgery for a right temporal lobe stereotactic biopsy.
Pathology of the specimen revealed noncaseating granulomatous inflammation consistent with neurosarcoidosis. The patient had improvement of her symptoms after treatment with solumedrol and methotrexate. She was discharged to a rehabilitation facility without further event or complication.
Discussion
Neurologic affliction in sarcoidosis ranges from 1% to 27% of cases,18 but it generally occurs in 5% of cases.1 Neurosarcoidosis is a multisystem granulomatous disease with sarcoid-type granulomas and epithelial cells, with macrophages being centered in noncaseating granulomas, and with lymphocytes, plasma cells, and mast cells found in the periphery.10 In clinical practice, a diagnosis of neurosarcoidosis is made by the clinical determination of multisystem diseases in combination with histologic confirmation of tissue.18 If there is no other evidence of other system involvement, diagnosis is made via biopsy of neural tissue or positive Kveim test.2, 10, 18
Diverse clinical studies have described the presentation of neurosarcoidosis.2, 3, 10–12, 14–16, 18, 19 In a study by Zajicek et al, the most common neurologic presentation was optic nerve disease followed by involvement of other cranial nerves, spinal cord lesion, brain stem or cerebellar lesion, meningeal lesion, cognitive decline, hydrocephalus, and pituitary or hypothalamic involvement, respectively.10 Cerebral (CNS) and cranial nerve lesions were the most common neurologic manifestations in a study of 50 patients by Oksanen, who found a lower incidence of infratentorial, spinal cord, peripheral nerve, and muscular lesions.18 James and Sharma found that facial nerve palsy and papilledema were the most common signs.19 Stern et al cited 33 patients who presented with neurologic manifestations of sarcoidosis, most commonly cranial neuropathy, CNS parenchymal lesions, and aseptic meningitis.2 Generally, facial and optic nerves are most commonly involved, followed by CNS parenchymal lesions.
From a radiologic perspective, neurosarcoidosis has been described by a number of different modalities, including single-photon emission CT, CT, and MR imaging.4–9, 12, 13, 17 CT findings of neurosarcoidosis are generally nonspecific and include hydrocephalus, periventricular hypoattenuation and contrast enhancement, calcification (within granulomata), meningeal contrast enhancement, white matter lesions, and lesions at the optic nerves or chiasm.9, 10, 17, 20
MR imaging presentations of neurosarcoidosis are quite variable.4, 5, 7–10, 17 In an MR imaging study by Lexa and Grossman, patients most commonly exhibited white matter periventricular and periaqueductal hyperintensity on long TR/long TE sequences.5 Leptomeningeal enhancement and parenchymal mass with enhancement were also seen. The spectrum of presentations was wide, but it included hydrocephalus, masses, and enhancing nerve roots. There was one pontine infarct, which was not described as acute or chronic change. There was no evidence of diffusion-weighted sequences.
Christoforidis et al found that MR imaging findings usually consisted of hypointensity or mixed intensity on T2-weighted sequences and enhancement with gadolinium.4 There were no instances of stroke, and DWI was not performed. Seltzer et al demonstrated hypothalamic and pituitary thickening with enhancement as well as a case of sagittal sinus thrombosis.8 Similar findings were present in a study by Miller et al.9
Okamoto et al studied one case of neurosarcoidosis with DWI that showed a markedly hypointense, CSF-like signal intensity.6 There were no instances of restricted diffusion mentioned. Other authors have studied patients with strokelike symptoms with a variety of modalities and have found hyperintensity on T2-weighted images and gadolinium enhancement.7, 13, 17 None of these studies of strokelike episodes mentioned the use of DWI or the presence of restricted diffusion.
Our particular case is of interest for 2 reasons. The first is from a clinical perspective because the patient’s sentinel presentation was with a focal neurologic deficit without other evidence of sarcoidosis (including systemically) and imaging consistent with stroke. To our knowledge, in all other cases reported with stroke or strokelike symptoms, the patient had evidence for other stigmata of sarcoidosis, most commonly bilateral hilar enlargement on chest radiographs.11–16 The second reason our case of is interest is that we are unaware of a documented case of sarcoidosis presenting with infarction with the added characterization of infarction on DWI.
The method of infarction and ischemia in neurosarcoidosis is not completely understood but is thought to result from small-vessel vasculitis, embolus, or large-vessel inflammation.15, 21 Brown et al hypothesized “granulomatous invasion of blood vessel walls” with “ disruption of the media and internal elastic lamina” that caused infarction with rare reports of large-vessel affliction.16
In summary, to our knowledge this is the first reported case of confirmation of acute cerebral infarction with DWI in neurosarcoidosis. Additionally, the infarction was the sentinel event leading to biopsy and a diagnosis that had not been suspected previously because of the lack of concordant symptoms and absence of abnormal laboratory studies. In retrospect, the patient’s first presentation with MR imaging findings of T2 hyperintensity in the subcortical white matter should have raised the suspicion of sarcoidosis; however, it is uncertain if the diagnosis still would have been entertained with unremarkable peripheral imaging and unremarkable laboratory studies, which were seen at the most recent presentation. Ultimately, this case once again highlights the fact that sarcoidosis, especially neurosarcoidosis, has a multitude of clinical and imaging presentations. Previously, sarcoidosis has been found in a differential consideration of multiple sclerosis, meningioma, tuberculous meningitis, normal pressure hydrocephalus, and vasculitis. Albeit rarely, neurosarcoidosis should be considered in the presentation of acute stroke with restricted diffusion with unknown etiology in a young person, even if other stigmata of sarcoidosis are not present.
References
- 1.Siltzbach LE, James DG, Neville E, et al. Course and prognosis of sarcoidosis around the world. Am J Med 1974;57:847–52 [DOI] [PubMed] [Google Scholar]
- 2.Stern BJ, Krumholz A, Johns C, et al. Sarcoidosis and its neurological manifestations. Arch Neurol 1985;42:909–17 [DOI] [PubMed] [Google Scholar]
- 3.Delaney P. Neurologic manifestations in sarcoidosis. Ann Int Med 1977;87:336–45 [DOI] [PubMed] [Google Scholar]
- 4.Christoforidis GA, Spickler EM, Recio MV, et al. MR of CNS sarcoidosis: correlation of imaging features to clinical symptoms and response to treatment. AJNR Am J Neuroradiol 1999;20:655–6 [PMC free article] [PubMed] [Google Scholar]
- 5.Lexa FJ, Grossman RI. MR of sarcoidosis in the head and spine: spectrum of manifestations and radiographic response to steroid therapy. AJNR Am J Neuroradiol 1994;15:973–82 [PMC free article] [PubMed] [Google Scholar]
- 6.Okamoto K, Ito J, Ishikawa K, et al. Diffusion-weighted echo-planar MR imaging in differential diagnosis of brain tumors and tumor-like conditions. Eur Radiol 2000;10:1342–50 [DOI] [PubMed] [Google Scholar]
- 7.Larner AJ, Ball JA, Howard RS. Sarcoid tumour: continuing diagnostic problems in the MRI era. J Neurol Neurosurg Psychiatry 1999;66:510–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seltzer S, Mark AS, Atlas SW. CNS sarcoidosis: evaluation with contrast-enhanced MR imaging. AJNR Am J Neuroradiol 1991;12:1227–33 [PMC free article] [PubMed] [Google Scholar]
- 9.Miller DH, Kendell BE, Barter S, et al. Magnetic resonance imaging in central nervous system sarcoidosis. Neurology 1988;38:378–83 [DOI] [PubMed] [Google Scholar]
- 10.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis—diagnosis and management. Q J Med 1999;92:103–17 [DOI] [PubMed] [Google Scholar]
- 11.Das SK, Sinha I, Kundu TN, et al. Two cases of neurosarcoidosis presenting as peripheral neuropathy and stroke in young. J Assoc Physicians India 1998;46:479–81 [PubMed] [Google Scholar]
- 12.Michotte A, Dequenne P, Jacobovitz D, et al. Focal neurological deficit with sudden onset as the first manifestation of sarcoidosis: a case report with MRI follow-up. Eur Neurol 1991;31:376–79 [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Awata S, Matsuoka H, et al. Chronological changes in brain MRI, SPECT, and EEG in neurosarcoidosis with stroke-like episodes. Psychiatry Clin Neurosci 1998;52:629–33 [DOI] [PubMed] [Google Scholar]
- 14.Duffey P, Bates D. Transient focal neurological deficits in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1997;14:171–72 [PubMed] [Google Scholar]
- 15.Corse AM, Stern BJ. Neurosarcoidosis and stroke. Stroke 1990;21:152–53 [PubMed] [Google Scholar]
- 16.Brown MM, Thompson AJ, Wedzicha JA, et al. Sarcoidosis presenting with stroke. Stroke 1989;20:400–05 [DOI] [PubMed] [Google Scholar]
- 17.Ketonen L, Oksanen V, Kuuliala I. Preliminary experience of magnetic resonance imaging in neurosarcoidosis. Neuroradiology 1987;29:127–29 [DOI] [PubMed] [Google Scholar]
- 18.Oksanen V. Neurosarcoidosis: clinical presentations and course in 50 patients. Acta Neurol Scand 1986;73:283–90 [DOI] [PubMed] [Google Scholar]
- 19.James DG, Sharma OP. Neurological complications of sarcoidosis. Proc R Soc Med 1967;60:1169–706060718 [Google Scholar]
- 20.Morehouse H, Danziger A. CT findings in intracranial neurosarcoid. Comput Tomogr 1980;4:267–70 [DOI] [PubMed] [Google Scholar]
- 21.Younger D, Hays A, Brust J, et al. Granulomatous angiitis of the brain. Arch Neurol 1988;45:514–1 [DOI] [PubMed] [Google Scholar]